Preparation method of high-purity 16 alpha-hydroxyprednisolone
A technology of hydroxyprednisolone and hydroxyprednisolone acetate, which is applied in the field of preparation of high-purity 16α-hydroxyprednisolone, can solve problems such as difficult refining and difficult removal of impurities, and achieve improved purity and operating costs The effect of low, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
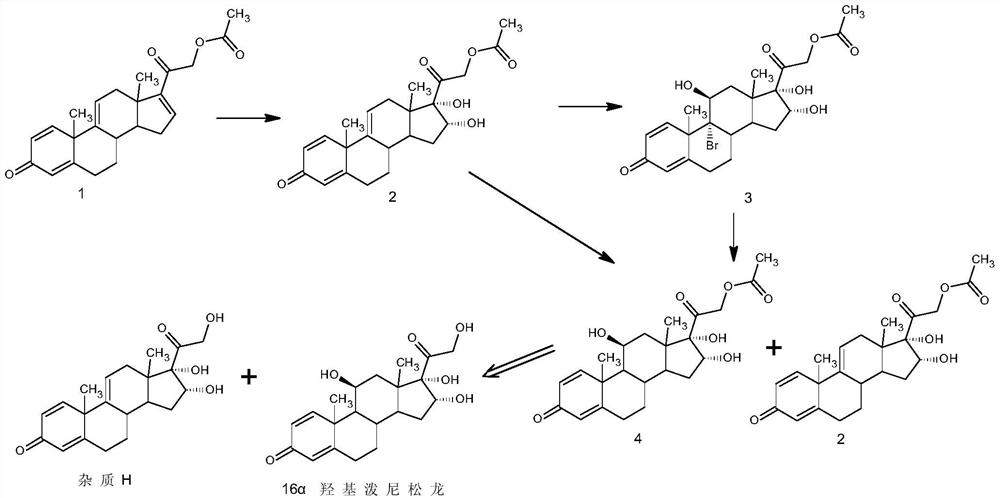
Method used
Image
Examples
Embodiment 1
[0026] The preparation of embodiment 1 high-purity 16α-hydroxyprednisolone
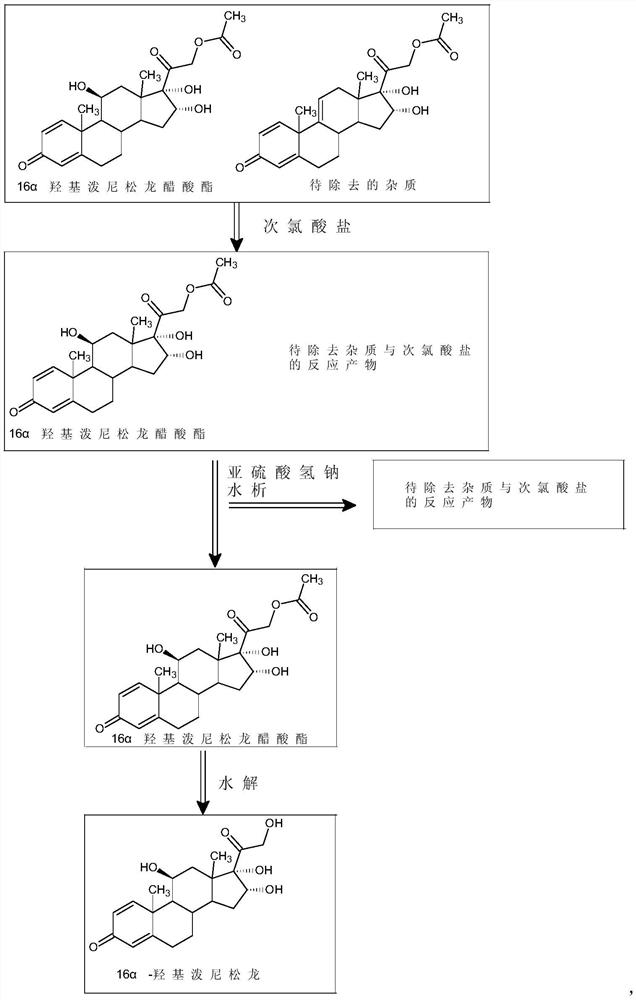
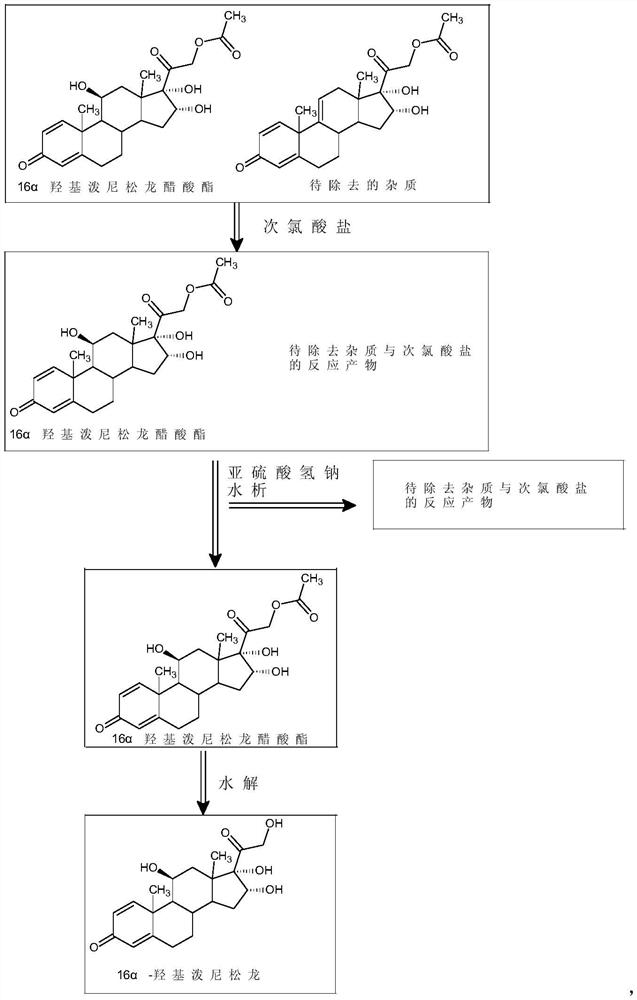
[0027] 50 grams of 16α-hydroxyprednisolone acetate crude product was dissolved in 700 milliliters of dichloromethane and 700 milliliters of ethanol, then 1.5 milliliters of formic acid was added, 24 milliliters of 5% sodium hypochlorite aqueous solution was added dropwise, and the temperature was controlled at 15°C to stir the reaction to obtain the preliminary treatment product ;
[0028] Add 150 milliliters of 5% sodium bisulfite aqueous solution to the above-mentioned preliminary treatment product, concentrate to remove the solvent, add water for water analysis, and filter to obtain 45 grams of 16α-hydroxyprednisolone acetate fine product;
[0029] Dissolve 45 grams of 16α-hydroxyprednisolone acetate refined product obtained above in a mixed organic solvent of 250 milliliters of dichloromethane and 250 milliliters of methanol, stir and cool to 0° C., add 90 milliliters of 10% sodium sulfite aqueous...
Embodiment 2
[0030] The preparation of embodiment 2 high purity 16α-hydroxy prednisolone
[0031] 50 grams of 16α-hydroxyprednisolone acetate crude product was dissolved in 200 milliliters of dichloromethane and 300 milliliters of methanol, then added 4.8 milliliters of propionic acid, 12 milliliters of 10% sodium hypochlorite aqueous solution was added dropwise, and the temperature was controlled to stir at 25°C to obtain preliminary treatment Taste;
[0032] Add 100 milliliters of 15% sodium bisulfite aqueous solution to the above-mentioned preliminary treatment product, concentrate to remove the solvent, add water for water analysis, and filter to obtain 46 grams of 16α-hydroxyprednisolone acetate fine product;
[0033] Dissolve 46 grams of 16α-hydroxyprednisolone acetate refined product obtained above in a mixed solvent of 600 milliliters of dichloroethane and 350 milliliters of isopropanol, stir and cool to -10°C, add 80 milliliters of 20% sodium sulfite aqueous solution, stir After ...
Embodiment 3
[0034] The preparation of embodiment 3 high-purity 16α-hydroxyprednisolone
[0035] 50 grams of crude product of 16α-hydroxyprednisolone acetate was dissolved in 500 milliliters of dichloromethane and 400 milliliters of isopropanol, then 3 milliliters of glacial acetic acid was added, 5 milliliters of 13% sodium hypochlorite aqueous solution was added dropwise, and the temperature was controlled at 40° C. to stir the reaction, get primary treatment;
[0036] Add 25 milliliters of 30% sodium bisulfite aqueous solution to the above-mentioned preliminary treatment product, concentrate to remove the solvent, add water for water analysis, and filter to obtain 45.5 grams of 16α-hydroxyprednisolone acetate fine product;
[0037] 45.5 grams of 16α-hydroxyprednisolone acetate fines obtained above were dissolved in 850 milliliters of ethanol and 500 milliliters of tetrahydrofuran mixed organic solvent, stirred and cooled to 10 ℃, added 70 milliliters of 30% sodium sulfite aqueous soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com