Freeze-dried powder preparation for preventing and improving fundus macular degeneration and preparation method thereof
A technology for freeze-dried powder and macular disease, which is applied in freeze-dried delivery, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. effect of care
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
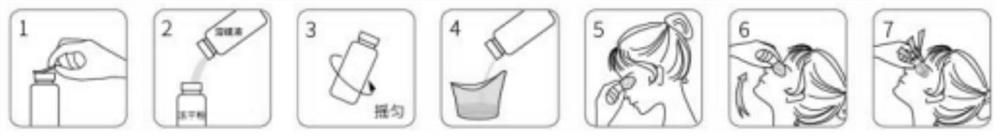
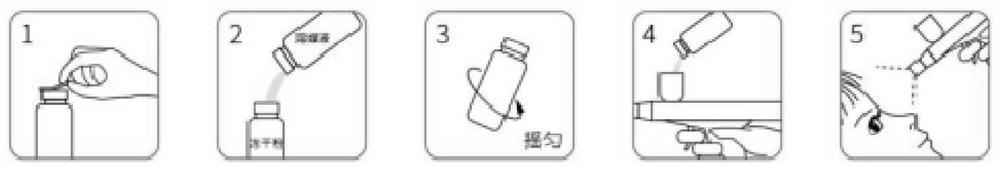
[0085] As a specific technical solution, the second aspect of the present invention provides a method for preparing the above-mentioned lyophilized powder preparation, including the preparation of the lyophilized powder and the preparation of the vehicle. Proceed as follows:
[0086] S1. Preparation of freeze-dried powder:
[0087] (1) Stir and dissolve the preparation raw materials of the freeze-dried powder evenly;
[0088] (2) after the sampling test is qualified, filter the discharge to obtain the lyophilized liquid;
[0089] (3) Take the freeze-dried liquid for physical, chemical and microbiological index inspection, quantitatively fill 1.5mL / bottle or 2mL / bottle into cleaned and sterilized 5mL or 10mL vials, and put them into plates and half-stoppered, then put into freeze dryer;
[0090] (4) freeze-drying process parameters are set, and the freeze-drying machine is started;
[0091] (5) Pre-cooling, freezing the lyophilized liquid;
[0092] (6) heat up, and dry and...
Embodiment 1
[0128] Embodiment 1 provides a kind of freeze-dried powder preparation that is used for the prevention and improvement of fundus macular disease, is made of two parts of freeze-dried powder and solvent solution, and its composition of mass percent is respectively:
[0129] Freeze-dried powder: based on the total mass percentage of freeze-dried powder, including sodium hyaluronate 0.03%, lutein 0.01%, riboflavin 0.01%, pyridoxine 0.05%, cyanocobalamin 0.05%, lactoferrin 0.5% %, oligopeptide-1 0.0005%, oligopeptide-50.0005-%, mannitol 3.0%, sodium chondroitin sulfate 0.03%, hydrogenated lecithin 0.1%, deionized water balance;
[0130] Solvent solution: based on the total mass percentage of the solvent solution, including 0.1% taurine, 0.5% sweetophylline, 0.05% dipotassium glycyrrhizinate, 0.03% polysorbate-80, 0.01% sodium chloride, and 0.01% lysozyme , Deionized water balance;
[0131] The packaging quality of the freeze-dried powder is 0.15g, and the solvent solution is 5mL;...
Embodiment 2
[0150] Embodiment 2 provides a kind of freeze-dried powder preparation that is used for the prevention and improvement of fundus macular disease, is made up of two parts of freeze-dried powder and solvent liquid, and its composition of mass percent is respectively:
[0151] Freeze-dried powder: based on the total mass percentage of freeze-dried powder, including sodium hyaluronate 0.04%, lutein 0.02%, riboflavin (vitamin B2) 0.01%, pyridoxine (vitamin B6) 0.06%, cyanocobalamin Ammonium (vitamin B12) 0.06%, lactoferrin 0.8%, oligopeptide-1 0.0007%, oligopeptide-5 0.0006%, mannitol 3.5%, sodium chondroitin sulfate 0.03%, hydrogenated lecithin 0.15%, deionized water margin;
[0152] Solvent solution: based on the total mass percentage of the solvent solution, including 0.15% taurine, 0.8% sweetophylline, 0.08% dipotassium glycyrrhizinate, 0.04% polysorbate-80, 0.02% sodium chloride, and 0.015% lysozyme , Deionized water balance;
[0153] The packaging quality of the freeze-drie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com