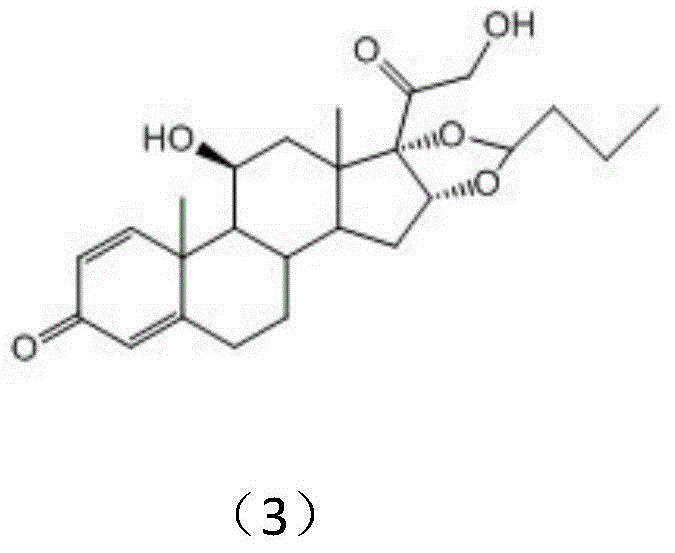
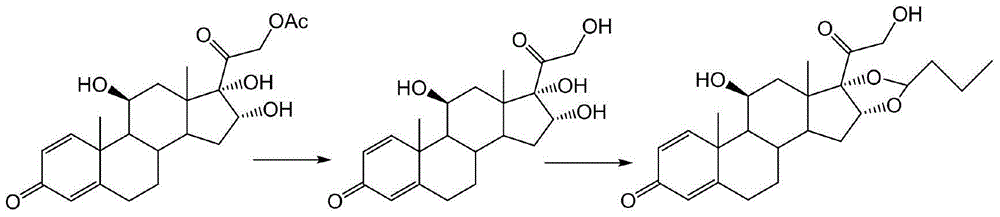
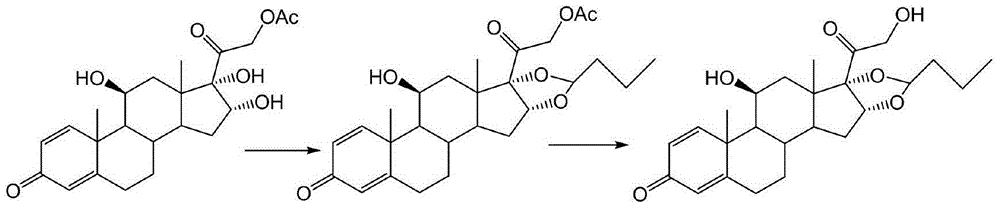
Preparation method of budesonide
A monohydric alcohol and solvent technology, applied in the field of preparation of budesonide, can solve the problems of low total product yield, rearrangement of pinacol, instability of 16alpha-hydroxyprednisolone, etc., to achieve easy operation, controllability, The effect of improving the total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Add 50 milliliters of acetonitrile and 5 milliliters of n-butyraldehyde in the reaction kettle, drop into 10 grams of 16alpha-hydroxyprednisolone acetate after passing through nitrogen once, continue to add 1 milliliter of 70% perchloric acid under stirring with nitrogen, control The reaction was carried out at an internal temperature of 45° C. for 2 hours, and TLC detected that the reaction of the raw materials was complete. Cool down to room temperature, and adjust the pH to neutral with an appropriate amount of triethylamine. Then it was concentrated to 10% of the original volume under negative pressure at about 50 degrees; cooled to room temperature.
[0041] Add 50 milliliters of methanol and 10 milliliters of dichloromethane into the reactor, stir and dissolve the materials, then cool down to minus 5°C, keep warm, slowly add 10 milliliters of methanol solution with 1 gram of potassium hydroxide dropwise, and control The inner temperature must not exceed zero, and...
Embodiment 2
[0044] Add 50 milliliters of tetrahydrofuran and 5 milliliters of n-butyraldehyde to the reaction kettle, and then put in 10 grams of 16alpha-hydroxyprednisolone acetate after nitrogen replacement once, continue to stir with nitrogen and add 1.5 milliliters of 70% perchloric acid to control the internal temperature. The reaction was carried out at 40°C for 2 hours, and TLC detected that the reaction of the raw materials was complete. Cool down to room temperature, and adjust the pH to neutral with an appropriate amount of triethylamine. Then it was concentrated to 10% of the original volume under negative pressure at about 40 degrees; cooled to room temperature.
[0045] Add 50 milliliters of methanol and 10 milliliters of dichloromethane into the reactor, stir and dissolve the materials, then lower the temperature to minus 5 degrees, keep warm, slowly add 10 milliliters of methanol solution with 1 gram of potassium hydroxide dropwise, and control the internal temperature. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com