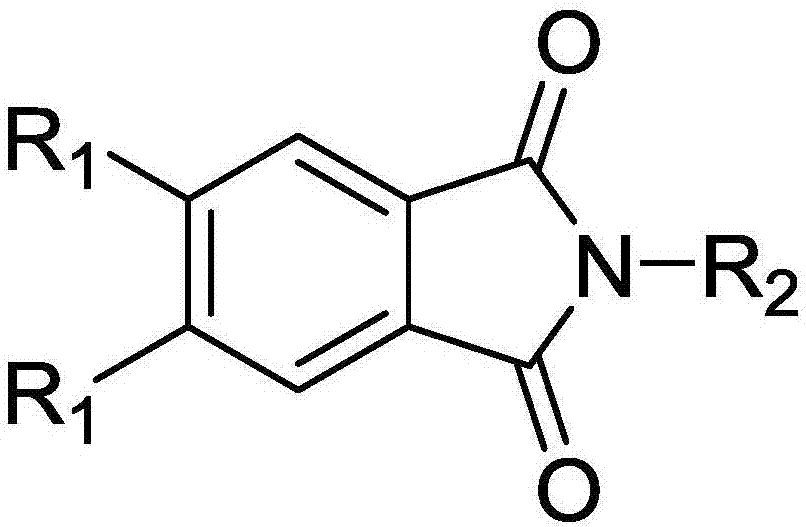
Thermally-activated delay fluorescent material and organic electroluminescence device
A technology for thermally activated delayed and fluorescent materials, applied in the field of thermally activated delayed fluorescent materials and organic electroluminescent devices, can solve problems such as difficult scale preparation, low preparation efficiency, long synthesis steps, etc., and achieve high scientific research value and application value , The effect of cheap raw materials and low driving voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The reaction formula is as follows:
[0040]
[0041] The specific steps of the reaction are as follows:
[0042] In a 5000mL round bottom flask, add 600g of A 1 , 364g of aniline and 2000mL of glacial acetic acid, heated and refluxed at 130°C for 4 hours, distilled off 1000mL of glacial acetic acid after the reaction system was cooled to room temperature, then placed the remaining reaction system at 4°C for low-temperature recrystallization, and filtered to obtain crude Product, then recrystallized with a small amount of glacial acetic acid to obtain 720g of white needle-like crystals. The white crystal is compound B 1 , the yield was 85%.
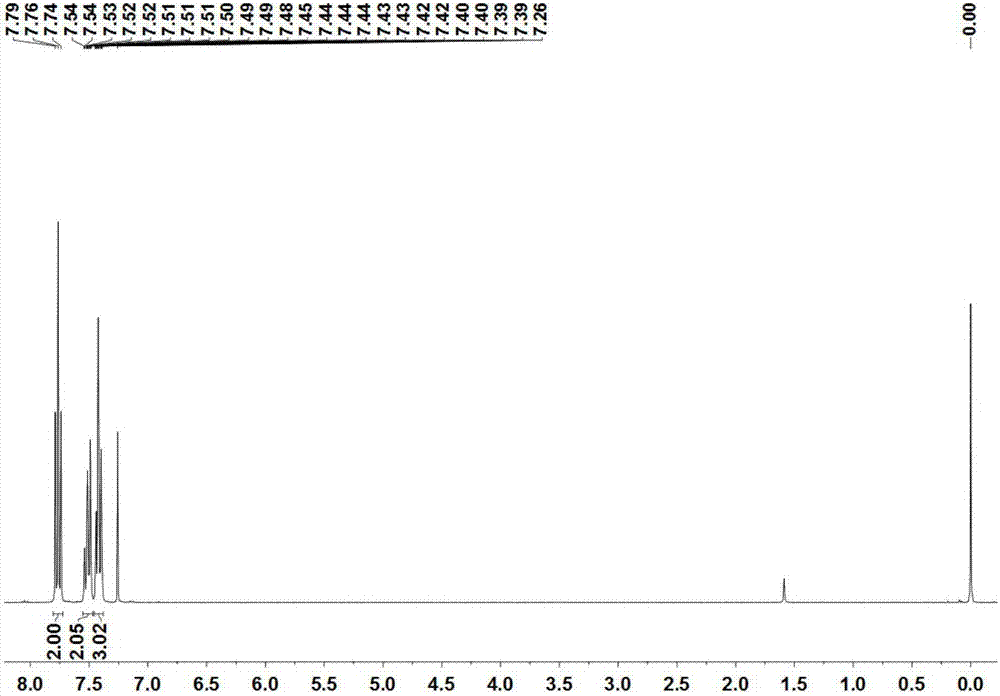
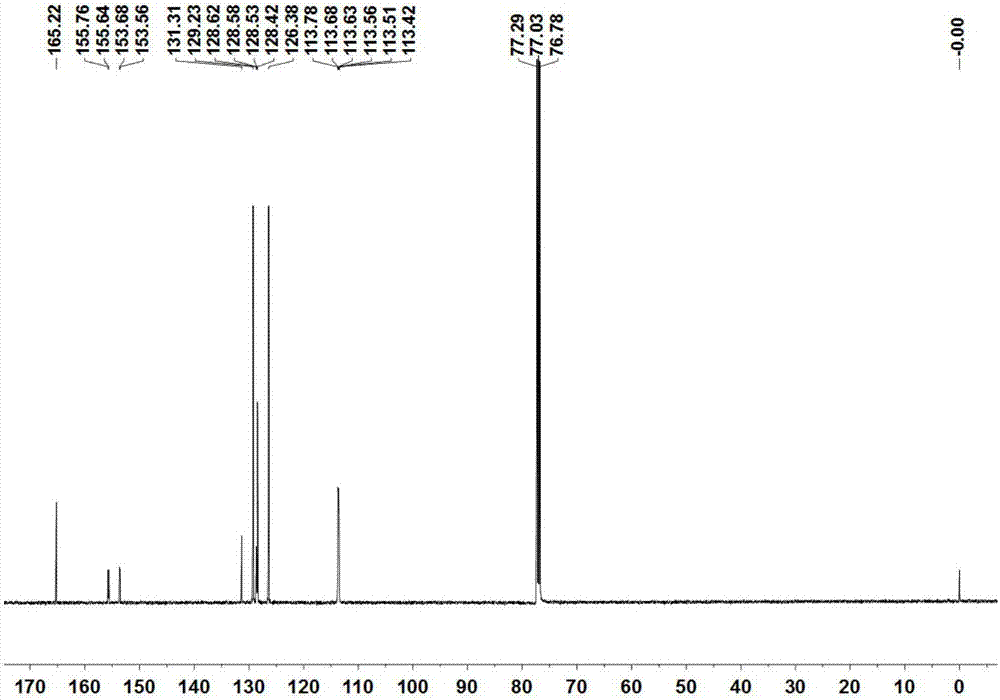
[0043] The compound B 1 The structure detection results are as follows:
[0044]1 H NMR (300MHz, CDCl 3 )δ7.76(t,J=7.3Hz,2H),7.55–7.47(m,2H),7.46–7.38(m,3H). 13 C NMR (126MHz, CDCl 3 )δ165.2, 155.8, 155.6, 153.7, 153.6, 131.3, 129.2, 128.62, 128.58, 128.5, 128.4, 126.4, 113.8, 113.7, 113.63, 113.56, 113.5, 113.4. 19 F N...
Embodiment 2
[0047] The reaction formula is as follows:
[0048]
[0049] The specific steps of the reaction are as follows:
[0050] Under argon protection, add 1.72g of 60% sodium hydride and 20mL of anhydrous tetrahydrofuran to a 200mL reactor equipped with a reflux device to obtain the first mixed solution; dissolve 6.0g of carbazole in 20mL of anhydrous Tetrahydrofuran, get the second mixed solution; 4.66g A 1 Dissolve in 20mL of anhydrous tetrahydrofuran to obtain the third mixed solution; slowly add the first mixed solution to the second mixed solution, stir until there are no bubbles, then add the third mixed solution, and then raise the temperature to 40°C and stirred for 4 hours to obtain the fourth mixed solution; the fourth mixed solution was cooled to room temperature, and a large amount of water and dichloromethane were added thereto for extraction, and the extracted organic phase was dried with anhydrous sodium sulfate and filtered. Then the organic liquid phase was rem...
Embodiment 3
[0054] The reaction formula is as follows:
[0055]
[0056] Under argon protection, 1.72g of 60% sodium hydride and 20mL of anhydrous tetrahydrofuran were added to a 200mL reactor equipped with a reflux device to obtain the first mixed solution; 10.0g of 4,5-bis(3 ,6-Di-tert-butyl)carbazole was dissolved in 20mL of anhydrous tetrahydrofuran to obtain the second mixed solution; 4.66g A1 was dissolved in 20mL of anhydrous tetrahydrofuran to obtain the third mixed solution; the first mixed solution was slowly added into the second mixed solution, stir until there are no bubbles, then add the third mixed solution, then raise the temperature to 25°C and stir for 4 hours to obtain the fourth mixed solution; cool the fourth mixed solution to room temperature, A large amount of water and dichloromethane were added thereto for extraction, the extracted organic phase was dried with anhydrous sodium sulfate, filtered, and then the organic liquid phase was distilled off to obtain a cr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Current efficiency | aaaaa | aaaaa |
| Current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com