Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
362 results about "Phthalimides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
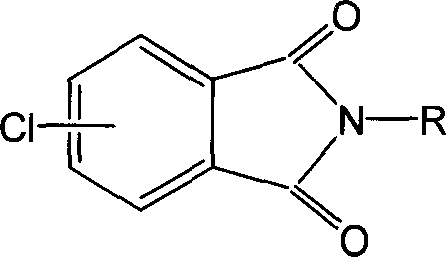
The imide of phthalic acids.
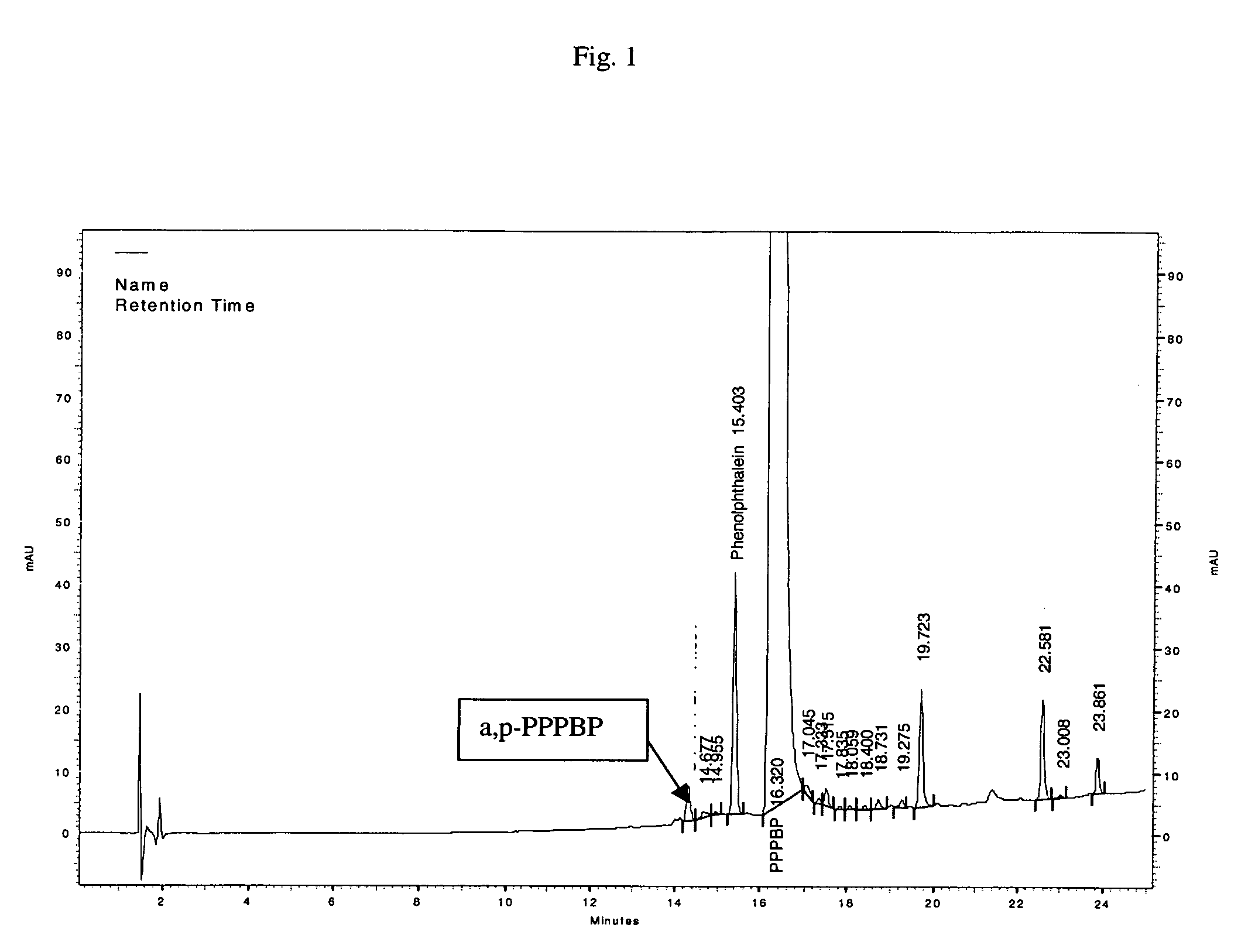
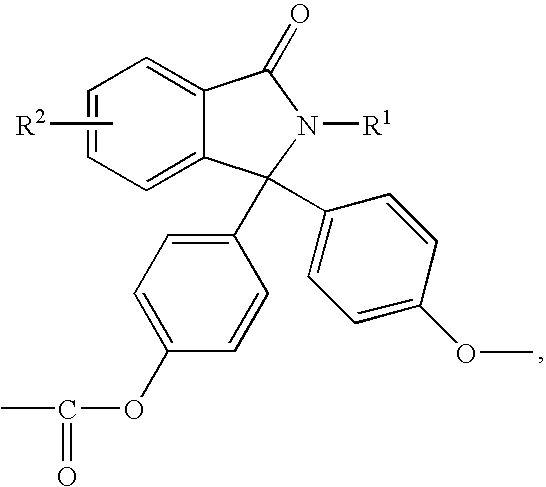
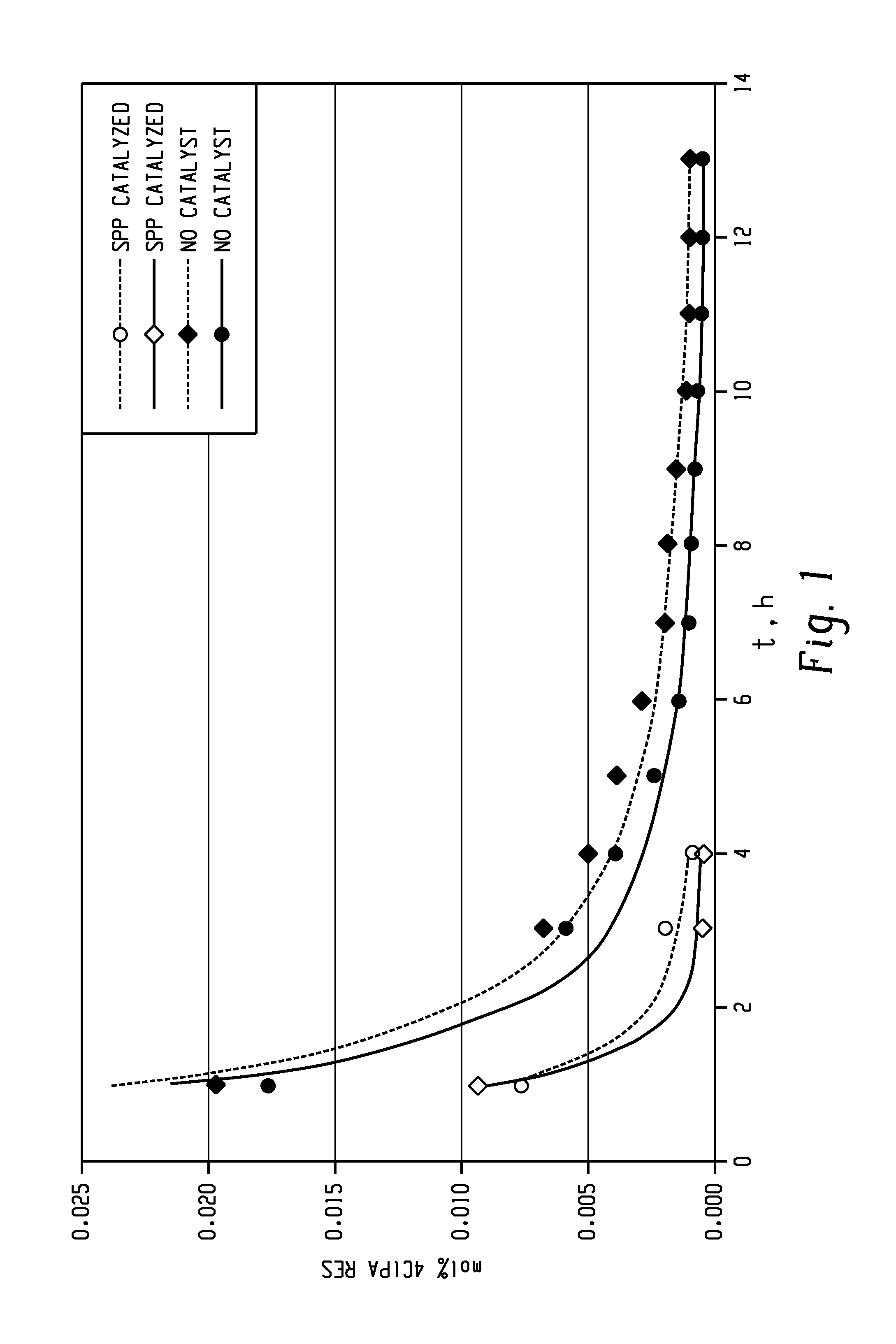
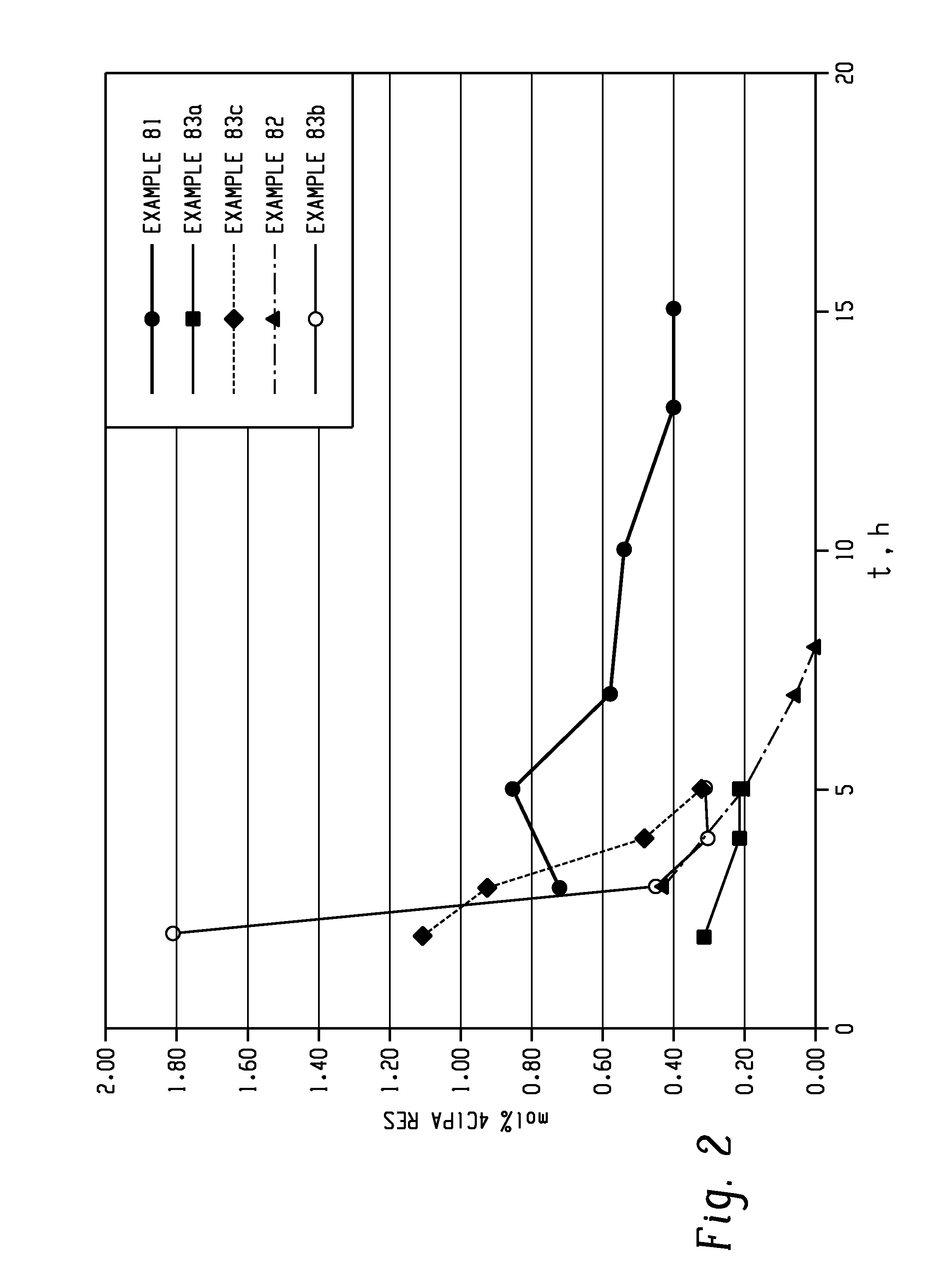
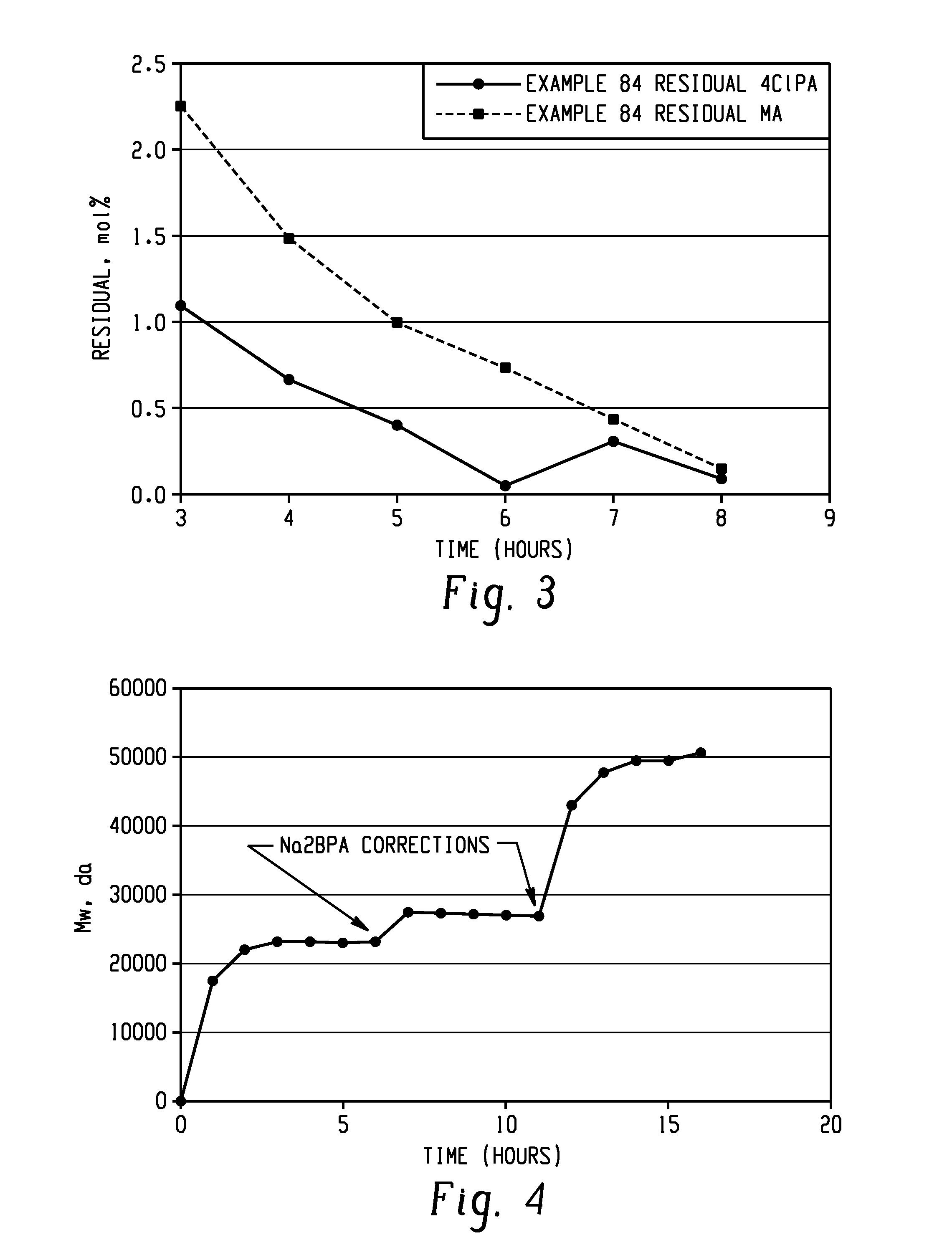
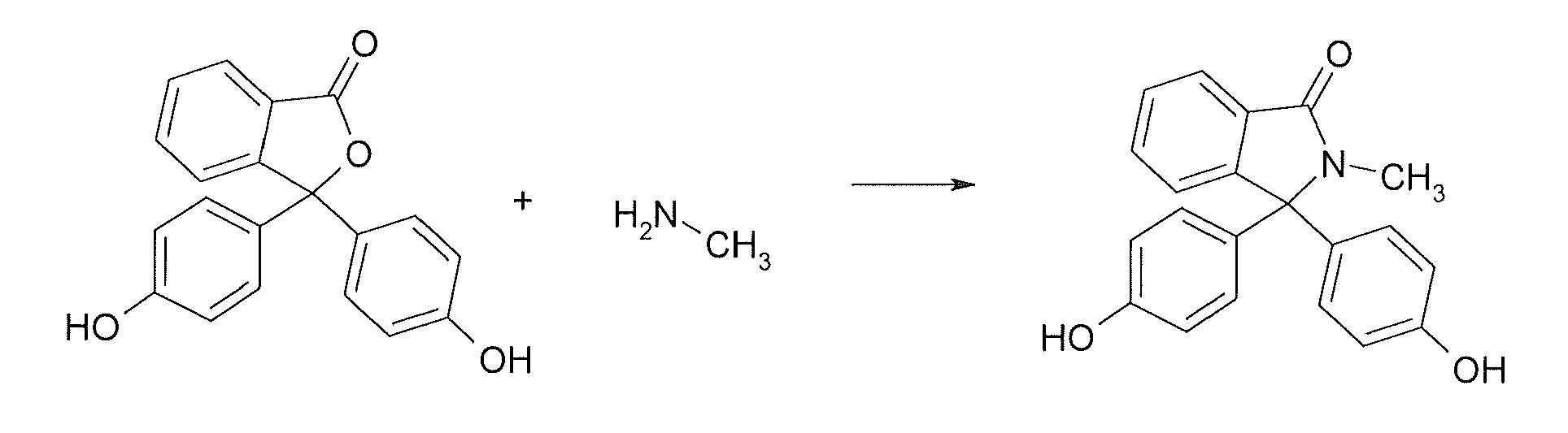
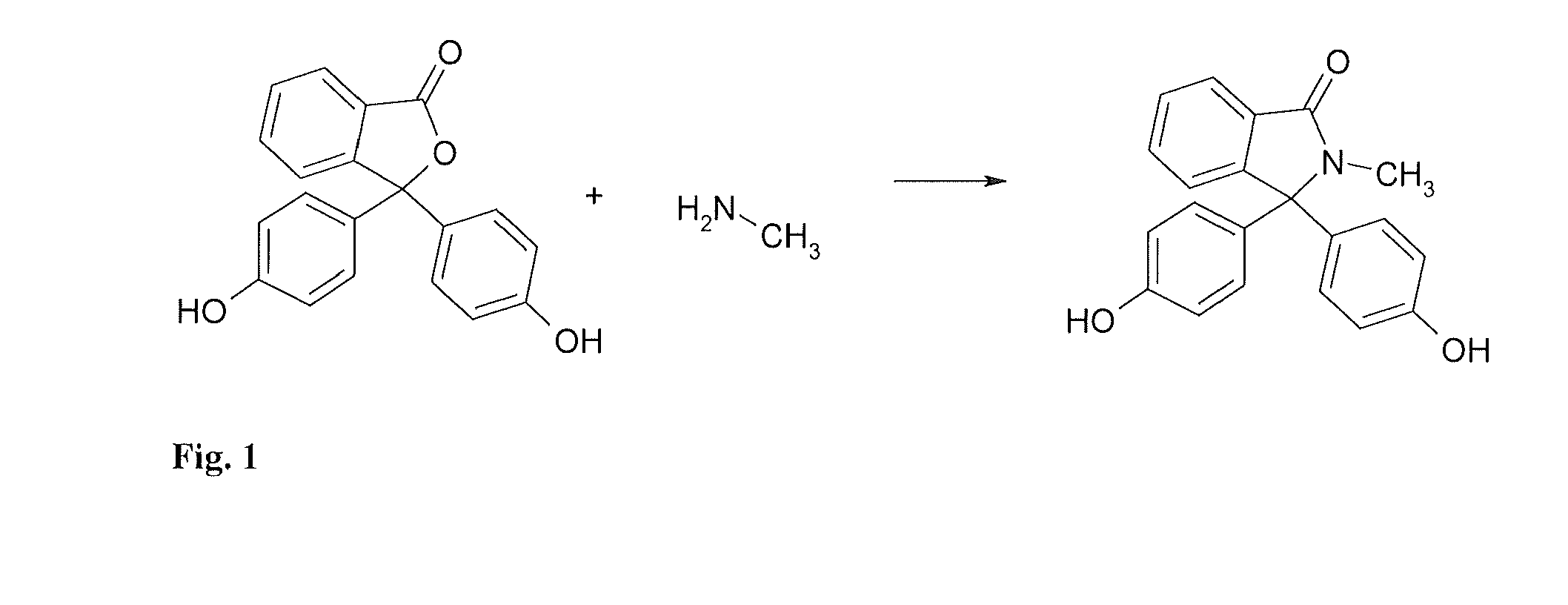
Methods for producing and purifying 2-hydrocarbyl-3,3-bis(4-hydroxyaryl)phthalimidine monomers and polycarbonates derived therefrom
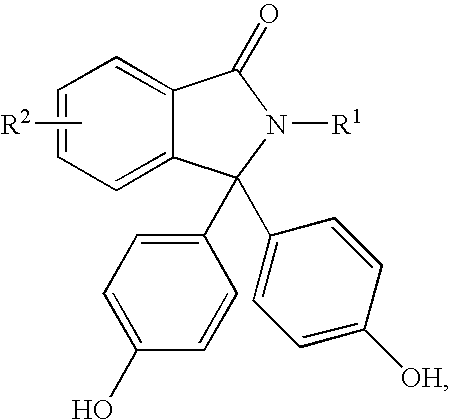
Disclosed herein is a method for producing a 2-hydrocarbyl-3,3-bis(4-hydroxyaryl)phthalimidine. The method comprises forming a reaction mixture comprising at least one substituted or unsubstituted phenolphthalein, at least one substituted or unsubstituted primary hydrocarbyl amine, and an acid catalyst; and heating the reaction mixture to a temperature of less than 180° C. to remove a distillate comprising water and form a crude 2-hydrocarbyl-3,3-bis(4-hydroxyaryl)phthalimidine product; where the 2-hydrocarbyl-3,3-bis(4-hydroxyaryl)phthalimidine has a formula: where R1 is selected from the group consisting of a hydrogen and a hydrocarbyl group, and R2 is selected from the group consisting of a hydrogen, a hydrocarbyl group, and a halogen.
Owner:SHPP GLOBAL TECH BV
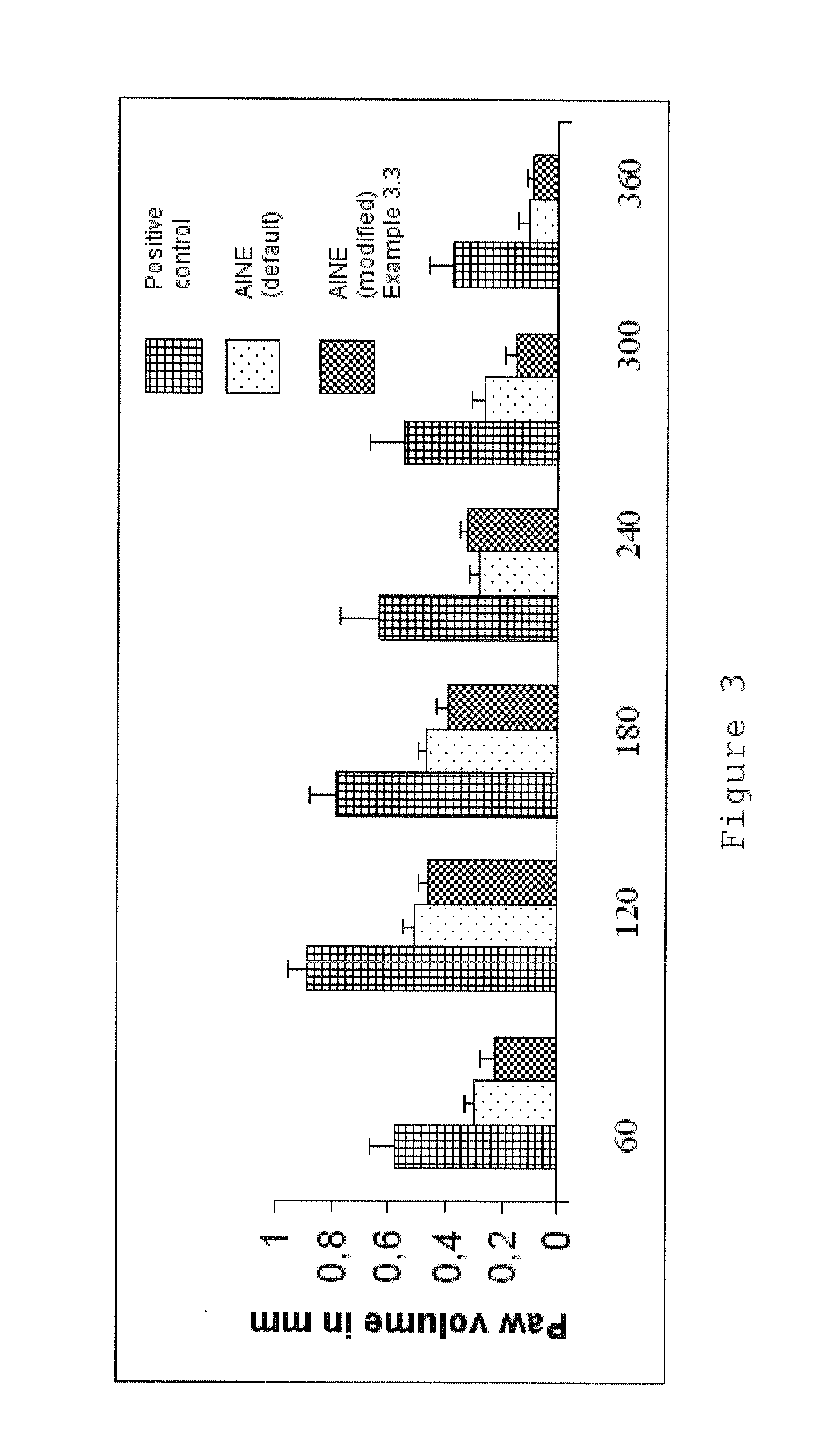
Phthalimide derivatives of non-steroidal Anti-inflammatory compounds and/or tnf-alpha modulators, method for producing same, pharmaceutical compositions containing same and uses thereof for the treatment of inflammatory diseases
InactiveUS20120115817A1Inhibit inflammationMinimizing major limitation and complicationBiocideMonoazo dyesNon steroidal anti inflammatoryRheumatoid arthritis
The present invention relates to phthalimide derivatives of non-steroidal and / or TNF-α modulating anti-inflammatory compounds as well as the process of obtaining the so-called derivatives, pharmaceutical compositions containing such derivatives and their uses, including use in the treatment of inflammatory diseases, especially those related to chronic inflammatory processes, such as rheumatoid arthritis and intestinal inflammatory diseases (for instance, Chron's disease) and the use of the referred to pharmaceutical compositions as antipyretic, analgesic and platelet antiaggregating medications.
Owner:EMS +1
Methods for producing and purifying 2-hydrocarbyl-3,3-bis(4-hydroxyaryl)phthalimidine monomers and polycarbonates derived therefrom
Owner:SHPP GLOBAL TECH BV
Methods of manufacture of bis(phthalimide)s and polyetherimides, and bis(phthalimide)s, and polyetherimides formed therefrom
A method of manufacture of a bis(phthalimide) composition includes reacting, in the presence of a solvent and a catalytically active amount of an imidization catalyst selected from quaternary ammonium salts, quaternary phosphonium salts, and combinations thereof, a substituted phthalic anhydride with an organic diamine, wherein conversion to the bis(phthalimide) is 99% complete in less than 6 hours.
Owner:SABIC GLOBAL TECH BV
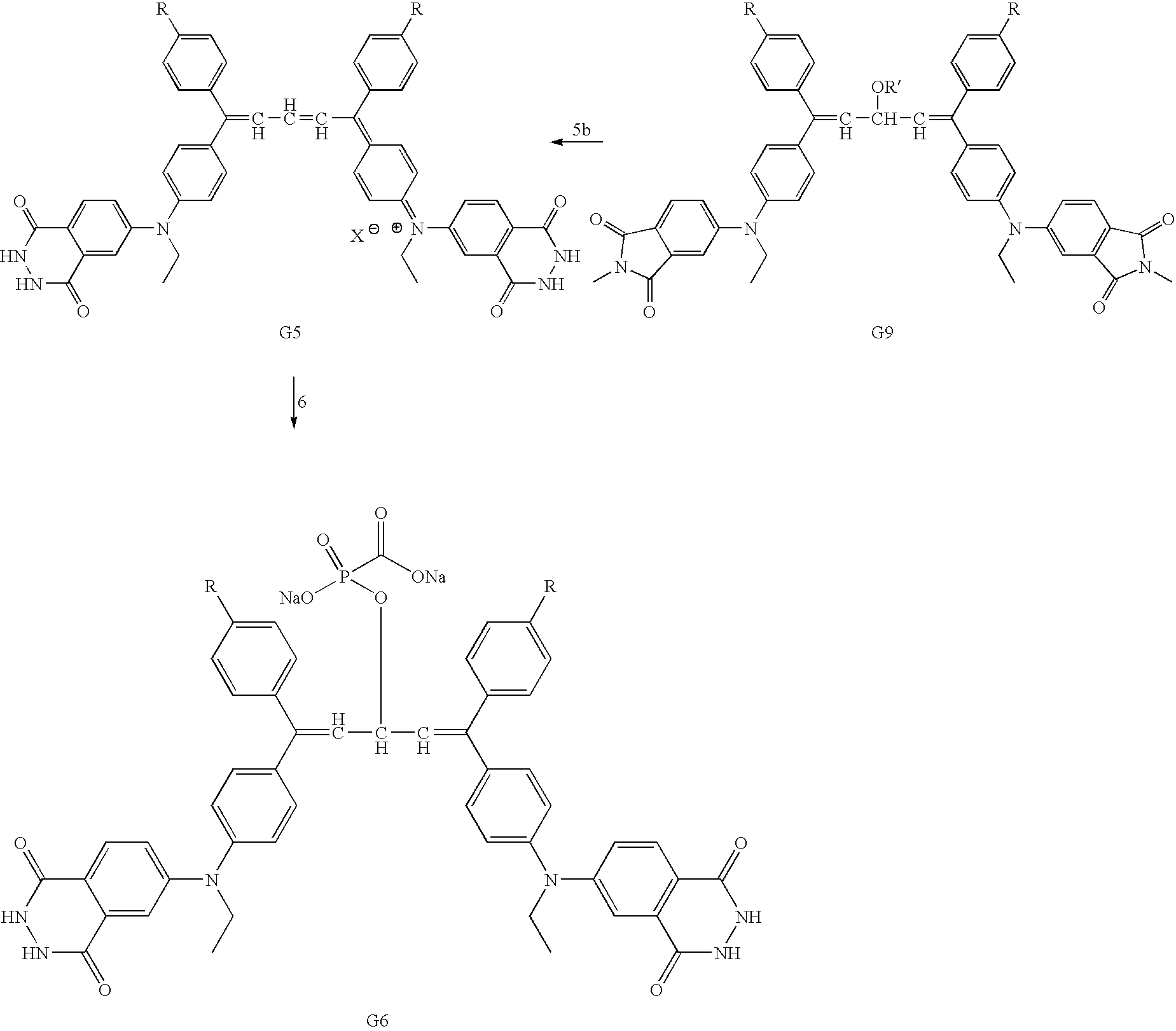
Preparation of prodrugs for selective drug delivery
InactiveUS20050080260A1Good effectRaise the ratioOrganic chemistryOrganic compound preparationAminationPhthalimides
Synthesis of a chemical compound having the formula A-B-C that may serve for applications such as drug delivery where A is a chemiluminescent, moiety, B is a photochromic moiety, and C is a biologically active moiety where A-B-C may serve as a prodrug. Novel synthetic methods of the present invention to form the prodrug comprised the steps of (1) forming a benzophenone, (2) forming a diaryl ethylene, (3) attaching a phthalimide moiety to at least one of the aryl groups of the ethylene to form a phthalimide-ethylene conjugate, (4) condensing two ethylene-phthalimide conjugates to form a phthalimide-pentadiene conjugate, (5) converting the phthalimide to the phthalhydrazide by reaction with hydrazine to form a carrier compound according to the present invention, and (6) reacting the carrier compound with an nucleophilic moiety of the drug to form the corresponding prodrug. Alternatively the carrier can be prepared by using the halo-substituted diaryl ethylene to make the corresponding cationic leuco dye-like compound with known methods. The cationic compound then is protected by reacting with a nucleophile and coupled with the aminophathalimide by palladium-catalyzed amination to form the protected phthalimide-pentadiene conjugate. The latter is refluxed with hydrazine to convert its phthalimide to the phthalhydrazide and acidified to give the carrier. An additional aspect of the present invention relates to the use of these compounds as antiviral agents for the treatment of viral infections such as HIV and as anticancer agents for the treatment of cancers such as bowel, lung, and breast cancer.
Owner:LUMINIDE
Methods for producing and purifying 2-hydrocarbyl-3,3-bis(4-hydroxyaryl)phthalimidine monomers and polycarbonates derived therefrom
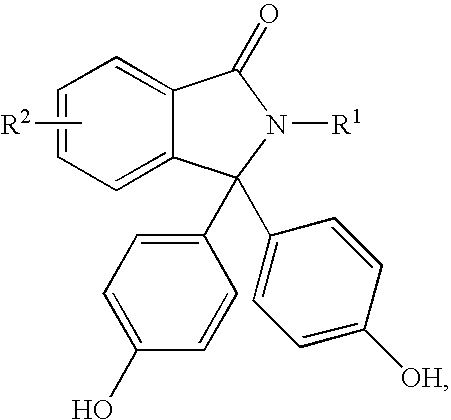
Disclosed herein is a method for producing a 2-hydrocarbyl-3,3-bis(4-hydroxyaryl)phthalimidine. The method comprises forming a reaction mixture comprising at least one substituted or unsubstituted phenolphthalein compound, at least one substituted or unsubstituted primary hydrocarbyl amine, and an acid catalyst; and heating the reaction mixture to form 2-hydrocarbyl-3,3-bis(4-hydroxyaryl)phthalimidine. An adduct of the 2-hydrocarbyl-3,3-bis(4-hydroxyaryl)phthalimidine is formed either by using an excess of the primary hydrocarbyl amine in the first heating step, or by isolating crude 2-hydrocarbyl-3,3-bis(4-hydroxyaryl)phthalimidine after the heating step and then reacting with a further amount of the primary hydrocarbyl amine. The 2-hydrocarbyl-3,3-bis(4-hydroxyaryl)phthalimidine has a formula:where R1 is selected from the group consisting of a hydrogen and a hydrocarbyl group, and R2 is selected from the group consisting of a hydrogen, a hydrocarbyl group, and a halogen.
Owner:SHPP GLOBAL TECH BV
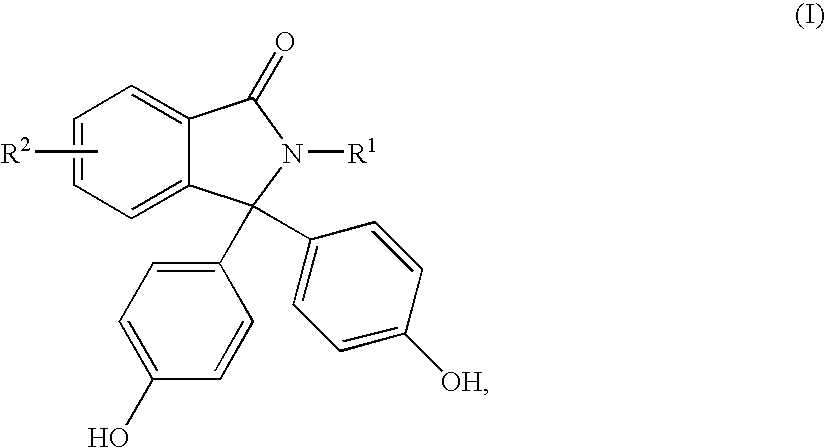
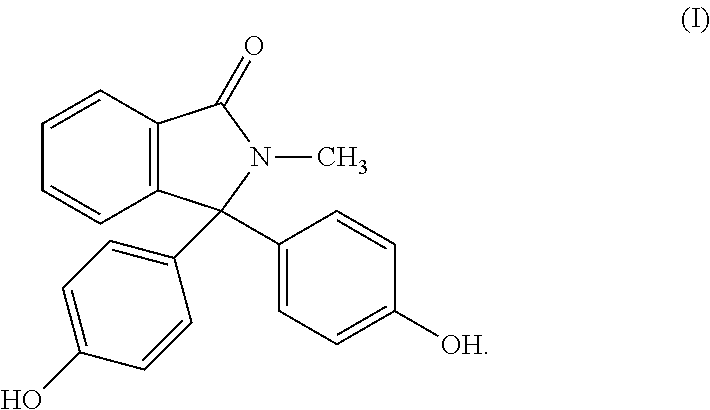
Polycarbonate having improved thermal and mechanical properties and reduced coefficients of thermal expansion
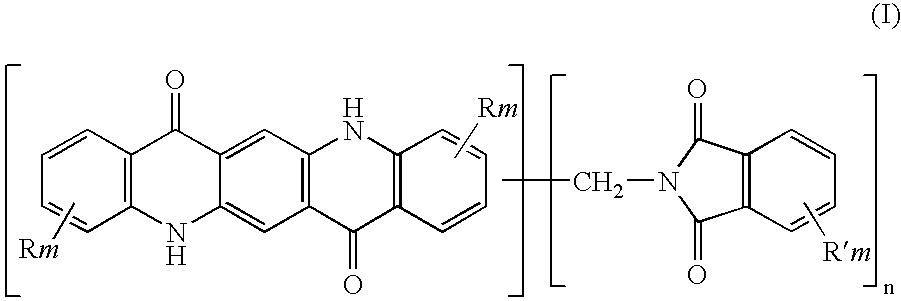
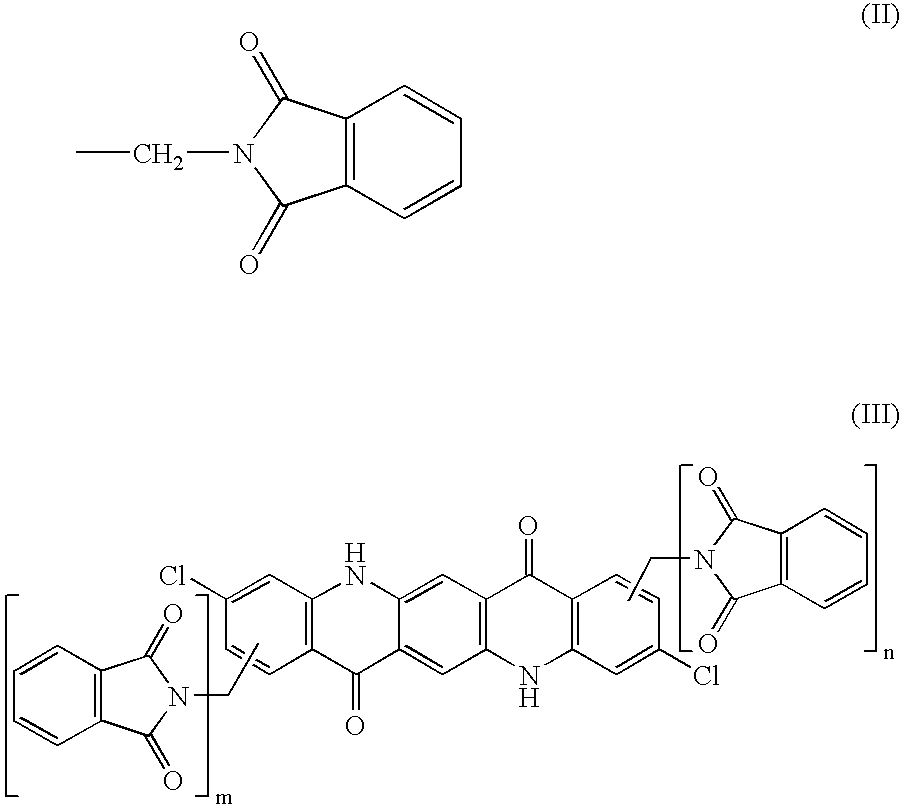
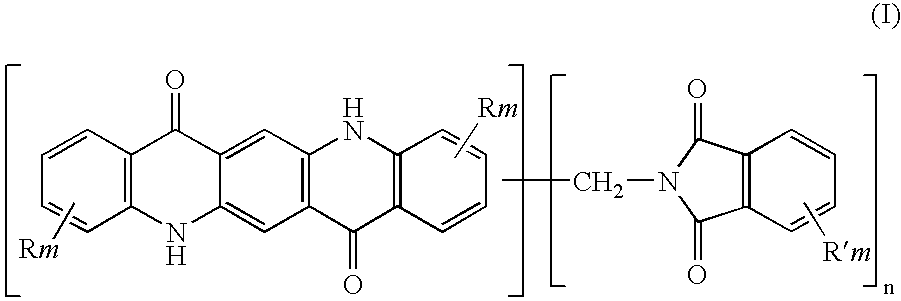
The present invention relates to thermoplastic high-Tg polycarbonates and moulding materials which are distinguished by improved thermal properties and improved mechanical properties, in particular by reduced thermal expansion. The present invention furthermore relates to a process for the preparation of these polycarbonates. In particular, this invention relates to polycarbonates which the structural unit which derives from phthalimide of the formula (I) and polycarbonate compositions and moulding materials therefrom as well as a process for the preparation of these polycarbonates, and the use thereof, in particular as reflectors and display substrates.
Owner:COVESTRO DEUTSCHLAND AG
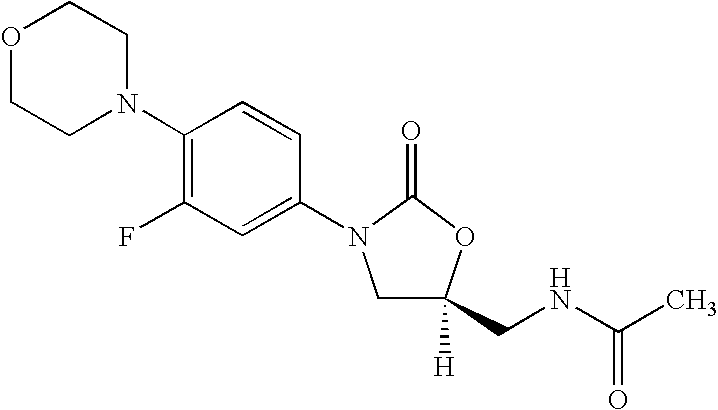
Novel process for the preparation of linezolid and related compounds
The present invention provides a novel process for preparation of 5-aminomethyl substituted oxazolidinones, key intermediates for oxazolidinone antibacterials including linezolid. Thus linezolid is prepared by a) reacting 3-fluoro-4-morpholinyl aniline with R-epichlorohydrin; b) subjecting N-[3-Chloro-2-(R)-hydroxypropyl]-3-fluoro-4-morpholinyl aniline produced above to carbonylation; c) reacting (5R)-5-(chloromethyl)-3-[3-fluoro-4-(4-morpholinyl)phenyl]-2-oxazolidinone produced above with potassium phthalinide; d) reacting (S)-N-[[3-[3-Fluoro-4-[4-morpholinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]phthalimide produced above with hydrazine hydrate; and e) reacting S-N-[[3-[3-Fluoro-4-[4-morpholinyl]phenyl]-2-oxo-5-oxazo-lidinyl]methyl]amine produced above with acetic anhydride to produce linezolid.
Owner:HETERO USA INC
Antibacterial polypropylene plastic and preparation method thereof
InactiveCN102838809ABroad-spectrum antibacterialImmediate effectPolymer sciencePolyhexamethylene guanidine
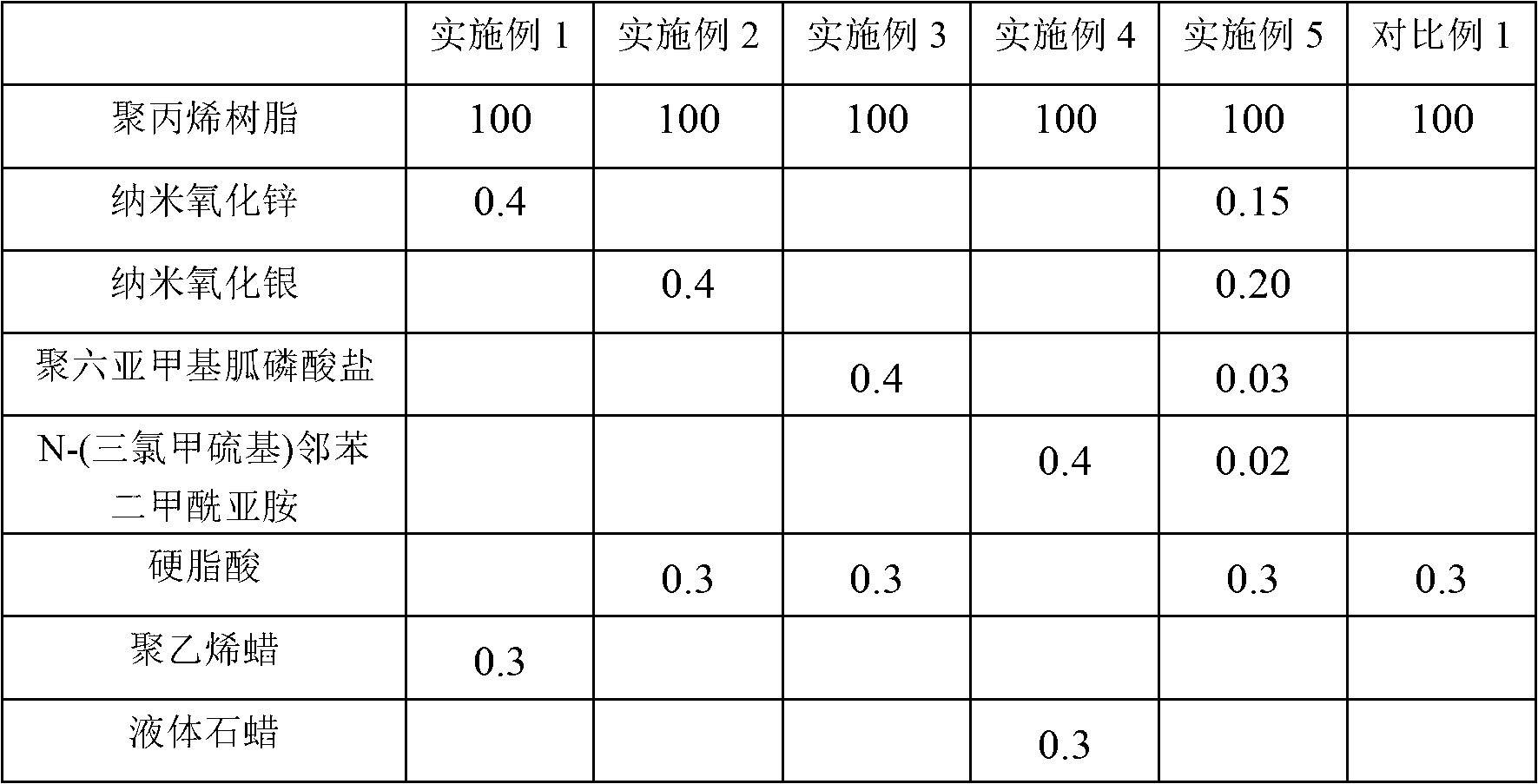
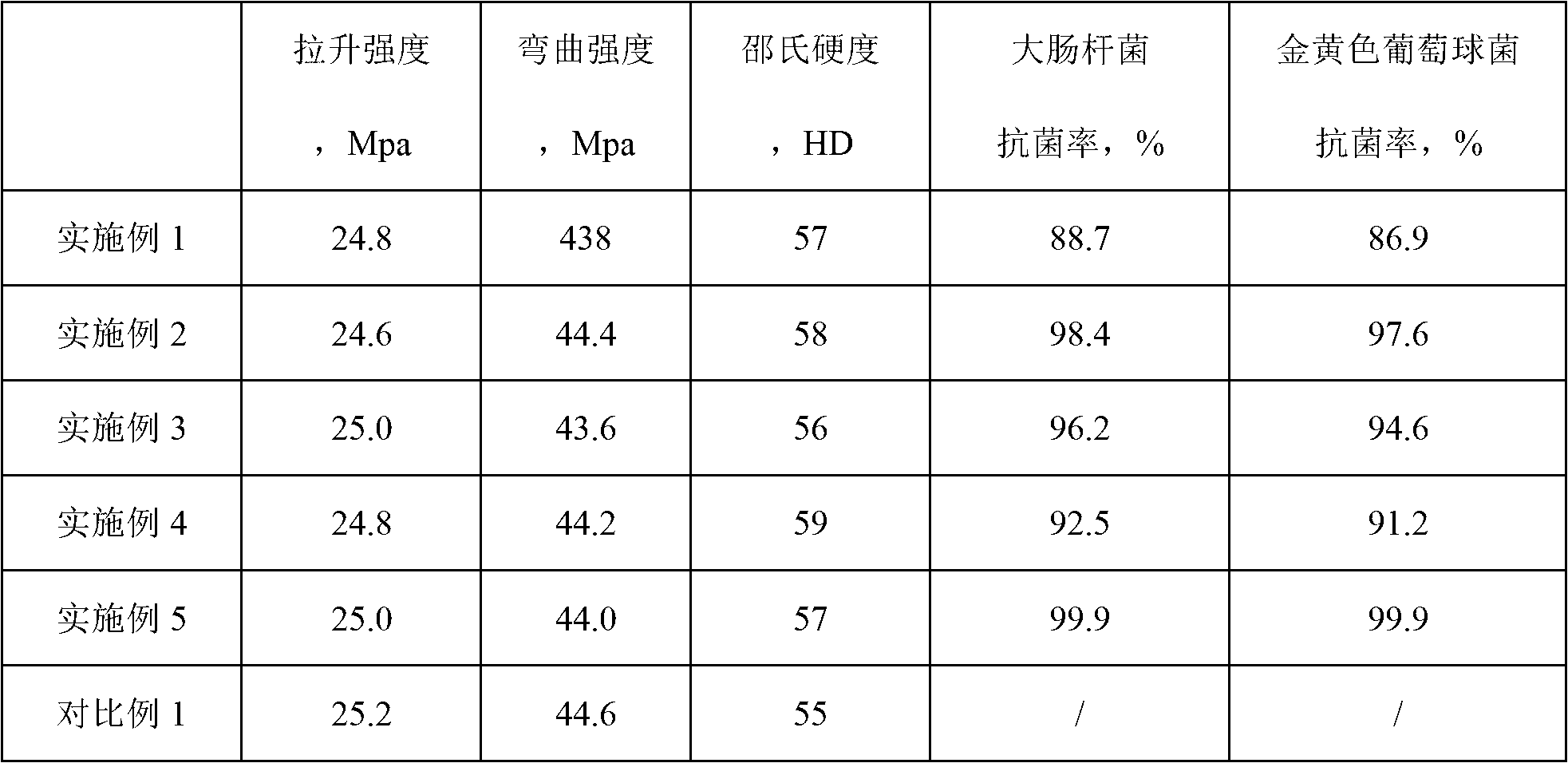
The invention discloses an antibacterial polypropylene plastic and a preparation method of the antibacterial polypropylene plastic. The antibacterial polypropylene plastic comprises the following components by weight: 100 parts of polypropylene, 0.2-0.6 parts of antibacterial agent and 0.1-0.5 parts of dispersant, wherein the antibacterial agent is one or a mixture of nanometer zinc oxide, nanometer silver oxide, polyhexamethyleneguanidine phosphate and N-(trichloromethylthio) phthalimide. The antibacterial polypropylene plastic still has good effects of inhibiting and killing bacteria and preventing mould after being used for a long time, and is not influenced by factors such as illumination and temperature. The antibacterial polypropylene plastic can be easily processed by an injection process into automotive upholsteries in various shapes, such as an inner cover plate for a glove box, a glove box hopper, an auxiliary instrument board body, a storage box gasket and a panel storage box.
Owner:SHANGHAI HAN MOLDING SHAPE CO LTD
Antibacterial ABS plastic
The invention discloses antibacterial ABS (acrylonitrile-butadiene-styrene) plastic which comprises the following components in parts by weight: 100 parts of ABS, and 0.3-0.5 parts of composite antibacterial, wherein the composite antibacterial comprises the following components in parts by weight: 2-6 parts of cedar wood oil, 4-8 parts of chitosan, 10-20 parts of nano titanium oxide, 15-25 parts of nano silver oxide, 30-50 parts of polyhexamethylene biguanidine hydrochloride, and 2-4 parts of N-(trichloro methylthio) phthalimide. The antibacterial ABS plastic has strong bacterium inhibiting, bacterium killing and mould proof effects, and can be processed into automotive interior trim parts in various shapes, such as inner cover plates of glove boxes, glove box buckets, subpanel bodies, storage case pads, and panel storage cases, by an injection molding technology easily.
Owner:上海佳谷模具有限公司
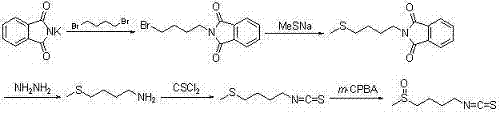
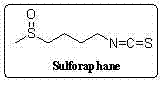
Synthetic method for sulforaphane
ActiveCN102249968AAvoid hydrazinolysisSimple and fast operationOrganic chemistryBulk chemical productionSulforaphaneSodium methanethiolate
The invention provides a synthetic method for sulforaphane and belongs to the field of drug synthesis. The method comprises the following steps that: after amino in 4-amino-1-butanol is protected by Boc groups, hydroxy in 4-amino-1-butanol is changed into methanesulfonyl ester by methanesulfonyl chloride, and then the resultant reacts with sodium methyl mercaptide to produce 4-methylthio butyl-1-tert-butoxycarbonylamide; Boc protective groups are removed under acidic condition to obtain 4-methylthio-1-butylamine; 4-methylthio-1-butylamine reacts with carbon disulfide for one hour in the presence of triethylamine and p-toluenesulfonyl chloride is added for treatment for half an hour to produce 4-methylthio butyl-1-isothiocyanate; and at last 4-methylthio butyl-1-isothiocyanate is oxidized by m-CPBA to produce sulforaphane. According to the invention, complex hydrazinolysis of phthalimide in aftertreatment is avoided and toxic thiophosgene is not needed in the preparation of isothiocyanate; overall yield of sulforaphane is 64%, substantially higher than the overall yield of 8% reported in literature; the whole preparation process is simple and time-saving and is suitable for large scale production.
Owner:江苏宁录科技股份有限公司
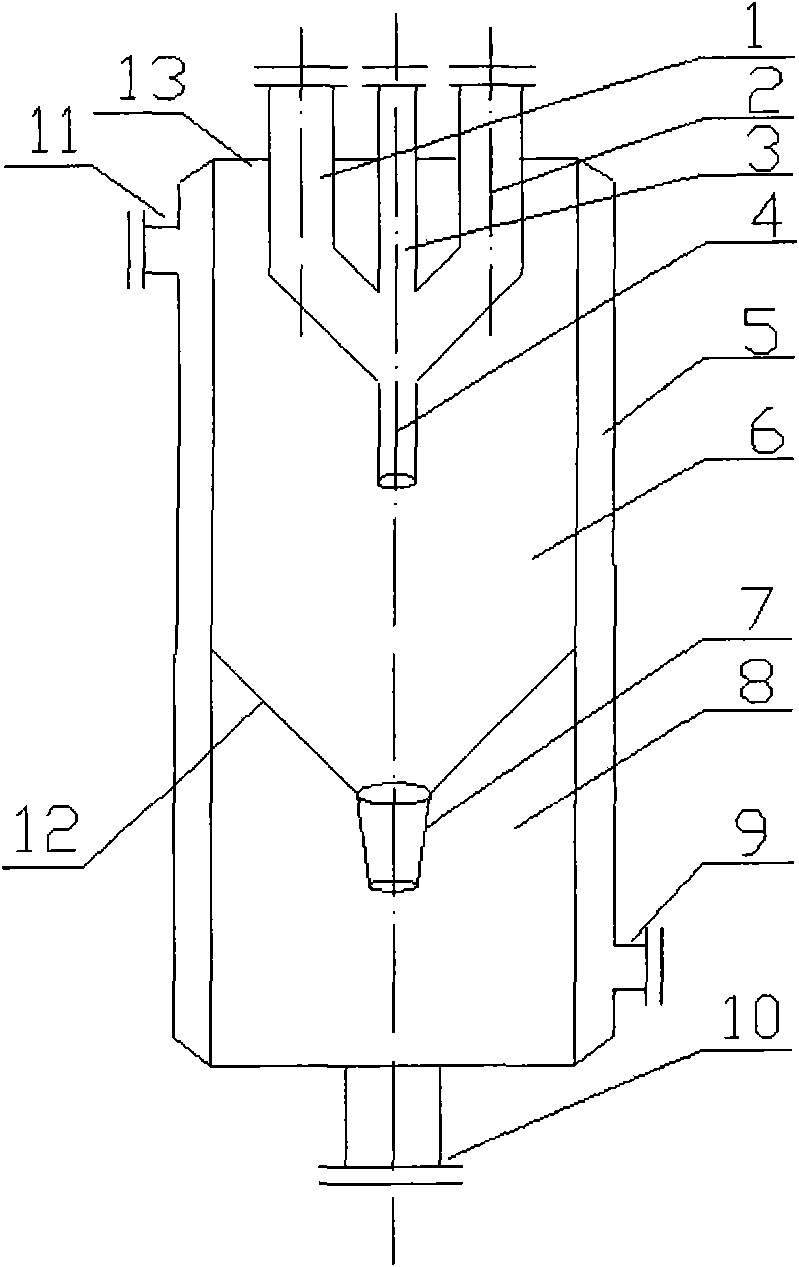
Technique for using methylbenzene to prepare benzaldehyde and benzene methanol by multistage oxidation and equipment
InactiveCN101607867APreparation by oxidation reactionsOrganic compound preparationBenzaldehydeFractionating column
The invention discloses a technique for using methylbenzene to prepare benzaldehyde and benzene methanol by air oxidation and equipment. In a reaction separating system consisting of a multistage oxidation reactor as well as a gas-liquid separator, a lateral line fractionating column or a fractionating column system, 0.1 to 50ppm of monometallic porphyrin or u-oxygen bimetallic porphyrin is separately used as a catalyst, or metallic porphyrin and phthalimide (or salt) are used as a mixed catalyst according to the weight ratio of 1:20 to 100; when the reaction temperature is 80 to 200 DEG C, the methylbenzene is maintained in a multistage oxidizer for 60 to 180 minutes and prepared into the benzaldehyde and benzene methanol by catalytic oxidation; and the gas-liquid separation and purification are carried out. Compared with the prior art, the technique and equipment can obviously improve the conversion rate of the methylbenzene, and the conversion rate of the methylbenzene can reach to be more than 95% by experiments; in addition, the technique and equipment also can improve the selection of the benzaldehyde and benzene methanol, and the selection of the benzaldehyde and benzene methanol can reach to be more than 50%.
Owner:HUNAN UNIV
Thermo-stable, arsenic-free synergistic biocide concentrate composition for polymer matrices and process for preparing same
InactiveUS20130310428A1High processing temperatureHigh bactericidal activityBiocideDead animal preservationImideThio-
A thermo-stable, arsenic-free synergistic biocide concentrate composition to afford enhanced antimicrobial properties to a polymer matrix comprising (i) a mixture of trihalomethyl-thio-phthalimide analogue and a second biocide; (ii) an antioxidant; (iii) a carrier; and (iv) optionally, one or more additives. The composition is capable of withstanding high processing temperatures up to about 250° C., substantially without thermal degradation of biocides and discoloration of the polymer matrix employed.
Owner:TROY TECH II
Thermally-activated delay fluorescent material and organic electroluminescence device
InactiveCN106966954AGood chemical stabilityHigh fluorescence quantum yieldOrganic chemistrySolid-state devicesQuantum yieldDerivatization
The invention relates to a thermally-activated delay fluorescent material. The thermally-activated delay fluorescent material is a 4,5-position substituted phthalimide derivative, R1 is an electron-rich aromatic amine substituent group containing at least one nitrogen, amino nitrogen of the electron-rich aromatic amine substituent group is connected with phthalimide, and R2 is any one of saturated aliphatic group, unsaturated aliphatic group, aryl and hetero aryl. The 4,5-position substituted phthalimide derivative has the advantages of having thermally-activated delay property, high fluorescence quantum yield and easy derivatization characteristics and the advantages of good stability and the like. A preparation method of the thermally-activated delay fluorescent material is simple in synthesis, the raw materials are cheap, the product yield is high, and the material can be prepared in a large-scale mode. The invention provides an organic electroluminescence device. The organic electroluminescence device has the advantages of high efficiency, low driving voltage, long service life, stable light emitting and the like. The thermally-activated delay fluorescent material provided with a 4,5-position substituted phthalimide structure and the organic electroluminescence device based on the material have a very good application prospect.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Use of phthalimide derivative in preparation of medicament for resisting angiogenesis
InactiveCN1961876APrevent proliferationOrganic active ingredientsMetabolism disorderAngiogenesis growth factorNeo angiogenesis
The invention relates to the action of phthalimide derivatives of formula I and II in the preparation of medicament against angiogenesis, wherein the definitions of each substituent group are described in the specification.
Owner:北京恩华医药研究院有限公司 +1
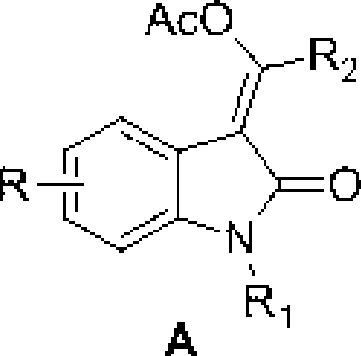
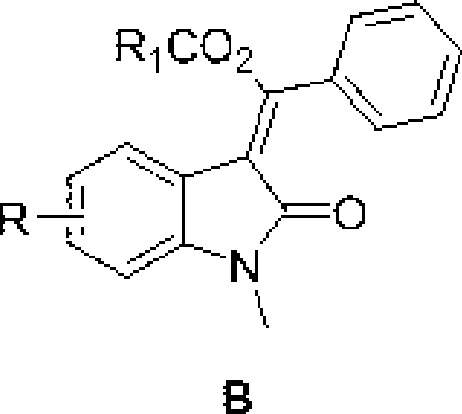
3-methylene-indol-2-one derivates and preparation method thereof
The invention relates to a 3-methylene-indole-2-ketone derivative and a preparation method thereof. The derivative is synthesized by taking N-methyl-N-phenyl-hydrocinnamamide or N-aryl phenylpropargyl acylamide and phthalic imide as substrates through tandem reactions such as C-H activation / acetylene hydrocarbon palladinization / cross coupling and so on, based on the catalysis of Pd<II> / Pd<IV> under the coaction of a palladium catalyst and an oxidizing agent, then ethyl acetate is added, the mixture is scrubbed by common salt, an aqueous phase is then extracted by the ethyl acetate, an organic layer is collected, and the 3-methylene-indole-2-ketone derivative is prepared through drying, condensation and column chromatography. The preparation method has the advantages of simple and convenient reaction operation, short reaction steps, simple conditions, short time, low cost, and high yield, and is worthy to be popularized and applied.
Owner:WENZHOU UNIVERSITY
Oxo anion-adsorbing ion exchangers
InactiveUS20070241057A1Simple preparationImprove arsenic adsorptionIon-exchanger regenerationOrganic anion exchangersIron oxyhydroxideBrown iron oxide
The present invention relates to a process for the preparation of iron oxide / iron oxyhydroxide-containing weakly basic anion exchangers prepared according to the phthalimide process and their use for removing oxo anions and their thio analogues, preferably of arsenic, from water and aqueous solutions and to a regeneration process.
Owner:LANXESS DEUTDCHLAND GMBH
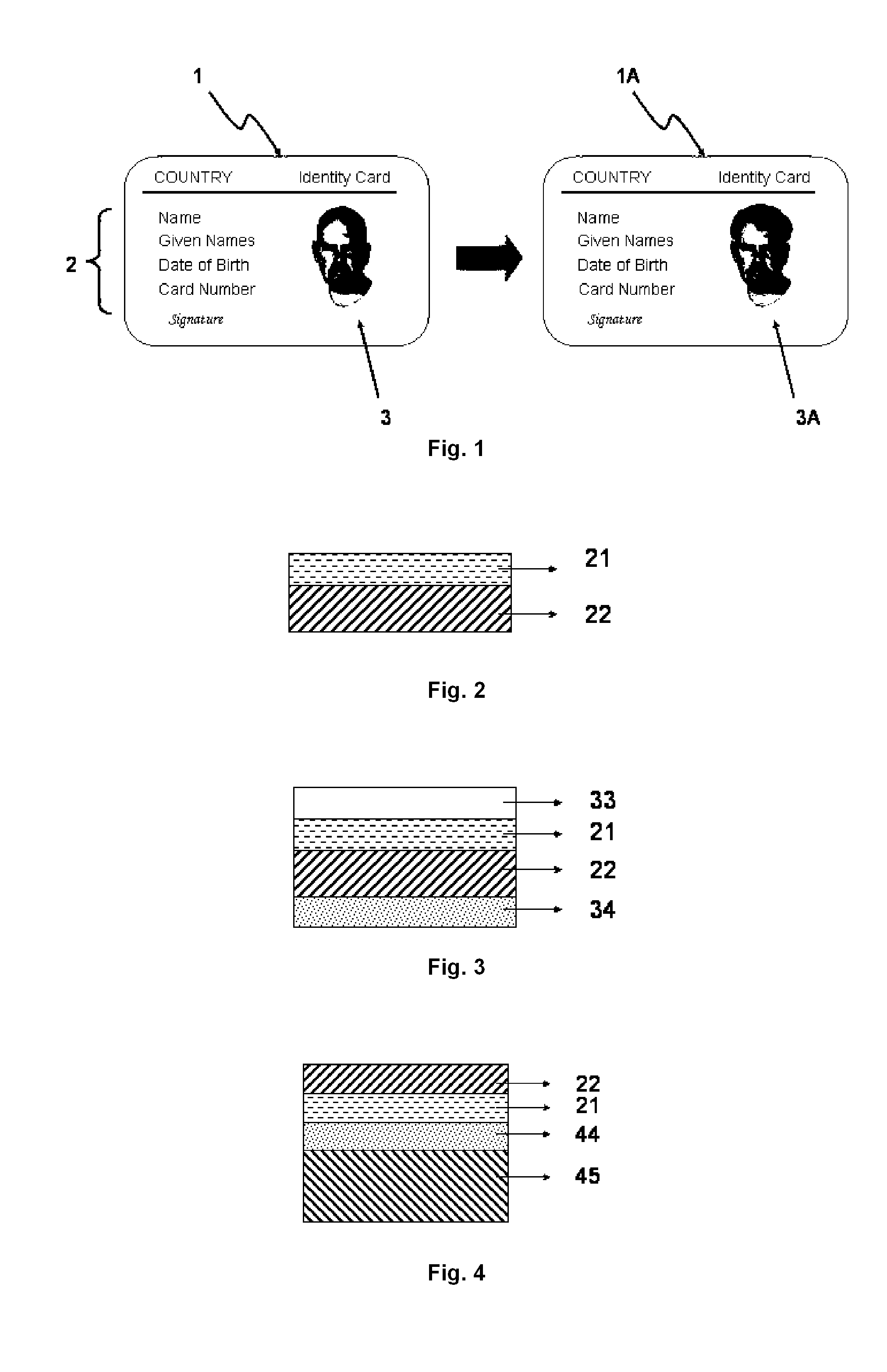
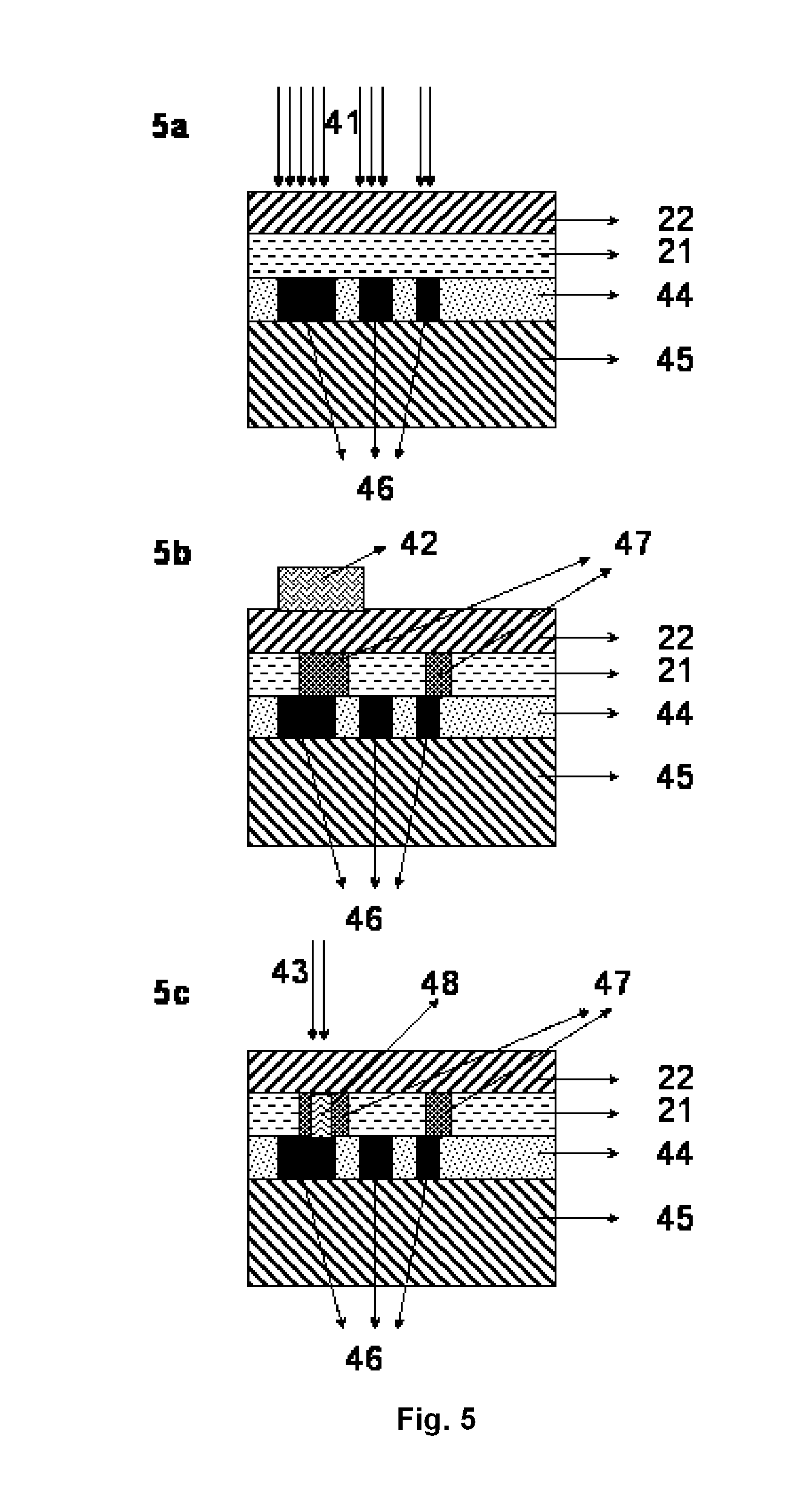
Security laminates for security documents
InactiveUS20110200765A1Simple and cost-effective to implementDifficult to falsifyPhotosensitive materialsCellulose adhesivesPolyethylene terephthalate glycolPolyethylene terephthalate
A security laminate for securing a security document comprising:a) a biaxially stretched polyethylene terephthalate support; andb) a thermosensitive layer coated on the support;wherein the thermosensitive layer contains a binder, a substantially light-insensitive organic silver salt, an organic reducing agent and a toning agent selected from the group consisting of phthalimides, phthalazinones, benzoxazine diones and naphthoxazine diones.Methods for manufacturing security documents are also disclosed.
Owner:AGFA GEVAERT AG
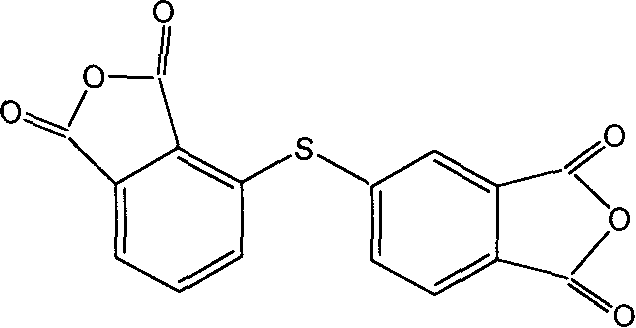
Preparation methd of 2,2',3,4'-diphenyl thioether tetra acid dianhydride
The invention belong to the art of preparation technique of 2, 3', 3, 4'-diphenyl sulfide tetra-acid dianhydride. It adopts sodium hydrosulfide as a sulfuration agent to react N- substituted- 4- or- 3- phthalimide chloride non-protonic polar solvent to generate intermediate product N- substituted- 4- or- 3- phthalimide sulfhydryl substituted; the latter reacting N- substituted- 3- or- 4- phthalimide chloride non-protonic polar solvent in faintly alkaline condition to generate asymmetric N, N'- double substituted- 2, 3', 3, 4'-diphenyl sulfide tetra imide, hydrolyzing, acidifying, by toluene and dimethylbenzene with water to prepare directly asymmetric2,3',3,4'- diphenyl sulfide tetra-acid dianhydride, which can be used to synthesize polyimide.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
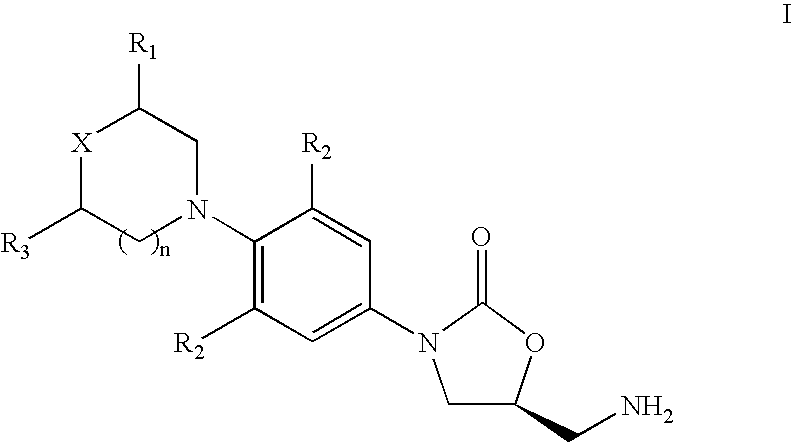
4-[4-(substituted phenyl) piperazine piperazinyl-1]-butylcarbamic acid substituted aromatic ester derivative and preparation method thereof
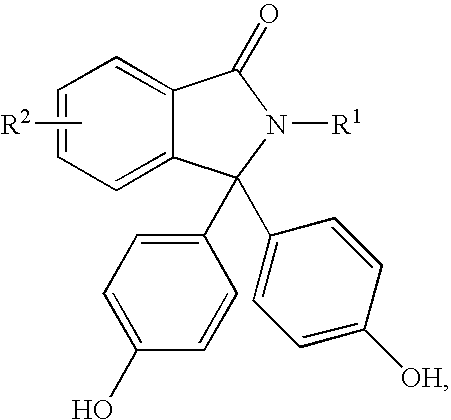
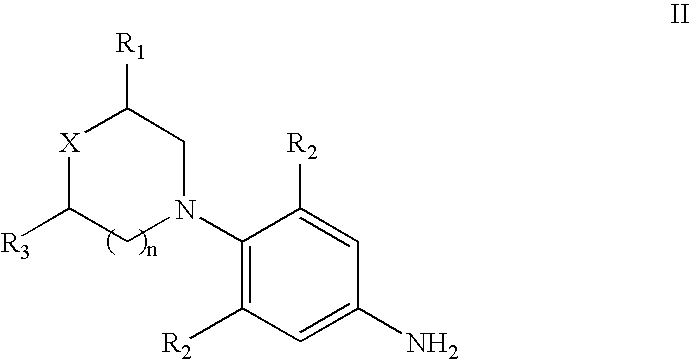
ActiveCN103073524AHigh affinityEffective treatmentNervous disorderOrganic chemistryReaction intermediateStructural formula
The invention discloses a phenyl piperazidine heterocyclic medicinal compound. The compound has high affinity to a dopamine D3 receptor, so that the compound can be used for treating addiction to and dependence on medicines such as cocaine, and a central nervous system disorder relevant to the addiction and the dependence. The compound is a 4-[4-(substituted phenyl) piperazine piperazinyl-1]-butylcarbamic acid substituted aromatic ester derivative with a structural formula as Formula (1) as shown in the specification. A synthetic method of the derivate comprises the steps that substituted aniline and 2-(beta-chloroethyl) amine hydrochloride reacts in a solvent by taking inorganic base as an acid-binding agent to form corresponding substituted phenyl piperazidine hydrochloride 1; substituted phenyl piperazidine hydrochloride 1 and N-(delta-bromobutyl) phthalimide react in acetonitrile by taking K2CO3 as an acid-binding agent and under catalysis of KI to form a reaction intermediate 2; the intermediate 2 is subjected to hydrazinolysis to form an intermediate 3; and the intermediate 3 and an intermediate 5 are condensed by taking triethylamine as an acid-binding agent and a catalyst to form a target product I. The intermediate 5 is obtained in a manner that triphosgene and substituted aromatic phenol conduct partial condensation reaction in methylene chloride.
Owner:宁波市微循环与莨菪类药研究所 +1
CTP synthesis technology of rubber scorch retarder and device thereof
ActiveCN101624364AReduce chance of decompositionAdequate responseOrganic chemistryChemical/physical/physico-chemical stationary reactorsImideSulfuryl
The invention relates to a CTP synthesis technology of rubber scorch retarder and a device thereof. The technology comprises the following steps of: continuously adding cyclohexyl sulfenyl chloride and phthalimide sodium salt into a tube type reactor, continually adding with nitrogen, momentary completely mixing the cyclohexyl sulfenyl chloride and the phthalimide sodium salt at the tube type reactor and reacting, adding materials into a condensation kettle, and continuously stirring to have a condensation reaction under consistent temperature. The invention also provides a special tube type reactor. By adding a pipeline condensation reaction, the invention can reduce the decomposition probability of the cyclohexyl sulfenyl chloride, thereby heightening yield efficiency to be more than 92% and purity to be more than 98%.
Owner:SHANDONG YANGGU HUATAI CHEM
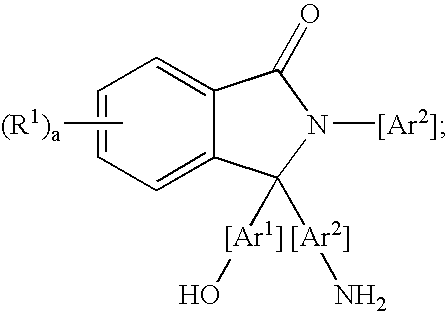
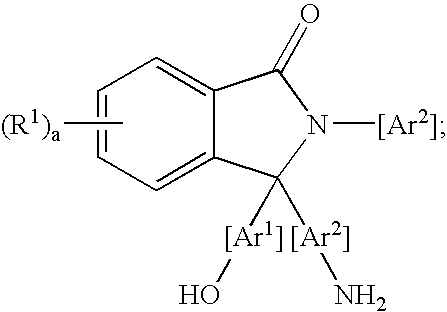
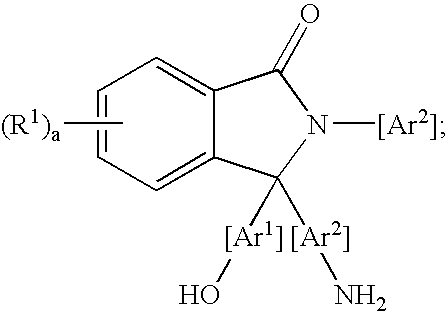
Methods for purifying 2-aryl-3,3-bis(hydroxyaryl)phthalimidines
A method for purifying a 2-aryl-3,3-bis(hydroxyaryl)phthalimidine comprises contacting a crude 2-aryl-3,3-bis(hydroxyaryl)phthalimidine with a purification agent, removing a 2-aryl-3-(aminoaryl)-3-(hydroxyaryl)phthalimidine compound from the crude 2-aryl-3,3-bis(hydroxyaryl)phthalimidine, and producing a purified 2-aryl-3,3-bis(hydroxyaryl)phthalimidine product comprising less than 200 parts per million of the 2-aryl-3-(aminoaryl)-3-(hydroxyaryl)phthalimidine compound. The purification agent is selected from the group consisting of an acidic material, an organic acid chloride, an organic anhydride, or a combination thereof. The 2-aryl-3-(aminoaryl)-3-(hydroxyaryl)phthalimidine compound has a formula: wherein each R1 is independently selected from a group consisting of a hydrocarbyl radical, a nitro radical, and a halogen atom; “a” is an integer from 0 to 4; and Ar1 and Ar2 are independently at each occurrence an aromatic radical. The purified 2-aryl-3,3-bis(hydroxyaryl)phthalimidines have low color, and are useful for preparing polymers, such as polycarbonates having a low color. The polycarbonates are useful for producing articles.
Owner:SHPP GLOBAL TECH BV
Substituted 2-(2,6-dioxopiperidin-3-yl)-phthalimides and -1-oxoisoindolines and method of reducing TNFα levels
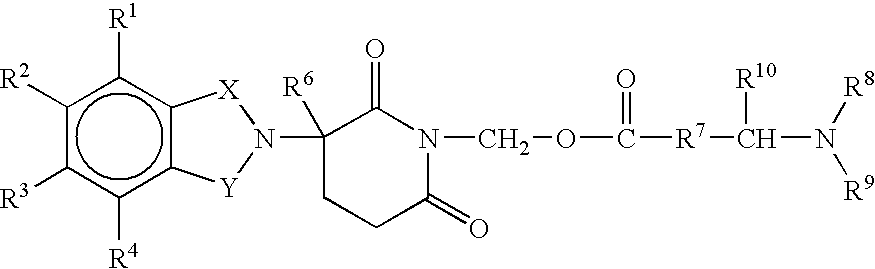
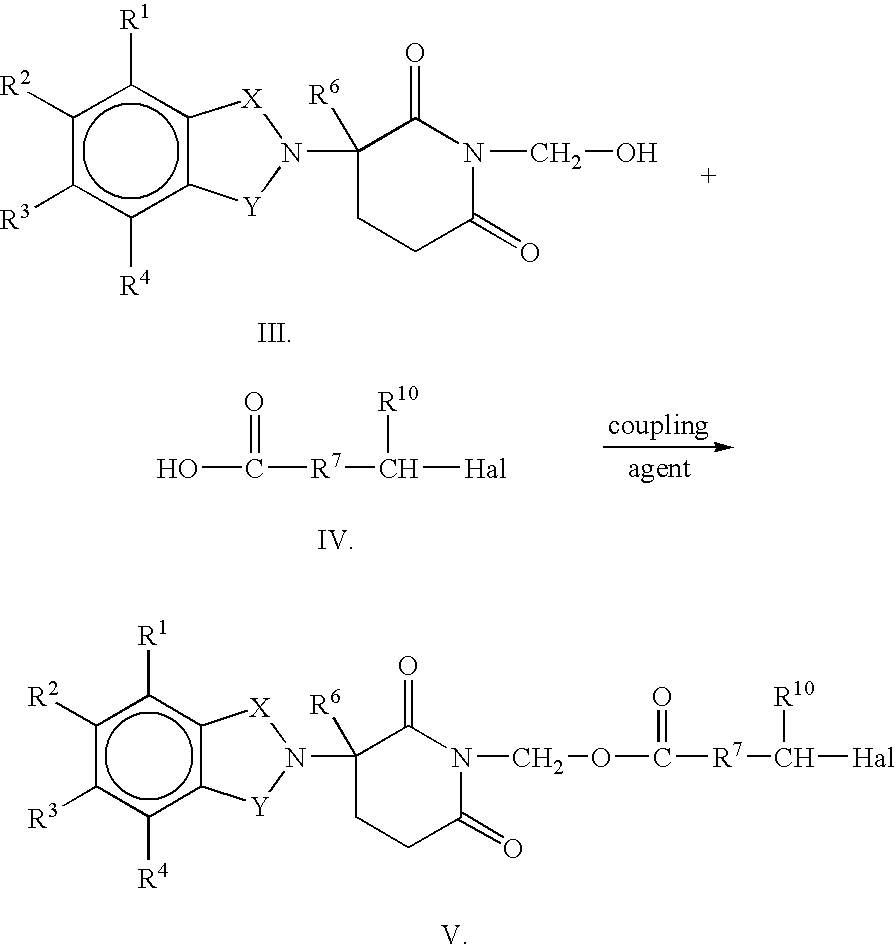
Substituted 2-(2,6-dioxopiperidin-3-yl)-phthalimides and 1-oxo-2-(2,6-dioxopiperidin-3-yl)isoindolines reduce the levels of TNFα in a mammal. A typical embodiment is 1-oxo-2-(2,6-dioxo-3-methylpiperidin-3-yl)-4,5,6,7-tetrafluoroisoindoline.
Owner:CELGENE CORP
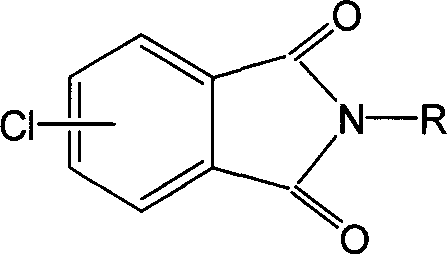
Sheathing material for power cables and preparation method thereof
InactiveCN103788517AAvoid premature vulcanizationHigh tensile strengthRubber insulatorsInsulated cablesPotassiumPolyethylene glycol
The invention discloses a sheathing material for power cables. The sheathing material comprises the following raw materials in parts by weight: 12-16 parts of chlorosulfonated polyethylene, 3-6 parts of butadiene-acrylonitrile rubber, 6-9 parts of chlorinated butyl rubber, 0.3-0.5 part of zinc dimethyldithiocarbamate, 0.3-0.7 part of dibenzothiazyl disulfide, 1.2-1.7 parts of an activator zinc oxide, 2-3 parts of stearic acid, 0.6-1 part of N-(cyclohexylthio)phthalimide, 0.5-1 part of chlorinated paraffin, 1-2 parts of a surfactant polyethylene glycol, 1.5-2 parts of potassium chloroplatinate, 1-4 parts of dioctyl phthalate, 4-7 parts of a reinforcing agent carbon black 330, 4-6 parts of a filler ultrafine talcum powder, and 2.5-4 parts of a filler calcium carbonate. The invention also discloses a preparation method of the sheathing material for power cables. According to the invention, the sheathing material for the power cables is good in mechanical properties, high in tensile strength, good in tear strength and wear resistance, excellent in aging resistance, simple in preparation process, and low in production cost.
Owner:ANHUI ZHONGTONG CABLE TECH
Composition for wire coating material, insulated wire, and wiring harness
InactiveUS20130273367A1Improve heat resistanceGood peeling effectOrganic chemistryPlastic/resin/waxes insulatorsPolymer sciencePtru catalyst
To provide a composition for a wire coating material, requiring no electron irradiation crosslinking and a less filler, that can produce a crosslinked coat having a high heat resistance, gel fraction and peel property at high temperature, and provide an insulated wire, and a wiring harness. A wire coating material composition contains (A) silane-grafted polyolefin, (B) undenatured polyolefin, (C) functional-group modified polyolefin modified by functional groups of a carboxylic acid group, an acid anhydride group, an amino group and an epoxy group, (D) a bromine flame retardant having a phthalimide structure or the flame retardant and an antimony trioxide, (E) a crosslinking catalyst batch containing a resin containing a crosslinking catalyst, and (F) a zinc oxide and an imidazole compound or a zinc sulfide, (G) a triazine hindered phenolic antioxidant having a melting point of 150 degrees C. or more, and (H) a triazole derivative or a hydrazide metal deactivator.
Owner:AUTONETWORKS TECH LTD +2
Synthesizing method of 5-fluoro-3-methyl isobenzofuran-1(3H)-ketone
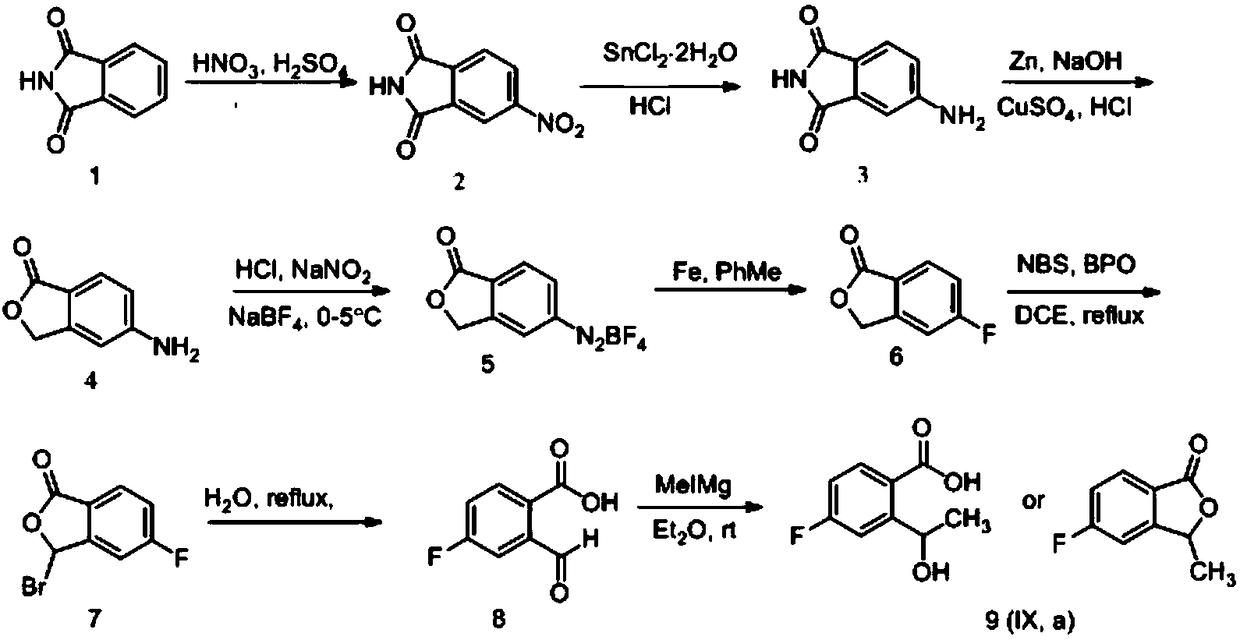
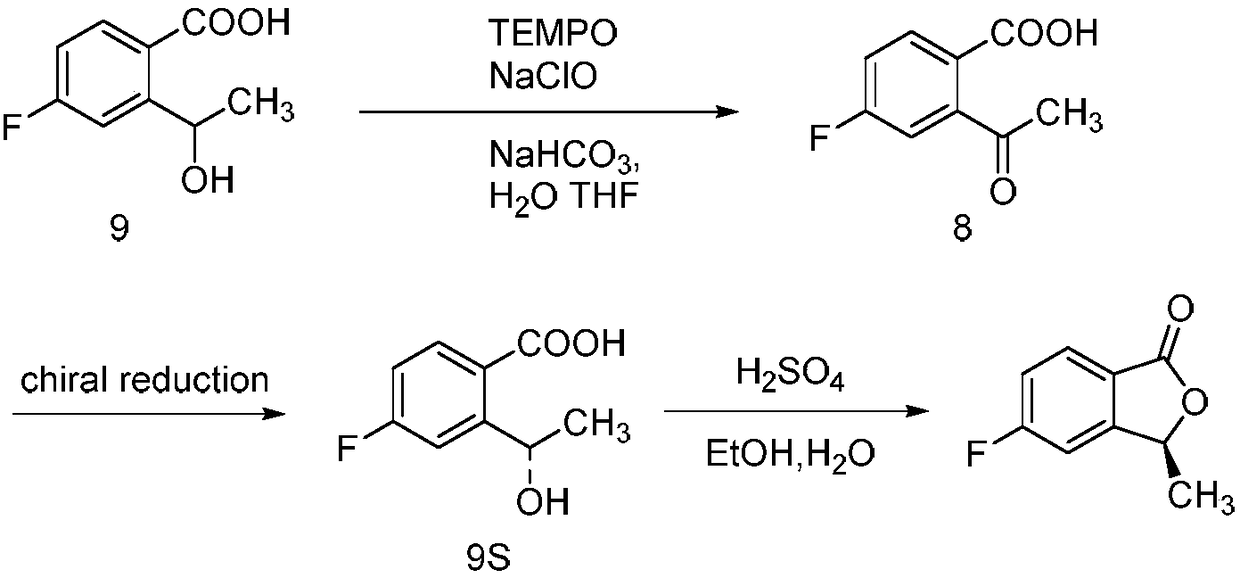
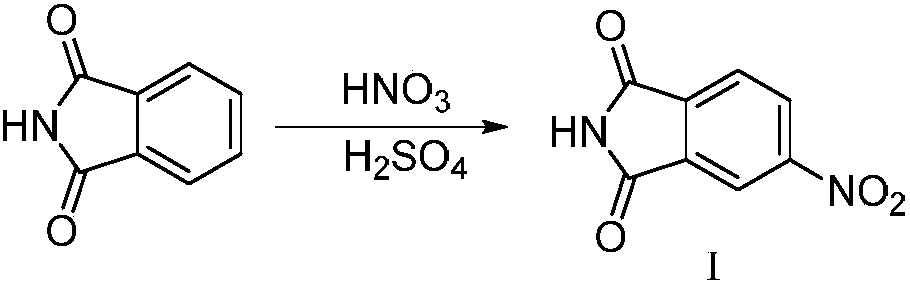
ActiveCN109134410AEasy to operateEasy post-processingPreparation from carboxylic acid esters/lactonesOptically-active compound separationIsobenzofuranNitration
The invention relates to the technical field of medicine and relates to a synthesizing method of 5-fluoro-3-methyl isobenzofuran-1(3H)-ketone. The 5-fluoro-3-methyl isobenzofuran-1(3H)-ketone is synthesized by subjecting the initial raw material phthalimide to 8 steps such as nitration, reduction, cyclizing, diazotization, bromination and esterification. The method has the advantages that the preparation of the 5-fluoro-3-methyl isobenzofuran-1(3H)-ketone which is the key intermediate of the antitumor drug Lorlatinib (PF-06463922), total yield can reach 7.0% or above, and the method is simpleto operate, convenient in post-processing, low in time consumption, low in cost and beneficial to industrialization; the Lorlatinib is synthesized by subjecting the intermediate and 1-methyl-3-(( methylamino)methyl)-1H-pyrazol-5-nitrile to ammonolysis, substitution, coupling, chiral resolution and the like, and a new method is provided for the synthesizing of the antitumor drug Lorlatinib.
Owner:SHENYANG PHARMA UNIVERSITY
Process for producing aromatic diaether dianhydride monomer
The invention relates to a method for synthesizing a dianhydride monomer, in particular to a method for synthesizing an aromatic HQEDA monomer. The method adopts a 3(4) substituted phthalimide to prepare HQEDA and provides novel middle steps for preparing the HQEDA. The method comprises the following steps: preparing a 3 (4) substituted-N-alkyl (aryl) phthalimide from the 3(4) substituted phthalimide through Gabriel reaction principle or by adding a salifying agent and halocarbon into an apolar aprotic solvent to react; and using the prepared 3 (4) substituted-N-alkyl (aryl) phthalimide to prepare bis imide and the bis ether anhydride. The method has the advantages that raw materials are easily available, preparation method is simple and easy to operate, and the purity and yield of the prepared 3 (4) substituted-N-alkyl (aryl) phthalimide are obviously higher than that of the prior method.
Owner:NANJING UNIV OF TECH
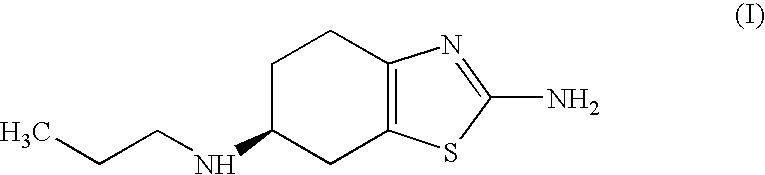
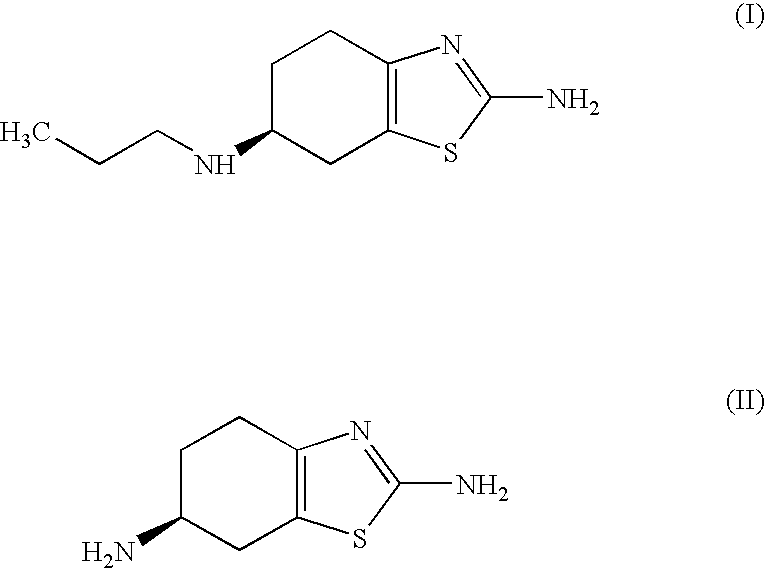
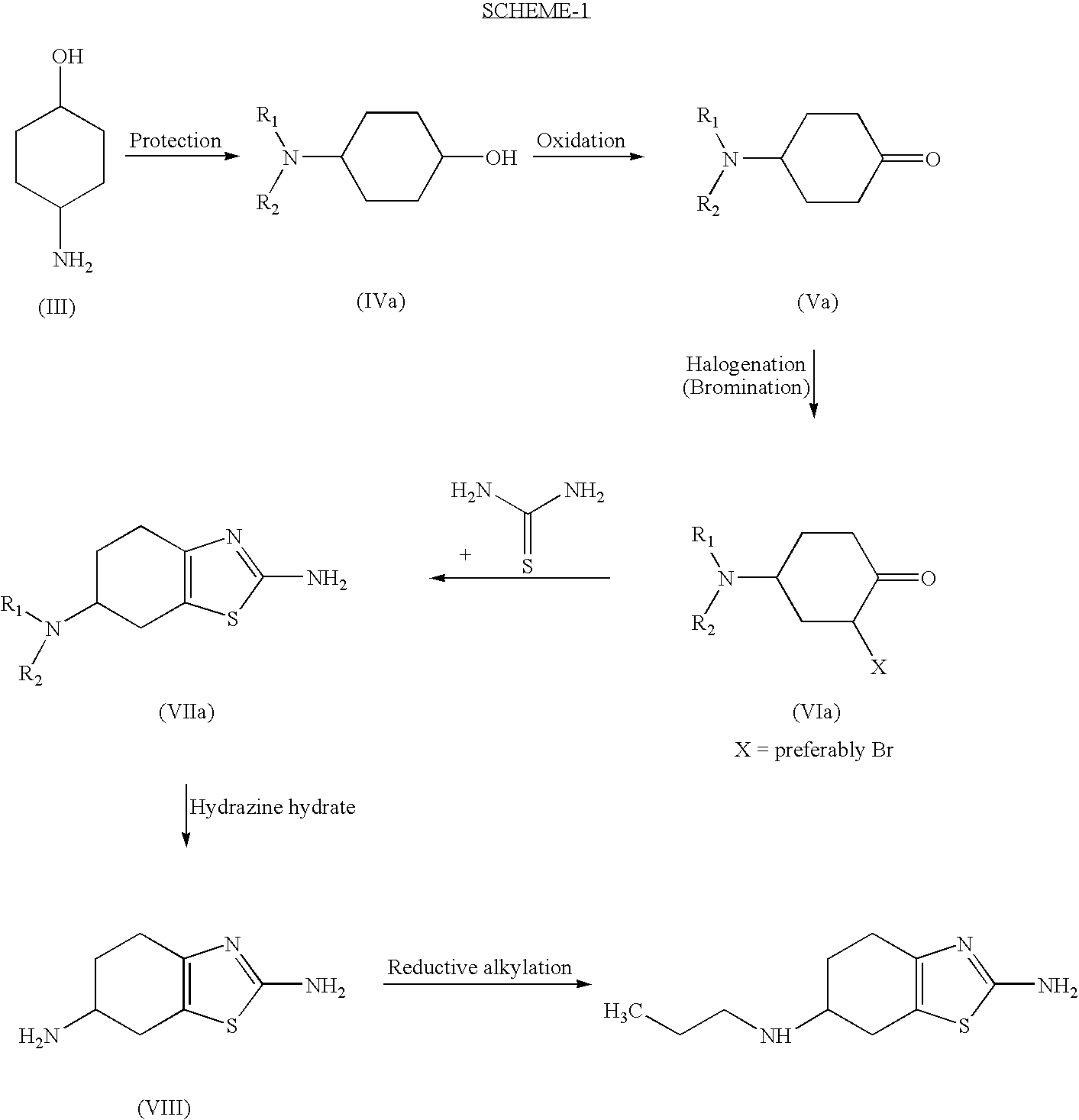
Process for the preparation of biologically active tetrahydrobenzthiazole derivative
InactiveUS20070123573A1Shorten the setting timeOrganic active ingredientsBiocideCyclohexanoneThiourea
Improved process for the preparation of the intermediate compound of formula II for formation of biological active tetrahydrobenzothiazole compound of formula (I) as well as the biological active tetrahydrobenzothiazole compound of formula (I) and / or its pharmaceutically acceptable salts or solvates. The process comprises reacting 4-amino cyclohexanol of formula (III) or its acid addition salts with phthalic anhydride in presence of acid catalyst and their salts, in polar aprotic solvent or its mixture with organic solvent, capable of removing water azeotropically to give 4-(phthalimido)-cyclohexanol of formula (IV); oxidizing 4-(phthalimido)-cyclohexanol of formula (IV) to give 4-(phthalimido)-cyclohexanone of formula (V); brominating 4-(phthalimido)-cyclohexanone of formula (V) with brominating agent in organic solvent in presence of Lewis acid catalyst to prepare 2-bromo-4-(phthalimido)-cyclohexanone of formula (VI); treating 2-bromo-4-(phthalimido)-cyclohexanone of formula (VI) with thiourea in organic solvent in presence of base to give 2-amino-6-phthalimido-4,5,6,7-tetrahydro benzothiazol of formula (VII); reacting compound of formula (VII) with hydrazine hydrate and base in polar solvent to give racemic 2,6-diamino-4,5,6,7-tetrahydro-1,3-benzothiazole of formula (VIII); resolving racemic 2,6-diamino-4,5,6,7-tetrahydro-1,3-benzothiazole of formula (VIII) to prepare (6S)-2,6-diamino-4,5,6,7-tetrahydro-1,3-benzothiazole of formula (II). To form the compound of Formula I and if desired its salts / solvates the above process is carried out with further steps of coupling (6S)-2,6-dimino-4,5,6,7-tetrahydro-1,3-benzothiazole of formula (II) with propionaldehyde in presence of mineral acid in polar organic solvent and reducing agent to prepare (S)-(−)-2-Amino-6-(n-propylamino)-4,5,6,7-tetrahydrobenzothiazole of formula (I);and if desired converting (S)-(−)-2-Amino-6-(propylamino)-4,5,6,7-tetrahydrobenzothiazole to its pharmaceutically acceptable salts or solvates.
Owner:ALEMBIC LTD
Preparation method of 1-substituted homotaurine
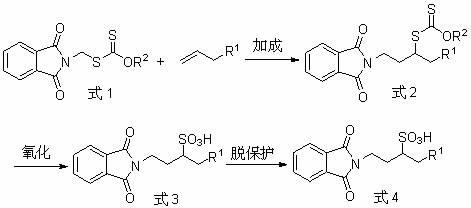
InactiveCN102424665AHas medicinal valueEasy to operateSulfonic acid preparationEnzyme Inhibitor AgentHomotaurine
The invention provides a preparation method of N-phthaloyl protected 1-substituted homotaurine. The method comprises: conducting free radical addition to terminal olefin by dithiocarbonic acid-O-alkyl-S-phthalimidemethyl ester so as to obtain corresponding xanthate, then carrying out oxidation to prepare N-phthaloyl protected 1-substituted homotaurine, then performing acid or base catalyzed hydrolysis or hydrazinolysis, thus obtaining 1-substituted homotaurine. The preparation method of the invention has simple and easily available raw materials, convenient operation, and no need for a tedious desalting and purifying process, thus being especially suitable for large scale industrial production. And the prepared compound can be use as a nutrient, a medicament, an enzyme inhibitor, an antimicrobial agent, a surfactant, a plant growth regulator, and a raw material for preparing sulfono-peptides, etc.
Owner:BEIJING UNIV OF CHEM TECH
Water-based pigment dispersion for ink-jet recording, ink composition for ink-jet recording and method of producing the same
ActiveUS20060235106A1Improve adsorption capacitySmall particle sizeOrganic chemistryDuplicating/marking methodsWater basedPigment dispersion
Disclosed are an aqueous pigment dispersion for inkjet ink, a method for producing the same, and an ink composition for inkjet recording which mainly contains the aqueous pigment dispersion for inkjet ink. The method for producing the aqueous pigment dispersion for inkjet ink is characterized by comprising a kneading step wherein a mixture including a styrene resin, a quinacridone pigment, a phthalimidomethylated quinacridone compound, an alkali metal hydroxide and a wetting agent is kneaded for producing a solid colored kneaded material, and a dispersing step wherein the thus-obtained solid colored kneaded material is dispersed into an aqueous medium.
Owner:DAINIPPON INK & CHEM INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
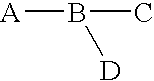
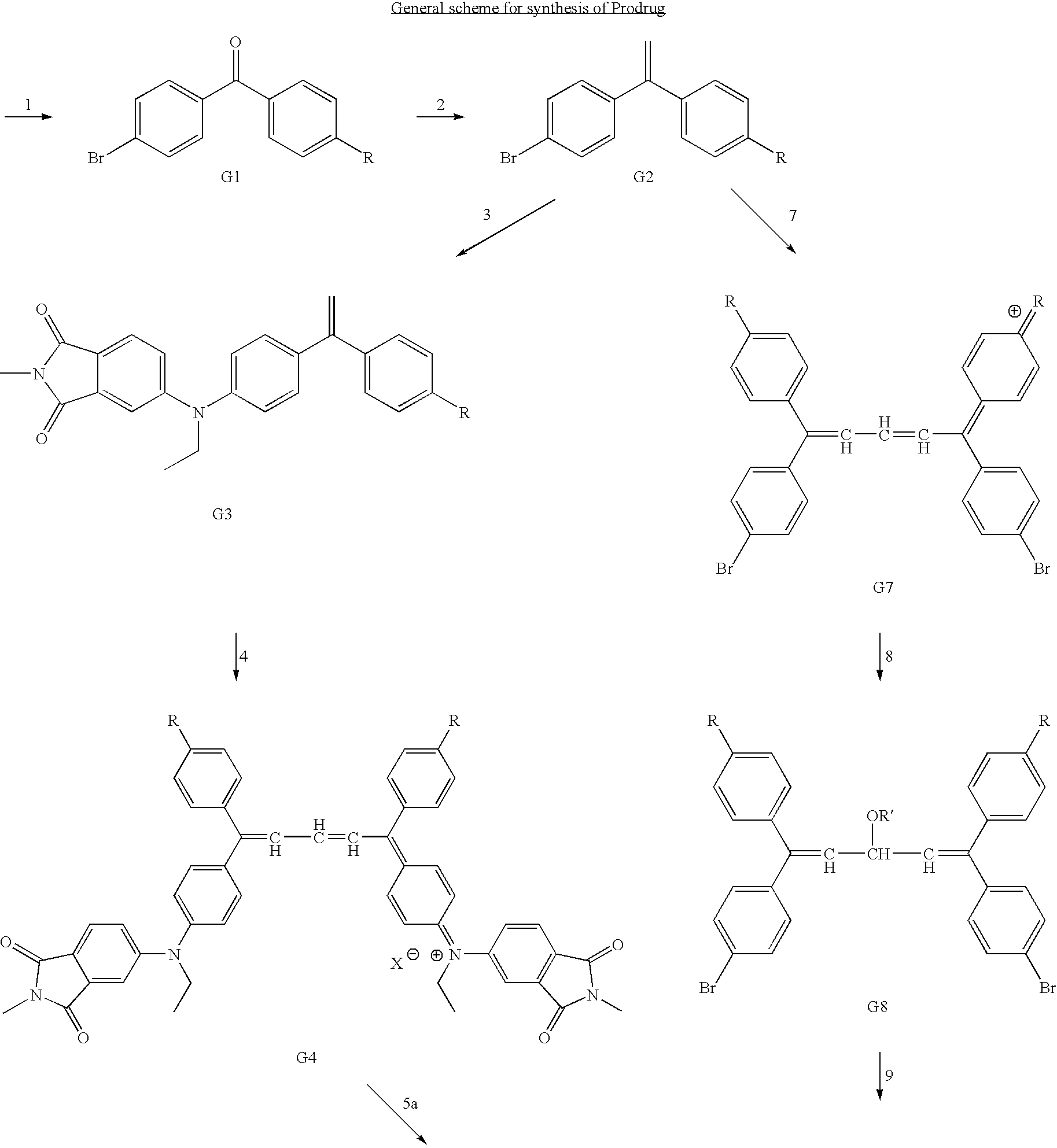


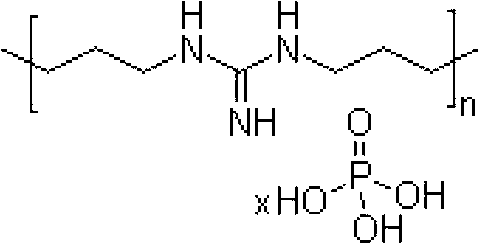
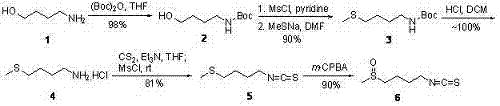


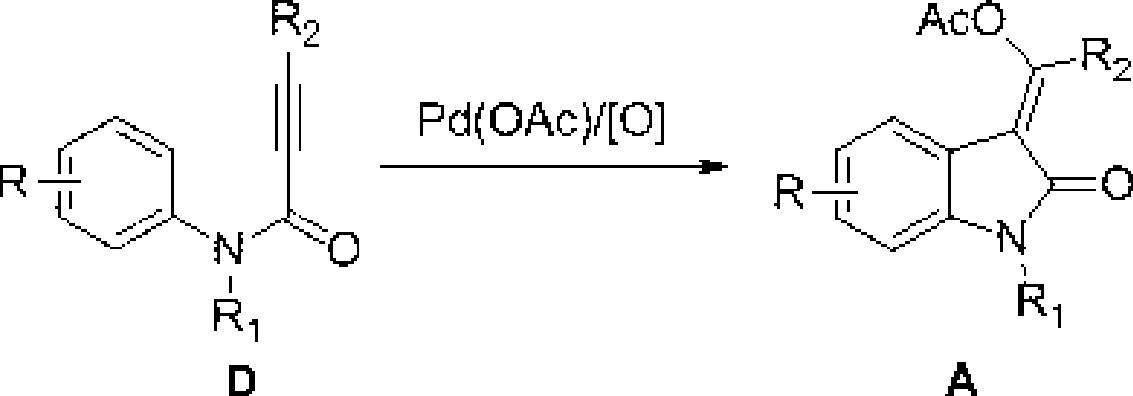

![4-[4-(substituted phenyl) piperazine piperazinyl-1]-butylcarbamic acid substituted aromatic ester derivative and preparation method thereof 4-[4-(substituted phenyl) piperazine piperazinyl-1]-butylcarbamic acid substituted aromatic ester derivative and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ccda6d25-26d1-4761-b15f-5f57af727bcc/BDA00002781366000011.PNG)
![4-[4-(substituted phenyl) piperazine piperazinyl-1]-butylcarbamic acid substituted aromatic ester derivative and preparation method thereof 4-[4-(substituted phenyl) piperazine piperazinyl-1]-butylcarbamic acid substituted aromatic ester derivative and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ccda6d25-26d1-4761-b15f-5f57af727bcc/BDA00002781366000021.PNG)
![4-[4-(substituted phenyl) piperazine piperazinyl-1]-butylcarbamic acid substituted aromatic ester derivative and preparation method thereof 4-[4-(substituted phenyl) piperazine piperazinyl-1]-butylcarbamic acid substituted aromatic ester derivative and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ccda6d25-26d1-4761-b15f-5f57af727bcc/BDA00002781366000041.PNG)