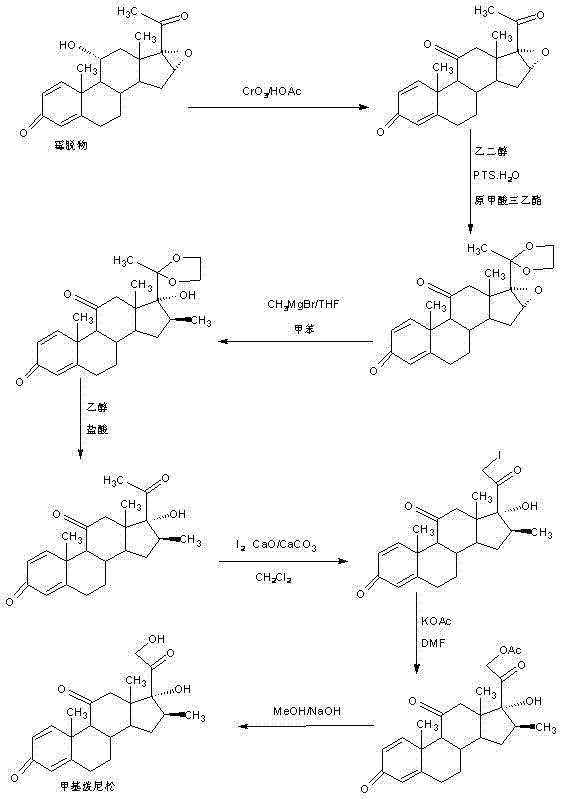
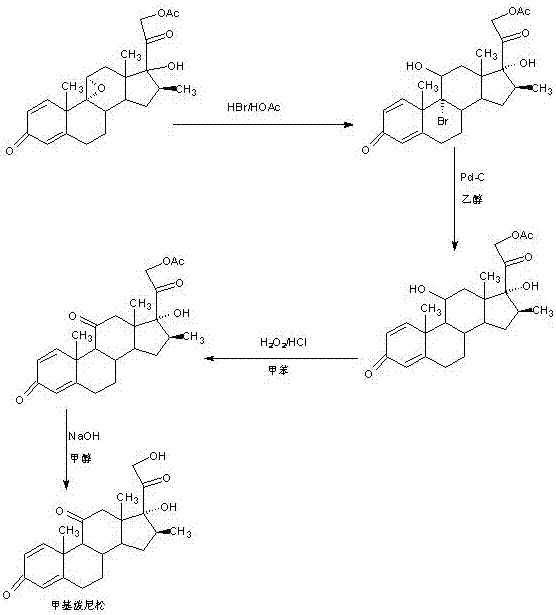
Preparation method of methyl metacortandracin
A technology of methylprednisone and methylprednisone acetate, applied in the field of steroid hormone drug preparation, can solve the problems of uneconomical and environmentally friendly synthesis, complex process operation, expensive production raw materials, etc. Economic and environmental protection, easy production and operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A. Synthesis of bromohydryl compounds
[0025] In a 1000ml three-neck flask, add 100g DB11, 250ml glacial acetic acid, 100g 40% hydrobromic acid, keep warm at 20-25 degrees and stir for 4-6 hours. TLC detects the reaction end point. After the reaction, add 100g 20% caustic soda Neutralize the solution, then concentrate under reduced pressure to recover the solvent, cool, add 500ml of tap water, stir and crystallize for 60-90 minutes, centrifuge, wash, spin filter, discharge the filtrate and washing liquid to the waste water treatment pool, and dry the filter cake to obtain bromohydroxyl Product: 9a bromo-16b methyl-prednisolone acetate 122g, HPLC content 98.5%, weight yield 122%;
[0026] B. Synthesis of debrominated compounds
[0027] In a 2000ml three-necked flask, add 100g bromohydroxide, 1000ml DMF, completely dissolve bromohydroxide at 20-25 degrees, feed nitrogen, stir, add 60g ammonium acetate and 5g 1% palladium carbon catalyst, replace the air, Keep warm at...
Embodiment 2
[0033] A. Synthesis of bromohydryl compounds
[0034] In a 1000ml three-necked flask, add 100g DB11, 250ml chloroform, and 100g 40% hydrobromic acid, keep warm at 20-25 degrees and stir for 4-6 hours. TLC detects the reaction end point. After the reaction, add 100g 20% caustic soda solution Neutralize, separate water, wash, dry with anhydrous sodium sulfate, then concentrate under reduced pressure to recover the solvent, cool, add 500ml tap water, stir and crystallize for 60-90 minutes, centrifuge, wash, spin filter, and discharge the filtrate and lotion to Waste water treatment pond, filter cake is dried to obtain bromohydroxyl product: 9a bromo-16b methyl-prednisolone acetate 120g, HPLC content 99.0%, weight yield 120%;
[0035] B. Synthesis of debrominated compounds
[0036] In a 2000ml three-necked flask, add 100g bromohydroxide, 1000ml 90% ethanol, completely dissolve the bromine hydroxy substance at 20-25 degrees, feed nitrogen, stir, add 60g sodium bicarbonate and 5g...
Embodiment 3
[0042] A. Synthesis of bromohydryl compounds
[0043] In a 1000ml three-neck flask, add 100g DB11, 250ml alcohol, 100g 40% hydrobromic acid, keep warm at 20-25 degrees and stir for 4-6 hours. TLC detects the reaction end point. After the reaction, add 100g 40% sodium carbonate Neutralize the solution, then concentrate under reduced pressure to recover the solvent, cool, add 500ml of tap water, stir and crystallize for 60-90 minutes, centrifuge, wash, spin filter, discharge the filtrate and washing liquid to the waste water treatment tank, and dry the filter cake to obtain the bromohydroxyl compound : 9a bromo-16b methyl-prednisolone acetate 121g, HPLC content 99.2%, weight yield 121%;
[0044] B. Synthesis of debrominated compounds
[0045]In a 2000ml three-necked flask, add 100g of bromohydrin and 1000ml of 90% ethanol, completely dissolve the bromohydrin at 20-25 degrees, blow in nitrogen, stir, add 60g of triethylamine and 15g of Raney nickel catalyst, and replace the air ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com