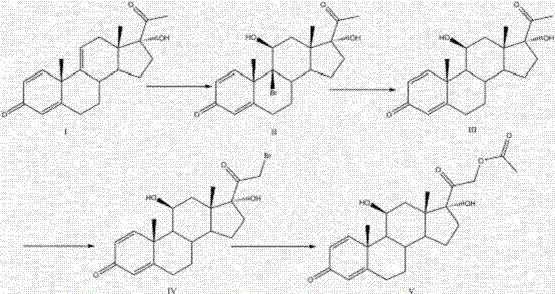
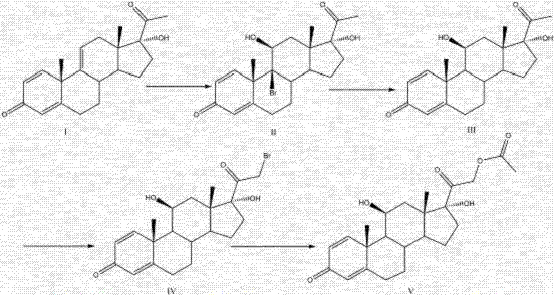
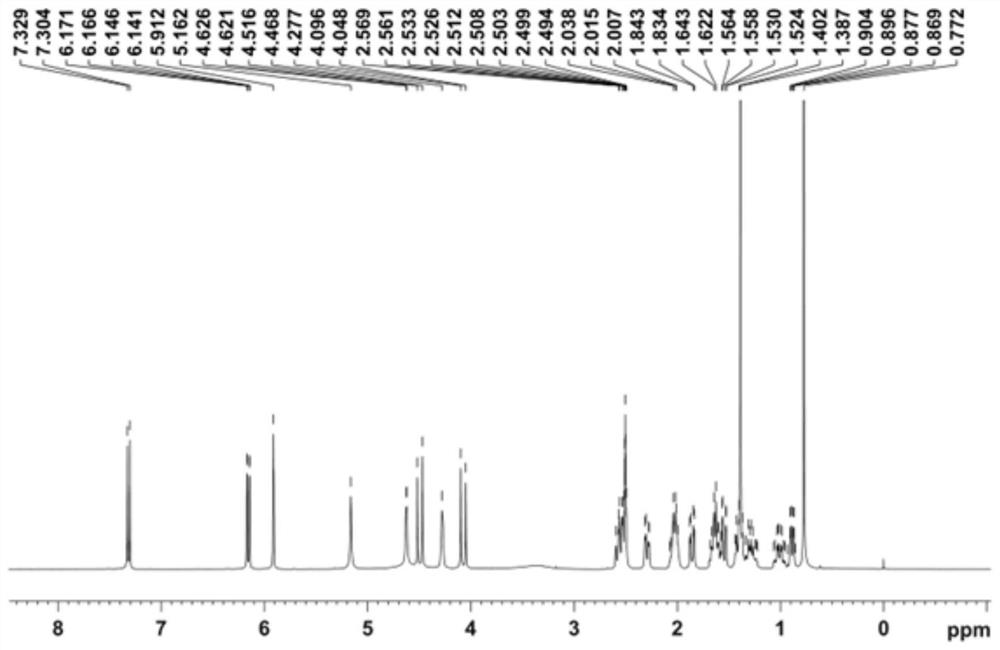
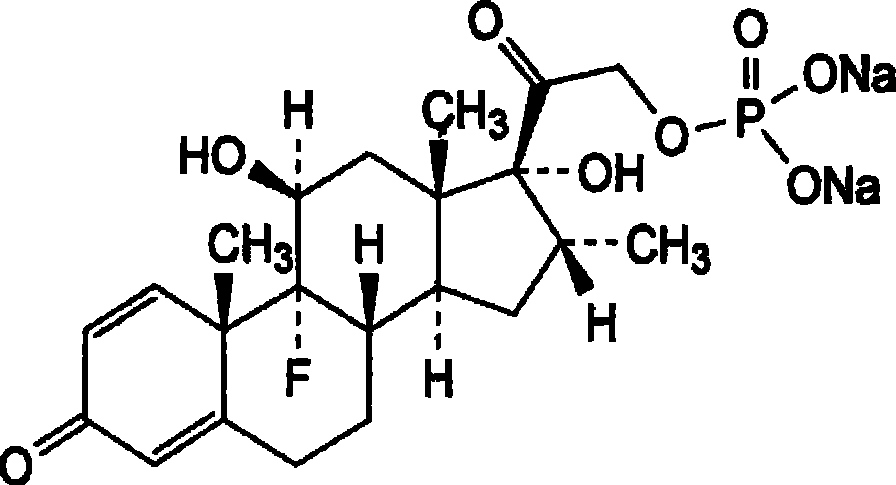
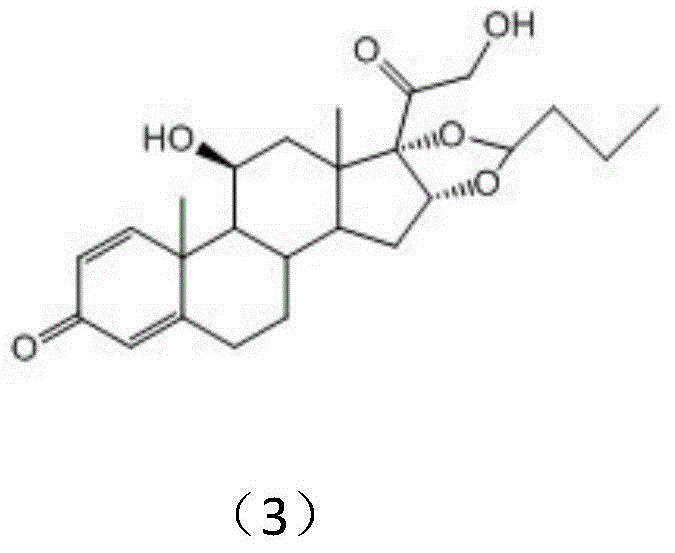
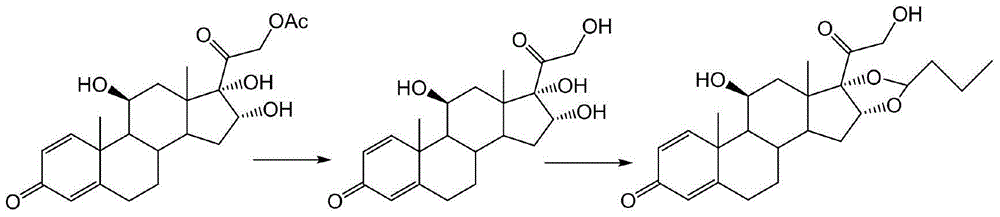
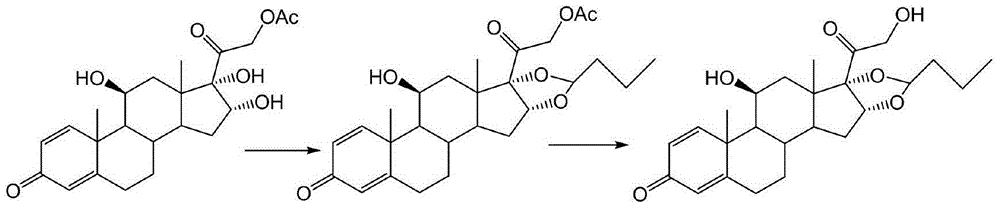
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
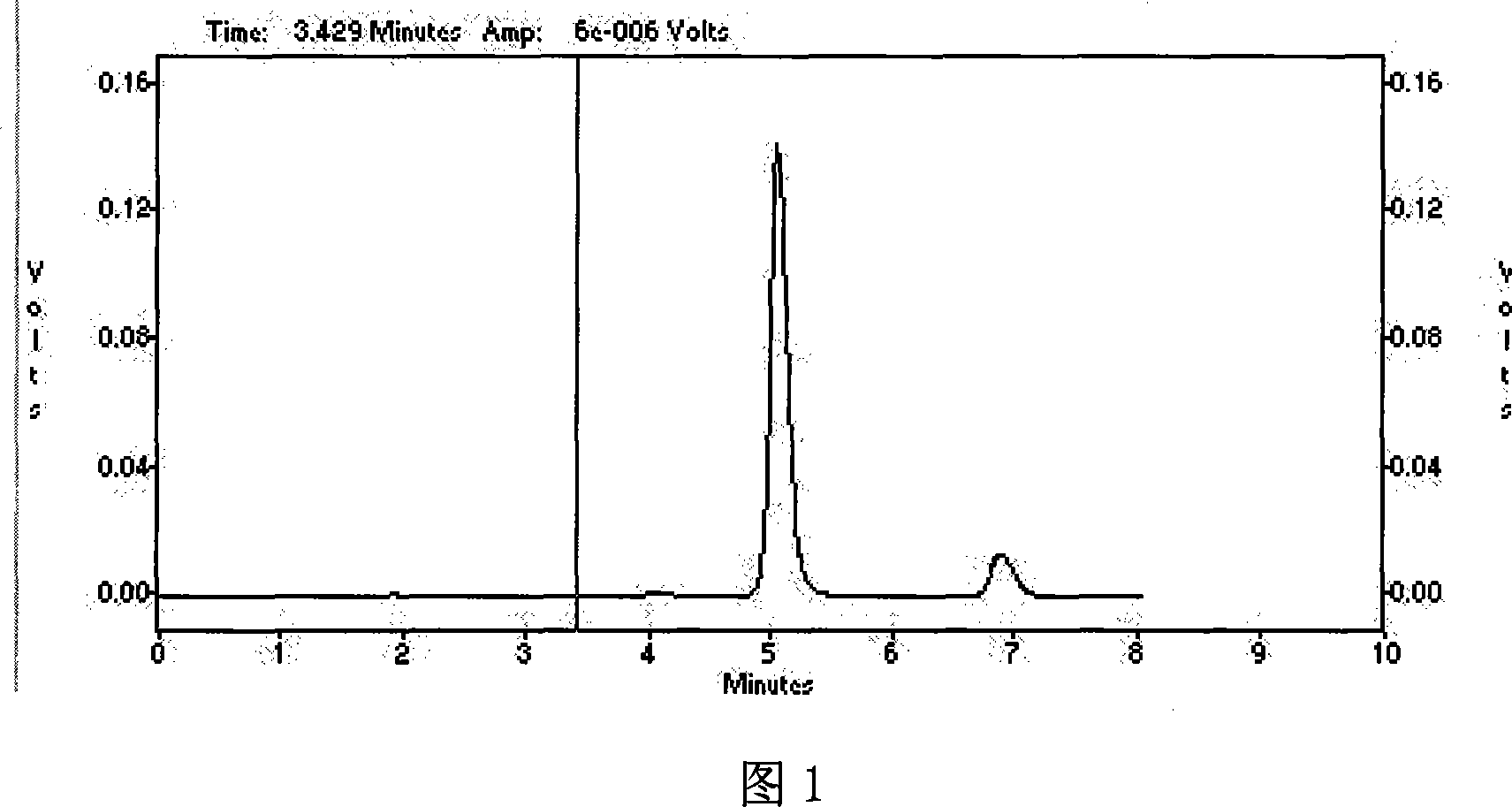
59 results about "Prednisolone acetate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
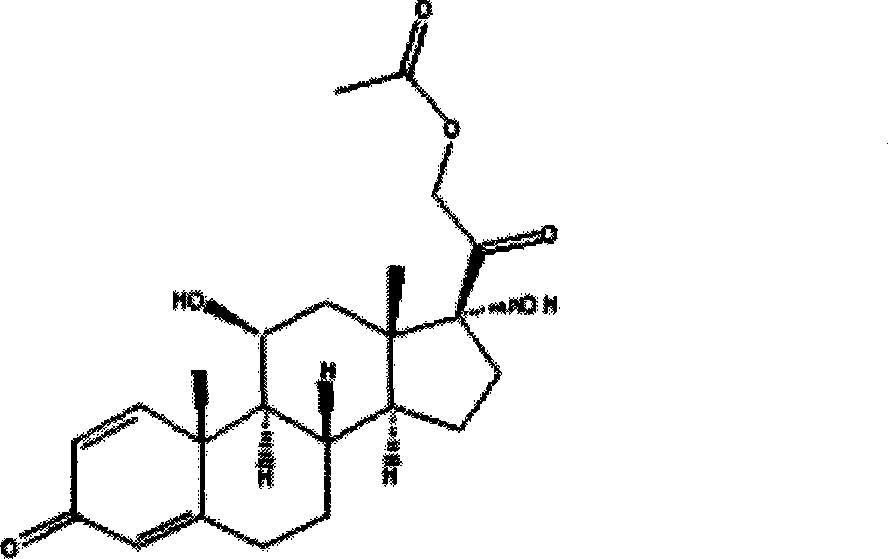
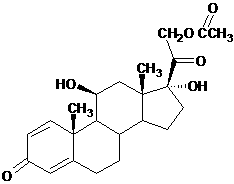
Prednisolone acetate is a synthetic glucocorticoid corticosteroid and a corticosteroid ester. It is the 21-acetate ester of prednisolone.
Oral suspension of prednisolone acetate
The present invention relates to novel oral suspension formulation comprising prednisolone acetate, a pharmaceutically acceptable vehicle and a thickening agent. The present invention further provides a method of treating patients in need of prednisolone with the novel formulation.
Owner:TARO PHARMA
Method for producing prednisolone acetate
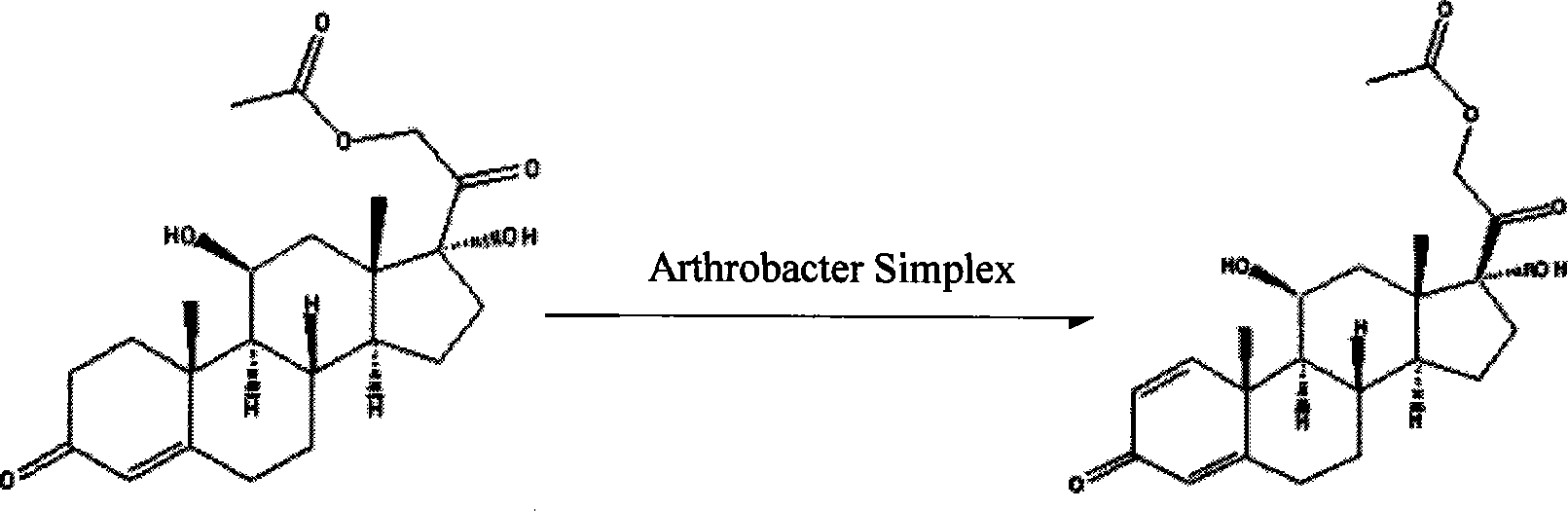
InactiveCN101210259AStrong response specificityEasy to operateMicroorganism based processesFermentationMicrobial transformationArthrobacter simplex
The invention belongs to the field of microbial pharmaceutics and pharmaceutical engineering, specifically relates to a production method of prednisone acetate by microbial transformation with Arthrobacter simplex as bacteria strain and hydrocortisone acetate as substrate. The method uses Arthrobacter simplex as bacteria strain and comprises the following steps of: performing primary seed culture, performing second fermentation culture, adding hydrocortisone acetate into the fermentation liquid of Arthrobacter simplex to transform hydrocortisone acetate into prednisone acetate, filtering, and collecting cake to obtain prednisone acetate. The bacteria can be prepared into double liquid phase, broken cells or protoplast, each of which has high transformation ratio. The inventive production method replaces cortisone acetate with hydrocortisone acetate as raw material, and has the advantages of high yield, simple process, good economical and practical performance, and less use of harmful reagents; and is important for steroids production with biotransformation method.
Owner:TIANJIN UNIV OF SCI & TECH
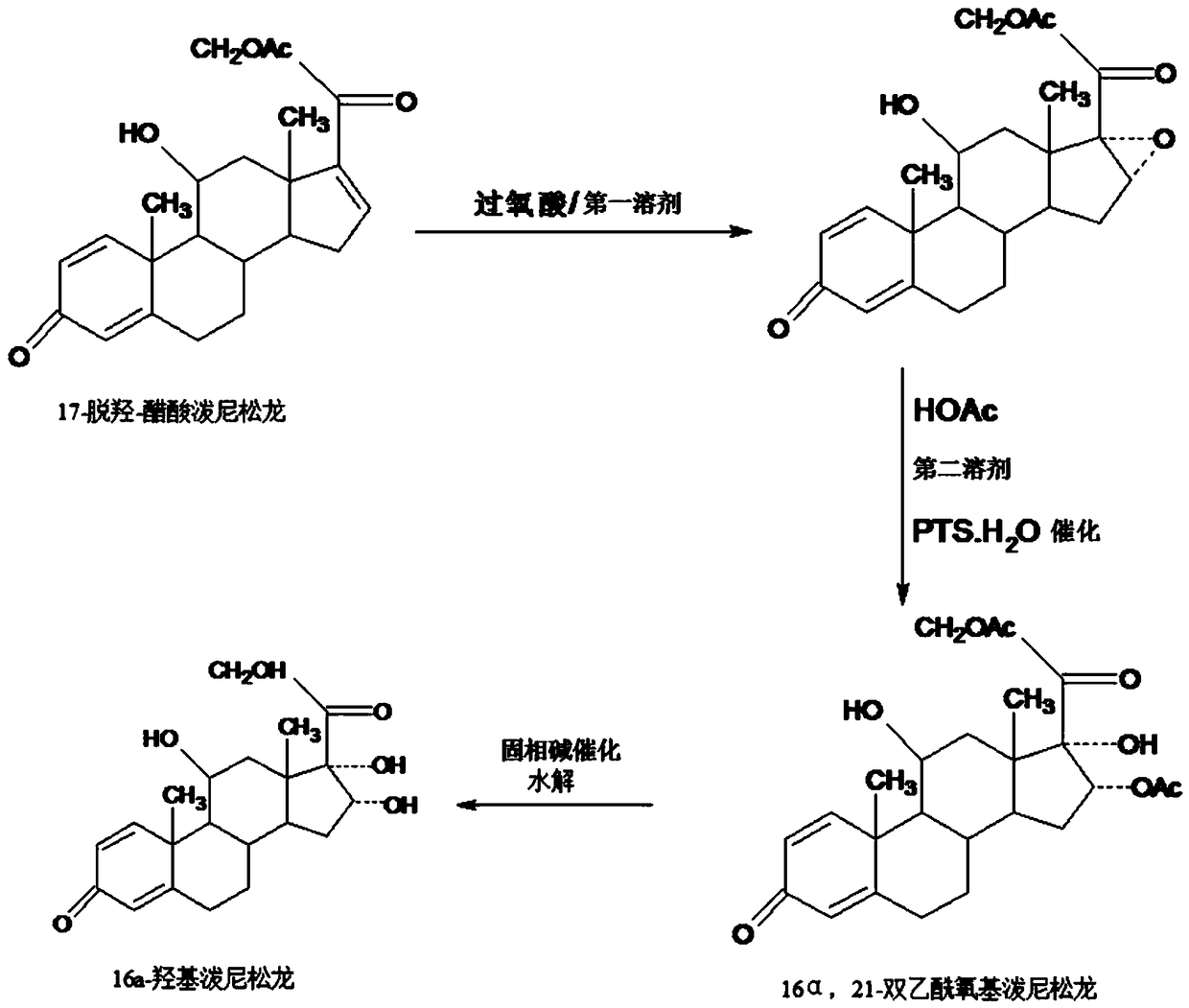
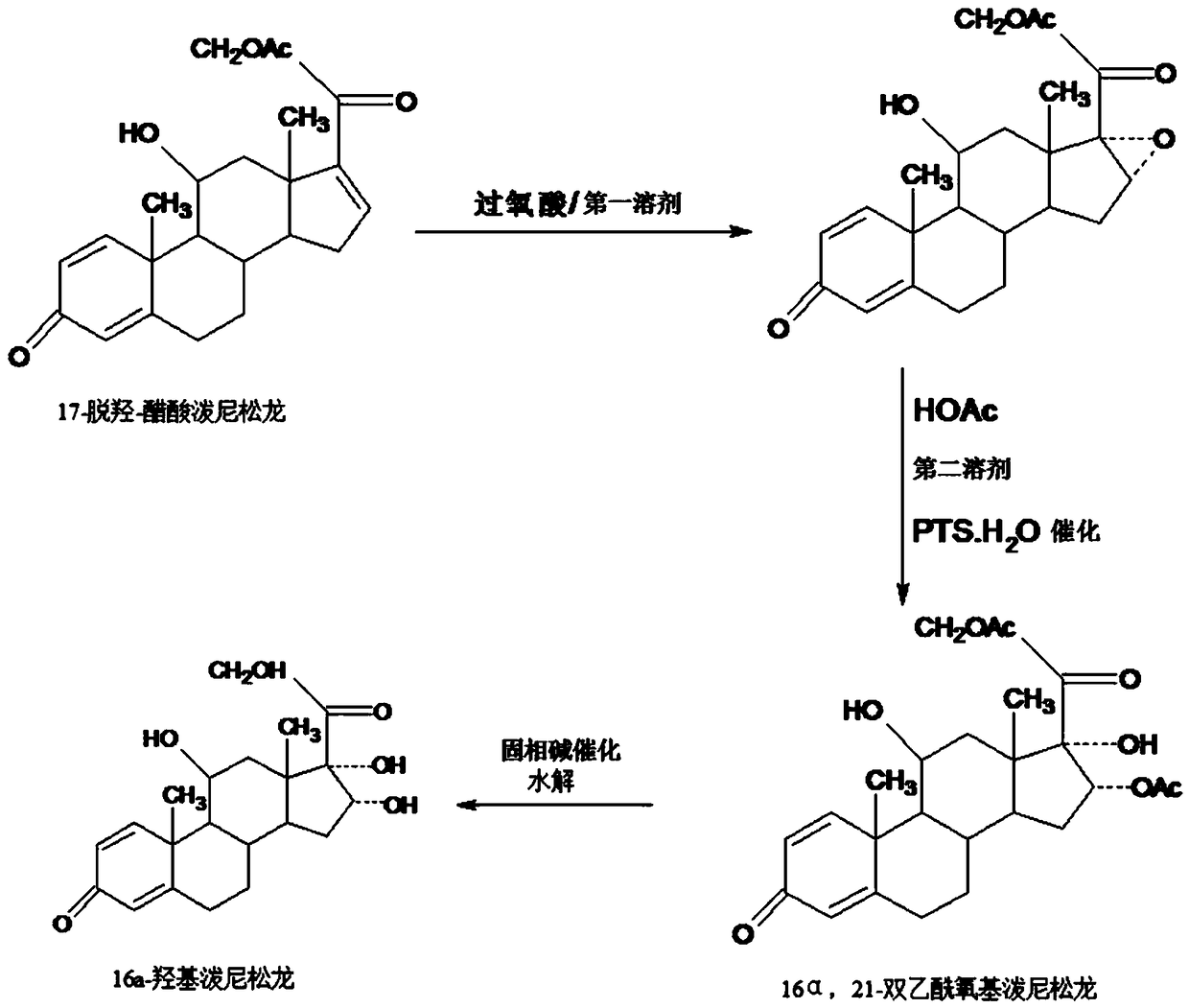
Preparation method of 16a-hydroxy prednisolone
ActiveCN107488203AInhibition of rearrangement and ring expansion side reactionsReduce generationPhysical/chemical process catalystsOrganic chemistry methodsOrganic solventAlcohol
The invention discloses a preparation method of 16a-hydroxy prednisolone. The preparation method comprises the following steps: dissolving 16a-hydroxy prednisolone acetate into an organic solvent, adding an inert solid carrier adsorbed with strong base as a hydrolysis reaction solid-phase base catalyst, and hydrolyzing 21-acetate to obtain a 16a-hydroxy prednisolone crude product; and carrying out lower alcohol recrystallization on the crude product under the condition of below C4 to obtain a 16a-hydroxy prednisolone competitive product, wherein the refined weight yield is 85% to 90%, and the preparation weight total yield is 75% to 80%. The solid carrier is selected from aluminum oxide, silica gel or calcium carbonate; the base catalyst is selected from sodium carbonate; and the organic solvent is selected from methylbenzene or chloroform. Compared with a traditional method, the method disclosed by the invention is simple and convenient in production operation, impurities generated in traditional production can be greatly reduced, and the total yield for synthesis is greatly improved; compared with the traditional method, the production cost is reduced by 10% to 15%; and a synthetic reaction solvent can be recycled, and industrial production is facilitated.
Owner:HUNAN KEREY BIOTECH
Ophthalmic preparation of levofloxacin and prednisolone acetate and preparation method thereof
ActiveCN102085203ALess irritatingReduce secretionAntibacterial agentsOrganic active ingredientsOphthalmologyLevofloxacin
The invention relates to an ophthalmic preparation of levofloxacin and prednisolone acetate and a preparation method thereof. Particularly, the invention relates to an ophthalmic preparation containing levofloxacin and prednisolone acetate with an effective amount for treatment and / or prevention, a high polymer material, a surfactant, a complexing agent and water. The invention also relates to anophthalmic preparation containing the ophthalmic preparation provided by the invention and a medicinal excipient mixed with the ophthalmic preparation provided by the invention before application, and a preparation method of the ophthalmic preparation. The ophthalmic preparation provided by the invention not only has a favorable effect of treating eye diseases, but also has very low stimulation to the eyes.
Owner:SHENYANG XINGQI PHARM CO LTD
Oral suspension of prednisolone acetate
Owner:TARO PHARMA
Ceftiofur hydrochloride cream and preparation method thereof
ActiveCN104042619AImprove immunityGood treatment effectAntibacterial agentsOrganic active ingredientsMonoglycerideEthylic acid
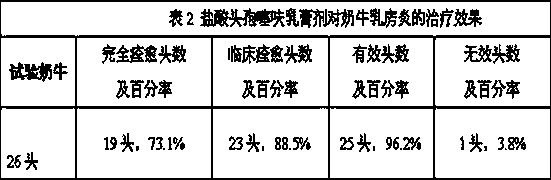
The invention relates to ceftiofur hydrochloride cream and a preparation method thereof. The ceftiofur hydrochloride cream comprises ceftiofur hydrochloride, prednisolone acetate, vitamin E, monoglyceride stearate, albolene, lanolin, liquid paraffin, span 80, polysorbate 80, ethylparaben and purified water. The preparation method comprises the following steps of preparing an oil phase and a water phase, adding ceftiofur hydrochloride and prednisolone acetate into the oil phase and the water phase, and carrying out mixing to obtain a uniform mixture. The ceftiofur hydrochloride cream can be used for treating cow mastitis.
Owner:LINYI UNIVERSITY
Method for preparing 16a-hydroxyl prednisolone product
InactiveCN109232696AAvoid many difficulties such as difficult purificationEasy to operateSteroidsAcetic acidOrganic solvent
The invention provides a method for preparing a 16a-hydroxyl prednisolone product. The method comprises the steps: firstly, subjecting 17a-deshydroxy prednisolone acetate, which serves as a starting raw material, and organic peroxy acid to an epoxidation reaction at 16,17 sites in a first organic solvent, so as to prepare epoxide; subjecting the epoxide and glacial acetic acid to a ring-opening reaction under the catalysis of an acid catalyst in a second organic solvent, so as to prepare 16a,21-diacetoxyl prednisolone; then, dissolving the 16a,21-diacetoxyl prednisolone in a third organic solvent, and hydrolyzing acetate of two positions under the catalysis of a solid-phase alkali catalyst, so as to prepare 16a-hydroxyl prednisolone; finally, subjecting the crude 16a-hydroxyl prednisoloneobtained through solid-phase alkali-catalyzed hydrolysis to heated refluxing, decoloring and recrystallization by low carbon alcohols of C4 or less, thereby obtaining the 16a-hydroxyl prednisolone product. The 16a-hydroxyl prednisolone is prepared by the efficient, environment-friendly and cheap method.
Owner:HUNAN KEREY BIOTECH
Dry cow mamma perfusion agent for preventing and curing cow mastitis and preparation method thereof
InactiveCN1286463CDecreased cell countReduce incidenceAntibacterial agentsAntipyreticAmpicillinVegetable oil
The invention discloses a dried cow breast perfusion agent and its process method for treating cow mastitis. It is in the characterized in that: perfusion agents of each 10 ml prescriptions comprise ampicillin or amoxicillin of 100-150 mg; prednisolone acetate is 4-7 mg; sodium chloride is 400-550 mg; vegetable oil is 6-6.5 ml; polysorbate os 0.75-0.82 ml; left is distiller water. And the process method is: vegetable oil. Sodium chloride, and distilled water are mix uniformly to be added polysorbate for emulsion; ampicillin or amoxicillin are added to mixed after prednisolone acetate being added, then emulsified in low temperature; aseptic package to attain finish products. Said invention has significantly effect on prevention and treatment of dried cow occult mastitis without waste, and it has low process cost and low treatment cost.
Owner:NANJING AGRICULTURAL UNIVERSITY
Method for preparing steroids and novel intermediate compound used therein
InactiveCN101117350AHigh yieldThe yield of iodine replacement is improvedSteroidsBulk chemical productionColor ScaleGlucocorticoid
The present invention relates to an improved preparation method of glucocorticoids steroid, in particular to the preparation methods of prednisolone acetate, cortisone, prednisolone or hydrocortisone. The present invention also relates to a new intermediate compound used in the process of preparing the compounds. The present invention provides a new synthetic route of iodo first and opening the ring after, improving the selectivity of final products, reducing the impurity content of the products, and satisfying the color scale of glucocorticoids drugs, thereby conquering the disadvantages of preparing 17 alpha- hydroxyl at first, and then preparing 21-hydroxyl or the derivatives during the process of preparing 17 alpha, 21-hydroxyl steroid or the derivatives of prior art.
Owner:TIANJIN PHARMA GROUP CORP
Preparation of prednisolone acetate
The invention discloses a preparation of prednisolone acetate and belongs to the technical field of chemical pharmacy. In the method, prednisolone and acetic anhydride are employed as raw materials for preparing the prednisolone acetate through a synthetic reaction which is carried out in a dipolar aprotic solvent. The prednisolone and the acetic anhydride are reacted with each other under an alkali metal acetate catalyst to prepare the prednisolone acetate, wherein the dipolar aprotic solvent is dimethyl formamide or dimethyl sulfoxide. According to the technical scheme, not only is a problem of toxicity due to pyridine solved, but also the yield and the quality are increased and the reaction time is reduced.
Owner:HENAN LIHUA PHARMA
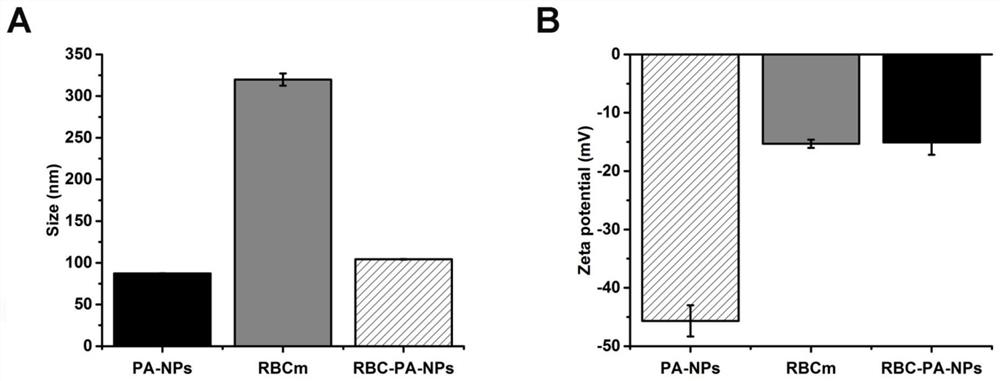
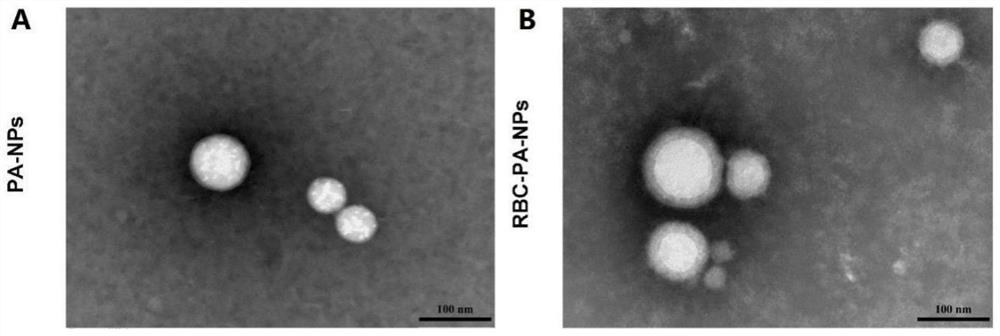
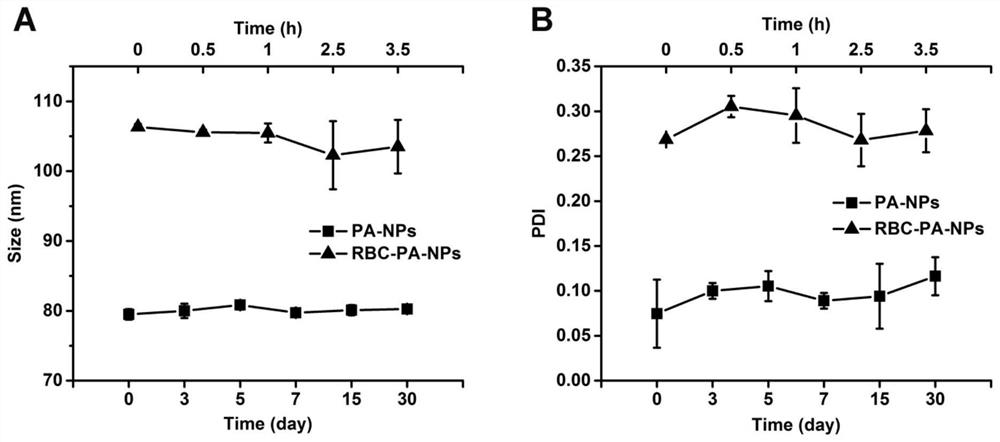
Novel kidney-targeted nano drug delivery system with biomimetic modification of erythrocyte membrane as well as preparation method and application of novel kidney-targeted nano drug delivery system
ActiveCN113133988AOrganic active ingredientsPharmaceutical non-active ingredientsEthylic acidCell membrane
The invention belongs to the technical field of biological medicines, and particularly relates to a novel kidney-targeted nano drug delivery system with biomimetic modification of an erythrocyte membrane as well as a preparation method and application of the novel kidney-targeted nano drug delivery system. According to the application, polylactic acid-glycolic acid copolymer (PLGA) is taken as a carrier material, prednisolone acetate (PA) is taken as a model drug, and a novel bionic nano drug delivery system (RBC-PA-NPs) with kidney targeting is constructed by utilizing a strategy of coating an erythrocyte membrane on the surface. The drug delivery system combines the biocompatibility of the erythrocyte membrane and the targeting property of the nanoparticles, so that the kidney targeting property of PA is improved, and the glomerulonephritis is treated more effectively. Therefore, evaluation is carried out from the aspects of a preparation process, physicochemical properties, cytotoxicity and uptake, in-vivo targeting and the like of the drug delivery system.
Owner:THE AFFILIATED HOSPITAL OF SOUTHWEST MEDICAL UNIV
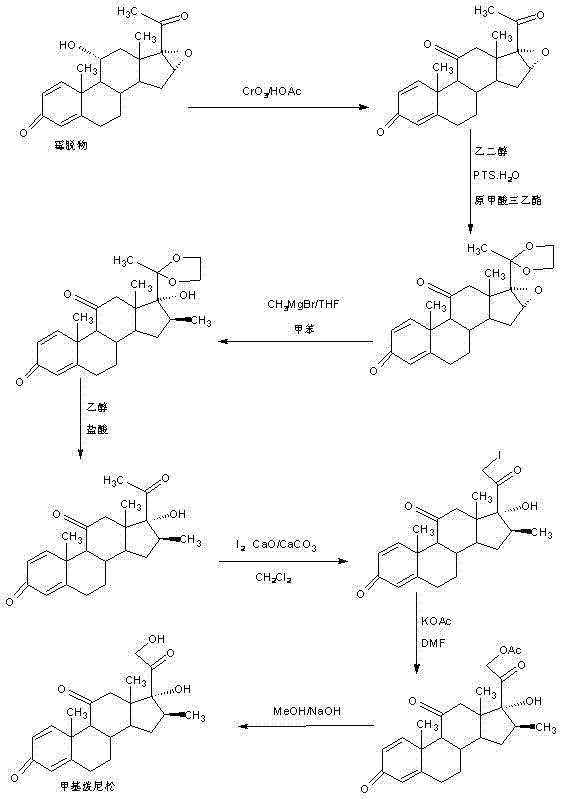
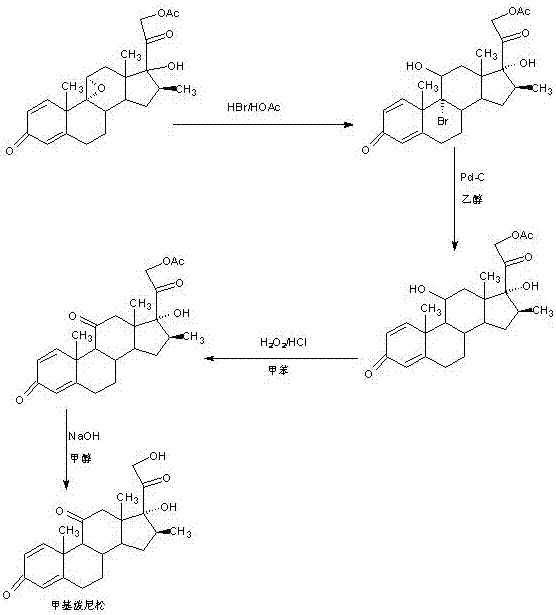
Preparation method of methyl metacortandracin
The invention discloses a preparation method of methyl metacortandracin. The methyl metacortandracin is prepared by taking 16-b methyl-9 (11) a-epoxy-prednisolone acetate (called as DB11 for short) as a raw material; dissolving the raw material in an organic solvent and reacting with hydrobromic acid to obtain bromine hydroxyl content 9a bromine-16b methyl-prednisolone acetate; performing catalytic hydrogenation and 9-bit debromination on bromine hydroxyl content in the organic solvent by acid-binding agent and palladium carbon to obtain a debromination matter 16b methyl-metacortandracin; oxidizing the debromination matter with strong oxidant in the organic solvent to obtain prednisolone acetate; finally, hydrolyzing the prednisolone acetate in the organic solvent by catalyst to obtain methyl metacortandracin. The total weight yield through four-step synthesis is 52-55%. The production method is wide in raw material source, economic and environment-friendly in technique, short in synthesis route, and high in production yield; the production cost is 30-40% lower than that of the traditional method, and 15-20% lower than the method of application number 201610952306.6; the technique is simple and convenient; the solvent can be recycled and used indiscriminately; the preparation method is easy to practice the industrial production.
Owner:HUNAN KEREY BIOTECH
Prednisolone acetate preparation method
The present invention relates to a prednisolone acetate preparation method which is as follows: prednisolone acetate can be obtained by successive 3-site and 20-site keto-protection reaction, 11-site keto-reduction reaction, 21-site hydroxyl esterification reaction and 3-site and 20-site keto-deprotection reaction of prednisone acetate as a raw material. A new synthetic route of first esterification and then deprotection is provided, nitrosification quenching reaction and resin hydrolysis reaction can be omitted in the deprotection step, the technical process is greatly simplified, the cost of production is reduced, and the method is suitable for mass production.
Owner:HUAZHONG PHARMA
Dry cow mamma perfusion agent for preventing and curing cow mastitis and preparation method thereof
InactiveCN1666744ADecreased cell countReduce incidenceAntibacterial agentsOrganic active ingredientsVegetable oilAmpicillin
The invention discloses a dried cow breast perfusion agent and its process method for treating cow mastitis. It is in the characterized in that: perfusion agents of each 10 ml prescriptions comprise ampicillin or amoxicillin of 100-150 mg; prednisolone acetate is 4-7 mg; sodium chloride is 400-550 mg; vegetable oil is 6-6.5 ml; polysorbate os 0.75-0.82 ml; left is distiller water. And the process method is: vegetable oil. Sodium chloride, and distilled water are mix uniformly to be added polysorbate for emulsion; ampicillin or amoxicillin are added to mixed after prednisolone acetate being added, then emulsified in low temperature; aseptic package to attain finish products. Said invention has significantly effect on prevention and treatment of dried cow occult mastitis without waste, and it has low process cost and low treatment cost.
Owner:NANJING AGRICULTURAL UNIVERSITY
Compound lincomycin hydrochloride injection and reparation method thereof
InactiveCN101991594AReduce chance of drug resistanceBroad spectrum antibacterialAntibacterial agentsOrganic active ingredientsAntibiotic resistanceDissolution
The invention relates to compound lincomycin hydrochloride injection and a preparation method thereof. The preparation method comprises the following steps: (1) adding aminopyrine and prednisolone acetate into 95% ethanol and stirring for 10-25 min until the solution is clear; (2) fetching 300 L of injection water, adding kanamycin sulfate and lincomycin hydrochloride in turn and stirring for dissolution; and (3) mixing the solutions obtained in steps (1) and (2), adding injection water until the solution volume is 1,000 L, uniformly stirring and maintaining for 15-20 min until the liquid medicine is clear to obtain the compound lincomycin hydrochloride injection. The invention has the advantages that the compound preparation of kanamycin sulfate and lincomycin hydrochloride, the aminopyrine with adjuvant therapy effect and prednisolone acetate with adjuvant therapy effect are adopted and combined for use, thus achieving antibacterial action on gram-negative bacteria and gram-positive bacteria due to the joint application of the four medicines; and the antimicrobial spectrum is wide, the antimicrobial range is expanded, pig paratyphus and certain complications can be comprehensively treated, the curative effect is good and quick, the drug resistance rate of bacteria is reduced and the curative effect is enhanced.
Owner:TIANJIN BIJIA PHARMA CO LTD
Method for preparing steroid anti-inflammatory medicine prednisolone acetate by adopting enzyme process
InactiveCN105779552AThe solution steps are cumbersomeSettlement yieldFermentationPolyethylene glycolSolvent
The invention discloses a method for preparing steroid anti-inflammatory medicine prednisolone acetate by adopting an enzyme process. A compound 2 is taken as a substrate, the substrate reacts to generate a target product prednisolone acetate in presence of a hydrogen acceptor, a cosolvent and steroid 1,2-dehydrogenase, and the reaction is carried out in an aqueous phase buffer solution with the pH value of 5-9 and at the temperature of 10-45 DEG C, wherein the cosolvent is one or combination of more of dimethyl sulfoxide, Tween 60, Triton X 100 and polyethylene glycol, and the feeding volume of the cosolvent is 5-20% of the total volume of a reaction system. When the method disclosed by the invention is adopted for preparing the prednisolone acetate, the substrate concentration is high, the usage amount of enzyme is less, the operation is simple, the purity is high, and the conversion ratio is high.
Owner:ENZYMEWORKS
A kind of preparation method of prednisolone acetate
InactiveCN103396468BReduce initial investment costLow investment costSteroidsSynthesis methodsDouble bond
Owner:WUHAN LANGJI TECH DEV
Preparation method of prednisolone acetate
The invention relates to prednisolone acetate and a preparation method of prednisolone acetate, which are characterized in that the prednisolone acetate is prepared by sequentially carrying out a biological fermentation, an esterification reaction, a bromination reaction and a debromination reaction on the raw material, the prednisolone acetate is subjected to a hydrolysis reaction to obtain prednisolone, the overall yield is up to 81.75%, and the HPLC area normalization content of prednisolone is up to 99.5%. The preparation method is short in synthetic route and low in cost, is suitable forindustrial production, and has a very high industrial value.
Owner:HUNAN NORCHEM PHARMACEUTICAL CO LTD
Composition which promotes wound healing
InactiveCN102988960APeptide/protein ingredientsAmine active ingredientsWound healingChlorhexidine Acetate
The invention discloses a composition which promotes wound healing, comprising the components of 0.5-2.5% of chlorhexidine acetate, 1-3.5% of prednisolone acetate, 2-5% of dodecyl dimethyl benzyl ammonium chloride, 0.05-0.3% of vascular endothelial growth factor, 5-15% of sterile water and the balance of ointment matrix by weight percentage. The composition of the invention can obviously shorten the time of wound healing and effectively reduce the width of scar.
Owner:顾月燕
An ointment used for radically curing psoriasis
PendingCN106492198ANo side effectsPeptide/protein ingredientsHydroxy compound active ingredientsSide effectExternal application
An ointment used for radically curing psoriasis is provided and belongs to the field of medicines for external application. The ointment includes sulfadiazine tablets, prednisolone acetate tablets, doxycycline hydrochloride tablets, dexamethasone tablets, chlorpheniramine maleate tablets and Baochailing cream. The ointment includes 30% by weight of the sulfadiazine tablets, 5-9% by weight of the prednisolone acetate tablets, 5-1% by weight of the doxycycline hydrochloride tablets, 7-9% by weight of the dexamethasone tablets, 3-1% by weight of the chlorpheniramine maleate tablets and 50% by weight of the Baochailing cream. The ointment can effectively radically cure the psoriasis and relieve symptoms. The ointment is characterized by being nontoxic, tasteless and free of side effects so that the range of suitable crowds is wider. Raw materials of the prescription are simple and preparation is convenient.
Owner:冯炳养
Compound preparation of traditional Chinese and western medicines for treatment of chronic pelvic inflammation
InactiveCN105126088AHigh content of active ingredientsStable efficacyOrganic active ingredientsTripeptide ingredientsPronephrium penangianumSide effect
The invention belongs to the technical field of medicines and discloses a compound preparation of traditional Chinese and western medicines for treatment of chronic pelvic inflammation. The compound preparation comprises traditional Chinese medicine ingredients and western medicine ingredients. The traditional Chinese medicine ingredients include gynura bicolor, rhizome corydalis, salix alba, herb of common sow thistle, root of common buttonbush, bark of Chinese redbud, herb or fruit of little groundcherry, immature fruit of cassiabark tree, common fibraurea stem, hemsleya amabilis, ixeris chinensis, radix stephaniae tetrandrae, root of siberian cocklebur, jasminum giraldii diels, herb of oblongleaf betony, blood amber and pronephrium penangianum. The western medicine ingredients include glutathione, 1-hexacosyl alcohol, trimethoprim, chymotrypsin, limonene, prednisolone acetate, 3,3'-dithiodialanine and vitamin C. The compound preparation of traditional Chinese and western medicines for treatment of chronic pelvic inflammation is prepared by reasonable selection of Chinese herbal and western medicine compounds, effective ingredients of traditional Chinese medicines are extracted according to advanced pharmaceutical techniques to save active ingredients to the maximum extent and increase the content of effective ingredients in finished medicine products, and consequently treatment period is shortened. Furthermore, according to clinical verifications, the compound preparation has the advantages of remarkable curative effects, stability and avoidance of side effects, and chronic pelvic inflammation treated with the compound preparation is less prone to relapse.
Owner:卢连伟
Compound nose drops and its prepn.
InactiveCN1336175AReduce edemaAdjust immune functionOrganic active ingredientsRespiratory disorderCurative effectTherapeutic effect
The invented naristillae is composed of naphazoline hydrochloride nose drop 3-8 ml. contianing 0.1% naphazoline hydrochloride, lincomycin hydrochloride solution 2-6 ml. containing 0.6-1.8 gm licomycin hydrochloride, prednisolone aectate solution 1-5 ml. containing prednisolone acetate 25-125 mg. Advantages include: apparent therapeutic effect quick action, easy to use it, no recurrence etc.
Owner:杨俊爱
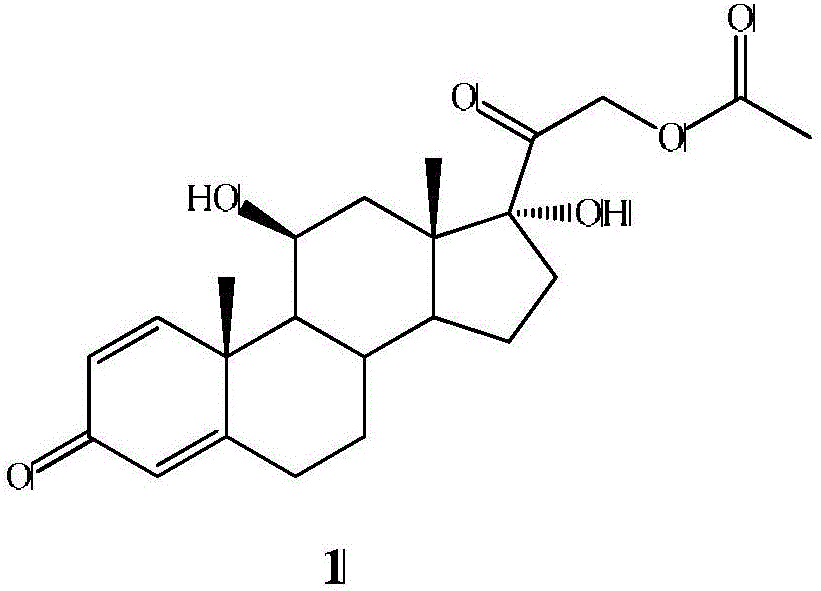
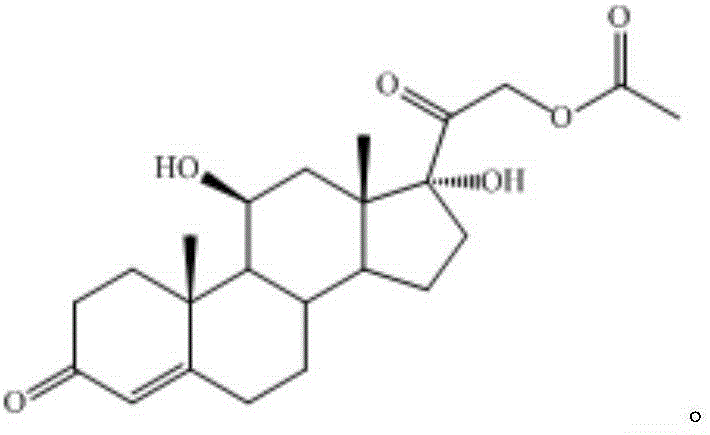
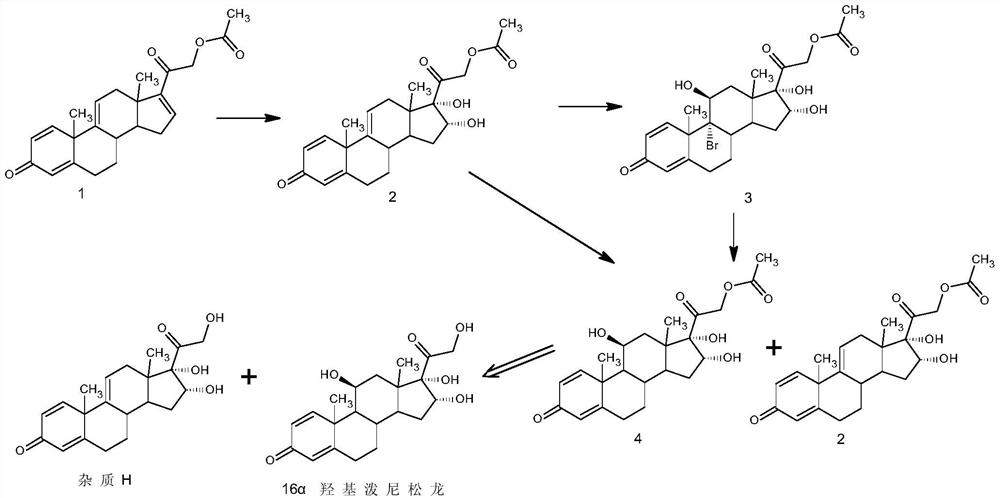
Preparation method of finished 16alpha,21-diacetoxy prednisolone
ActiveCN109081860AAvoid many difficulties such as difficult purificationEasy to operateSteroidsAcetic acidAlcohol
The invention provides a preparation method of finished 16alpha,21-diacetoxy prednisolone product, comprising: subjecting 17alpha-deshydroxy prednisolone acetate as an initial material to 16,17-epoxidation with an organic peroxy acid in a first organic solvent to obtain an epoxide; subjecting the epoxide, in a second organic solvent, to ring-opening reaction with glacial acetic acid under the catalysis of an acid catalyst to obtain the target product, 16alpha,21-diacetoxy prednisolone; subjecting the crude 16alpha,21-diacetoxy prednisolone to heating reflux discoloration and crystallization with a lower carbon alcohol of C4 and below so as to obtain the finished 16alpha,21-diacetoxy prednisolone. The intermediate, 16alpha,21-diacetoxy prednisolone, to 16alpha-hydroxy prednisolone is prepared herein via the method that is efficient, environmentally friendly and fair in cost.
Owner:HUNAN KEREY BIOTECH
Medicine for treating perennial allergic rhinitis
InactiveCN1357329AInhibitory responsePrevent proliferationOrganic active ingredientsRespiratory disorderNasal cavityAllergic pharyngitis
The present invention is one kind of medicine for treating perennial allergic rhinitis. Oral antiallergic medicine chromoglycate sodium, chlorphenamine and Prednisolone acetate and externally used rhinitis treating medicine Fuma liquid are compounded in certain proportion to prepare the medicine for treating allergic rhinitis through local application. The medicine can resist allergic reaction, inhibit excitation of sympathetic nerve, reduce the reaction of nasal mucous membrane to histamine, reduce the pathological reaction of nasal mucous membrane to various irritative damage, inhibit proliferation and exudation of connective tissue and relieve various rhinitis symptoms. It has obvious treating effects and no negative reaction.
Owner:曾咏梅
Compound preparation for treating ophthalmic infection and inflammation
InactiveCN101518649AGood treatment effectInfection applicableAntibacterial agentsOrganic active ingredientsRoxithromycinDexamethasone acetate
The invention relates to a compound preparation for treating ophthalmic infection and inflammation, which is characterized by comprising a large-ring lactone antimicrobial drug and steroidal anti-inflammatory drug and being used for treating ophthalmic infection and inflammation. The large-ring lactone antimicrobial drug comprises azithromycin, clarithromycin and roxithromycin and the like; and the steroidal anti-inflammatory drug comprises dexamethasone, dexamethasone acetate, dexamethasone sodium ascorbyl phosphate, prednisolone acetate, prednisolone, prednisolone sodium ascorbyl phosphate, betamethasone and betamethasone sodium ascorbyl phosphate and the like. The invention is a compound preparation which is superior to single drug-using and has good treatment effect.
Owner:WUHAN NUOAN PHARMACY
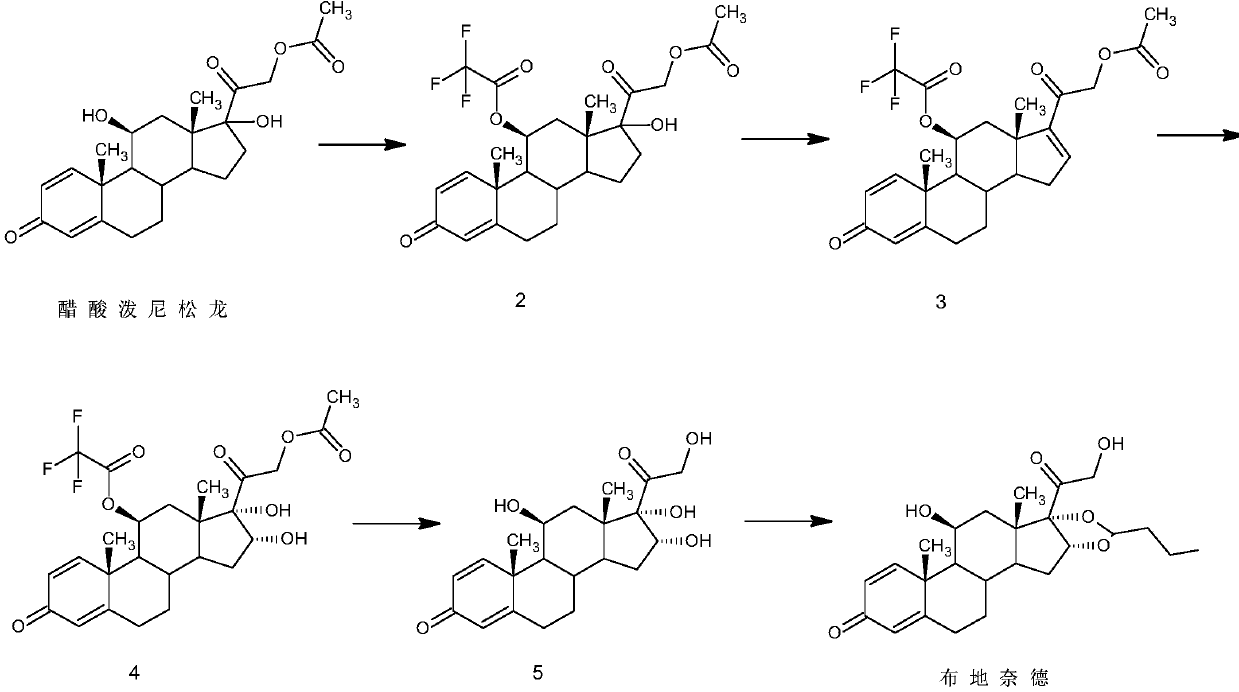
Preparation method of budesonide
The invention discloses a preparation method of budesonide. The preparation method comprises the following steps: (1) carrying out n-butyraldehyde aldolization for 16 alpha-hydroxyl prednisolone acetate as shown in formula (1) to obtain a budesonide crude prodct; (2) then hydrolyzing 21-acetates; and (3) collecting the product budesonide from reactant. By adopting the preparation method, the unstable midbody 16 alpha-hydroxyl prednisolone is avoided. The preparation method is easy to operate and control, and the total yield of the budesonide is greatly increased. The reaction formula is shown in the specification.
Owner:湖南玉新药业有限公司
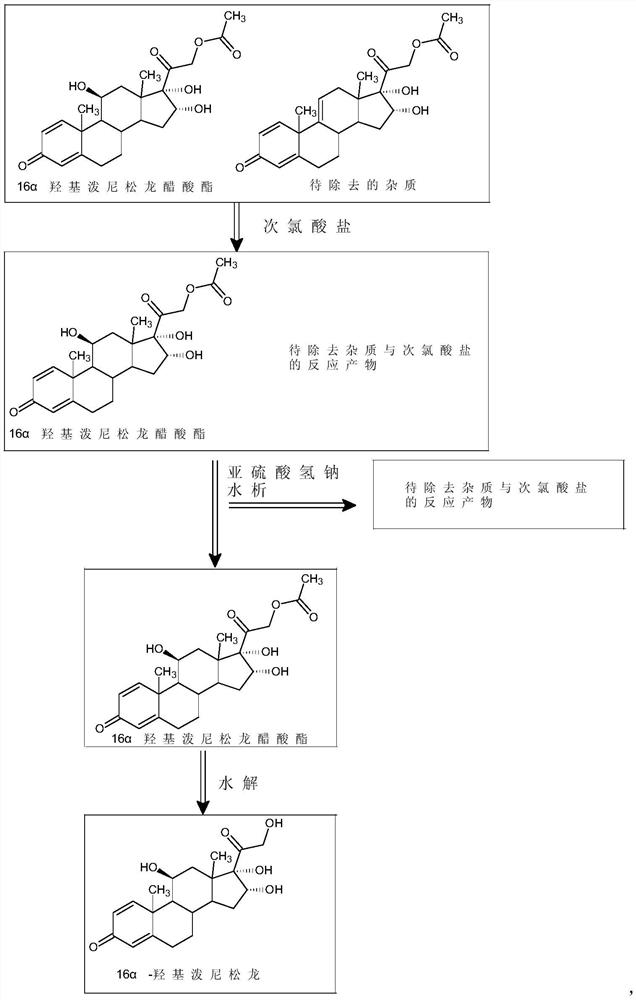
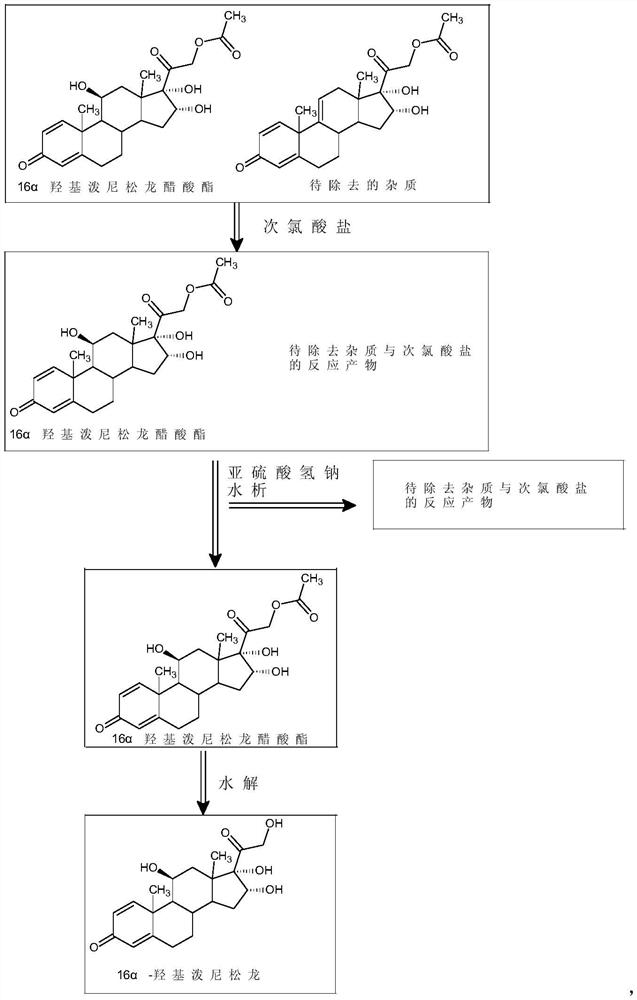
Preparation method of high-purity 16 alpha-hydroxyprednisolone
InactiveCN112125943AHigh purityMild reaction conditionsSteroidsD-alpha-Tocopheryl AcetateEthylic acid
The invention discloses a preparation method of high-purity 16 alpha hydroxyl prednisolone, and belongs to the technical field of medicine preparation and processing. The method comprises the following steps: completely dissolving a 16 alpha hydroxyl prednisolone acetate crude product in a mixed solvent of dichloromethane and alcohol, adding an organic acid, dropwise adding a hypochlorite aqueoussolution, controlling the temperature to be 15-40 DEG C, carrying out a stirring reaction to obtain a primary treatment product, removing the impurity through a hydration refining method, and hydrolyzing to obtain the high-purity 16 alpha hydroxyl prednisolone. The method is simple to operate, mild in reaction condition and short in reaction route, the purity of the final product prepared by the method is higher than 99.5%, the content of the impurity H is lower than 0.02%, and the requirement of the market on high-purity 16 alpha hydroxyl prednisolone can be easily met.
Owner:ZHEJIANG SHENZHOU PHARMA
Preparation method of budesonide
ActiveCN111560047AShort process routeHigh total mass yieldSteroids preparationBiochemical engineeringEthylic acid
The invention discloses a preparation method of budesonide, and belongs to the technical field of preparation and processing of medicines. According to the method, prednisolone acetate is used as an initial raw material, and is subjected to five steps of protection, dehydration, dihydroxy, hydrolysis and condensation to prepare the budesonide. According to the preparation method, the reaction process can be effectively shortened by improving the defects of a traditional process, reaction conditions are mild, osmium tetroxide which is high in toxicity and not environmentally friendly is avoided, environmental pollution is reduced, and the method is high in overall conversion rate, easy and convenient to operate, suitable for industrial production and wide in market prospect.
Owner:ZHEJIANG SHENZHOU PHARMA
Compound kanamycin sulfate injection and preparation method
InactiveCN104173362AReduce chance of drug resistanceBroad spectrum antibacterialAntibacterial agentsOrganic active ingredientsCombined treatmentAdjuvant therapy
The invention relates to a compound kanamycin sulfate injection and its preparation method. The composition of the compound kanamycin sulfate injection and its preparation steps are as follows: (1) aminopyrine and prednisolone acetate are put into 95% ethanol, and stirring is carried out for 10-25 min until the solution is clear; (2) 300L of injection water is taken, and kanamycin sulfate and lincomycin hydrochloride are successively added and stirred until dissolved; and (3) solutions prepared in the steps (1) and (2) are merged, injection water is added to 1000L, the solution is uniformly stirred, and standing lasts for 15-20 min until the liquid medicine is clear. A compound preparation of kanamycin sulfate and lincomycin hydrochloride is adopted, and aminopyrine and prednisolone acetate which have efficacy of adjuvant therapy are added. With combined utilization of the above four medicines, the compound kanamycin sulfate injection has an antibacterial effect on Gram-negative bacteria and Gram-positive bacteria, has wide antibacterial spectrum to widen antimicrobial range, can comprehensively cure swine paratyphoid and some complications and has high and fast curative effects. By the use of the compound kanamycin sulfate injection, drug resistance probability of bacteria is minimized, and curative effect is enhanced.
Owner:TIANJIN BIJIA BIOTECH
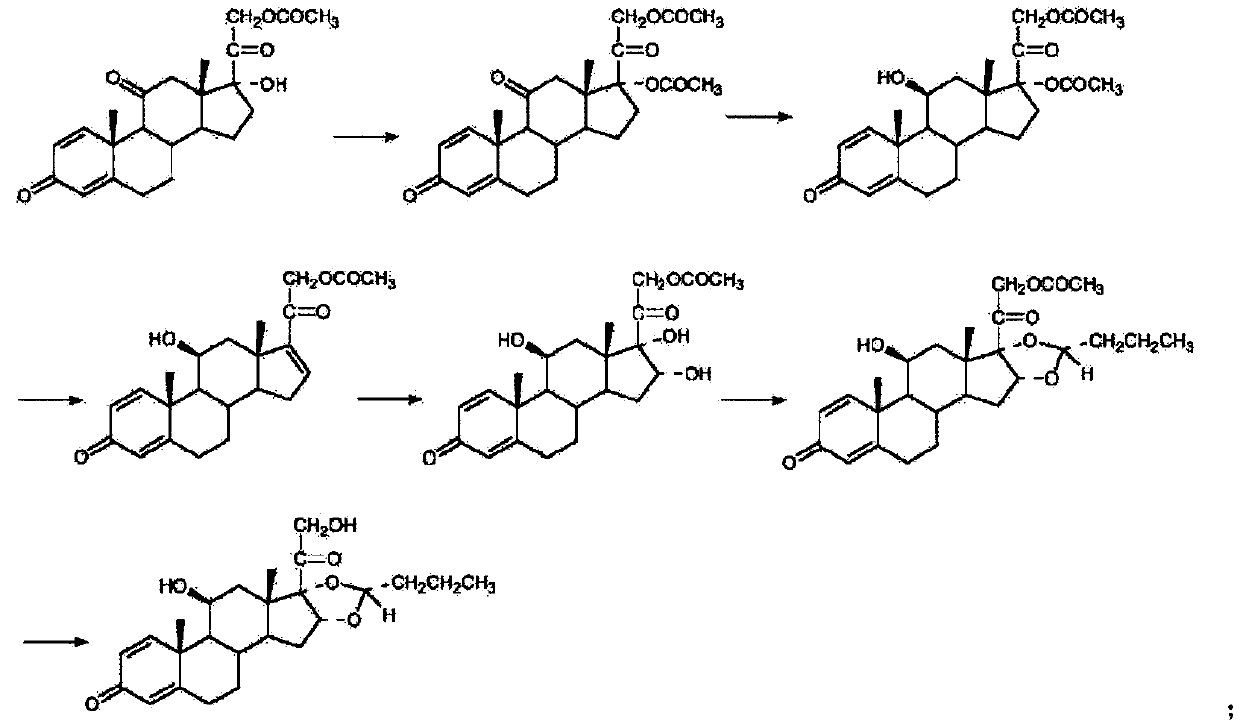
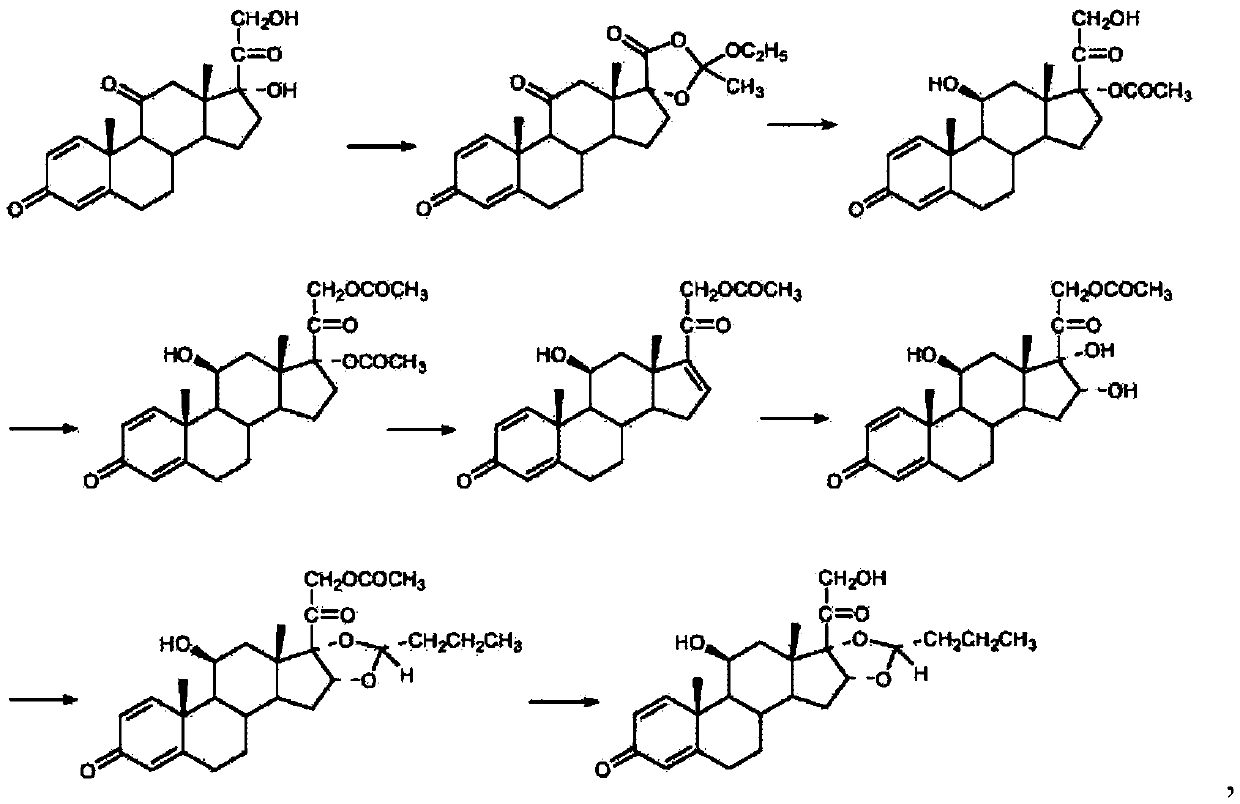
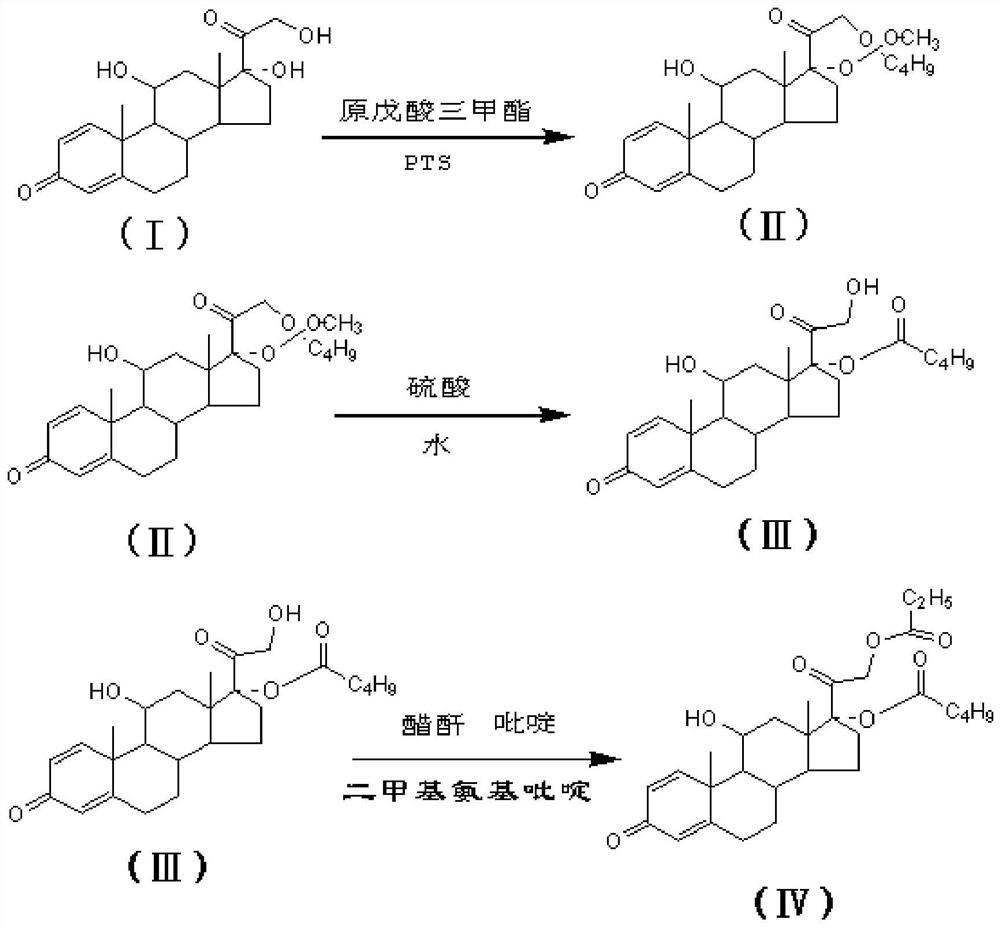
Chemical Synthesis Process of Prednisolone Acetate Valerate
ActiveCN109608511BSuitable for mass productionEasy to produceSteroidsChemical synthesisAcetic anhydride
The invention belongs to the technical field of chemical synthesis, and discloses a chemical synthesis process of prednisolone acetate valerate, using prednisolone, p-toluenesulfonic acid, trimethyl orthovalerate, dimethylaminopyridine, pyridine and acetic anhydride as raw materials, and synthesized prednisolone acetate valerate through fewer process steps, which not only simplifies the production process, significantly reduces the production cost, but also has high yield and product purity, and is suitable for large-scale production in factories , has good industrial development value and good market application prospect.
Owner:SHANGHAI NEW HUALIAN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com