Preparation method of 1-substituted homotaurine
A technology of high taurine and taurine, applied in the preparation of sulfonic acid, organic chemistry, etc., can solve the problems of troublesome desalination and purification process, limited scope of application, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
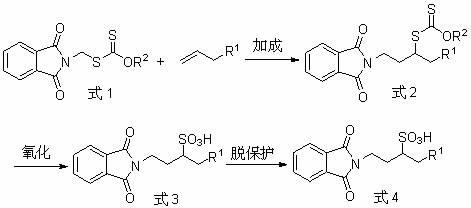
[0055] 1-phthalimido-3-nonylsulfonic acid 3a preparation of
[0056] Add dithiocarbonic acid- O -Ethyl- S - Phthalimide methyl ester (xanthate) (562 mg, 2 mmol), evacuated, N 2 A solution of n-octene (448 mg, 4 mmol) in 1 mL of 1,2-dichloroethane (DCE) was added under protection, and 21 mg of lauroyl peroxide (DLP) ( 2.5 mol%), the reaction was detected by TLC, and DLP (1.25-10 mol%) was added every 45 min until the reaction of the raw material xanthate was complete, and the reaction was stopped. The solvent was removed from the reaction solution by silica gel column separation to obtain the addition product shown in Formula 2.
[0057] Add the above addition product (353 mg 1 mmol) into a 25 mL round bottom flask, add 1 mL THF to dissolve, add 5 mL anhydrous formic acid, place in an ice-water bath for 10 min, slowly add 30% H 2 o 2 (1 mL), the reaction was stirred overnight. Spin off the solvent and separate on a silica gel column (eluent petroleum ether: ethyl ac...
Embodiment 2
[0059] 1-phthalimido-3-heptanesulfonic acid 3b preparation of
[0060] According to the method described in Example 1, with dithiocarbonic acid- O -Ethyl- S - Phthalimidomethyl ester and n-hexene were used as raw materials to obtain 1-phthalimido-3-heptanesulfonic acid as a brown oily liquid with a yield of 57%. 1 H NMR (D 2 o , 400MHz) d ppm: 0.63 (t, J = 7.0 Hz, 3H, CH 3 ), 1.03-1.26 (m, 4H, 2CH 2 ), 1.34-1.42 (m, 1H in CH 2 ), 1.62-1.72 (m, 2H in 2CH 2 ), 1.91 (dddd, J = 13.1, 6.6, 6.5, 6.3 Hz, 1H in NCH 2 ), 2.53-2.58 (m, 1H, SCH), 3.52 (t, J = 7.1 Hz, 2H, NCH 2 ), 7.50-7.57 (m, 4H, ArH). 13 C NMR (D 2 o , 100MHz) d ppm: 13.1, 21.9, 28.0, 29.0, 28.3, 35.9, 57.8, 123.2, 130.9, 134.7, 170.0.
Embodiment 3
[0062] 1-Cyclohexyl-4-phthalimido-2-butanesulfonic acid 3c preparation of
[0063] According to the method described in Example 1, with dithiocarbonic acid- O -Ethyl- S - Phthalimide methyl ester and 3-cyclohexyl-1-propene as raw materials to obtain 1-cyclohexyl-4-phthalimido-2-butanesulfonic acid, pale yellow oily liquid , yield 73%. 1 H NMR (D 2 o , 400MHz) d ppm: 0.50-1.48 (m, 13H, CH 2 & H in cyclohexyl), 1.69 (s, 1H in CH 2 ), 1.90 (s, 1H CH 2 ), 2.63 (s, 1H, SCH), 3.56 (s, 1H, 1H in NCH 2 ), 3.61 (s, 1H in NCH 2 ), 7.58 (s, 4H, ArH). 13 C NMR (D 2 o , 100MHz) d ppm: 25.6, 25.9, 26.0, 28.6, 31.8, 33.6, 34.5, 36.3, 37.3, 55.2, 123.2, 132.0, 134.7, 169.9, 214.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com