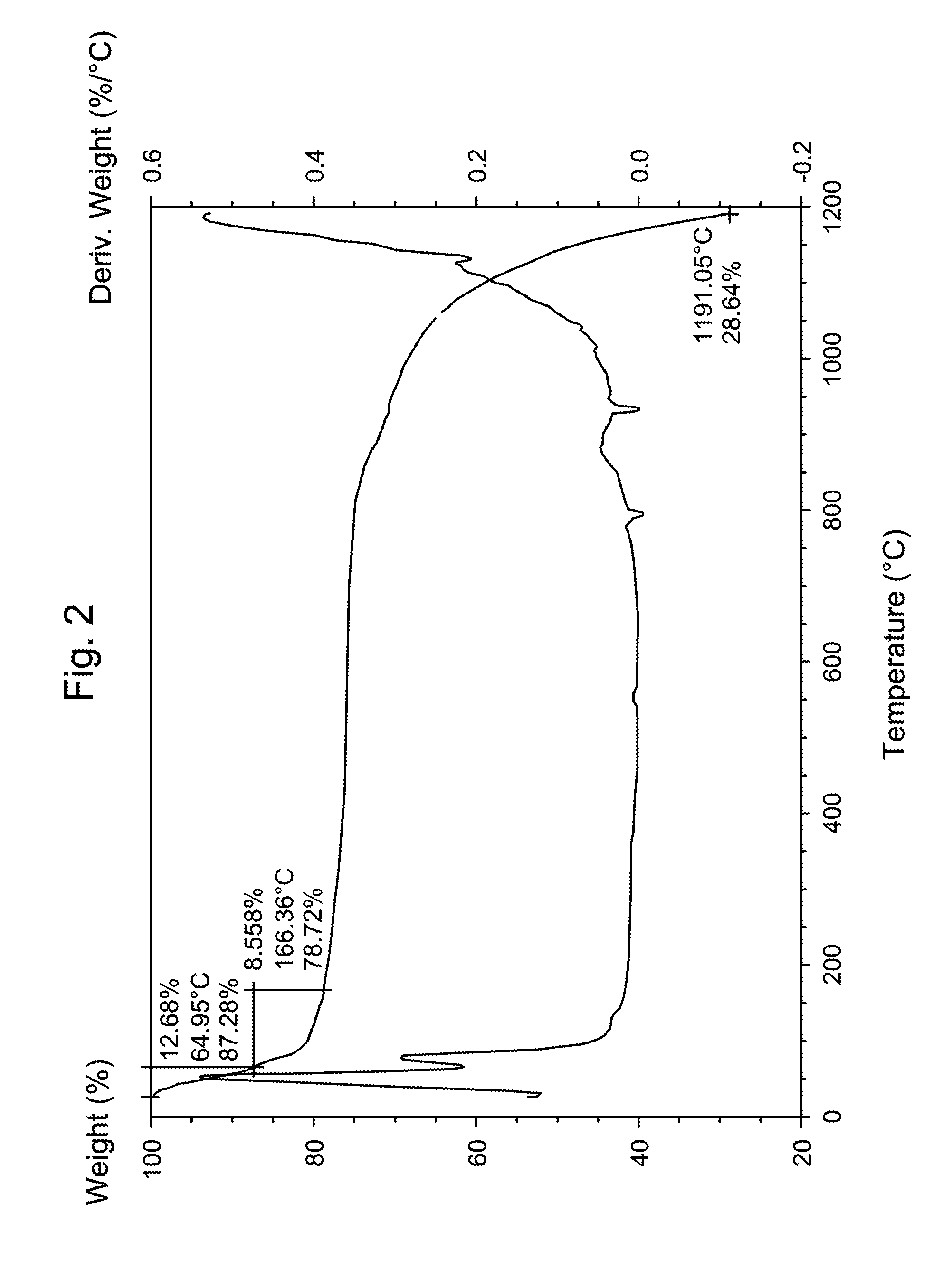
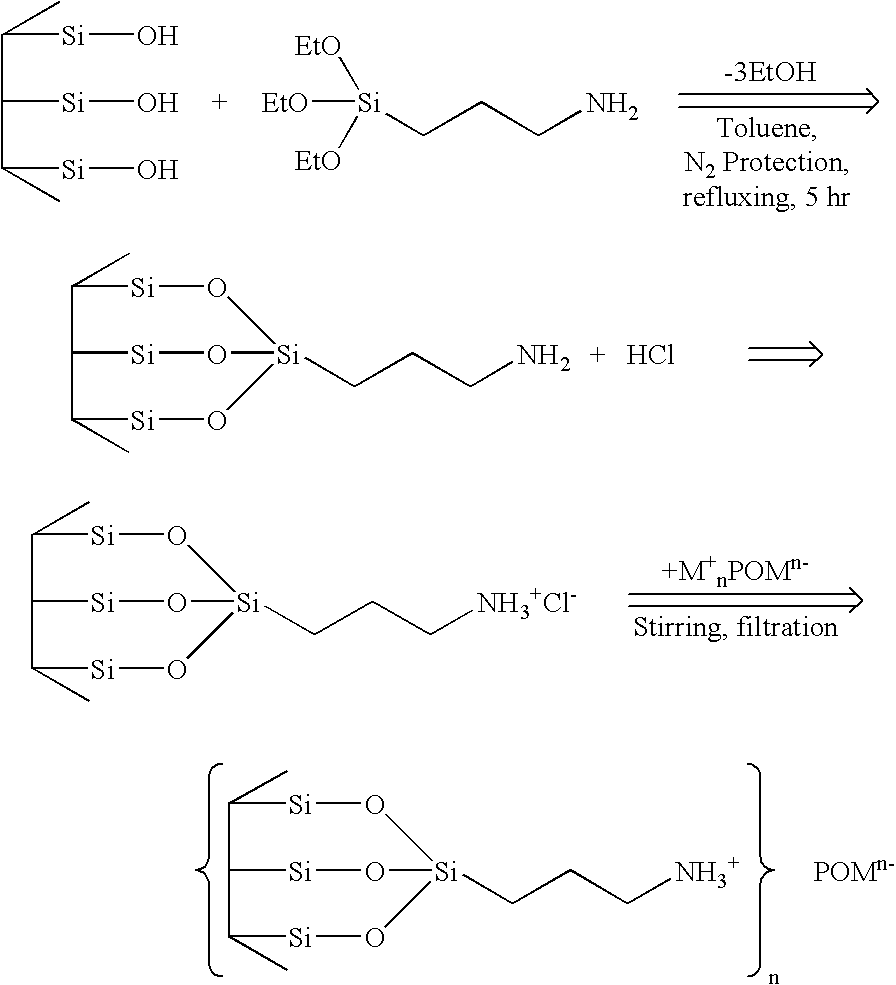
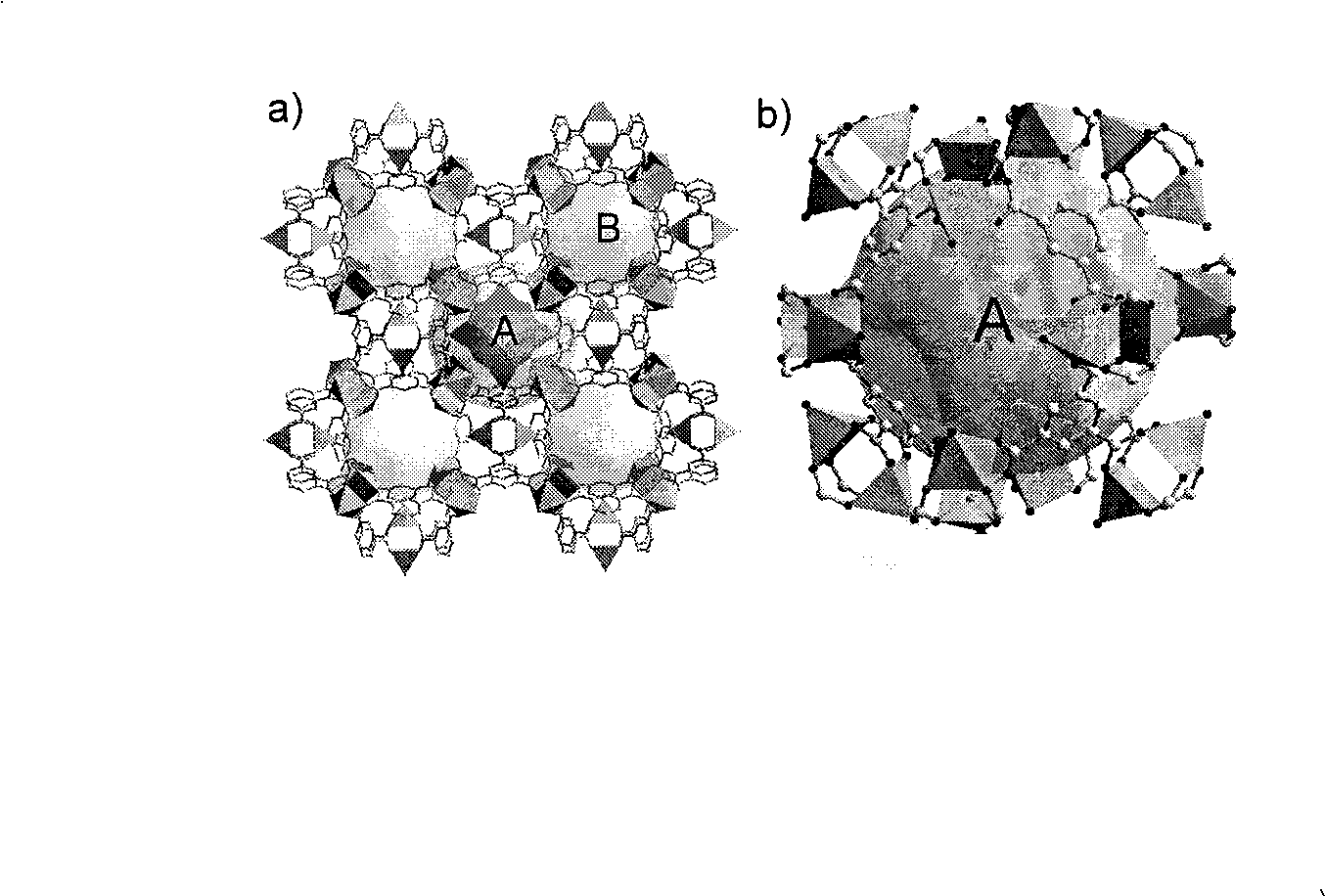
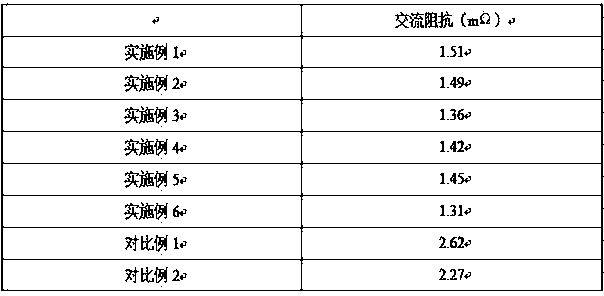
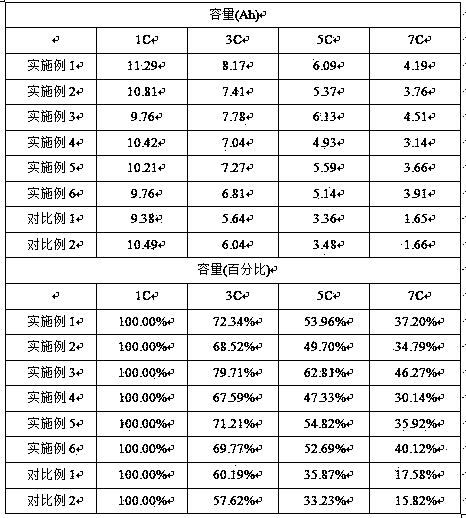
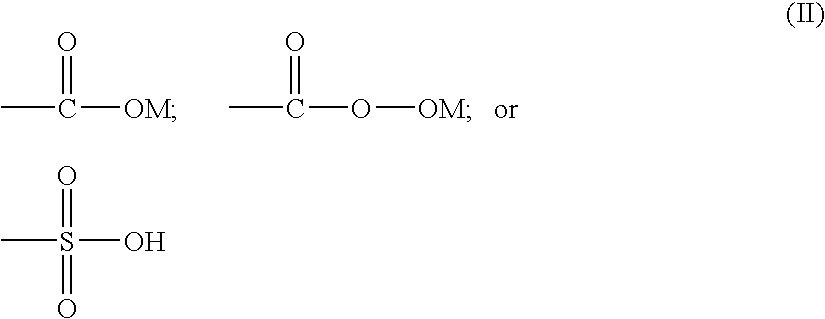Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
362 results about "Polyoxometalate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
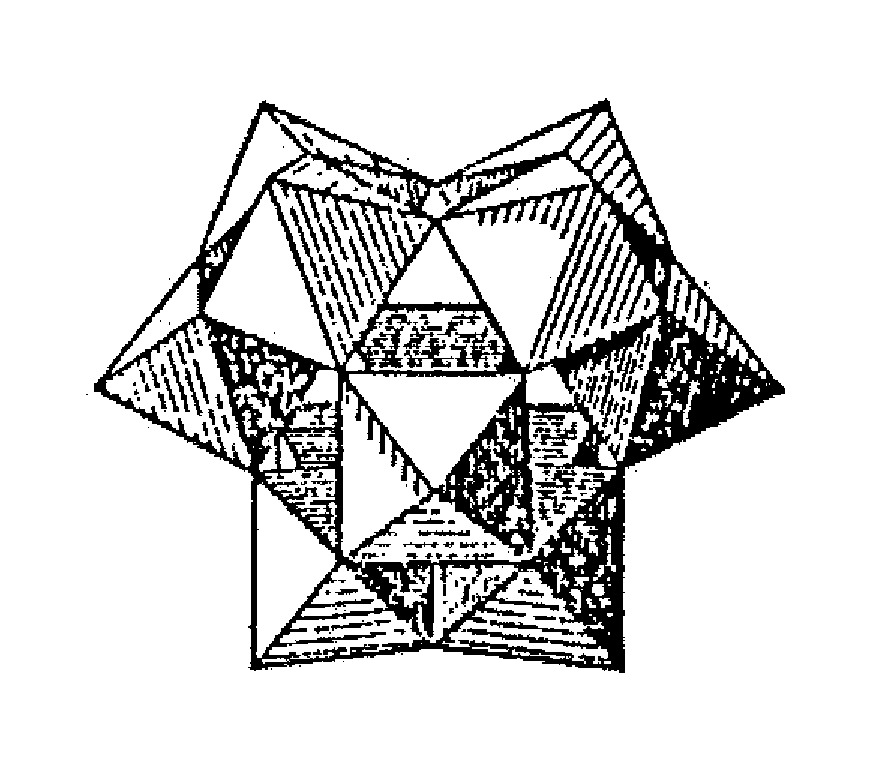
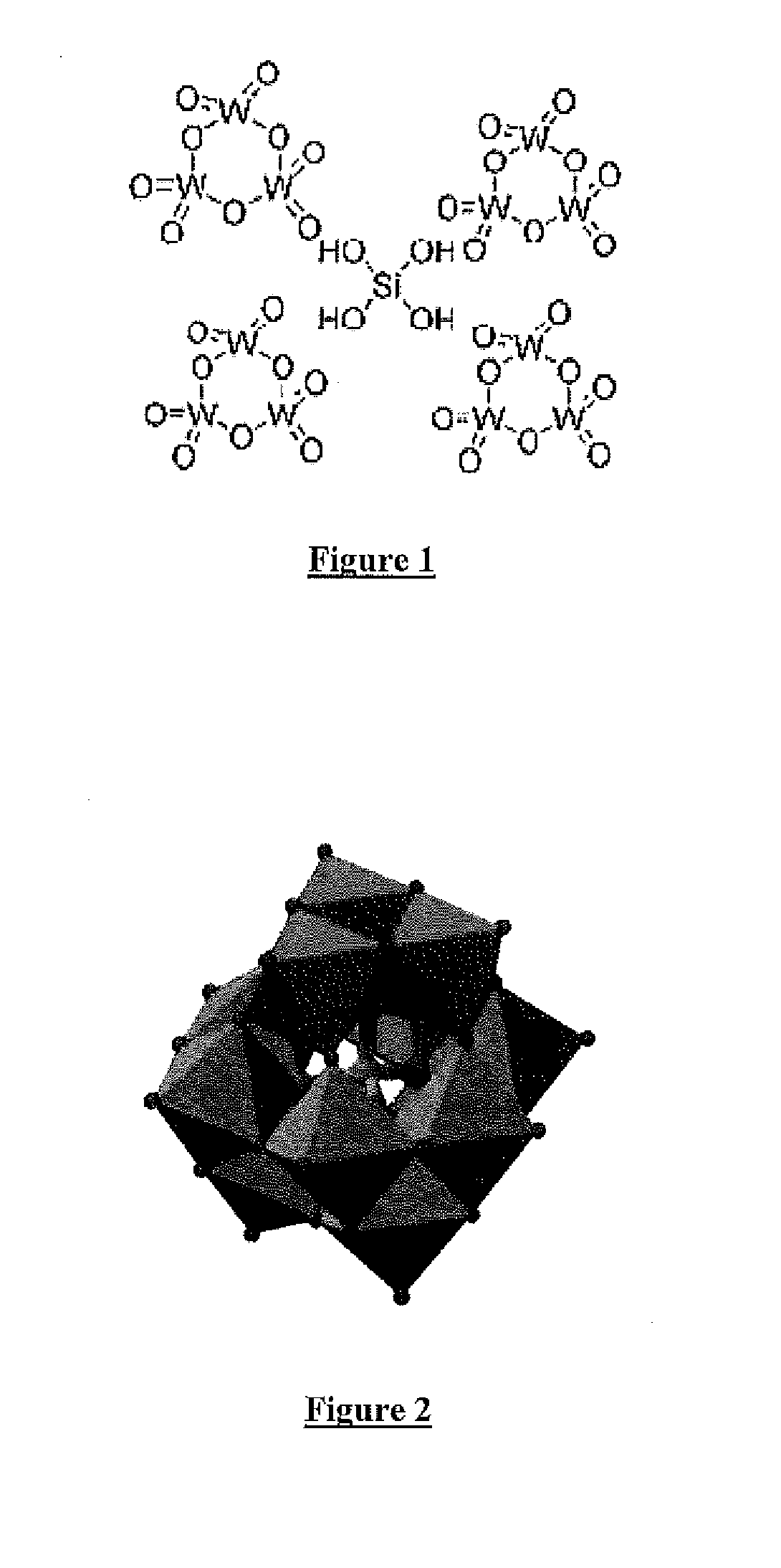
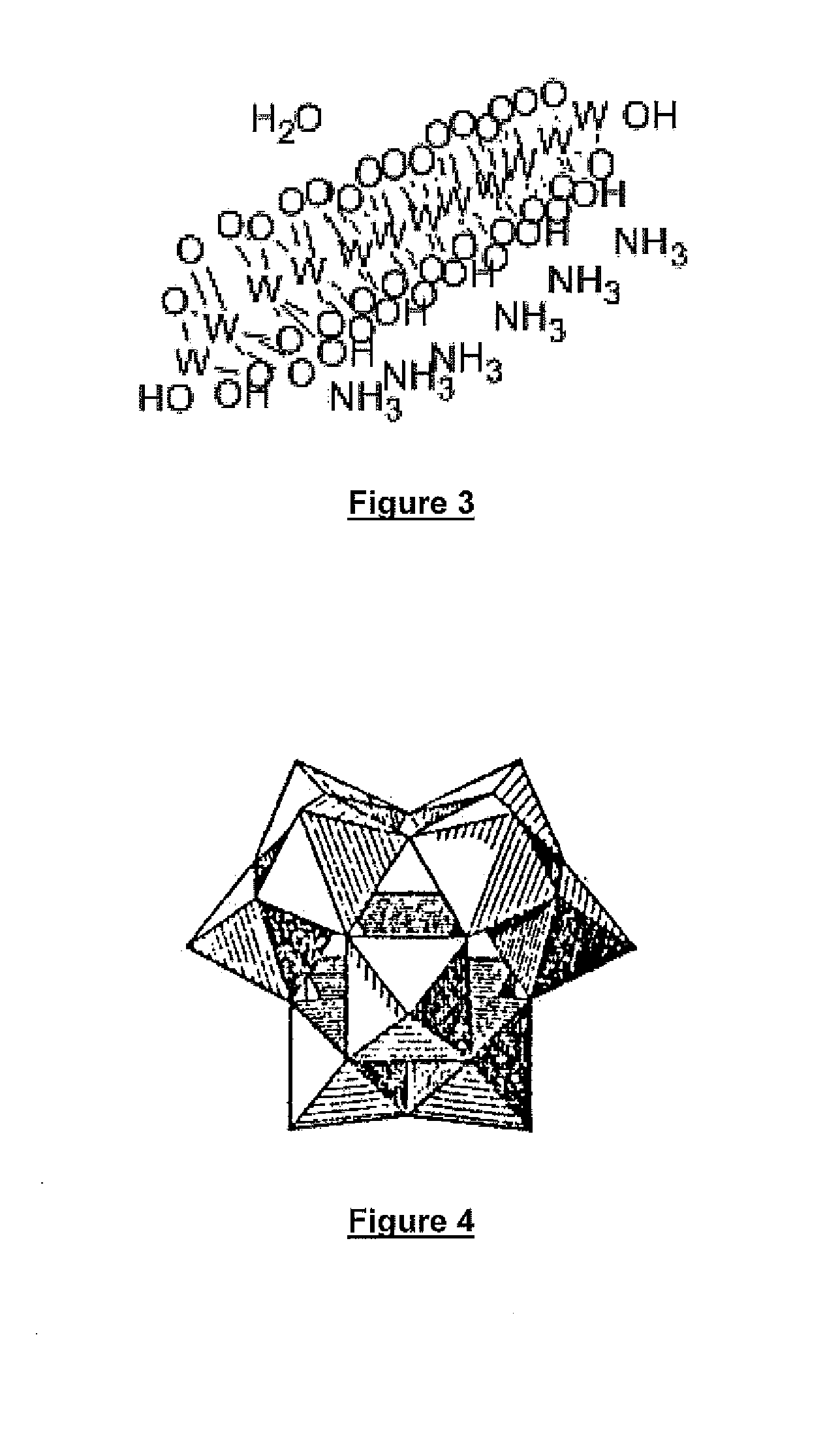
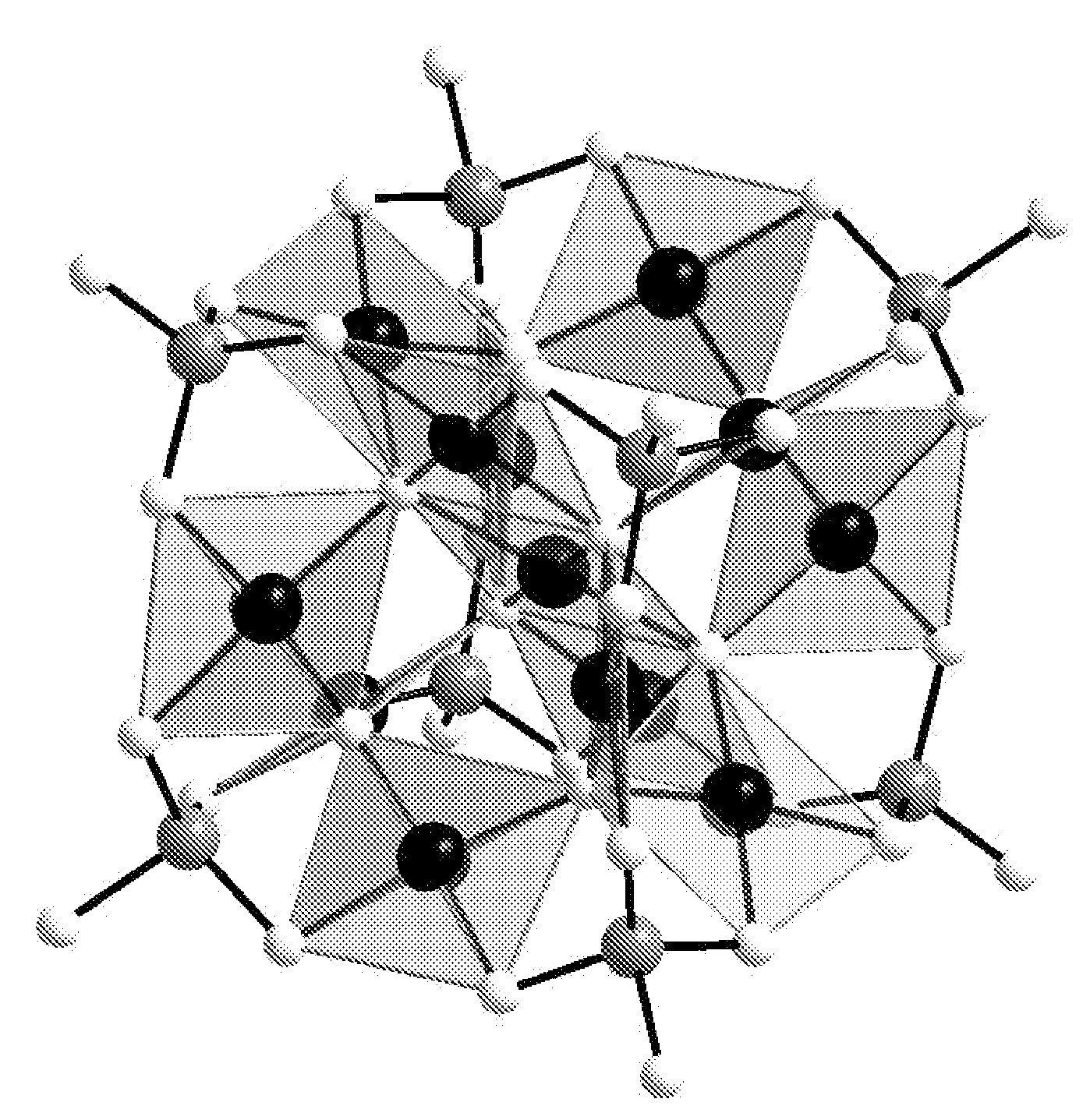
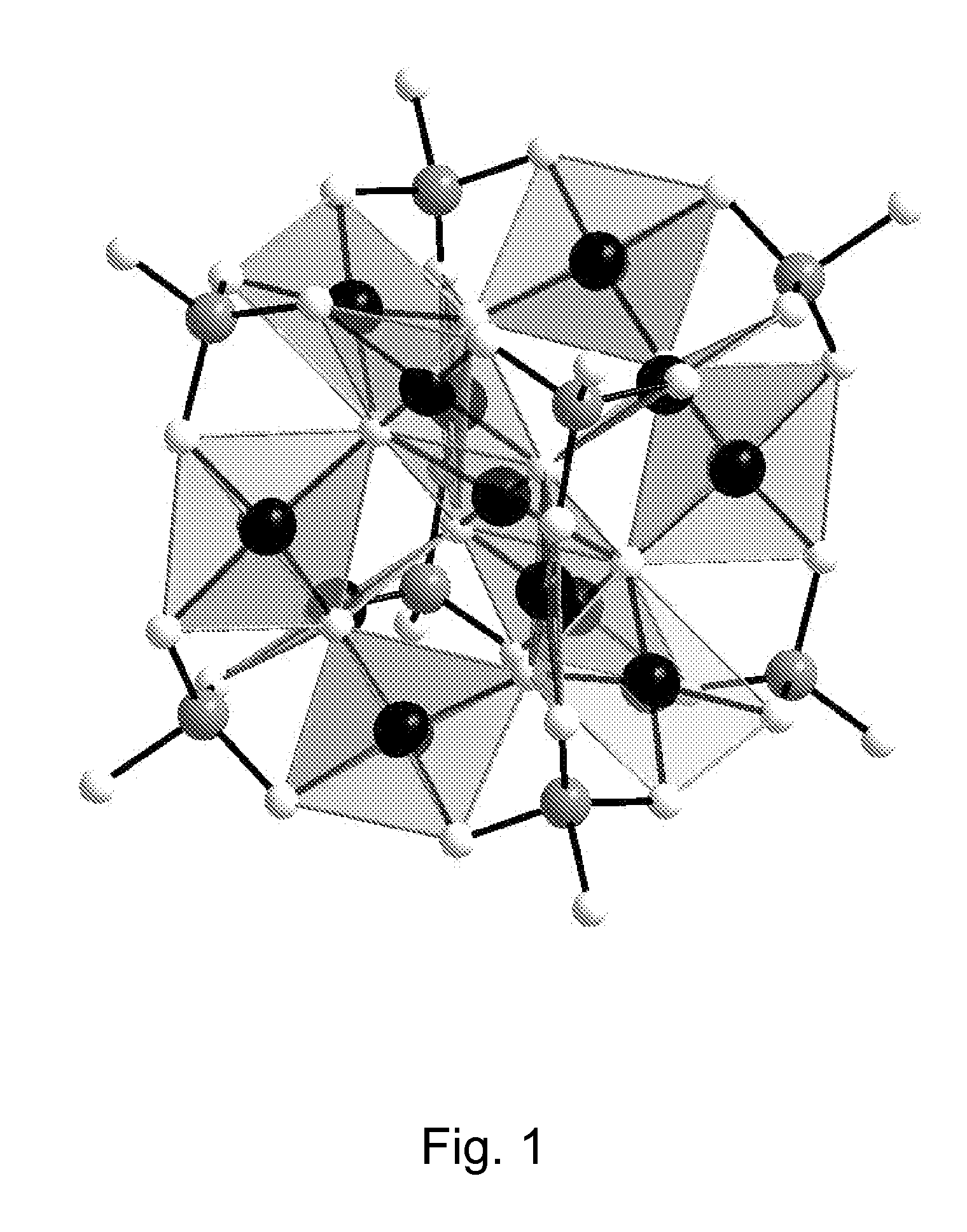
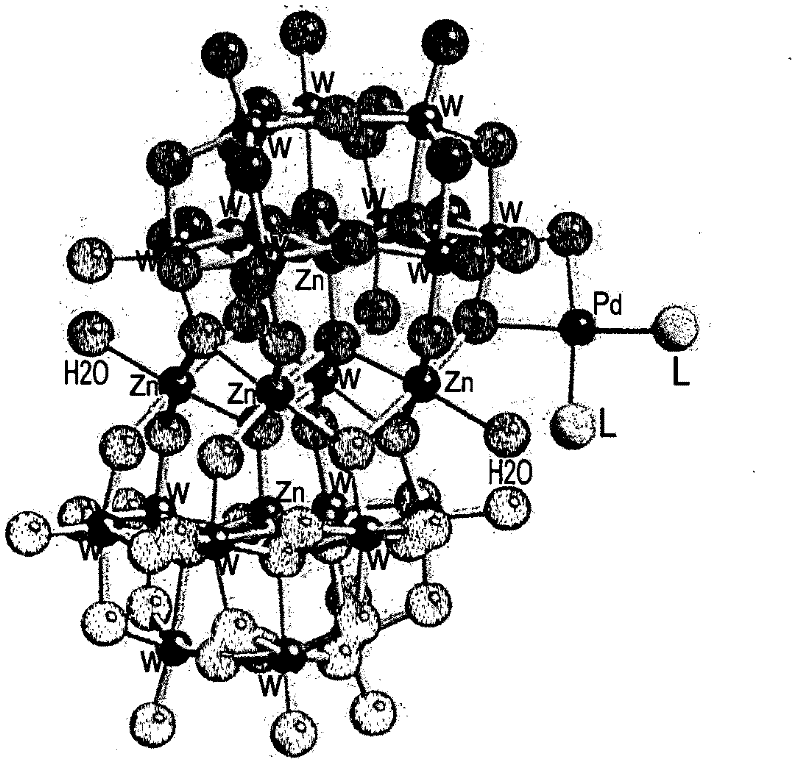
In chemistry, a polyoxometalate (abbreviated POM) is a polyatomic ion, usually an anion, that consists of three or more transition metal oxyanions linked together by shared oxygen atoms to form closed 3-dimensional frameworks. The metal atoms are usually group 6 (Mo, W) or less commonly group 5 (V, Nb, Ta) transition metals in their high oxidation states. They are usually colorless or orange, diamagnetic anions. Two broad families are recognized, isopolymetalates, composed of only one kind of metal and oxide, and heteropolymetalates, composed of one metal, oxide, and a main group oxyanion (phosphate, silicate, etc.). Many exceptions to these general statements exist.
Microspheres capable of binding radioisotopes, optionally comprising metallic microparticles, and methods of use thereof
One aspect of the present invention relates to a microsphere, comprising a hydrophilic polymer comprising a plurality of pendant anionic groups; a transition-metal, lanthanide or group 13-14 metal oxide, polyoxometalate or metal hydroxide or combination thereof; and a first radioisotope that emits a therapeutic β-particle. In certain embodiments, the microsphere further comprsies a second radioisotope that emits a diagnostic γ-ray; wherein the atomic number of the first radioisotope is not the same as the atomic number of the second radioisotope. In certain embodiments, the microsphere is composed of polymer impregnated with zirconia bound to 32p as the source of the therapeutic β-emissions and 67Ga as the source of the diagnostic γ-emissions. Another aspect of the present invention relates to the preparation of a microsphere impregnated with a radioisotope that emits therapeutic β-particles and a radioisotope that emits diagnostic β-emitting radioisotope and a γ-emitting radioistope; wherein the atomic number of the first radioisotope is not the same as the atomic number of the second radioisotope. In certain embodiments, said microspheres are administered to the patient through a catheter. In another embodiment, the microsphere is combined with the radioisotopes at the site of treatment.
Owner:BIOSPHERE MEDICAL INC
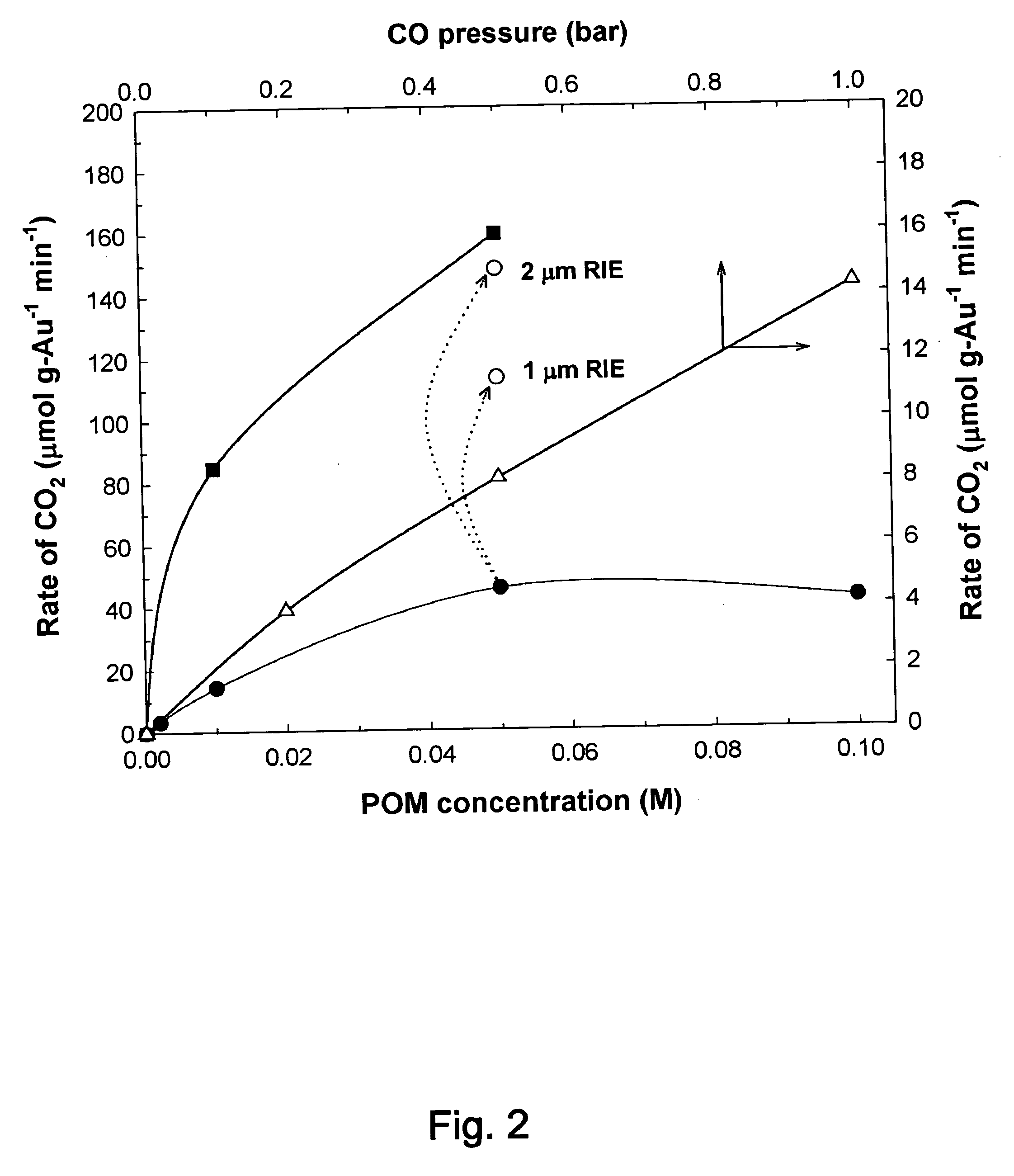
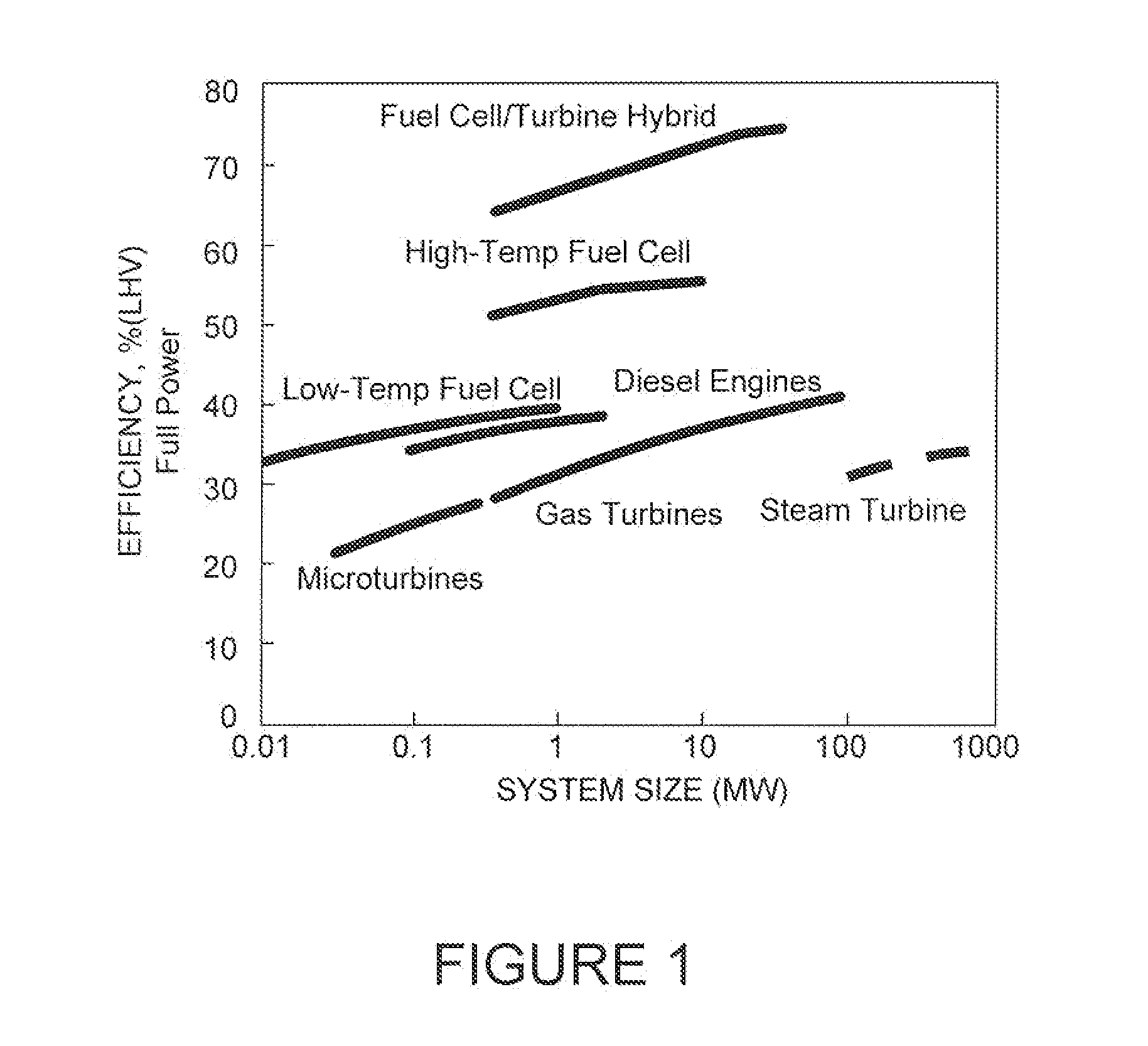
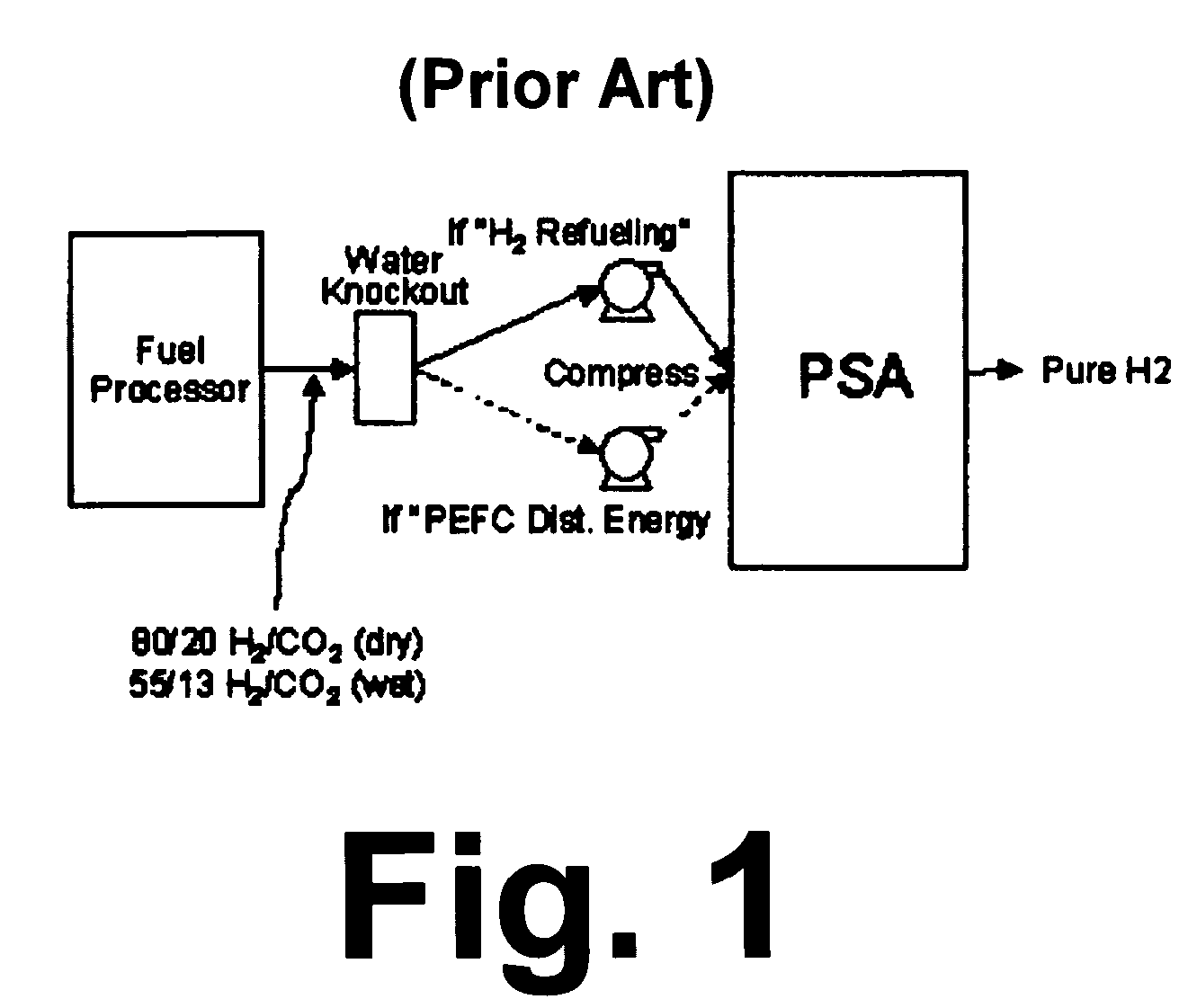
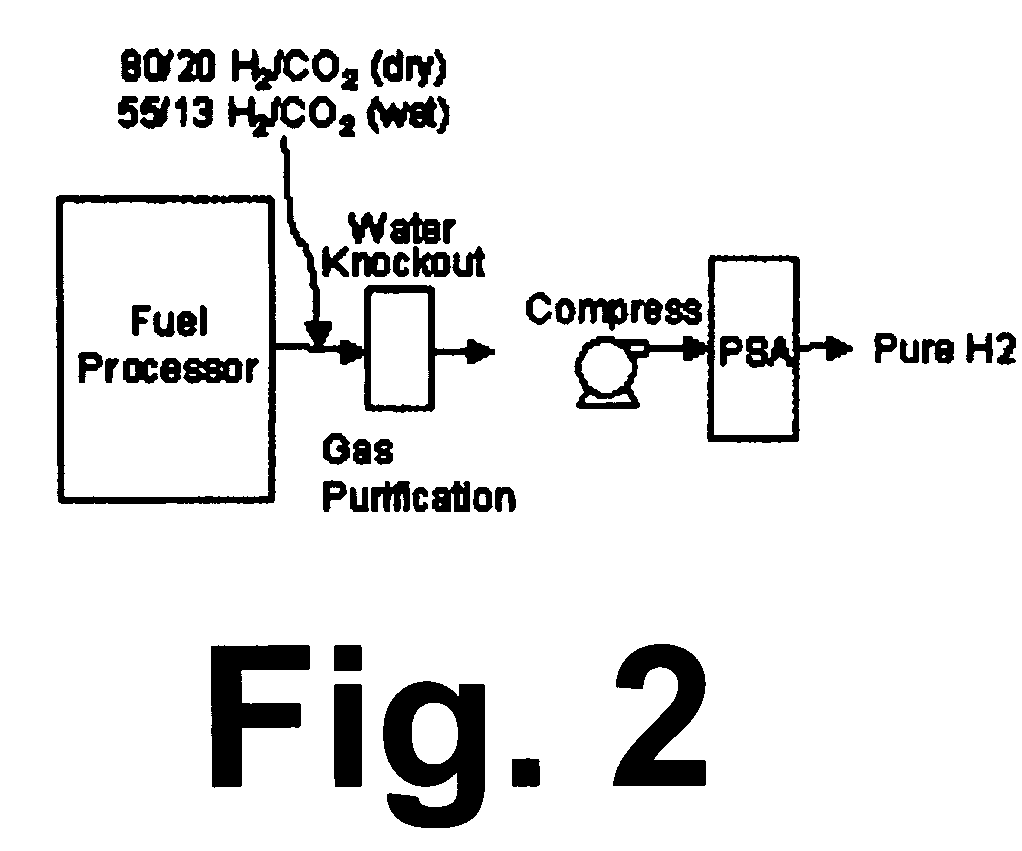
Catalytic method to remove CO and utilize its energy content in CO-containing streams
InactiveUS20060024539A1Low costUse minimizedMaterial nanotechnologyGas treatmentFuel cellsCatalytic method
Disclosed are a reactor and a corresponding method for producing electrical energy using a fuel cell by selectively oxidizing CO at room temperature using polyoxometalate compounds and transition metal compounds over metal-containing catalysts, thereby eliminating the water-gas shift reaction and the need to transport and vaporize liquid water in the production of H2 for fuel cells. The reactor also functions to deplete CO from an incoming gas stream.
Owner:WISCONSIN ALUMNI RES FOUND
Supported polyoxometalates and process for their preparation
InactiveUS7417008B2Group 4/14 element organic compoundsOxygen/ozone/oxide/hydroxideCatalytic oxidationMesoporous silica
Owner:EXXONMOBIL CHEM PAT INC
Preparation method and application of bimetallic photocatalytic functional POM/MOFs
ActiveCN104324754AOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsDiffusionReusability
Owner:DALIAN UNIV OF TECH
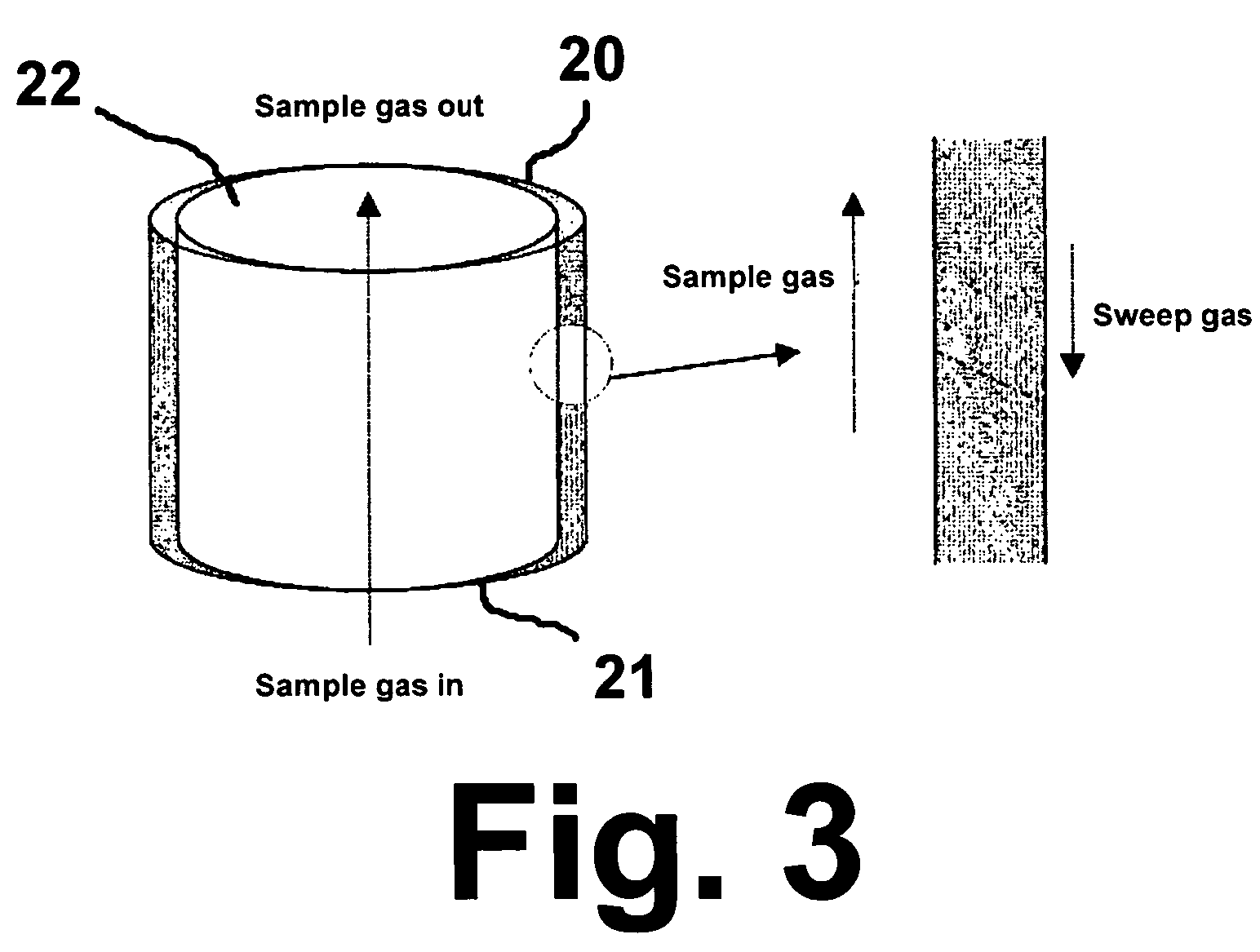
Adsorbent for carbon dioxide, method of preparing the same, and capture module for carbon dioxide including the same
ActiveUS20130260990A1Improve adsorption capacityImprove performance stabilityGas treatmentOther chemical processesSorbentMetal
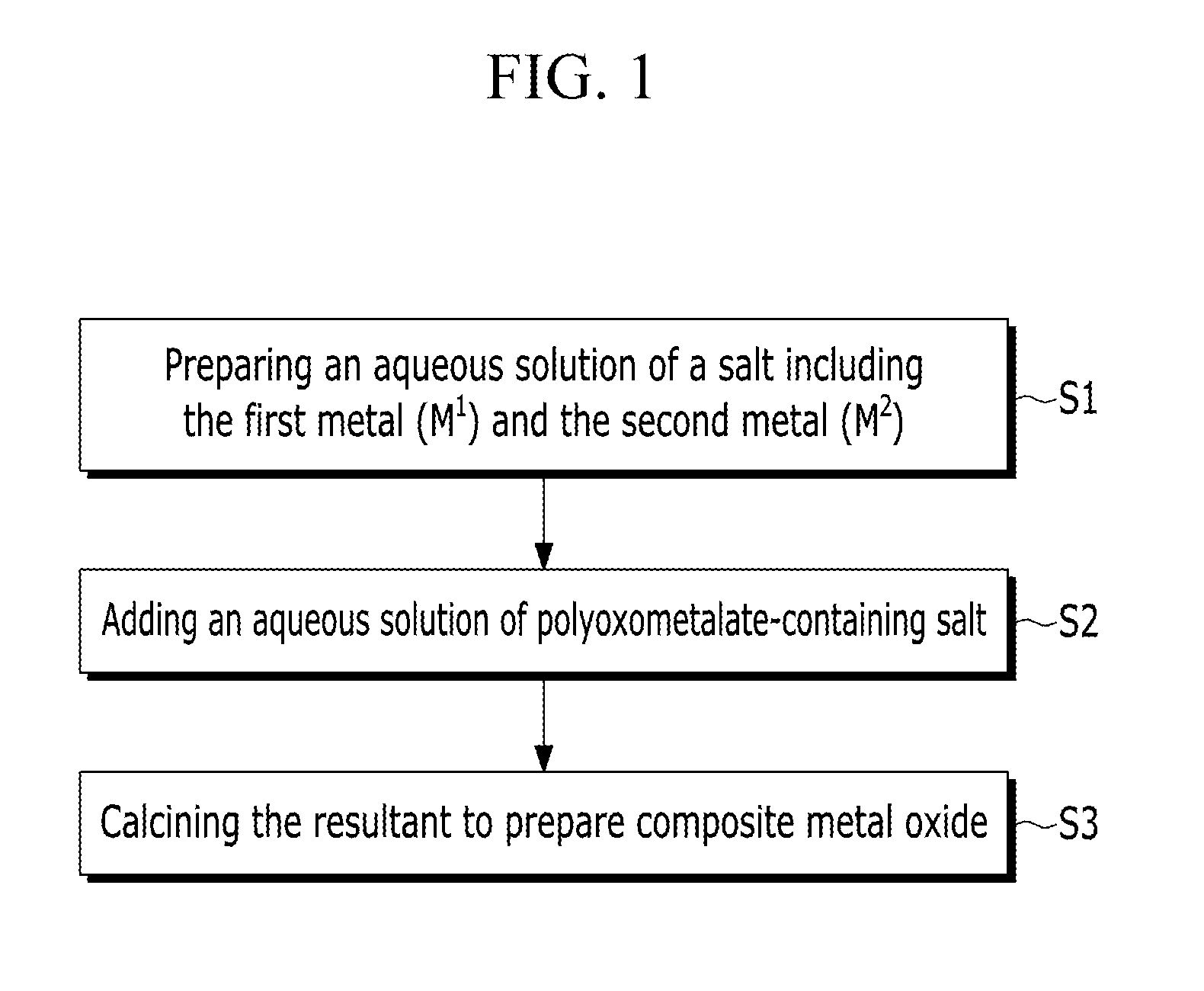
An adsorbent for carbon dioxide may include a composite metal oxide including a divalent first metal (M1), a trivalent second metal (M2), and at least one polyoxometalate (POM) ion selected from an anion represented by a first formula (e.g., Chemical Formula 1) and an anion represented by a second formula (e.g., Chemical Formula 2). A capture module for carbon dioxide may include the adsorbent.
Owner:SAMSUNG ELECTRONICS CO LTD
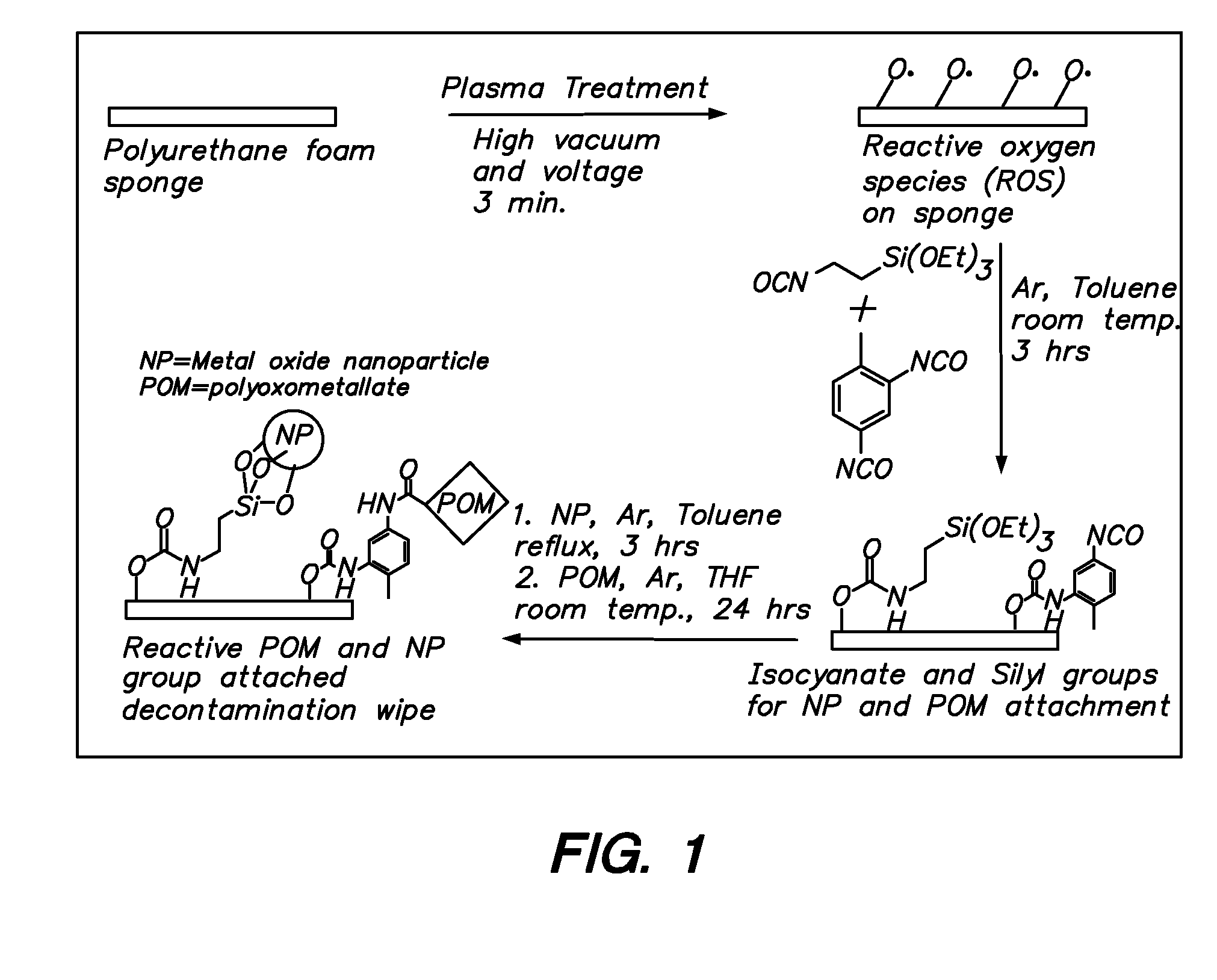
Functionalization of polymers with reactive species having bond-stabilized decontamination activity
Functionalized polymers and methods of functionalizing polymers with reactive species having decontaminating activity, such as polyoxometalates and metal oxides. Covalent bonding of the reactive species to the polymer securely immobilizes the reactive species and stabilizes the decontaminating activity of the reactive species. Specifically, the covalent bonding of the reactive species greatly reduces moisture deactivation during prolonged exposure to atmospheric moisture. Polyoxometalates are catalytically reactive through oxidative pathways and metal oxides are reactive through hydrolytic pathways. Both polyoxometalates and metal oxides having oxygen atoms available for covalent bonding with an appropriate bifunctional linking agent.
Owner:LYNNTECH
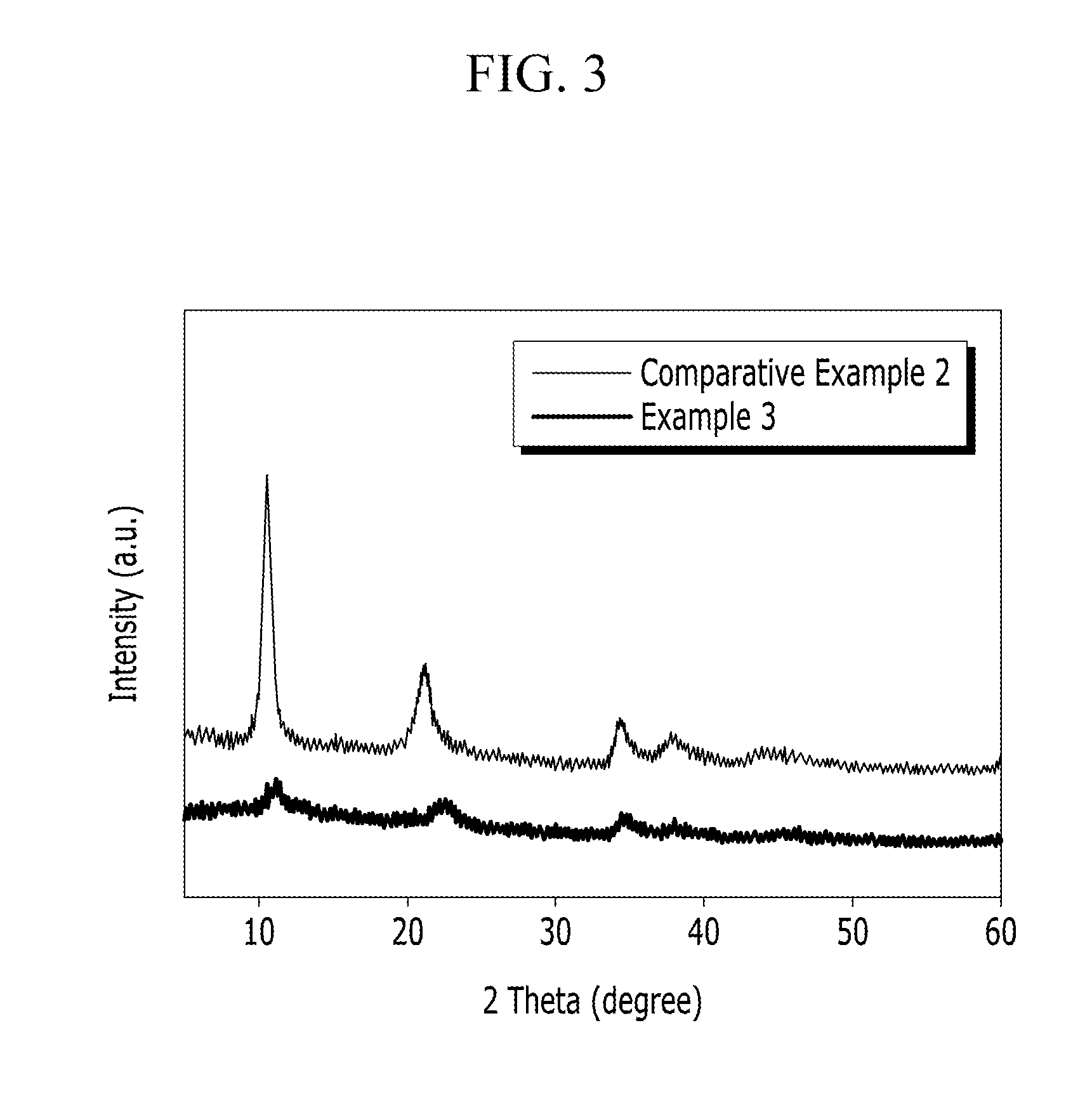
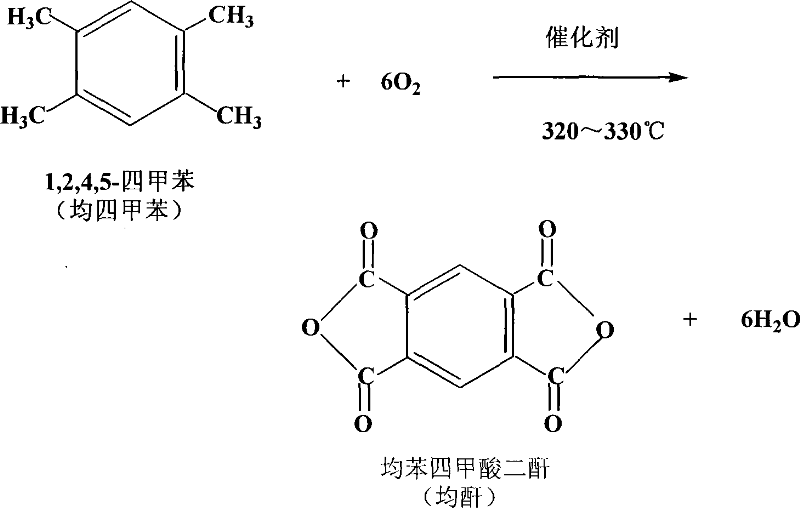
Production technique of benzenetetracarboxylic dianhydride by catalyzing carrier-type polyoxometalates
InactiveCN101037439AOvercome the difficulties, high production costs and other shortcomingsEasy to recycleOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsFixed bedIndustrial equipment
The invention discloses a craft for producing pyromellitic dianhydride catalyzed by a carrier-type poly-oxometalate, including melting the pyromellitic dianhydride by the groove, feeding to the mixing machine for evapouration after pre-heating with a feed concentration of 15.0-19.5g / m3, mixing with the air, entering into the fixed bed reactor for a oxidation, oxidating with an airspeed of 4500-6500 h-1, cooling the produced air, then condensing in the collector, getting coarse pyromellitic dianhydride. At 300-340 DEG C, the catalyzer is mostly poly-oxometalate which is fixed on the catalyst bed after loading. The mass ratio of the catalyzer to pyromellitic dianhydride is 0.2-1%. The invention has the durene in the C10 heavy aromatics as raw material, produces the pyromellitic dianhydride by oxidation in air, makes the C10 heavy aromatics be with a higher added value, uses the current resource and industrial equipments as more as possible, exploits the catalysis and synthesis path of the pyromellitic dianhydride. The invention has a low pollution and production cost, a wide development prospect and is suitable for commercial process.
Owner:NORTHEAST NORMAL UNIVERSITY
Polyoxometalate and heteropolyoxometalate compositions and methods for their use
ActiveUS20150200091A1Increase contentQuality improvementPhotomechanical apparatusSemiconductor/solid-state device manufacturingSemiconductorEnergy storage
The present invention relates to novel compositions comprising a metal component selected from a group chosen from at least one polyoxometalate, at least one heteropolyoxometalate and a mixture thereof; and, at least one organic component. The present invention also relates to methods of preparing the nanorod arrays and the nanorod materials and films. The present invention also relates to novel compositions to generate metal-oxide rich films, and also relates to processes for via or trench filling, reverse via or trench filling and imaging with underlayers. The materials are useful in wide range of manufacturing applications in many industries, including the semiconductor devices, electro-optical devices and energy storage industry.
Owner:MERCK PATENT GMBH
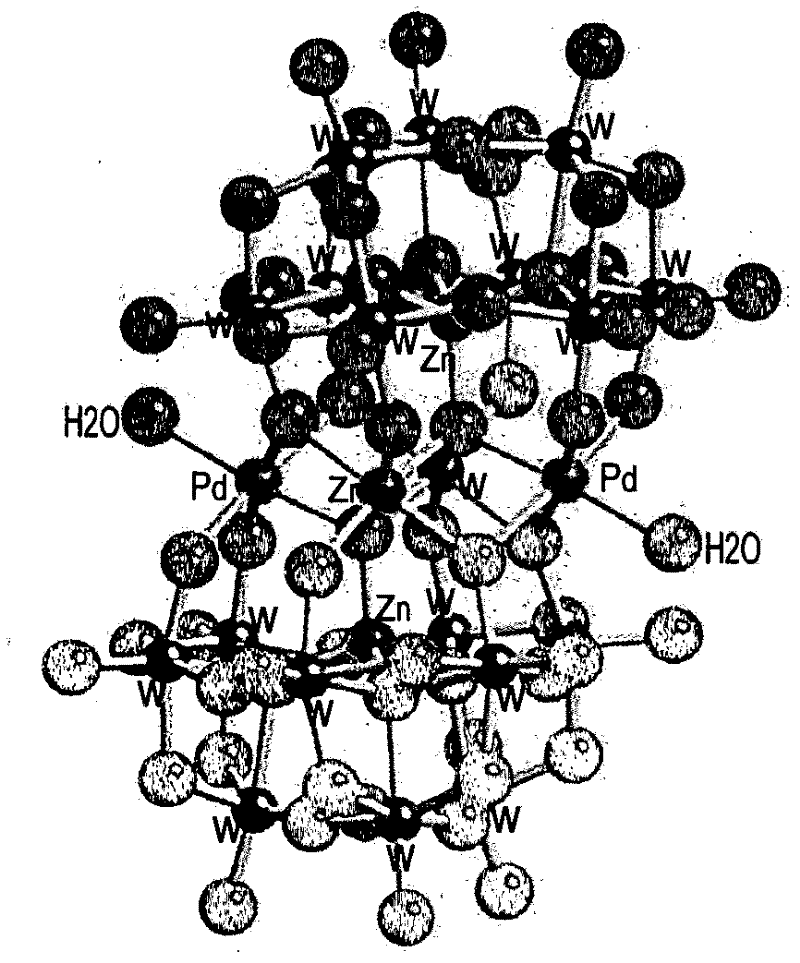
Novel Heteropolyanions With Late Transition Metal Addenda Atoms And Process For Their Preparation
The invention relates to polyoxometalates represented by the formula (An)m+ [M13X8RqOy]m− or solvates thereof, wherein A represents a cation, n is the number of the cations, m is the charge of the polyanion, M represents a transition metal selected from Pd, Pt, Au, Rh, Ir and mixtures thereof, X represents a heteroatom selected from As, Sb, Bi, P, Si, Ge, B, Al, Ga, S, Se, Te and mixtures thereof, and y is the number of oxygen atoms ranging from 32 to 40, a process for their preparation and their use for the catalytic oxidation of organic molecules.
Owner:EXXONMOBIL CHEM PAT INC
Catalyst for producing epoxy compound and process for producing epoxy compounds with the same
InactiveUS20030171604A1High yieldImprove utilization efficiencyOrganic chemistryOther chemical processesIndiumHafnium
The present invention provides a catalyst capable of producing an epoxy compound in high yield and improving the utilization efficiency of the oxidizing agent as well as a method of producing an epoxy compound using that catalyst. A catalyst for producing an epoxy compound by oxidizing a compound having at least one ethylenic double bond with an oxidizing agent, comprising a polyatom-containing heteropolyoxometalate anion (A1) having two defective and / or three defective structure sites and containing silicon as the heteroatom, and an element (E1) being at least one element selected from the group consisting of vanadium, tantalum, niobium, antimony, bismuth, chromium, molybdenum, selenium, tellurium, rhenium, cobalt, nickel, ruthenium, rhodium, palladium, osmium, platinum, iridium, silver, gold, zinc, aluminum, gallium, indium, scandium, yttrium, titanium, zirconium, hafnium, germanium, tin and lanthanoids, and being different from the polyatom.
Owner:NIPPON SHOKUBAI CO LTD
Supported polyoxometalates and process for their preparation
InactiveUS20070282138A1Group 4/14 element organic compoundsOxygen/ozone/oxide/hydroxideCatalytic oxidationMesoporous silica
The invention relates to supported polyoxometalates represented by the formula (An)m+ [M4(H2O)10(XW9O33)2]m− or solvates thereof, wherein A represents a cation, n is the number of cations, m is the charge of the polyoxoanion, M is a transition metal, and X is an element selected from the group consisting of As, Sb, Bi, Se and Te, characterized in that the polyoxometalate is supported on a solid support selected from the group consisting of Al2O3, MgO, TiO2, ZrO2, SiO2, mesoporous silica, active carbon, diatomite, clays, zeolites, polyoxometalate salts and mixtures thereof, with the proviso that the polyoxometalate salt supports are different from the supported polyoxometalates defined by the above formula, a process for their preparation and their use for the catalytic oxidation of organic molecules.
Owner:EXXONMOBIL CHEM PAT INC
Method for preparing high-dispersion solid-carrying Keggin type polyoxometallate crystalline-state catalyst
InactiveCN101406848AImprove stabilityOrganic-compounds/hydrides/coordination-complexes catalystsMetal/metal-oxides/metal-hydroxide catalystsChemical synthesisHeteropoly acid
The invention belongs to a chemical synthesis method, and relates to a method for utilizing a hydrothermal synthesis technique to prepare a highly-dispersed solid-supported Keggin-type polymetallic acid oxide salt crystalline catalyst. The method mixes certain amount of Keggin-type heteropoly acid or heteropolyacid salt, trimesic acid, copper salt and various methyl ammonium salts in water, and then obtains a crystal of the solid-supported catalyst under the condition of hydrothermal authigenic pressure. Polyacid catalysts are dispersed in a microporous metal-organic frame in a unimolecular form. The method can accurately determine the composition, structure and supported amount of the catalyst, and the supported amount of the catalyst is up to over 45 percent. The catalyst of the invention has the advantages that the catalyst can not drain away, is high in thermal stability, resistant to acid and alkali, stable and insoluble in water and common organic solvents, such as various solvents such as alcohol, cyanide, ketone, chloroform, and is an ideal catalyst for realizing heterogeneous catalytic reaction.
Owner:NORTHEAST NORMAL UNIVERSITY
Electrode material for polyoxometallate carbon nanotube lithium ion battery and preparation method of electrode material
ActiveCN104051734AImprove transmission characteristicsMeet lithium ion transportMaterial nanotechnologyCell electrodesCarbon nanotubeLithium-ion battery
The invention discloses an electrode material for a polyoxometallate carbon nanotube lithium ion battery. The electrode material is synthesized through oxidation of lithium polyoxometallate and functionalization of a carbon nanotube. The lithium polyoxometalate Li3XY12O40 has a three-dimensional skeleton structure, and lithium ions can be conducted in a three-dimensional skeleton; after oxidation and functionalization, the polyoxometalate is adsorbed on the wall of the carbon nanotube; and thus, the lithium ion transport characteristic is improved through the polyoxometalate, the electron transport characteristic is improved through the carbon nanotube, and double requirements of the electrode material on lithium ion transport and electron transport are met.
Owner:DONGFANG ELECTRIC CORP LTD
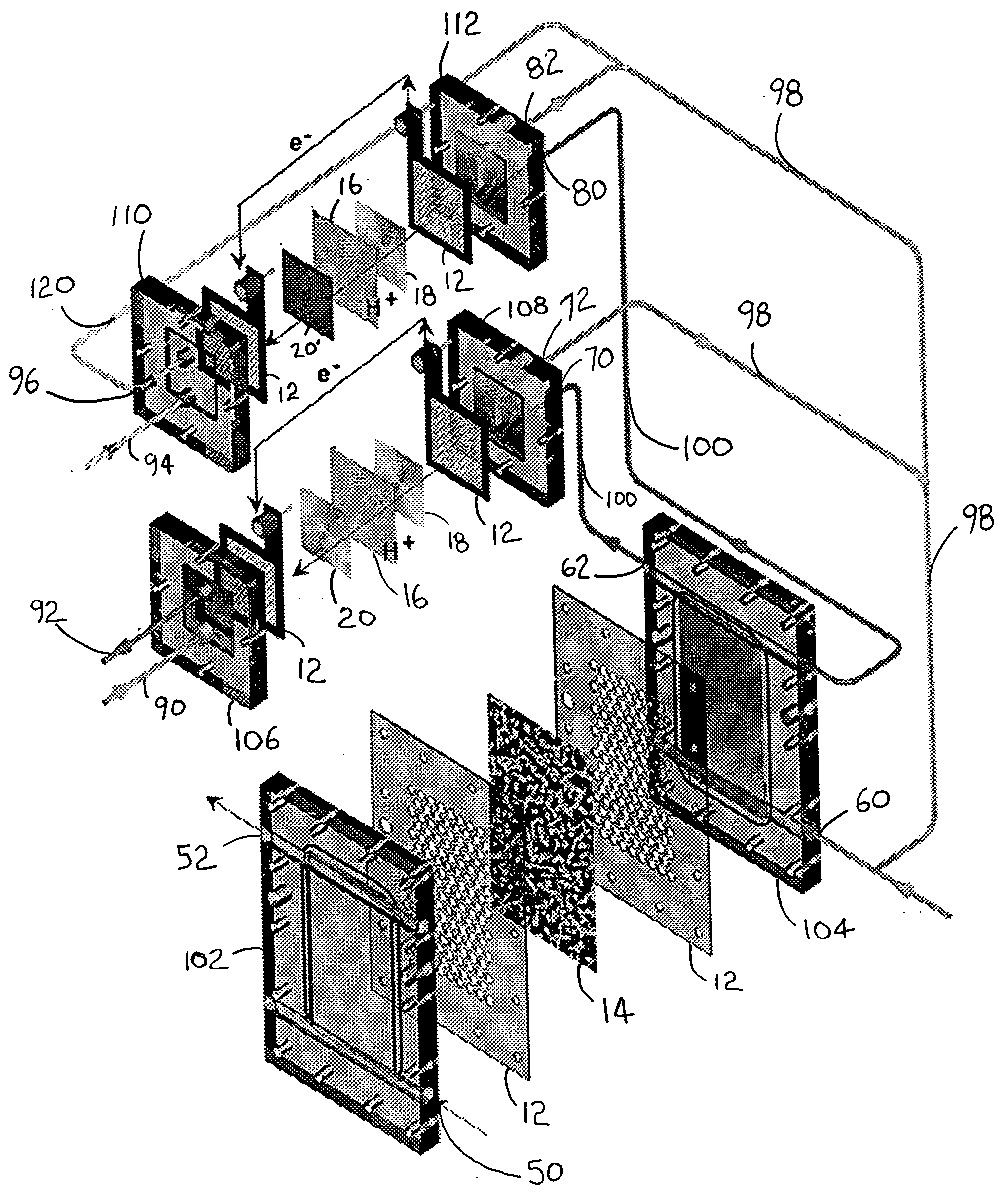
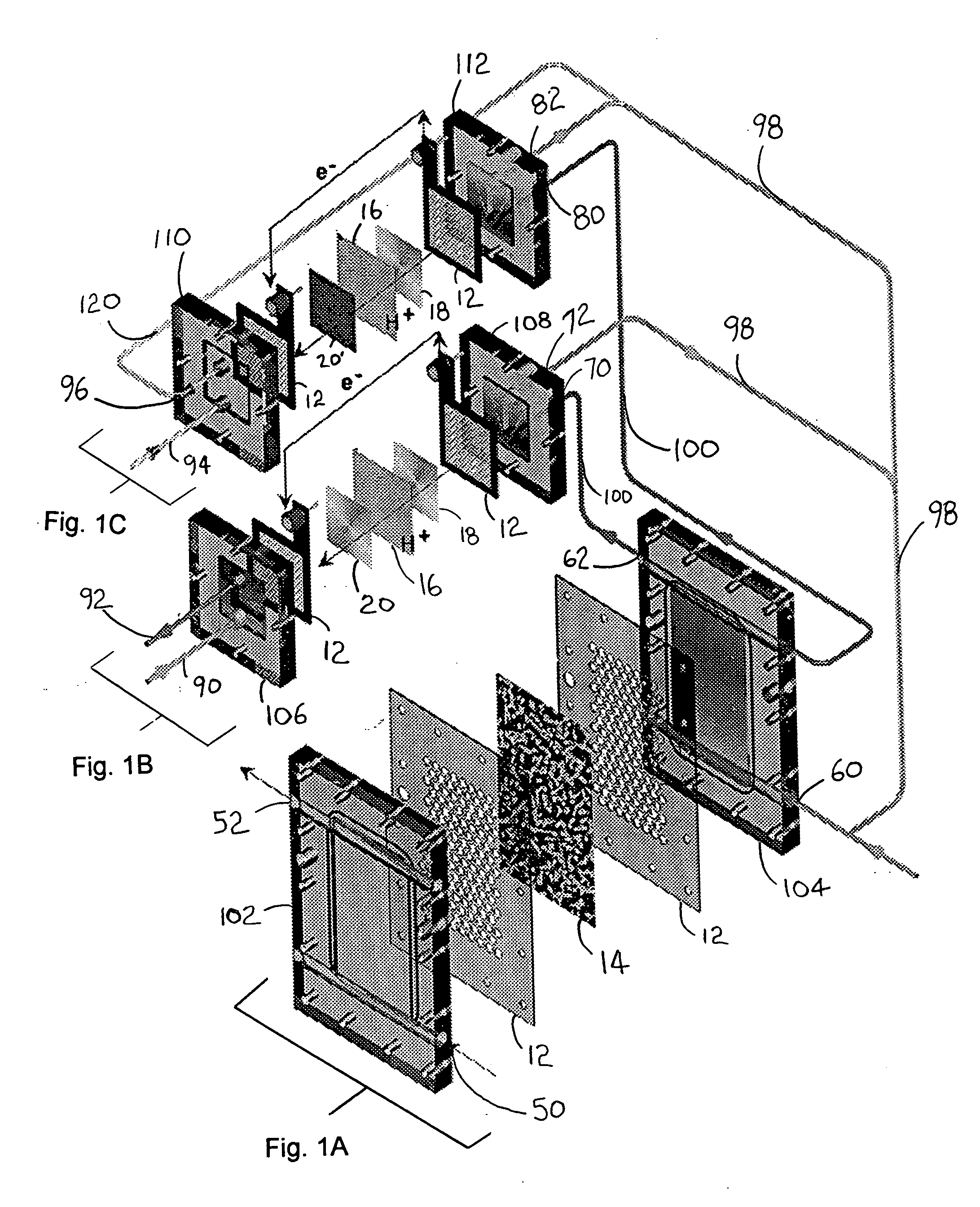
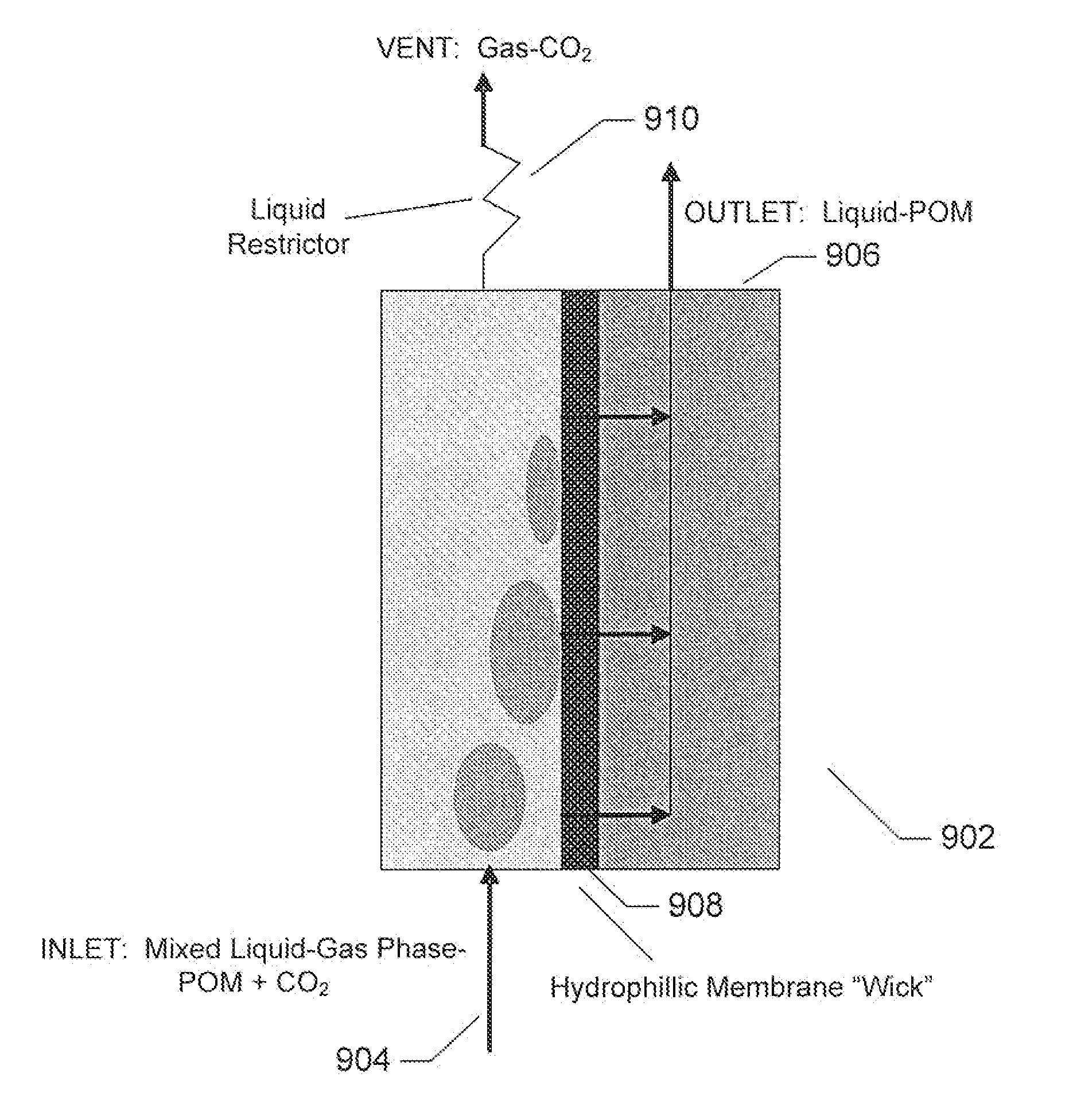
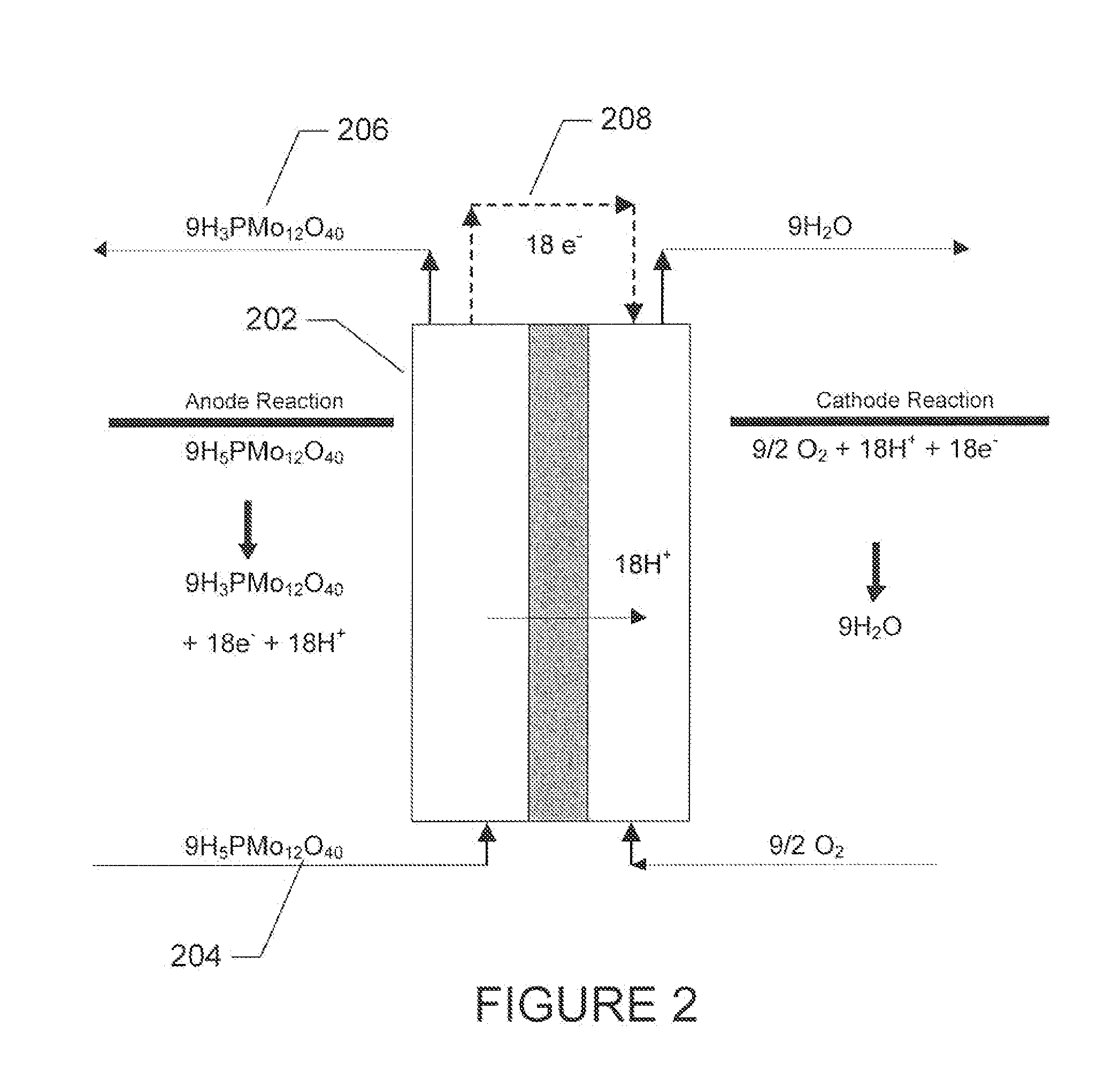
Polyoxometalate flow-cell power system
InactiveUS20110014527A1Improve electronic conductivityEfficiently oxidizedFuel and secondary cellsCell electrodesElectrochemical responseLiquid product
Embodiments of the present invention relate generally to redox flow batteries and, more specifically, to flow batteries that employ electron-ferrying redox compounds made from polyoxometalates (“POMs”). Embodiments of the present invention employ flow-battery technology that combines the fast electrochemical reaction of a battery with the fuel flexibility of a fuel cell to meet next-generation energy needs of a variety of power applications, including portable electronics used in military and commercial applications and large power modules that provide 550 W or more. To obtain a high-power-density stack, a reduced form of liquid POM is fed to the stack of cells, in certain embodiments of the present invention, where the reduced form of liquid POM is efficiently oxidized into liquid products at the anodes. Air is fed and reduced at the cathodes, generating water as a byproduct.
Owner:FC & ASSOC
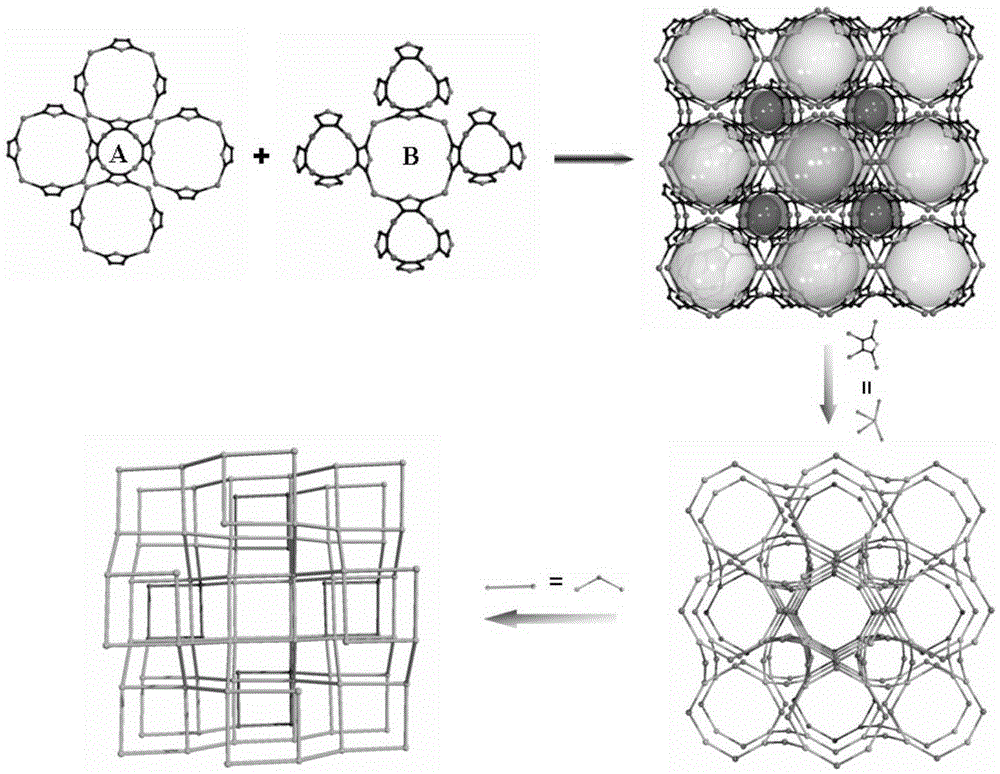
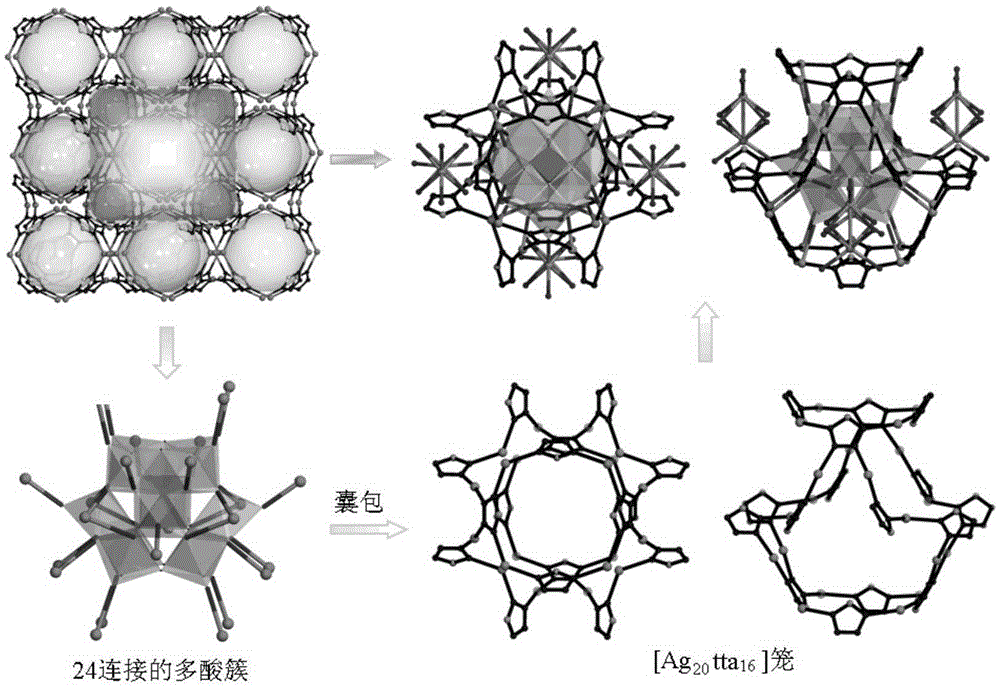
Polyoxometalate-based metal-organic frameworks crystalline material with nano-cage structure and preparation method and application thereof
InactiveCN105399779AImprove stabilityGood catalyticGroup 1/11 organic compounds without C-metal linkagesOrganic-compounds/hydrides/coordination-complexes catalystsOrganic dyeTetrazole
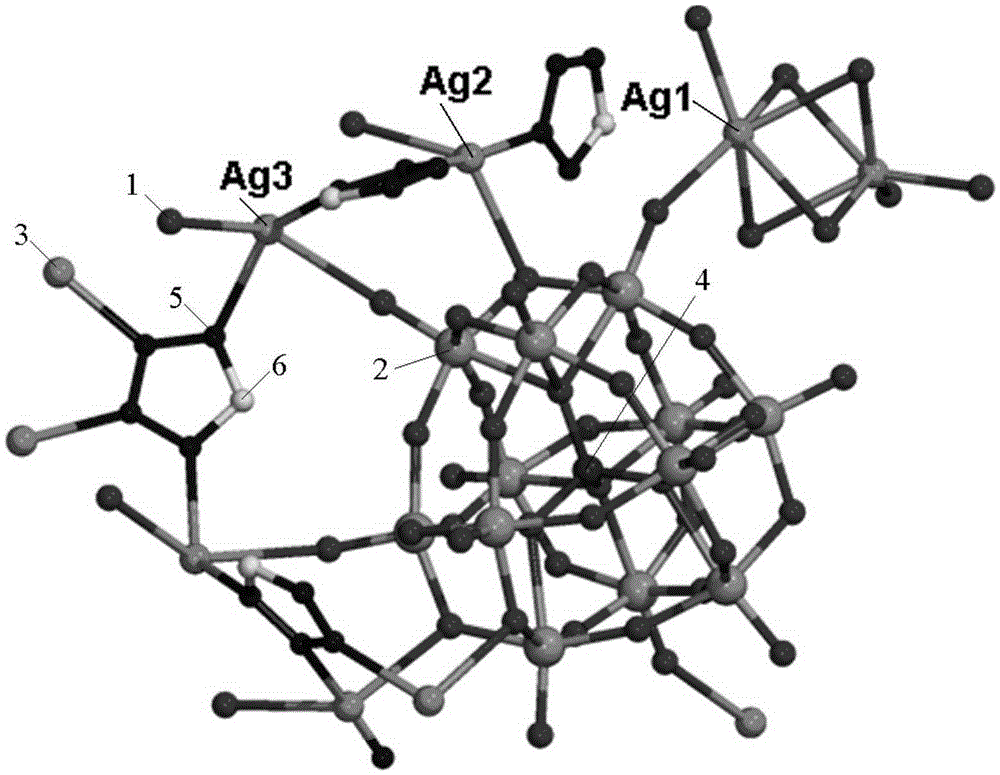
The invention relates to a metal-organic framework crystalline material with a nano-cage structure, in particular to a polyoxometalate (POM)-based metal-organic frameworks (POMOFs) crystalline material with a nano-cage structure and a preparation method and application thereof and aims to solve the problems in the prior art that a POMOFs crystalline material with a nano-cage structure is high in synthesis difficulty and poor in rhodamine B organic dye degradation effect. The POMOFs crystalline material has the chemical formula of Ag10(tta)4(H2O)4(SiW<IV>10W<V>2O40), the tetragonal system, and the space groups of I-4m2, alpha=90, beta=90, gamma=90 and Z=2. The preparation method comprises the following steps: dissolving silicotungstic acid, silver nitrate, tetrazole and a mineralizer in distilled water; regulating the pH value; carrying out a reaction for 3 days at 160 DEG C. The POMOFs crystalline material with the nano-cage structure can be obtained.
Owner:HARBIN UNIV OF SCI & TECH
Copper complex based on dipyridine bisamide organic ligand and Keggin type polyoxometalate, its synthetic method and its application
InactiveCN102513157AImprove hydrophilicityHigh affinityWater/sewage treatment by irradiationOrganic-compounds/hydrides/coordination-complexes catalystsHigh pressureCoordination complex
The invention relates to a copper complex based on a dipyridine bisamide organic ligand and Keggin type polyoxometalate, its synthetic method and its application. A molecular formula is one of the following formulas: [Cu2(L<1>)3(H2O)6(SiMo12O40)].2H2O; [Cu2(L<2>)3(H2O)6(SiMo12O40)].9H2O; [Cu2(L<2>)3(H2O)6(SiW12O40)].6H2O; [Cu2(L<3>)3(H2O)6(SiMo12O40)].6H2O; [Cu2(L<3>)3(H2O)6(SiW12O40)].6H2O; wherein L<1> is N, N'-di(3- pyridylformamido)-1,2-ethane; L<2> is N, N'-di(3-pyridylformamido)-1,4-butane; L<3> is N, N'-di(3-pyridylformamido)-1,6-hexane. The method comprises the following steps: addingdeionized water in the Cu(NO3).3H2O, Keggin type polyoxometalate, dipyridine diamide organic ligand, stirring under room temperature, regulating pH value, dumping in a high pressure reaction vessel and heating, insulating under hydrothermal condition, cooling to the room temperature to obtain bulk blue green crystals, alternatively cleaning by deionized water and ethanol, naturally drying under room temperature to prepare the copper complex based on dipyridine bisamide organic ligand and Keggin type polyoxometalate. The complex has the advantages of simple synthetic method, easy crystallization, high synthesis yield, strong affinity capability to water-soluble pollutants and good catalytic degradation effect, and can be used as photocatalysis materials.
Owner:BOHAI UNIV
Method for oxidation synthesis of adipic acid by epoxy cyclohexane
InactiveCN101177390AOrganic compound preparationCarboxylic compound preparationEpoxyCyclohexene oxide
The invention relates to a method for synthesizing adipate using cyclohexene oxide through oxidation, which uses the tungsten and molybdenum polyoxometalates as the catalyst, and the hydrogen peroxide solution as the oxidant to catalyze the oxidation of the cyclohexene oxide to synthesize the adipate. The invention is characterized in that the organic solvent is no longer used as the reacting medium, so as to avoid the environment pollution caused by using the nitric acid as the oxidant. The reacting temperature is 60 to 105 DEG C; the reacting time is 6 to 20 hours and the adipate yield can reach 46.9% to 83.2%.
Owner:JIANGSU UNIV
Polyoxometalate-based metal-organic frameworks crystal material with polyoxometalates as template
InactiveCN108997594AImprove photocatalytic performanceMaintain catalytic activitySemiconductor structureMetal-organic framework
The invention relates to polyoxometalate-based metal-organic frameworks crystal material assembled with polyoxometalates as a template, and relates to the polyoxometalate-based metal-organic frameworks crystal material assembled with the polyoxometalates as the template. The invention aims to solve the problem that a semiconductor structure formed between end oxygen of the polyoxometalates (POMs)in polyoxometalate-based metal-organic frameworks (POMOFs) crystal material synthesized by the prior art and metals in metal-organic frameworks (MOFs) has the conduction band greater than 0, resultingin a fact that the POMOFs material has no effect of hydrogen production by decomposing water under irradiation of a xenon lamp. The designed and developed POMOFs crystal material with the polyoxometalates as the template has the chemical formula of [CuI2(PPZ)4][H2GeW12O40].8H2O. A method comprises the steps: dissolving germanotungtic acid, copper chloride and 3-(pyridin-4-yl)pyrazole organic ligands in deionized water, adjusting the pH value, and then carrying out reaction for 3 d at the temperature of 160 DEG C. The POMOFs crystal material assembled with the polyoxometalates as the templatecan be obtained.
Owner:HARBIN UNIV OF SCI & TECH
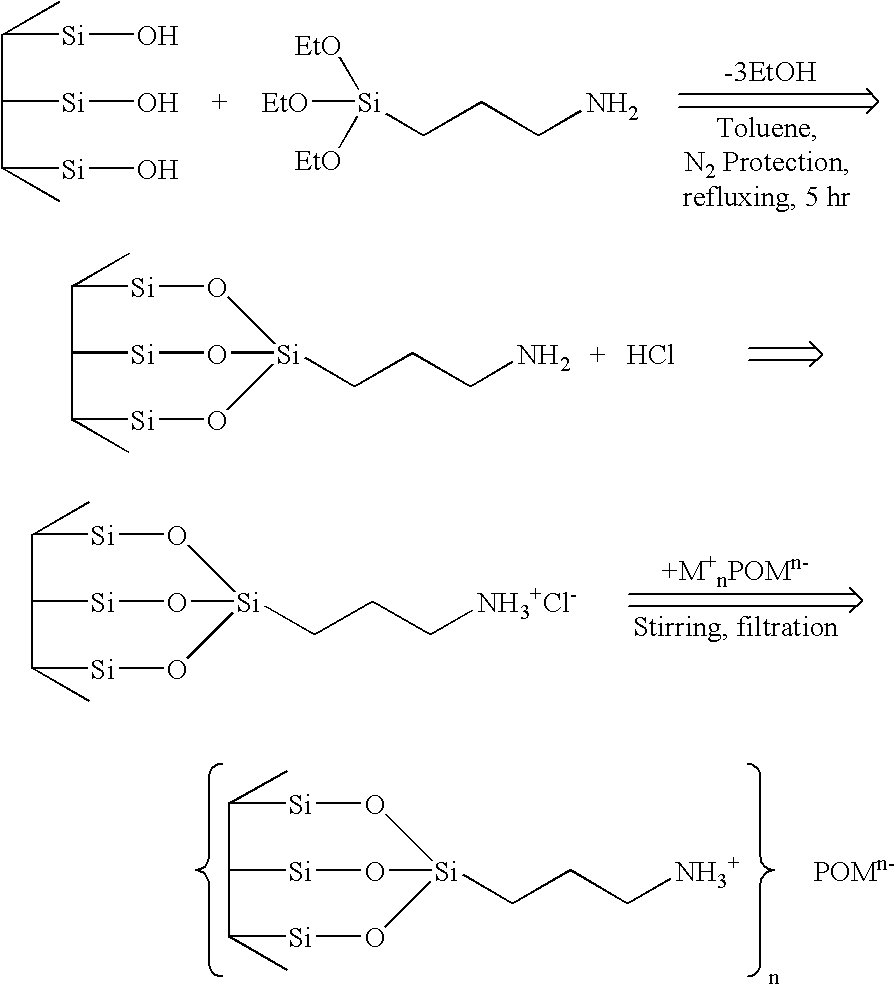
Process for Hydrodesulphuration of gasoil cuts using a catalyst based on heteropolyanions trapped in a mesostructured silica support
InactiveUS9340733B2Good dispersionHigh activityTreatment with hydrotreatment processesRefining to eliminate hetero atomsPtru catalystHydrodesulfurization
The invention relates to a process of hydrodesulphuration of at least one gasoil cut implementing a catalyst containing, in its oxide form, at least one metal from group VIB and / or at least one metal from group VIII of the periodic table, present in the form of at least one polyoxometalate of the formula (HhXxMmOy)q−, said polyoxometalates being present within a mesostructured silicon oxide matrix having a pore size within the range 1.5 to 50 nm and having amorphous walls of thickness within the range 1 to 30 nm, the said catalyst being sulphured before being implemented in the said process.
Owner:INST FR DU PETROLE +1
Hydrogenation Catalysts Prepared from Polyoxometalate Precursors and Process for Using Same to Produce Ethanol
InactiveUS20130245338A1Organic compound preparationHeterogenous catalyst chemical elementsAlcoholMetal
The present invention relates to hydrogenation catalysts prepared from polyoxometalate precursors. The polyoxometalate precursors introduce a support modifier to the catalyst. The catalysts are used for hydrogenating alkanoic acids and / or esters thereof to alcohols, preferably with conversion of the ester coproduct. The catalyst may also comprise one or more active metals.
Owner:CELANESE INT CORP
Catalyst for producing epoxy compounds and method of producing epoxy compounds using the same
InactiveUS6743748B2High yieldImprove utilization efficiencyOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsIridiumPtru catalyst
The present invention provides a catalyst capable of producing an epoxy compound in high yield and improving the utilization efficiency of the oxidizing agent as well as a method of producing an epoxy compound using that catalyst.A catalyst for producing an epoxy compound by oxidizing a compound having at least one ethylenic double bond with an oxidizing agent,comprising a polyatom-containing heteropolyoxometalate anion (A1) having two defective and / or three defective structure sites and containing silicon as the heteroatom, andan element (E1) being at least one element selected from the group consisting of vanadium, tantalum, niobium, antimony, bismuth, chromium, molybdenum, selenium, tellurium, rhenium, cobalt, nickel, ruthenium, rhodium, palladium, osmium, platinum, iridium, silver, gold, zinc, aluminum, gallium, indium, scandium, yttrium, titanium, zirconium, hafnium, germanium, tin and lanthanoids, and being different from the polyatom.
Owner:NIPPON SHOKUBAI CO LTD
Liquid cleaning compositions and their use
InactiveUS20030191040A1Prevent dye transferringAdequate levelInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsProteolytic enzymesStabilizing Agents
An aqueous liquid cleaning composition comprising a proteolytic enzyme and a primary stabiliser therefor, the composition further comprising a polyoxometalate.
Owner:UNILEVER HOME & PERSONAL CARE USA DIV OF CONOPCO IN C
Method for preparing graphene and polyoxometalate composite through electrochemical reduction
The invention relates to a method for reduction of graphene oxide by using a polyoxometalate as an electro-catalyst and for preparation of a graphene and polyoxometalate composite. The prepared composite is a powder material with a porous structure and has potential application values in the aspects like catalysis and electrode materials for a lithium ion battery and an electrochemical capacitor. The method comprises a first step of preparation of graphene oxide, a second step of preparation of mixed liquor of graphene oxide and the polyoxometalate, a third step of electrochemical reduction of graphene oxide by the polyoxometalate, a fourth step of separation of the graphene and polyoxometalate composite, etc. According to the invention, electrons are transferred from a working electrode to the polyoxometalate at first, so the polyoxometalate obtains the electrons and is reduced into a heteropoly blue; then the electrons are transferred from the heteropoly blue to graphene oxide, so graphene oxide is reduced and the heteropoly blue loses the electrons and turns into initial polyoxometalate. During the process of reduction, the polyoxometalate can spontaneously adhere onto the surface of produced graphene so as to form the graphene and polyoxometalate composite.
Owner:JILIN UNIV
Ion sieve for extracting uranium from water body and preparation method thereof
InactiveCN103894155AOther chemical processesRadioactive decontaminationPhosphomolybdic acidHydroxylamine
The invention provides an ion sieve for extracting uranium from a water body and a preparation method thereof. The ion sieve is prepared from pyrophosphate, molybdate, zirconium oxychloride, hexadecyl trimethyl ammonium bromide, acrylonitrile, and hydroxylamine hydrochloride. The preparation method comprises the steps: preparing a hydrogen ion exchanger of zirconyl-molybdopyrophosphate polyoxometalate by using zirconium oxychloride, the molybdate and the pyrophosphate, introducing a defined amount of uranium ions to the hydrogen ion exchanger, extracting through immobilizing the uranium ions, and baking for forming; then radiating and activating, making a product obtained by radiating and activating react with hexadecyl trimethyl ammonium bromide for performing organic modification, and then adding acrylonitrile and hydroxylamine hydrochloride for performing amine oximation; finally, performing solid-liquid separation, and then performing steps of high-temperature sintering, cooling and grinding, and the like to obtain the ion sieve for extracting uranium from the water body. The prepared ion sieve has a most suitable crystal structure of receiving the uranium ions, shows an efficient selective effect, and has a chelation function and a good selectivity to the uranium ions.
Owner:INST OF NUCLEAR PHYSICS & CHEM CHINA ACADEMY OF
Aerobic oxidation of primary aliphatic alcohols by using noble metal polyoxometalate complexes
InactiveCN102596399AOrganic compound preparationGroup 8/9/10/18 element organic compoundsPrimary alcoholPhotochemistry
A process of oxidizing primary alcohols to their corresponding aldehydes is disclosed. The process is effected in the presence of noble metal polyoxometalate complexes. A novel process for preparing noble metal polyoxometalate complexes, and novel noble metal polyoxometalate complexes are also disclosed.
Owner:YEDA RES & DEV CO LTD
Polyacid/polyaniline/carbon nano tube electrode material as well as preparation method and application thereof
ActiveCN104332597AImprove performanceImprove cycle stabilityHybrid capacitor electrodesCell electrodesCarbon nanotubeAniline
The invention discloses a preparation method of a series of polyacid, polyaniline and carbon nano tube electrode materials and application of the polyacid, polyaniline and carbon nano tube electrode materials to lithium ion batteries and super-capacitors. According to the technical scheme, the preparation method comprises the following steps: firstly, carrying out ultrasonic dispersion on a multi-wall carbon nano tube in hydrochloric acid; adding aniline and ammonium persulfate which are dissolved into hydrochloric acid for carrying out in-situ synthesis, so as to uniformly cover the multi-wall carbon nano tube with polyaniline; assembling polyacid on a polyaniline and carbon nano tube composite material by electrostatic bonding; and finally, forming an electrode material based on a polyacid / polyaniline / carbon nano tube. Compared with a reported polyaniline and carbon nano tube composite material and a pure carbon nano tube, the electrode material prepared by adopting the preparation method is used as a negative electrode of a lithium ion battery, so that the circulating stability is remarkably improved, and the discharging specific capacity and the speed capacity are obviously increased.
Owner:BEIJING UNIV OF CHEM TECH
Preparation and catalytic performance of polyacid-based metal organic framework crystal material with intercalation structure
ActiveCN109876865AImprove photocatalytic performanceStable structureOrganic-compounds/hydrides/coordination-complexes catalystsTungstateTetrazole
The invention aims to develop a polyacid-based metal organic framework crystal material with an intercalation structure, which effectively overcomes the technical bottlenecks that a simple polyacid material is wide in band gap (3 eV), can only absorb ultraviolet light, is soluble in water, and has a recover rate, and the like. Special intercalation structure characteristics of the material are utilized to improve the electron storage capacity of polyacid as an electron acceptor, accelerate the separation of catalytic photo-generated carriers, enhance the photocatalytic efficiency of a catalyst, and expand the spectral absorption range to visible light. The chemical formula of the polyacid-based metal organic framework crystal material with the intercalation structure is designed and developed as [CuI2CuII5(ptz)6(OH)2(4H2O)GeW12O40].6H2O. A method for preparing the material comprises the following steps: dissolving germanium tungstate, copper chloride and 5-(pyridine-2-yl)tetrazole organic ligand into deionized water to obtain a reaction solution, adjusting a pH value, and then reacting at a temperature of 160 DEG C for 3 days. . The metal organic framework crystal material with thepolyacid-based intercalation structure having visible light catalytic properties can be obtained.
Owner:HARBIN UNIV OF SCI & TECH
Process for preparation of cyclic carbonate
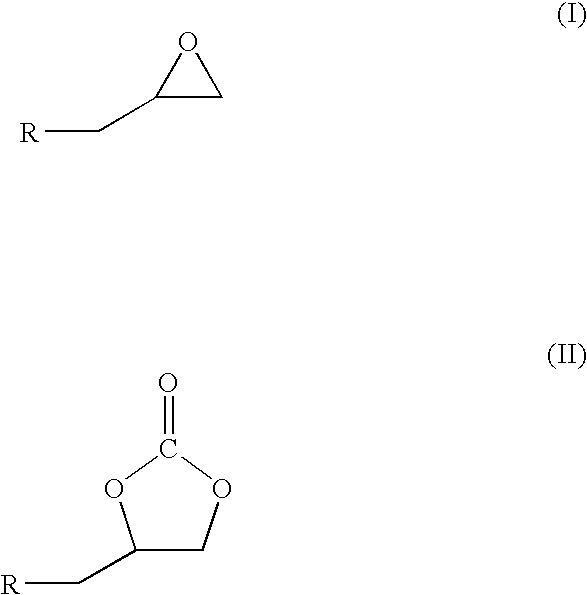
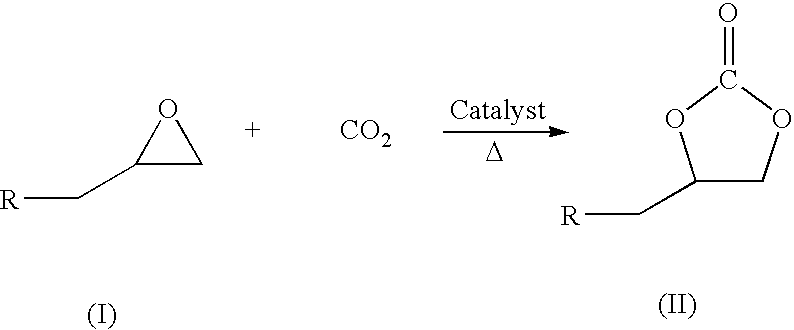
A catalytic system comprising of Zinc-substituted polyoxometalate, Na12[WZn3(H2O)2(ZnW9O34)2].48H2O and a Lewis base has been discovered to be efficient for chemical fixation of CO2 with epoxides (I) to form cyclic carbonates of structure II as given below: wherein R is selected from a halogen atom, aliphatic and aromatic group. The reaction was carried out at moderate CO2 pressure of 60 psig and in the temperature range 100-140° C. with very high turn number of more than 40,000, with nearly a quantitative conversion and 95-99% selectivity. The Zinc-substituted polyoxometalate catalyst is easy to separate after the reaction by filtration and reusable for many cycles. The reaction can be carried out without any solvent.
Owner:COUNCIL OF SCI & IND RES
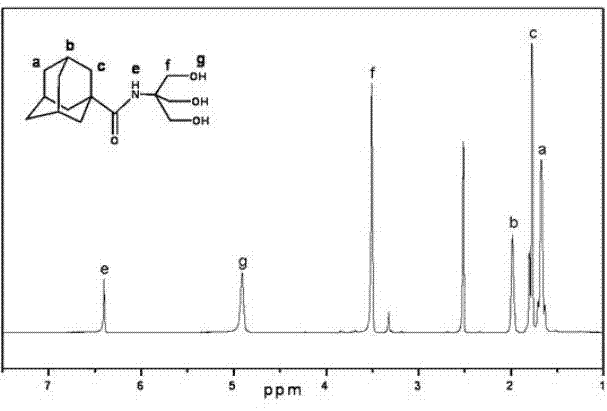
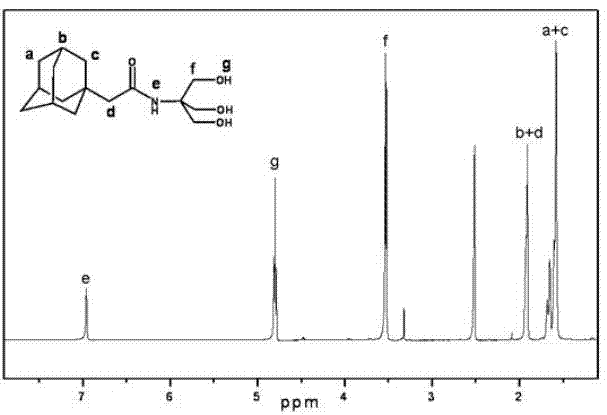
Molybdenum-containing polyoxometalate and adamantane hybrid compound and preparation method thereof
InactiveCN102304152AEasy to prepareReaction is easy to controlOrganic chemistrySynthesis methodsHybrid compound
The invention discloses a molybdenum-containing polyoxometalate and adamantane hybrid compound, which is prepared by connecting molybdenum-containing polyoxometalate and adamantane through a covalent bond. The preparation method comprises the following synthesis steps of: 1) performing amidation reaction on adamantane carboxylic acid serving as an initial raw material and trishydroxymethyl aminomethane to obtain N-trishydroxymethyl-adamantane carboxamide; and 2) reacting with the molybdenum-containing polyoxometalate to obtain the hybrid compound. By the invention, the molybdenum-containing polyoxometalate and the adamantine are connected through the covalent bond for the first time; and the synthesis method is simple and feasible, reaction conditions are easy to realize, purification is simple, and the yield is high. The hybrid molecule has potential application value in the aspects of medicines, lubricants, surfactants, catalysts and the like.
Owner:NANKAI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com