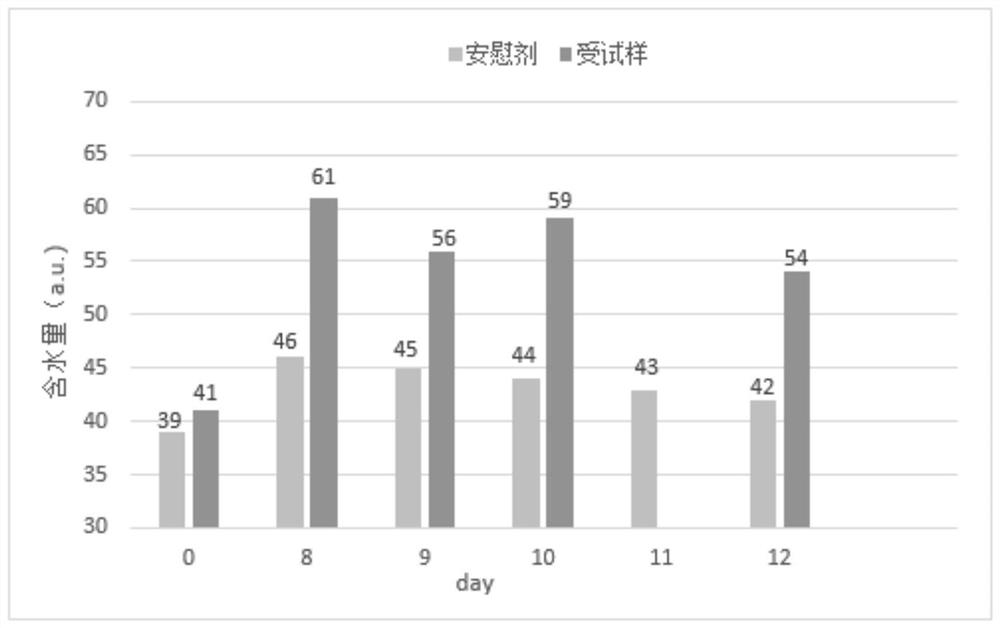
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
43 results about "Sodium chondroitin sulfate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sodium Chondroitin Sulfate is used for Arthritic, Knee osteoarthritis, Osteoarthritis, Joint pain caused by drugs used to treat breast cancer, Acid reflux, High cholesterol and other conditions. This salt may also be used for purposes not listed in this medication guide.
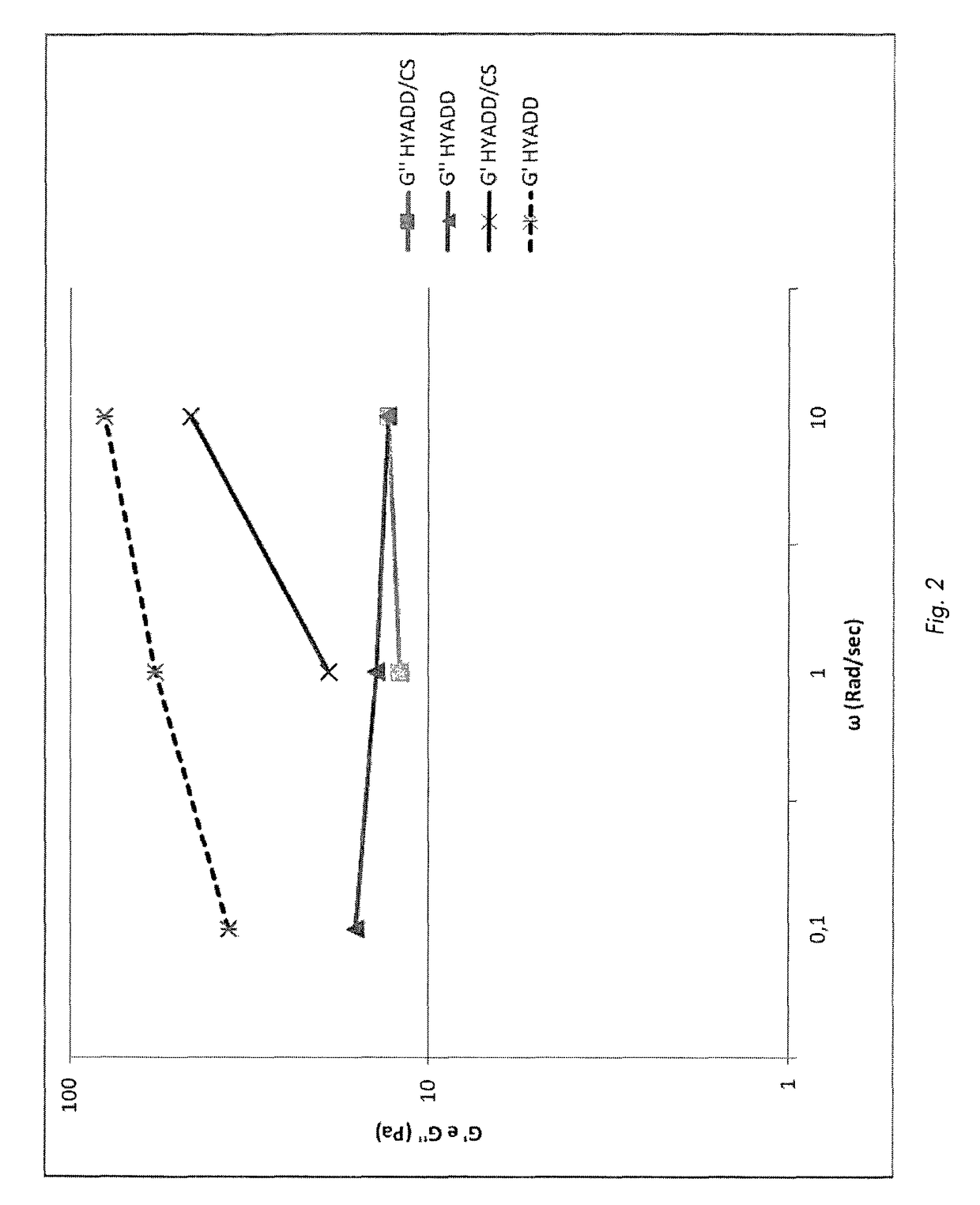
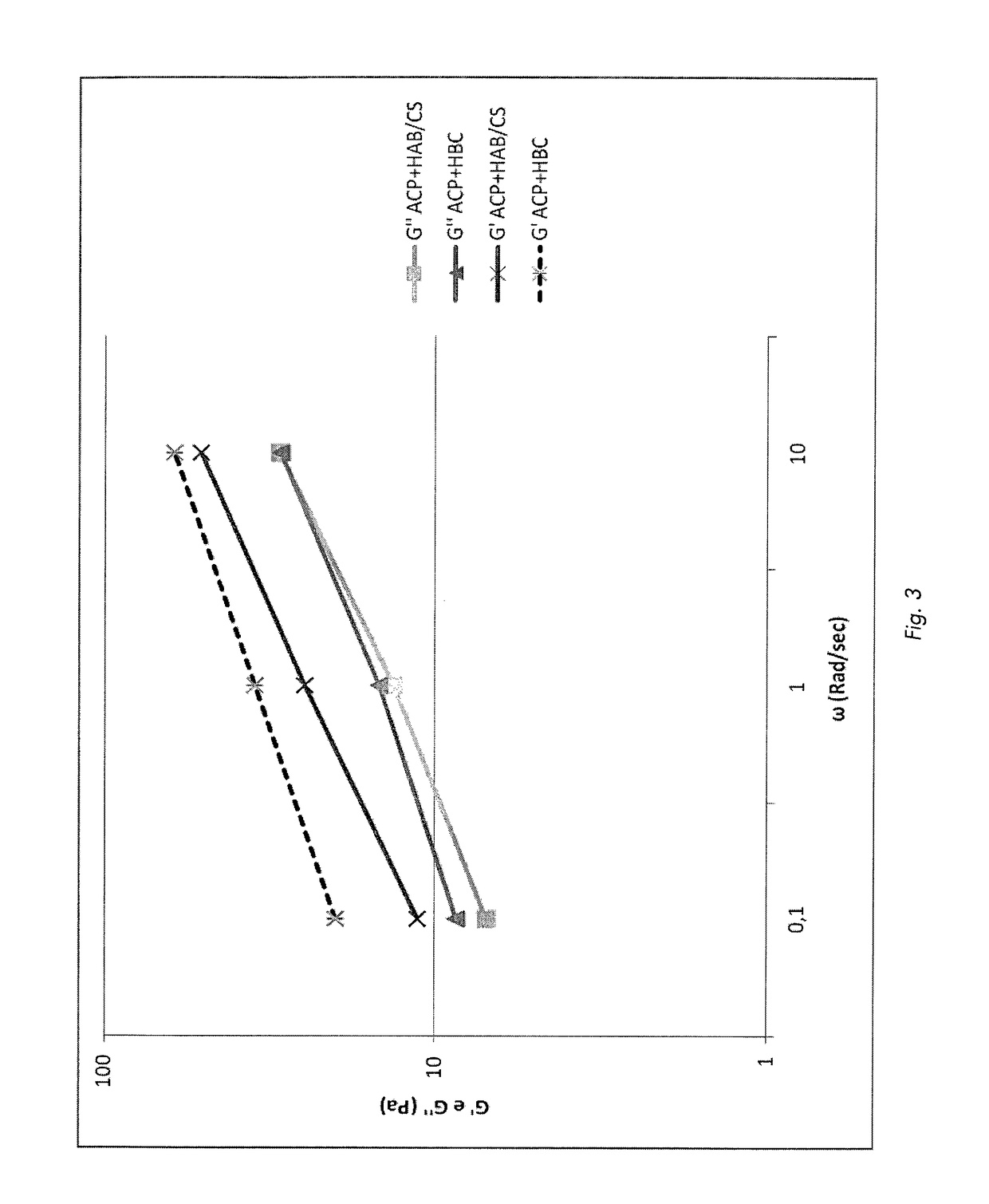
Preparation method of hyaluronic acid/gelatin/chondroitin sulfate bone repair bionic scaffold
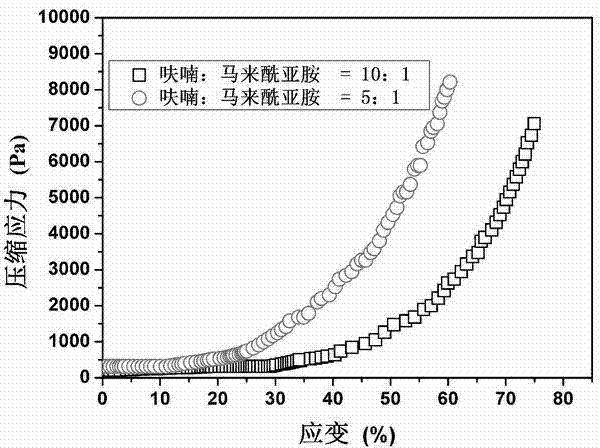
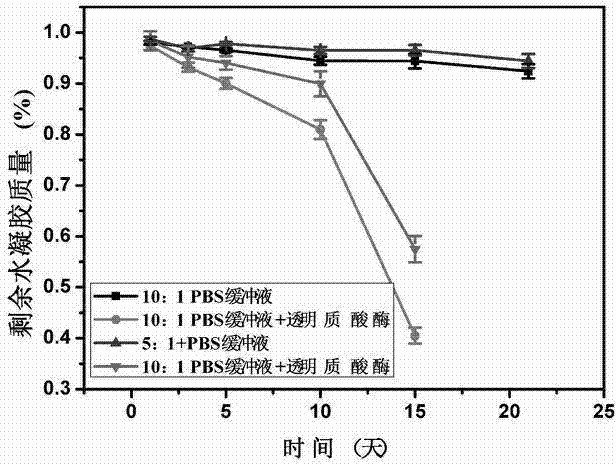
The invention discloses a preparation method of hyaluronic acid / gelatin / chondroitin sulfate bone repair bionic scaffold. The method comprises the steps of adding activator and furfurylamine into MES buffer solution of hyaluronic acid to obtain modified hyaluronic acid solid; adding EDC / NHS (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide / N-hydroxysuccinimide) into gelatin aqueous solution, and adding furancarboxylic acid to obtain modified gelatin solid; dissolving the above two solids into MES buffer solution to obtain a mixed solution; dissolving MAL-PEG-MAL (maleimide-polyethylene glycol-maleimide) into MES buffer solution, adding into the mixed solution, and reacting in 37 DEG C water bath to form transparent cross-linked hydrogel; soaking the hydrogel in MES buffer solution of sodium chondroitin sulfate, adding EDC / NHS, and reacting under stirring to obtain the cartilage repair bionic scaffold. The bionic scaffold has the advantages of interpenetrating network structure, excellent biocompatibility and bioactivity, better compression strength, anti-washout property and degradability, simple preparation process, and easy operation.
Owner:SOUTH CHINA UNIV OF TECH
Method for extracting calcium chondroitin sulfate in shark cartilage
A method for extracting shark calcium chondroitin sulfate from a shark cartilage comprises the processes in sequence such as curing, alkalization, enzymatic hydrolysis, deproteinization, crystallization, resin pretreatment, resin exchange, ultrafiltration dehydration, calcification of filtrate and crystallization dehydration dying. Because secondary crystallization is adopted, the covalent binding of protein and shark chondroitin sulfate is broken by hydrogen peroxide to enable the chondroitin sulfate in the protein to be dissociated, impurities such as the protein and the like can be effectively removed to improve the purity and the content of shark sodium chondroitin sulfate; cation resin is adopted to complete the sodium removal process by column chromatography, the exchanged shark chondroitin sulfate is high in concentration and the shark chondroitin sulfate which is produced during ion exchange can be accurately controlled, and the sodium content in the product is low; with dehydration of ultrafiltration, not only the concentration can be realized, but also the small molecular impurities can be removed to improve the purity of the shark chondroitin sulfate, at the same time, to improve the content of calcium. The method also can save the using amount of ethanol and reduce the production cost.
Owner:王清荣
Pharmaceutical composition for curing osteoarthritis
The invention relates to a pharmaceutical composition for the treatment of osteoarthritis, the composition of the invention consists of 5 to 10 weight parts of glucosamine hydrochloride, 5 to 10 weight parts of sodium chondroitin sulfate and 1 to 4 weight parts of sodium hyaluronate, and the needed oral formulation can be prepared by adopting the conventional preparation technology along with acceptable excipients in pharmacy. The pharmaceutical composition of the invention has the functions of prevention and treatment of osteoarthritis, so the invention can be used in the preparation of the drugs for the prevention and treatment of osteoarthritis and can also be used as the health care food which has the function of prevention of osteoarthritis.
Owner:BEIJING SUNHO PHARMA
Extraction of sodium chondroitin sulfate through combination of alkaline hydrolysis-enzymolysis method and flocculation precipitation method
The present invention relates to a sodium chondroitin sulfate extraction method, wherein sodium chondroitin sulfate is a mucopolysaccharide substance extracted from swine nasal bone and other animal cartilages. The traditional process method adopts alkaline hydrolysis, enzymolysis, salt hydrolysis, an ion exchange resin or a combination method thereof to extract, and the extraction solution contains a large amount of proteins, nucleic acids and other impurities so as to cause a certain difficult problem to further purification. According to the invention, the alkaline hydrolysis-enzymolysis method and the flocculation precipitation method are combined to remove impurities remained in the sodium chondroitin sulfate extraction solution, such that the process is reasonable, the removing efficiency is high, the subsequent refinement working hour is shortened, the product yield is improved, and the drug meets standards of the Chinese pharmacopoeia (2000 edition).
Owner:QINGDAO JIULONG BIO PHARMA
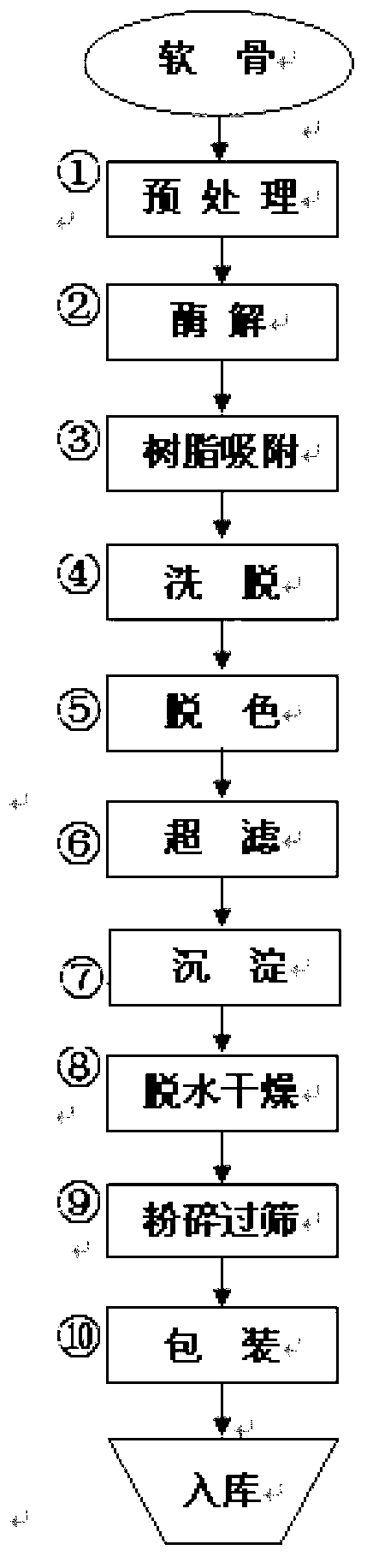
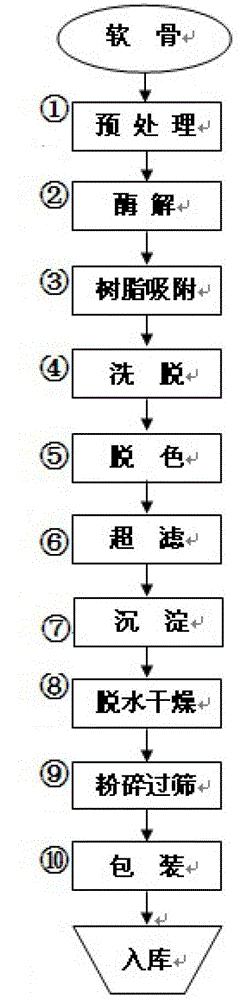
Preparation method of sodium chondroitin sulfate
The invention discloses a preparation method of sodium chondroitin sulfate, and the preparation method comprises the following steps of: (1) carrying out enzymolysis; (2) carrying out resin absorption; (3) eluting; (4) decoloring; (5) carrying out ultrafiltration; (6) depositing; and (7) dewatering and drying. The sodium chondroitin sulfate obtained by adopting the preparation method disclosed by the invention can be used for enhancing the yield of a product dry substance by 2% and increasing the content of the sodium chondroitin sulfate by 10%, thereby enhancing the product additional value, reducing the production cost and enhancing the economic benefit.
Owner:CHONGQING AOLI BIOPHARM
High purity low molecular weight sodium chondroitin sulfate preparation technology
The present invention discloses a high purity low molecular weight sodium chondroitin sulfate preparation technology, and the low molecular weight sodium chondroitin sulfate is prepared by preparation of a crude low molecular weight sodium chondroitin sulfate product and purifying of the crude low molecular weight sodium chondroitin sulfate product under certain conditions. Compared with the prior art, the preparation technology is simple in process, free of waste discharge and environmentally-friendly in process conditions; sources of raw materials are rich, product yield is high, product purity is high, quality is stable, cost is low, and the product is free of toxic and side effects, and can be further prepared into injections, capsules and the like.
Owner:QINGDAO JIULONG BIO PHARMA
Long-term preservation liquid for corneal tissue and preparation method thereof
InactiveCN109221093AFacilitate long-term maintenance of structureFacilitates long-term transparencyDead animal preservationFiberSodium bicarbonate
The invention discloses long-term preservation liquid for corneal tissue and a preparation method thereof. The long-term preservation liquid is prepared from the following components: 15 to 25g / L of sodium chondroitin sulfate, 0.5 to 4g / L of sodium hyaluronate, 0.5 to 3 g / L of dextran 40, 15 to 30 g / L of glycerin, 0.001 to 0.3 g / L of sodium pyruvate, 0.002 to 2 g / L of vitamin C, 0.05 to 0.7 g / L ofgentamicin sulfate, 1.4 to 2.2 g / L of sodium bicarbonate, 5.0 to 6.5 g / L of 4-hydroxyethylpiperazine ethanesulfonic acid, and 1 L of water for injection. A combination system of sodium chondroitin sulfate, sodium hyaluronate and dextran is adopted to obtain the preservation liquid which can be used for long-term preservation of corneal tissue, thereby being particularly advantageous for maintaining an original collagen fiber structure and transparency of a corneal lens for a long term; after the cornea tissue is preserved by the long-term preservation liquid, the cornea tissue can be used directly after rewarming, the operation is simple, and rehydration is not needed.
Owner:镇江雷音再生医学科技有限公司
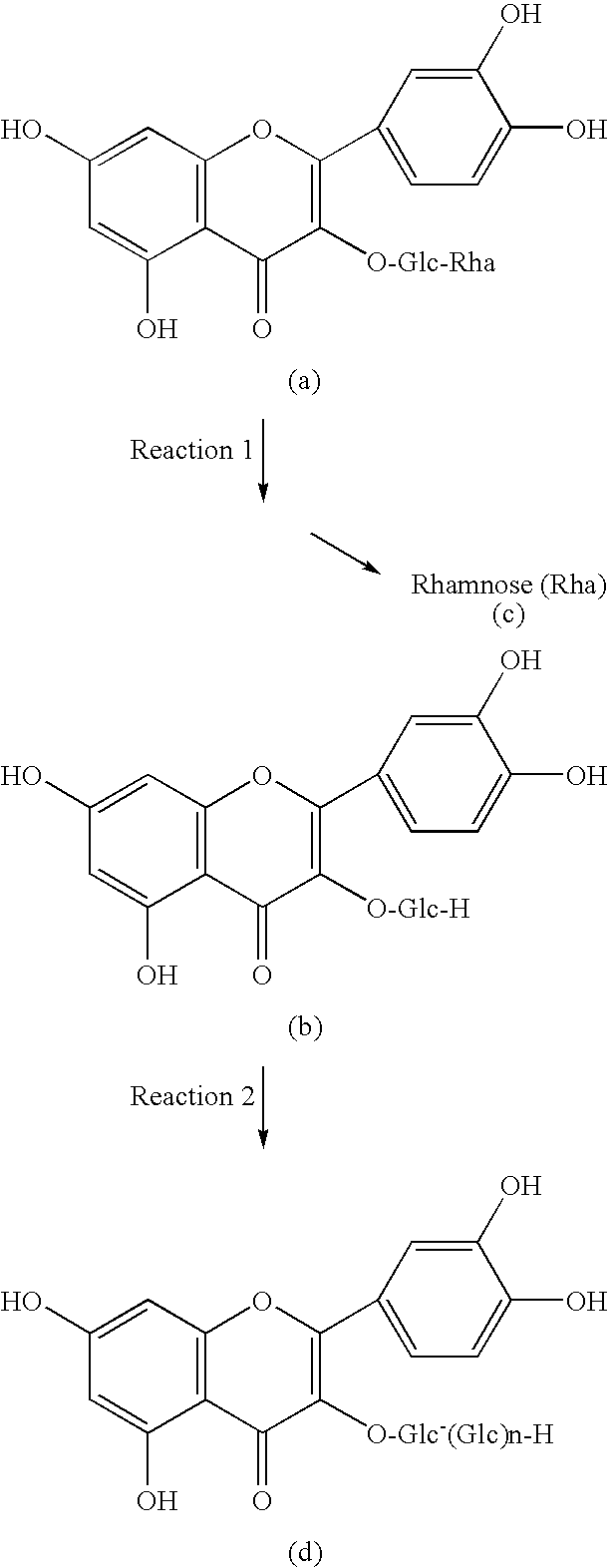
Method for manufacturing alpha-glycosylisoquercitrin, intermediate product and by-product thereof
ActiveUS20080187622A1Prevent oxidationOrganic active ingredientsDough treatmentSODIUM METAPHOSPHATECarrageenan
The present invention provides a method for producing isoquercitrin, α-glycosylisoquercitrin, and rhamnose, the method comprising a step of naringin-degrading enzyme treatment during the isoquercitrin production from rutin in the presence of an edible component, such as gelatin, wheat gluten, chitosan, lecithin, a glycerol fatty acid ester, xanthan gum, carrageenan, sodium chondroitin sulfate, casein, enzymatically decomposed gelatin, sodium alginate, konjac extract, gellan gum, guar gum, soybean protein, agar, pectin, yeast extract, egg-white peptide, cluster dextrin, gum arabic, arginine, sodium metaphosphate, karaya gum, locust bean gum, sodium pyrophosphate, glucosamine, chitin, sodium glutamate, dextrin, trehalose, or a grain-based food ingredients. According to the present invention, isoquercitrin and α-glycosylisoquercitrin, which are of use as antioxidants, anti-fading agents, flavor change inhibitors, etc., can be produced in enhanced yields.
Owner:SAN EI GEN F F I
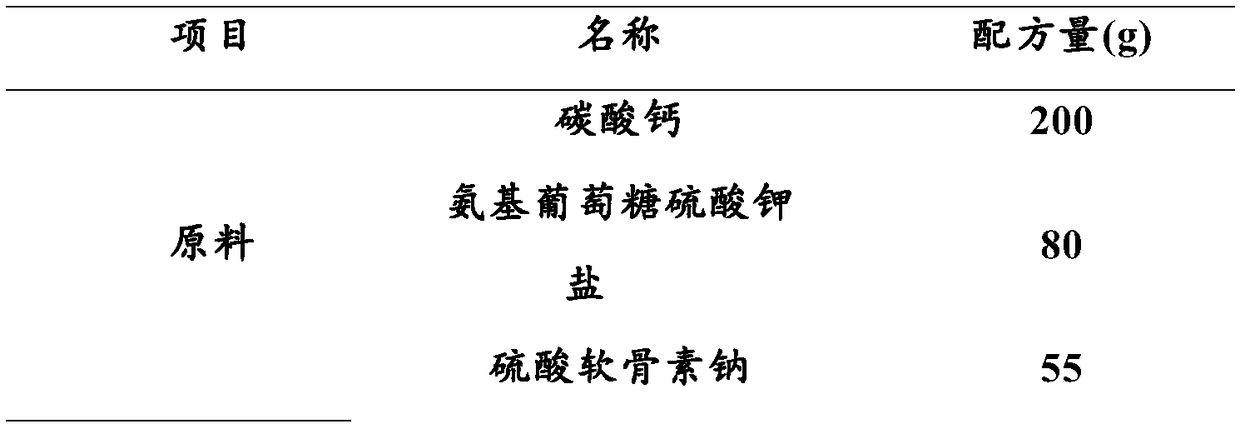
Composition capable of increasing bone density as well as preparation method and applications of composition
PendingCN109276710AImprove absorption rateIncrease bone densityOrganic active ingredientsPeptide/protein ingredientsGlucosamine / PotassiumOfficinalis
The invention discloses a composition capable of increasing bone density as well as a preparation method and applications of the composition. The composition capable of increasing the bone density provided by the invention is prepared from calcium carbonate, N-sulfo-glucosamine potassium salt, sodium chondroitin sulfate, collagen, vitamin D3, radix morindae officinalis and rhizoma curcumae longae.The composition provided by the invention is prepared according to the modern science theory in combination with the traditional Chinese medical theory prescription, and the bone density increase test and toxicity test prove that the composition is high in calcium absorption rate, can obviously increase the bone weight, bone density and bone calcium content, and has no bad influences on the growth and development.
Owner:INST OF MEDICINAL PLANT DEV CHINESE ACADEMY OF MEDICAL SCI HAINAN BRANCH
Composition for local anesthesia
InactiveUS20060189572A1Excellent durability of actionDurability of actionBiocidePharmaceutical delivery mechanismCatecholamineBULK ACTIVE INGREDIENT
A composition for local anesthesia which comprises a local anesthetic as an active ingredient and an agent for maintaining anesthetic action selected from the group consisting of acidic mucopolysaccharides such as sodium chondroitin sulfate and cellulose derivatives such as hydroxypropylmethylcellulose, and does not contain catecholamines, which has durability of anesthetic action suitable for minor dental operations such as tooth extraction, and can be used as a safe composition for local anesthesia used for oral surgery or dental treatment.
Owner:SHOWA YAKUHIN KAKO
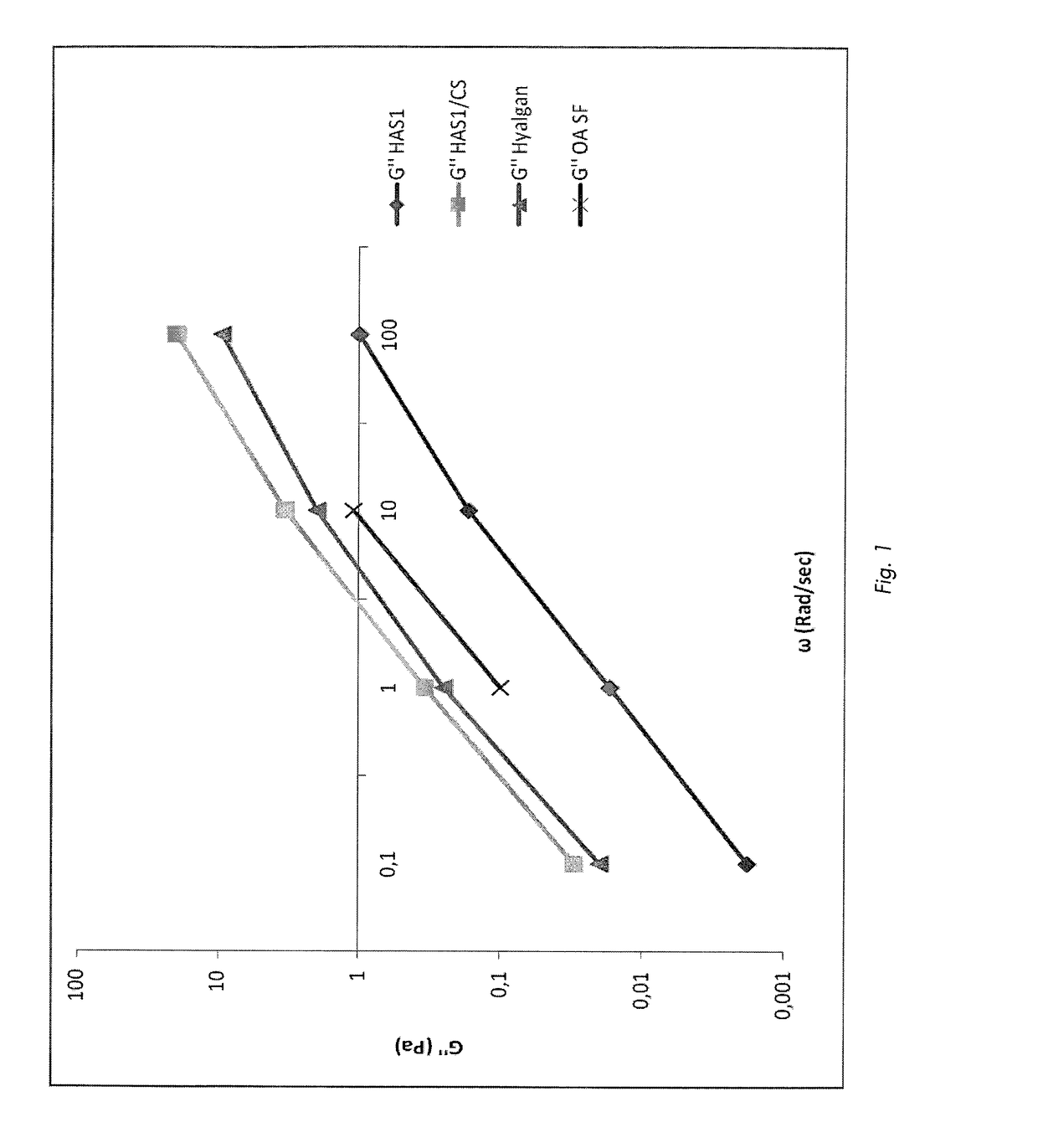
Pharmaceutical formulations comprising chondroitin sulfate and hyaluronic acid derivatives
ActiveUS20150072954A1Overcomes drawbackOrganic active ingredientsBiocideInterstitial cystitisTendinosis
The present invention relates to pharmaceutical formulations containing a combination of specific high-molecular weight hyaluronic acid derivatives and chondroitin sulfate to be used in the treatment of osteoarthritis, of subchondral damage, osteoporosis, synovitis, tenosynovitis, tendinitis, tendinosis, as an intra-articular washing liquid and as a viscous substitute of synovial fluid following osteochondral surgery. These formulations are also suitable for the treatment of interstitial cystitis.
Owner:FIDIA FARM SPA
Metal coordination supramolecular hydrogel and preparation method thereof
The invention belongs to the field of high molecular materials and in particular relates to metal coordination supramolecular hydrogel and a preparation method thereof. The preparation method providedby the invention comprises the following step: a) crosslinking sodium chondroitin sulfate and iron salt in water to obtain the metal coordination supramolecular hydrogel. The sodium chondroitin sulfate and Fe<3+> are used as raw materials and are cross-linked in the water to form the supramolecular hydrogel. The method provided by the invention is simple to operate and rapid in gel formation speed; the raw materials are green, environmentally friendly and cheap; an organic solvent and expensive instruments and equipment are not needed in a preparation process; the prepared supramolecular hydrogel has very good adhesion and can be injected; the supramolecular hydrogel can be self-healed at room temperature, without the need of a healing promoting agent or stimulation of other external conditions; furthermore, the Fe<3+> has a certain bleeding stopping function, so that the supramolecular hydrogel prepared by the preparation method can be clinically applied to a wound closure device, ahemostatic agent and a tissue sealing agent to control blood loss and promote tissue healing; therefore, the supramolecular hydrogel has a very great application prospect in the aspect of self healingof wounds.
Owner:GUANGDONG UNIV OF TECH
Cream preparation for preventing and treating joint diseases and preparation method thereof
ActiveCN105193837AAvoid first pass effectAvoid stimulationOrganic active ingredientsAerosol deliveryDiseaseTreatment effect
The invention relates to a cream preparation for preventing and treating joint diseases and a preparation method thereof. The cream preparation comprises active ingredients and auxiliary ingredients allowed in pharmacy; the active ingredients comprise, by weight, 3.0%-4.0% of glucosamine, 2.5%-4.0% of sodium chondroitin sulfate and 1.0%-2.5% of hyaluronic acid. The cream preparation not only can effectively prevent the joint diseases from occurring but also has the very good treatment effect on the joint diseases; the stable blood concentration can be better kept, the bioavailability is high, the administration times are decreased, and the administration dosage is accurate; in addition, the cream preparation is safer, has drug targeting, can be released to local lesion tissue at a constant speed and is significant in treatment effect, and administration can be temporarily stopped as needed if a reverse reaction occurs in the administration process.
Owner:ZHEJIANG CANDORLY PHARMA
Artificial lens implantation flexible hydrosol auxiliary agent and preparation method thereof
The invention discloses an artificial lens implantation flexible hydrosol auxiliary agent, which comprises the following components in percentage by mass: 0 to 5.0 percent of disodium hydrogen citrate, 0 to 2.5 percent of monosodium hydrogen citrate, 1.0 to 20 percent of carboxyl-propyl methylcellulose, 0.05 to 5.0 percent of sodium chondroitin sulfate, 0.1 to 10 percent of potassium chloride, 0.05 to 10 percent of magnesium chloride, 0.1 to 2.5 percent of sodium acetate, 0.1 to 1.0 percent of sodium chloride, and 44 to 99 percent of sterile double-distilled water. The artificial lens implantation flexible hydrosol auxiliary agent has excellent flow properties, can effectively reduce organism exclusion and prolong the service life of a lens, and particularly can better slow down postoperative lens turbidity, so that the artificial lens implantation flexible hydrosol auxiliary agent is expected to fundamentally reduce pains of patients and save expensive cost caused by repeated operations, and has a great expectancy of social benefits and economic benefits.
Owner:高家旗
Composition for oral cavity and food product, or beverage
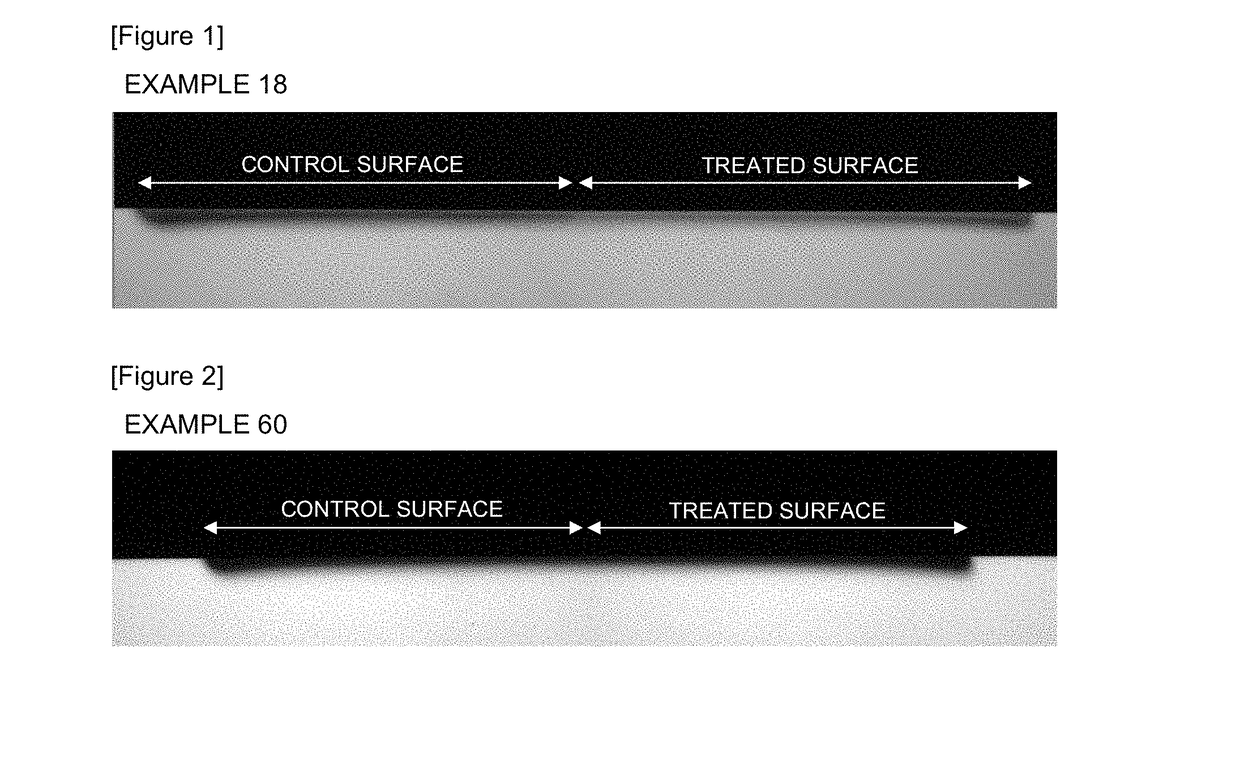
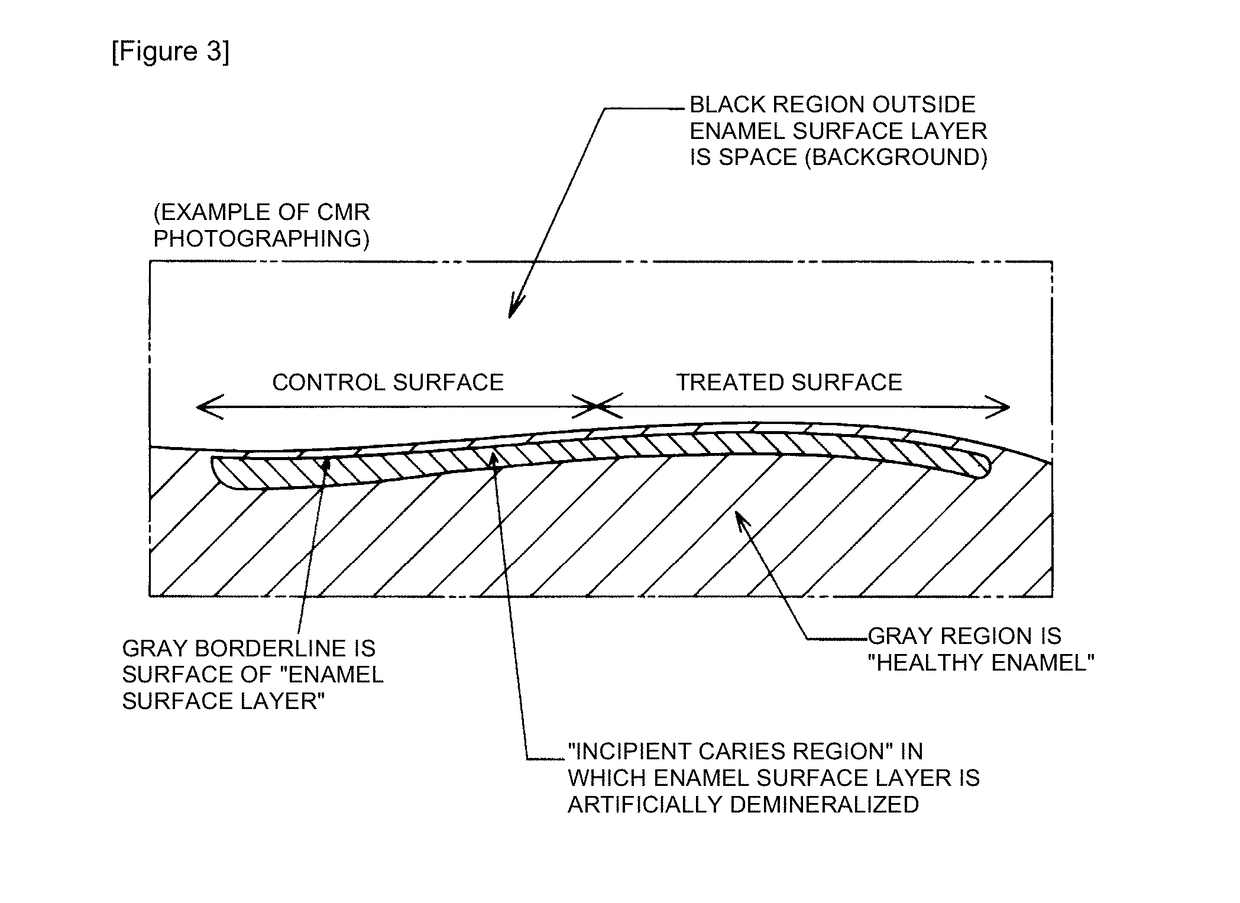
InactiveCN107613956AEnhanced remineralizationGood remineralizationCosmetic preparationsToilet preparationsDental enamelCalcium biphosphate
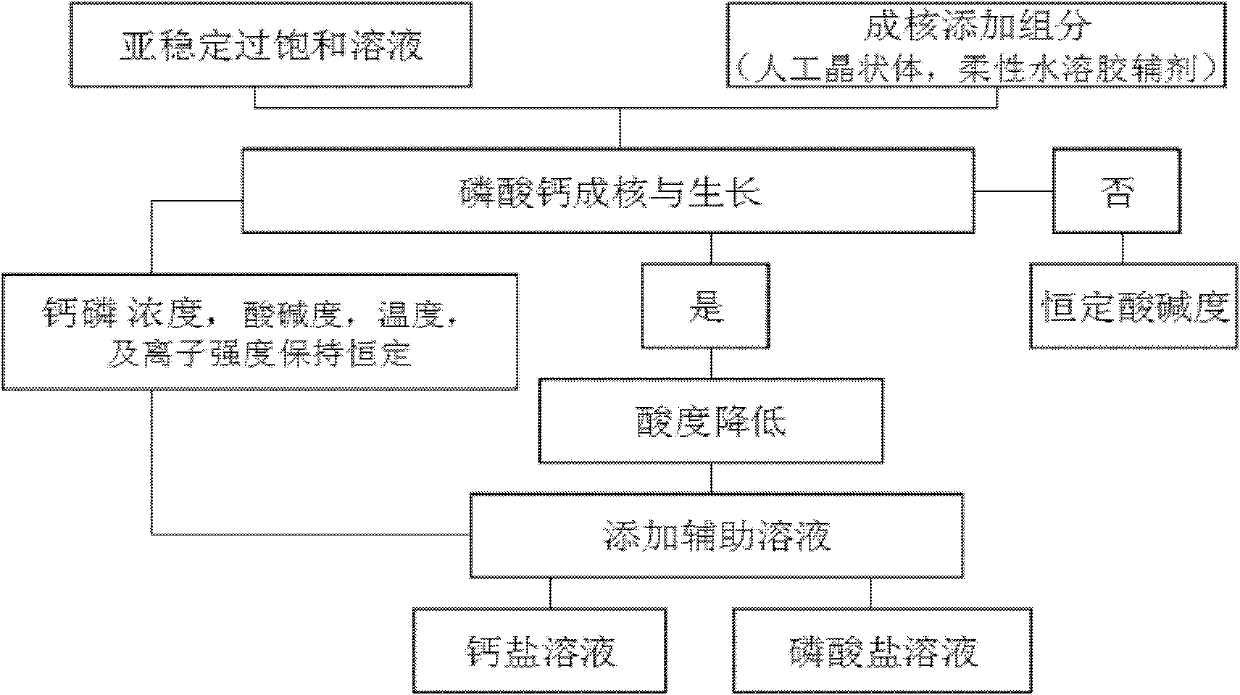
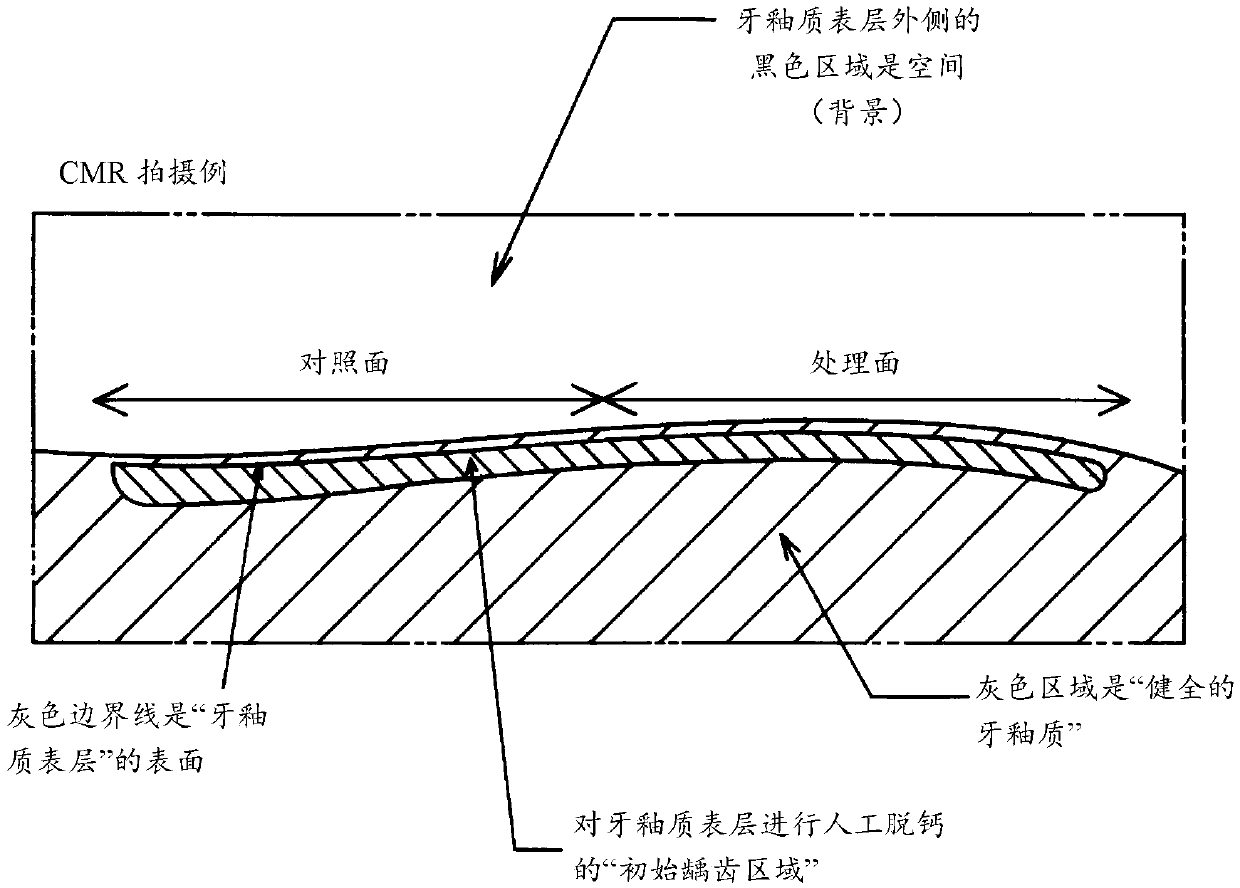
The present invention addresses the problem of providing a composition for the oral cavity, a food product or beverage, and a recalcification agent that have an excellent recalcifying effect on decalcified dental enamel. Provided are a composition for the oral cavity and a food product or beverage which are characterized in comprising sodium chondroitin sulfate and calcium phosphate. Provided is adental enamel recalcification agent which is characterized in comprising sodium chondroitin sulfate and calcium phosphate as active ingredients.
Owner:SANGI CO LTD
Pharmaceutical formulations comprising chondroitin sulfate and hyaluronic acid derivatives
ActiveUS9655918B2Overcomes drawbackOrganic active ingredientsInorganic non-active ingredientsInterstitial cystitisTendinosis
The present invention relates to pharmaceutical formulations containing a combination of specific high-molecular weight hyaluronic acid derivatives and chondroitin sulfate to be used in the treatment of osteoarthritis, of subchondral damage, osteoporosis, synovitis, tenosynovitis, tendinitis, tendinosis, as an intra-articular washing liquid and as a viscous substitute of synovial fluid following osteochondral surgery. These formulations are also suitable for the treatment of interstitial cystitis.
Owner:FIDIA FARM SPA
Extraction of Sodium Chondroitin Sulfate by Alkaline Hydrolysis-Enzyme Hydrolysis Combined with Flocculation and Precipitation
ActiveCN103601815BEfficient separationImprove the effect of enzymatic hydrolysisFlocculationDecomposition
The present invention relates to a sodium chondroitin sulfate extraction method, wherein sodium chondroitin sulfate is a mucopolysaccharide substance extracted from swine nasal bone and other animal cartilages. The traditional process method adopts alkaline hydrolysis, enzymolysis, salt hydrolysis, an ion exchange resin or a combination method thereof to extract, and the extraction solution contains a large amount of proteins, nucleic acids and other impurities so as to cause a certain difficult problem to further purification. According to the invention, the alkaline hydrolysis-enzymolysis method and the flocculation precipitation method are combined to remove impurities remained in the sodium chondroitin sulfate extraction solution, such that the process is reasonable, the removing efficiency is high, the subsequent refinement working hour is shortened, the product yield is improved, and the drug meets standards of the Chinese pharmacopoeia (2000 edition).
Owner:QINGDAO JIULONG BIO PHARMA
A method of purifying sodium chondroitin sulfate
The invention relates to a method of purifying sodium chondroitin sulfate. The sodium chondroitin sulfate is a mucopolysaccharide compound prepared by extracting pig nasal bone and other animal cartilage. A product of the sodium chondroitin sulfate, the content of which is higher than 95%, is prepared by subjecting the animal cartilage to alkaline hydrolysis-enzymolysis extraction, adding a reductant, reducing to obtain a decomposition solution, and performing two times of ethanol precipitation. The method is simple in process, mild in conditions and safe in production, and the cost of the method is lower than costs of adsorption, column chromatography and other methods. The method is suitable for large-scale industrial production.
Owner:QINGDAO JIULONG BIO PHARMA
The preparation method of chondroitin sulfate sodium
The invention discloses a preparation method of sodium chondroitin sulfate, and the preparation method comprises the following steps of: (1) carrying out enzymolysis; (2) carrying out resin absorption; (3) eluting; (4) decoloring; (5) carrying out ultrafiltration; (6) depositing; and (7) dewatering and drying. The sodium chondroitin sulfate obtained by adopting the preparation method disclosed by the invention can be used for enhancing the yield of a product dry substance by 2% and increasing the content of the sodium chondroitin sulfate by 10%, thereby enhancing the product additional value, reducing the production cost and enhancing the economic benefit.
Owner:CHONGQING AOLI BIOPHARM
Biological agent for preventing and relieving joint sub-health and preparation method thereof
InactiveCN113332405AIncrease painRelieve painHydroxy compound active ingredientsAntipyreticSodium hyaluronateProteoglycan
The invention discloses a biological agent for preventing and relieving joint sub-health and a preparation method thereof, and belongs to the technical field of biological agents. The biological agent of the present invention contains a plurality of bioactive components and a plurality of active ingredients of Chinese herbs. The bioactive components comprise S-adenosylmethionine, sodium hyaluronate, sodium chondroitin sulfate, vitrin and acetyl glucosamine, can prevent decomposition of proteoglycan by enzyme, provide exogenous hyaluronic acid for human joints, activate synthesis of mucopolysaccharide in the body, and promote synthesis of hyaluronic acid and collagen and cartilage regeneration. The bioactive components can improve content of hyaluronic acid in the body, reduce cartilage loss and maintain morphology of cartilage and synovial fluid. The active ingredients of Chinese herbs comprise fructus liquidambaris extract, herba lycopodii extract, fructus morinda citrifolia and ginger root extract, and have the effects of relaxing tendons and activating collaterals, promoting blood circulation to remove blood stasis, improving local microcirculation and relieving pain. According to the biological agent, Chinese medicines and western medicines are combined to fundamentally prevent and relieve joint inflammation.
Owner:东莞市容大生物科技有限公司
Shii-take soy sauce and preparation method thereof
InactiveCN106473085AIncreased ethyl acetate contentFood preservationNatural extract food ingredientsSmoked PlumEthyl acetate
The present invention provides shii-take soy sauce. The shii-take soy sauce is prepared from the following raw materials and an additive in parts by weight: 200-300 parts of soy sauce, 18-30 parts of shii-take, 20-40 parts of radix astragali, 18-25 parts of radix salviae miltiorrhizae, 10-20 parts of smoked plums, 12-20 parts of licorice and 6-15 parts of an additive. The additive comprises the following components in parts by weight: 1-4 parts of 2-ethyl-3,5-dimethylpyrazine, 2-4 parts of sodium alginate and 3-7 parts of sodium chondroitin sulfate. After placing, the shii-take soy sauce is relatively high in amino acids and ethyl acetate.
Owner:滁州先奇工业科技有限公司
Whole eyeball cornea preservation method
InactiveCN110384088ALong storage timeKeep aliveDead animal preservationChondroitin sulfate - sodium hyaluronateCorneal endothelial cell
The invention discloses a whole eyeball cornea preservation method. The method utilizes a sodium chondroitin sulfate-hyaluronate viscoelastic agent for carrying out eyeball preservation to prolong cornea preservation time, and is an improved method for preserving a cornea in a moist chamber. Specifically the cornea preservation method comprises the following steps: eyeball treatment: taking down an eyeball within 2 h after a donor dies, performing disinfection treatment as quickly as possible and then evacuating aqueous humor at the same time, and injecting a chondroitin sulfate-sodium hyaluronate solution into anterior chamber, wherein the chondroitin sulfate-sodium hyaluronate solution is a colorless transparent gelatinous solution consisting of 3% sodium hyaluronate, 4% sodium chondroitin sulfate and a physiological buffer balance salt; and placing the whole eyeball after treatment in a sterile tank, and placing the sterile tank in a 4 DEG C moist chamber environment for preservation. A cornea preservation time can be extended to 4 d by using the method, activity of corneal endothelial cells is not significantly decreased, and a moist chamber preservation time is greatly increased.
Owner:李冰
Composition for oral cavity and food product, or beverage
InactiveUS20180147131A1Improve actionEasy to moveCosmetic preparationsToilet preparationsCalcium biphosphateAdditive ingredient
It is an object to provide an oral composition, a food, or a beverage and a remineralizing agent having excellent action of remineralizing demineralized tooth enamel. The present invention is an oral composition, a food, or a beverage comprising sodium chondroitin sulfate and a calcium phosphate. The present invention is a tooth remineralizing agent comprising sodium chondroitin sulfate and a calcium phosphate as active ingredients.
Owner:SANGI CO LTD
Freeze-dried powder preparation for preventing and improving fundus macular degeneration and preparation method thereof
The invention belongs to the technical field of eye care products, and particularly relates to a freeze-dried powder preparation for preventing and improving fundus macular degeneration. The freeze-dried powder preparation is composed of freeze-dried powder and solvent liquid, wherein the freeze-dried powder comprises sodium hyaluronate, xanthophyll, riboflavin, pyridoxine, cyanocobalamin, lactoferrin, oligopeptide-1, oligopeptide-5, mannitol, sodium chondroitin sulfate, hydrogenated lecithin and deionized water, and the solvent liquid comprises taurine, bataine, dipotassium glycyrrhizinate, polysorbate-80, sodium chloride, lysozyme and deionized water. The invention further provides a preparation method of the freeze-dried powder preparation. The freeze-dried powder preparation prepared by using the preparation method is used for carrying out daily nutrition maintenance and cell injury repair on all organ functions of eyes, is safe and non-irritant, and has obvious effects on fundus macular conditions, such as visual deformation, macular disorder, middle-aged and elderly melasma acuminatum and senile macular deformation.
Owner:上海旷世医疗管理有限公司
Alkali-enzymolysis method for extracting sodium chondroitin sulfate
InactiveCN105461827AOptimize production process conditionsShorten the production cycleOral medicationAlkaline hydrolysis
The invention relates to a novel extraction method of sodium chondroitin sulfate, particularly sodium chondroitin sulfate extracted by alkali-enzymolysis. The method mainly solves the problems of complex process, high alkali / acid or salt consumption, higher production cost, long extraction period, low product quality stability, lower yield and the like in the current like product production technique. The method mainly comprises the following steps: by using animal cartilage as a raw material, carrying out alkaline hydrolysis and one-step enzymolysis, thereby extracting the sodium chondroitin sulfate from the animal cartilage. The product has the advantages of white color, loose powder, high yield (up to 30% or above) and high purity, and conforms to the quality standard for oral administration. The method has the characteristics of simple production procedure, short period, low cost and the like, is simple to operate and easy to implement, is helpful to relieving the environmental pollution, and is beneficial to environment friendliness.
Owner:QINGDAO JIULONG BIO PHARMA
Compound racanisodamine eye drops
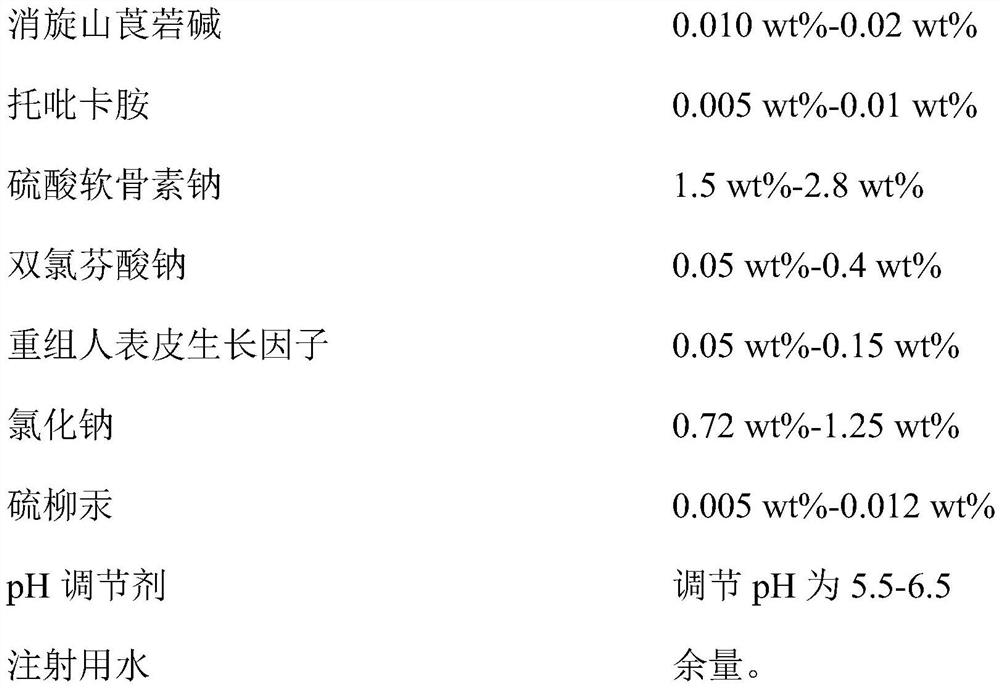
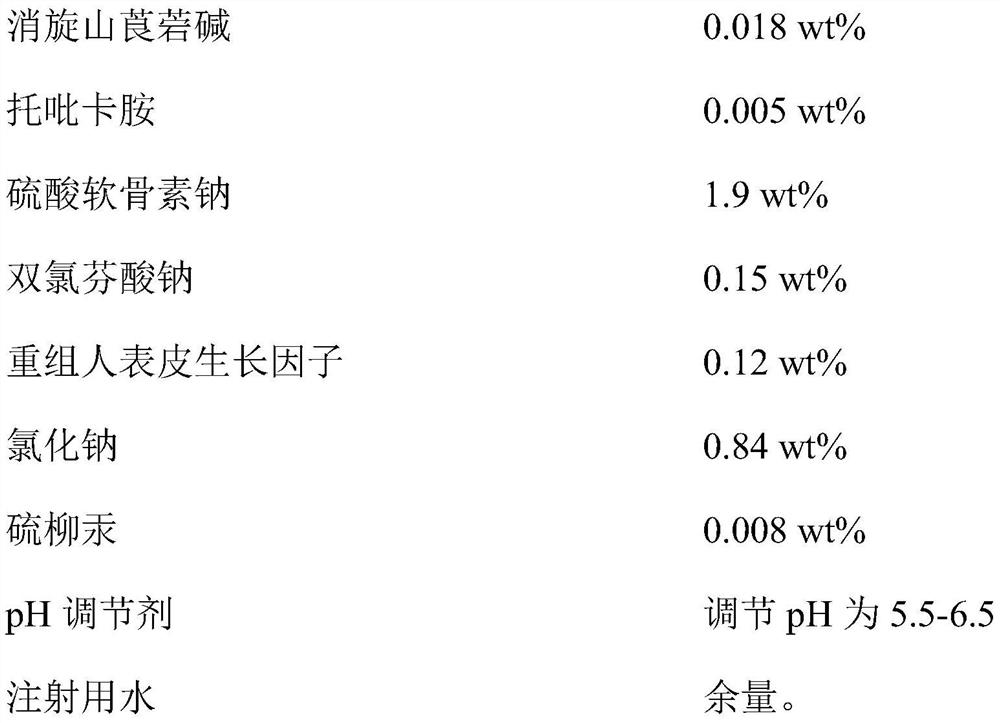
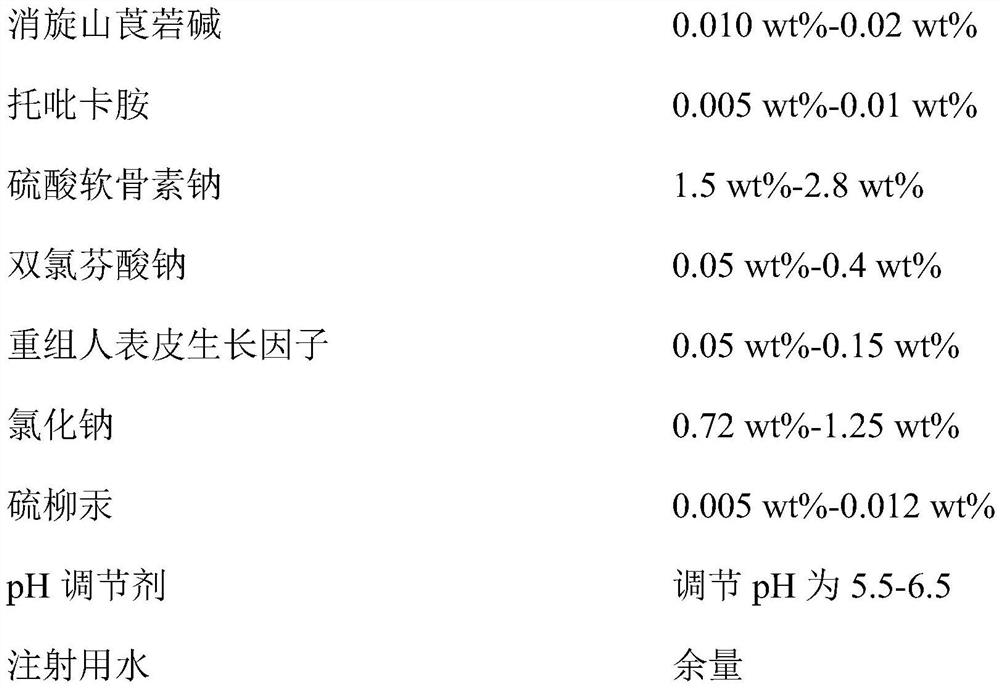
InactiveCN112353757ASignificant and effective delayObvious and effective control effectSenses disorderPeptide/protein ingredientsThiomersalateDrug biological activity
Provided is compound racanisodamine eye drops prepared from the following raw materials: 0.010-0.02 wt% of racanisodamine, 0.005-0.01 wt% of tropicamide, 1.5-2.8 wt% of sodium chondroitin sulfate, 0.05-0.4 wt% of diclofenac sodium, 0.05-0.15 wt% of a recombinant human epidermal growth factor, 0.72-1.25 wt% of sodium chloride, 0.005-0.012 wt% of thiomersalate, a pH regulator for adjusting pH to 5.5-6.5, and the balance being injection water. The compound racanisodamine eye drops disclosed by the invention are applied to adolescents, children and adults who often use eyes at a short distance, sothat the delaying and preventing effects on myopia of the adolescents and children are more obvious and effective after the compound racanisodamine eye drops are dripped for a long time, and for adults, visual fatigue can be significantly improved, and the elimination of eyeball discomfort and other symptoms can be accelerated; diclofenac sodium and racemic anisodamine are combined to achieve a good synergistic effect, diclofenac sodium is used as a ligand and matched with racemic anisodamine to generate a synergistic effect of biological activity, and meanwhile, diclofenac sodium and racemicanisodamine have the possibility of reducing toxicity, are low in price and easy to obtain and are worthy of popularization clinically.
Owner:ZHENJIANG HENGXIN PHARMA
Composition for improving bone density, and preparation method and application thereof
PendingCN111135284AIncrease bone densityImprove bone qualityOrganic active ingredientsHydrolysed protein ingredientsIncreased Bone DensityBiology
The invention discloses a composition for improving bone density. The composition is made of the following raw materials: a marine fish skin collagen peptide powder, calcium carbonate, glucosamine hydrochloride, sodium chondroitin sulfate, microcrystalline cellulose, and a vitamin D3 powder. The various raw materials are appropriately mixed to prepare the composition with the significant increasein bone density. Compared with the current preventive measure of simply supplementing calcium, the composite composition of the invention integrates effects of the various components to jointly enhance bone quality and increase bone density.
Owner:武汉跃莱健康产业有限公司
Preparation method of compound cerebroprotein hydrolysate tablets
InactiveCN107823626AGood dispersionGood adsorption load performanceNervous disorderHydrolysed protein ingredientsTreatment effectMicrosphere
The invention discloses a preparation method of compound cerebroprotein hydrolysate tablets. The preparation method includes: adding hydroxyapatite microspheres into a cerebroprotein hydrolysate solution for oscillating adsorption; freeze-drying, and loading part of cerebroprotein hydrolysate onto the hydroxyapatite microspheres to obtain cerebroprotein hydrolysate micropowder; mixing with auxiliary materials like sodium chondroitin sulfate, glutamic acid and microcrystalline cellulose, spraying, granulating, and drying to obtain cerebroprotein hydrolysate granules; tabletting, using a coatingmaterial for coating to obtain compound cerebroprotein hydrolysate tablets. By the preparation method, drug absorption can be promoted, and drug deactivation can be reduced, so that bioavailability of a cerebroprotein hydrolysate oral preparation is improved to realize good treatment effect.
Owner:安徽金太阳生化药业有限公司
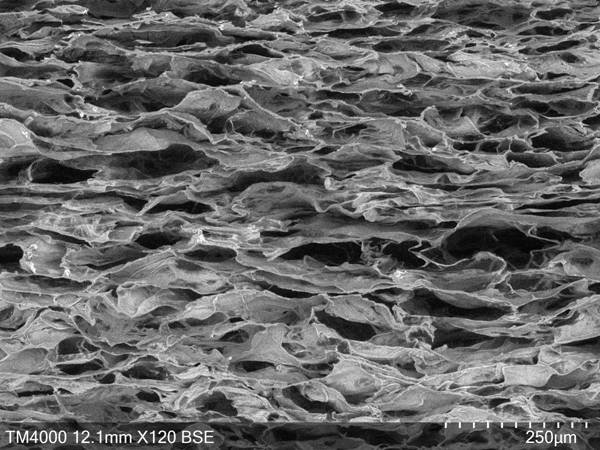
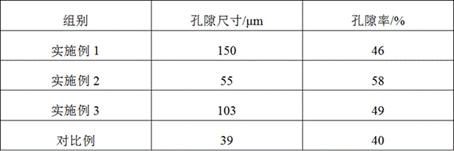
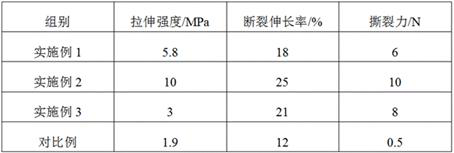
A tissue-guided regeneration collagen film and its preparation method
The invention relates to a tissue-guided regeneration collagen film and a preparation method thereof. The preparation method is as follows: swell type I collagen in an acetic acid solution, then add sodium chondroitin sulfate and stir evenly to obtain a collagen-chondroitin sulfate sodium slurry; dry the collagen-chondroitin sulfate sodium slurry, Obtain a dry film; mix the dry film with the cross-linking liquid, carry out the cross-linking reaction, and obtain a cross-linked collagen film after completion; clean the cross-linked collagen film and perform secondary drying to complete Then repress it. In the preparation method, high-concentration type I collagen is first swelled in acetic acid solution, and sodium chondroitin sulfate is added to stir, followed by drying, cross-linking, and secondary drying, and the obtained tissue-guided regeneration collagen film has excellent mechanical properties , and the pore size and porosity are conducive to cell growth and repair; in addition, its degradation cycle is long and it is not easy to cause allergies. The comprehensive effect is comprehensive and the applicability is wide.
Owner:TIANXINFU (BEIJING) MEDICAL APPLIANCE CO LTD
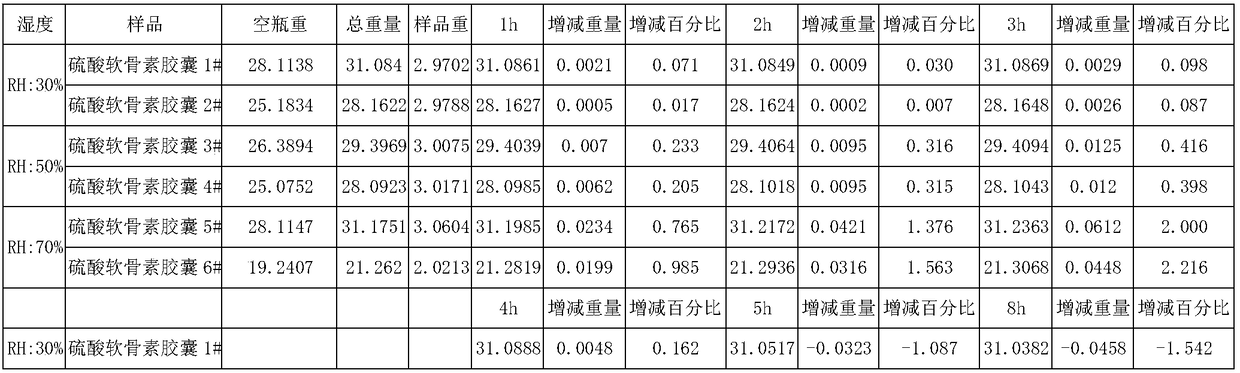
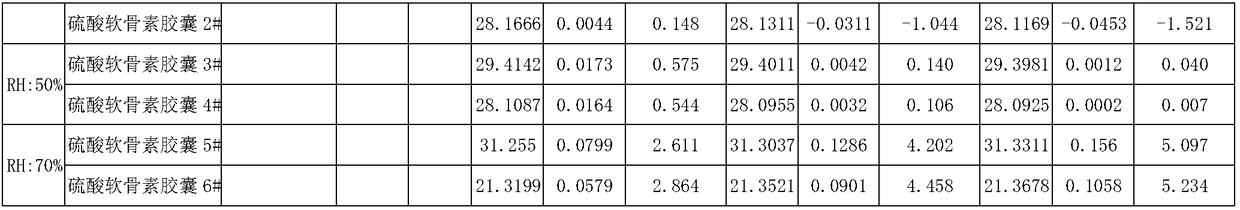
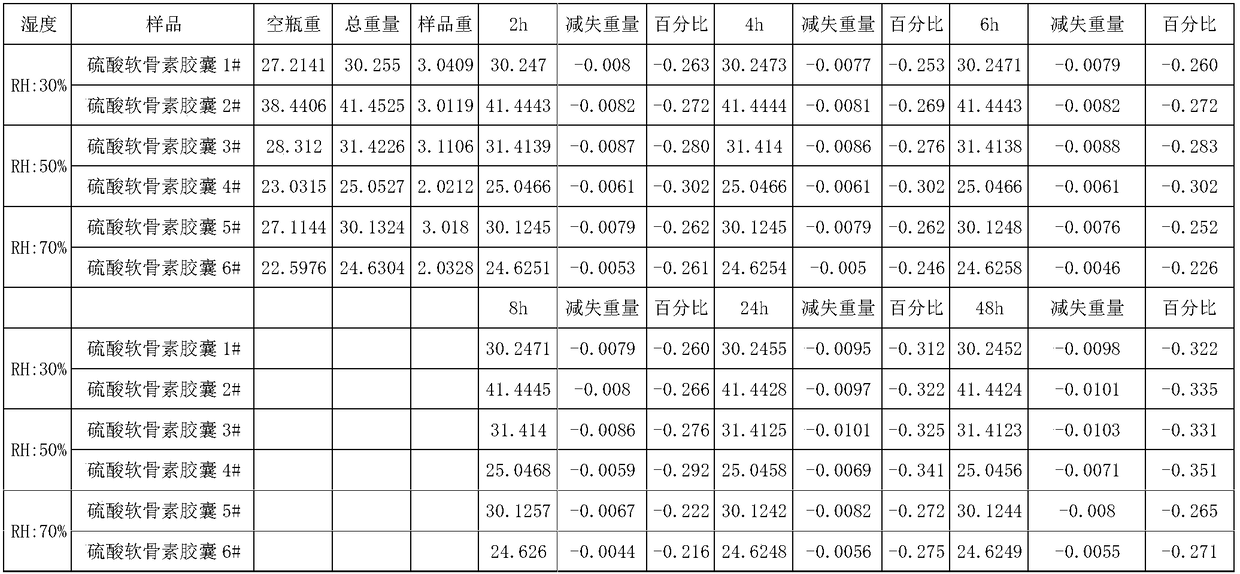
Sodium chondroitin sulfate capsule and preparation method thereof
ActiveCN108159015ASolve the problem of moisture absorptionGood quality and stabilityOrganic active ingredientsPharmaceutical non-active ingredientsAssurance qualityMoisture absorption
The invention discloses a sodium chondroitin sulfate capsule and a preparation method thereof. The sodium chondroitin sulfate capsule comprises following raw materials in parts by weight: 50 to 60 parts of sodium chondroitin sulfate, 30 to 40 parts of corn starch, 3 to 8 parts of low-substituted hydroxypropyl cellulose, 1 to 5 parts of silica, and 1 to 5 parts of talcum powder. The preparation method comprises following steps: (1) drying; (2) mixing; (3) filling; and (4) performing bubble cap and filling capsules in a moisture barrier bag. The problem of moisture absorption of a sodium chondroitin sulfate capsule is solved, the product quality stability is improved, and the product quality can be ensured within the shelf life.
Owner:CHENGDU TONGDE PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com