Compound kanamycin sulfate injection and preparation method
A technology of kanamycin sulfate and injection, which is applied in medical formulas, medical preparations containing active ingredients, drug delivery, etc., can solve problems such as the limitation of bactericidal effect, reduce the probability of drug resistance, reduce the number of medications, and improve the curative effect And the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] A compound kanamycin sulfate injection, every 1000L injection is made of the following raw materials:
[0021] Lincomycin hydrochloride 30kg, kanamycin sulfate 30kg, aminopyrine 50kg, prednisolone acetate 1kg, 95% ethanol 100L, the rest is water for injection.
[0022] A preparation method of compound kanamycin sulfate injection, the steps are as follows:
[0023] (1) Put aminopyrine and prednisolone acetate into 95% ethanol, stir for 10-25 minutes until clear;
[0024] (2) Get 300L of water for injection, add kanamycin sulfate and lincomycin hydrochloride successively and stir to dissolve;
[0025] (3) Combine the solutions prepared by "(1)" and "(2)", add water for injection to 1000L, stir evenly, keep for 15-20 minutes, and wait for the liquid to become clear.
Embodiment 2
[0027] A compound kanamycin sulfate injection, every 1000L injection is made of the following raw materials:
[0028] Lincomycin hydrochloride 110kg, kanamycin sulfate 110kg, aminopyrine 150kg, prednisolone acetate 20kg, 95% ethanol 400L, the rest is water for injection,
[0029] A preparation method of compound kanamycin sulfate injection, the steps are as follows:
[0030] (1) Put aminopyrine and prednisolone acetate into 95% ethanol, stir for 10-25 minutes until clear;
[0031] (2) Get 300L of water for injection, add kanamycin sulfate and lincomycin hydrochloride successively and stir to dissolve;
[0032] (3) Combine the solutions prepared by "(1)" and "(2)", add water for injection to 1000L, stir evenly, keep for 15-20 minutes, and wait for the liquid to become clear.
Embodiment 3
[0034] A compound kanamycin sulfate injection, every 1000L injection is made of the following raw materials:
[0035] Lincomycin hydrochloride 90kg, kanamycin sulfate 90kg, aminopyrine 100kg, prednisolone acetate 15kg, 95% ethanol 300L, the rest is water for injection,
[0036] A preparation method of compound kanamycin sulfate injection, the steps are as follows:
[0037] (1) Put aminopyrine and prednisolone acetate into 95% ethanol, stir for 10-25 minutes until clear;
[0038] (2) Get 300L of water for injection, add kanamycin sulfate and lincomycin hydrochloride successively and stir to dissolve;
[0039] (3) Combine the solutions prepared by "(1)" and "(2)", add water for injection to 1000L, stir evenly, keep for 15-20 minutes, and wait for the liquid to become clear.
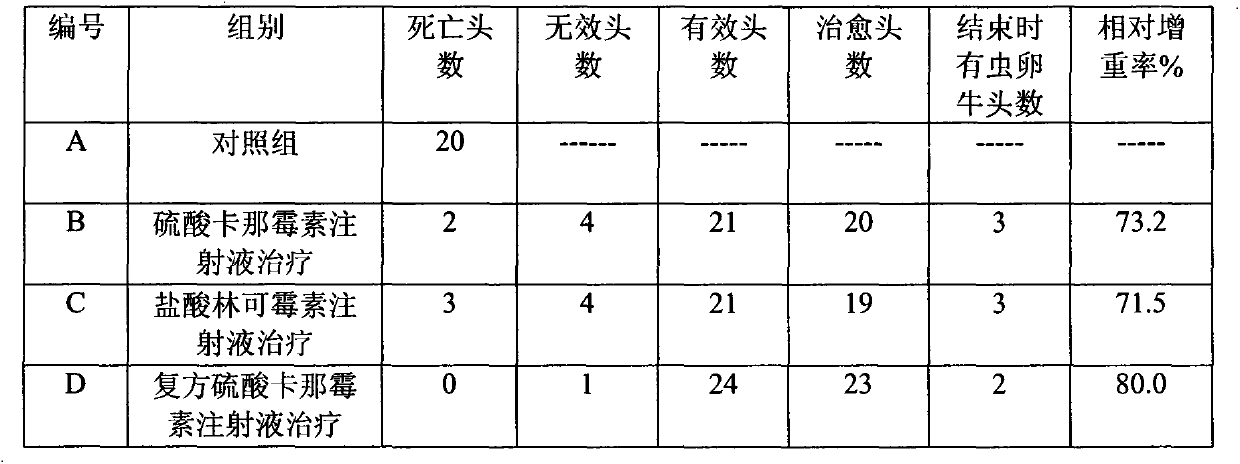
[0040] Clinical Trials:
[0041] 1. Experimental animals:
[0042] Randomly select 100 diseased adult pigs with a body weight of 40-80kg.
[0043] 2. Experimental drug:
[0044] Compound Kanamycin Sul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com