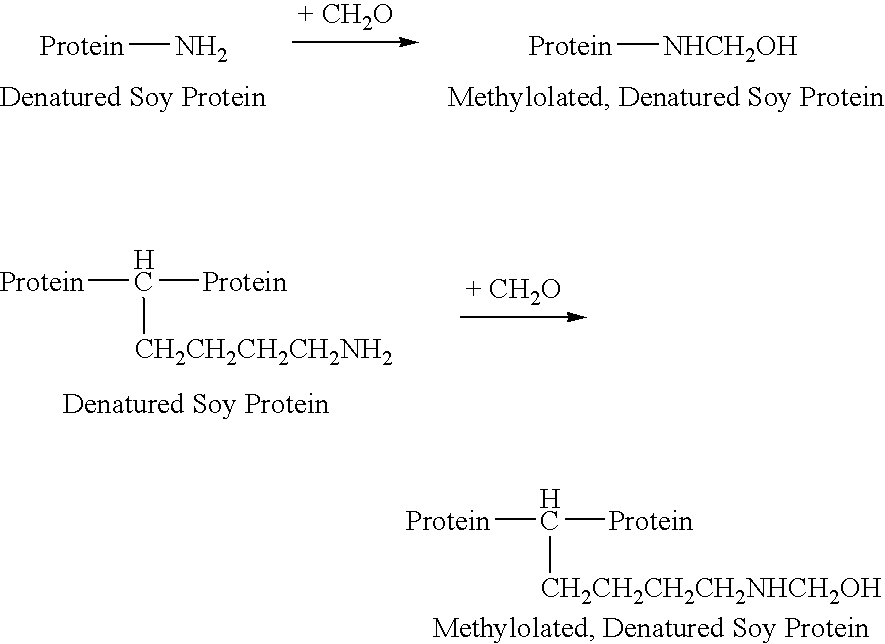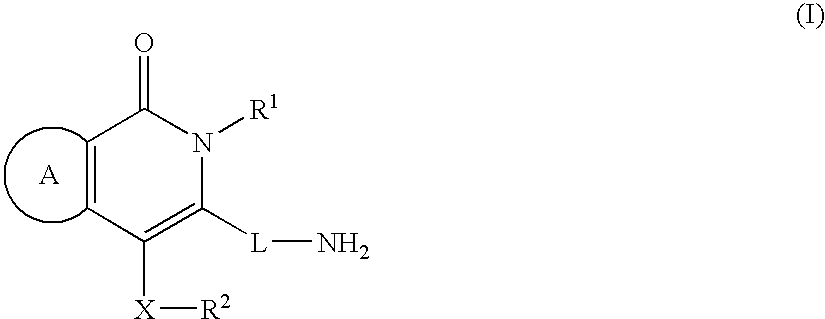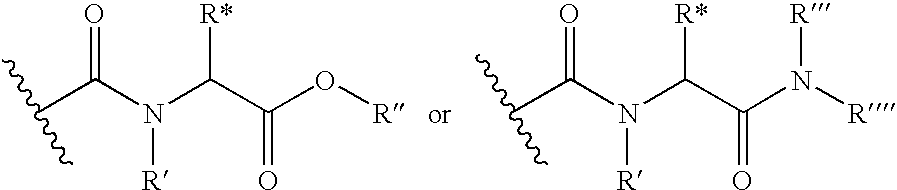Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1613 results about "Methyl Donors" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
S-adenosylmethionine is the methyl group donor for DNA methylation and several nutrients are required for the production of S-adenosylmethionine. These methyl nutrients include vitamins (folate, riboflavin, vitamin B12, vitamin B6, choline) and amino acids (methionine, cysteine, serine, glycine).
Monomethylvaline compounds capable of conjugation to ligands
ActiveUS20050238649A1Improve bioavailabilityImprove compoundAntibacterial agentsBiocideD-norephedrineBiochemistry
Owner:SEAGEN INC
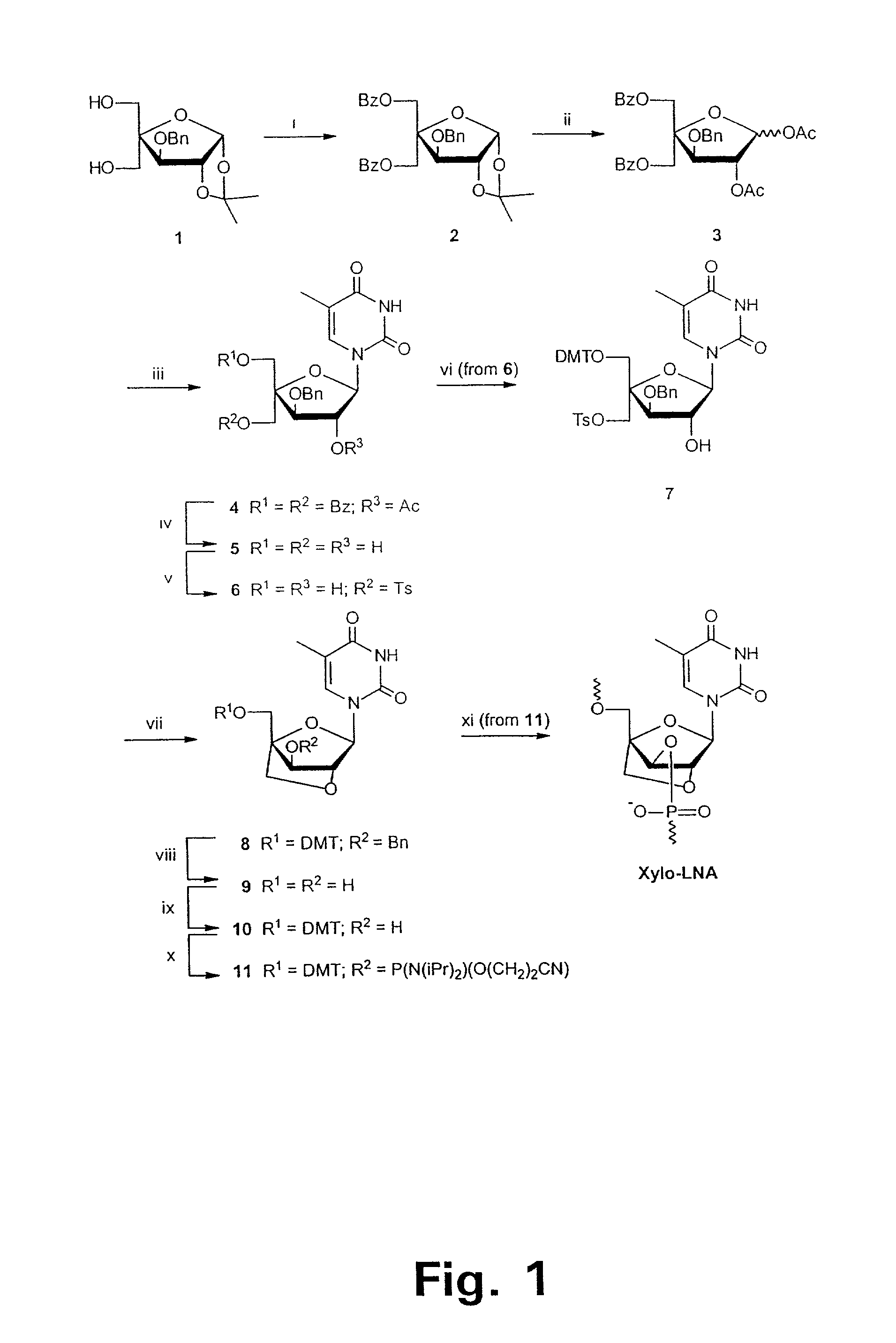
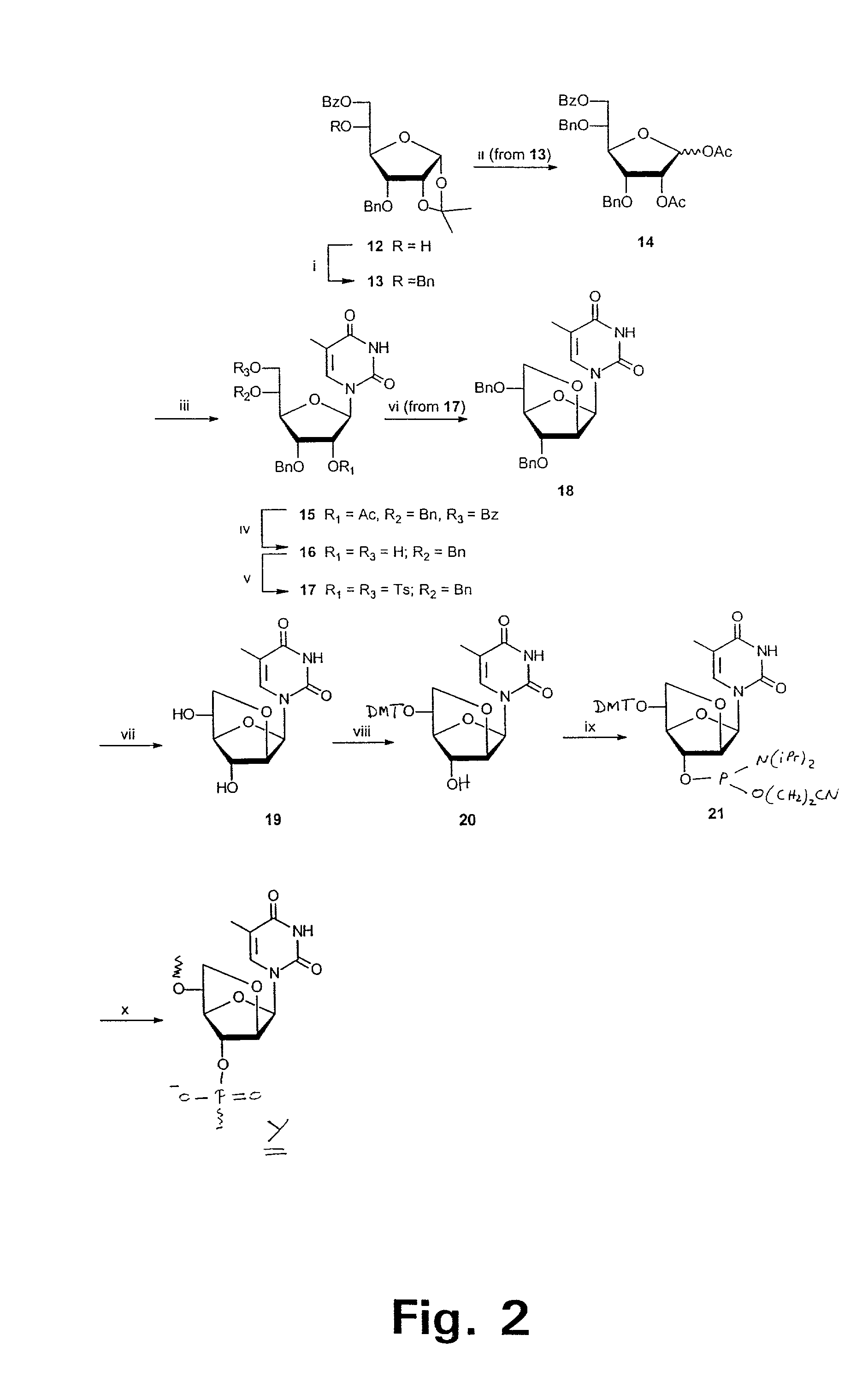
Xylo-LNA analogues
Based on the above and on the remarkable properties of the 2′-O,4′-C-methylene bridged LNA monomers it was decided to synthesise oligonucleotides comprising one or more 2′-O,4′-C-methylene-β-D-xylofuranosyl nucleotide monomer(s) as the first stereoisomer of LNA modified oligonucleotides. Modelling clearly indicated the xylo-LNA monomers to be locked in an N-type furanose conformation. Whereas the parent 2′-deoxy-β-D-xylofuranosyl nucleosides were shown to adopt mainly an N-type furanose conformation, the furanose ring of the 2′-deoxy-β-D-xylofuranosyl monomers present in xylo-DNA were shown by conformational analysis and computer modelling to prefer an S-type conformation thereby minimising steric repulsion between the nucleobase and the 3′-O-phopshate group (Seela, F.; Wömer, Rosemeyer, H. Helv. Chem. Acta 1994, 77, 883). As no report on the hybridisation properties and binding mode of xylo-configurated oligonucleotides in an RNA context was believed to exist, it was the aim to synthesise 2′-O,4′-C-methylene-β-D-xylofuranosyl nucleotide monomer and to study the thermal stability of oligonucleotides comprising this monomer. The results showed that fully modified or almost fully modified Xylo-LNA is useful for high-affinity targeting of complementary nucleic acids. When taking into consideration the inverted stereochemistry at C-3′ this is a surprising fact. It is likely that Xylo-LNA monomers, in a sequence context of Xylo-DNA monomers, should have an affinity-increasing effect.
Owner:QIAGEN GMBH
Method for producing complex DNA methylation fingerprints
InactiveUS6214556B1Accurately determineSugar derivativesMicrobiological testing/measurementFingerprintGenomic DNA
Method for characterizing, classifying and differentiating tissues and cell types, for predicting the behavior of tissues and groups of cells, and for identifying genes with changed expression. The method involves obtaining genomic DNA from a tissue sample, the genomic DNA subsequently being subjected to shearing, cleaved by means of a restriction endonuclease or not treated by either one of these methods. The base cytosine, but not 5-methylcytosine, from the thus-obtained genomic DNA is then converted into uracil by treatment with a bisulfite solution. Fractions of the thus-treated genomic DNA are then amplified using either very short or degenerated oligonucleotides or oligonuclcotides which are complementary to adaptor oligonucleotides that have been ligated to the ends of the cleaved DNA. The quantity of the remaining cytosines on the guanine-rich DNA strand and / or the quantity of guanines on the cytosine-rich DNA strand from the amplified fractions are then detected by hybridization or polymerase reaction, which quantities are such that the data generated thereby and automatically applied to a processing algorithm allow the drawing of conclusions concerning the phenotype of the sample material.
Owner:EPIGENOMICS AG
1h-Indazole-3-carboxamide compounds as cyclin dependent kinase (cdk) inhibitors
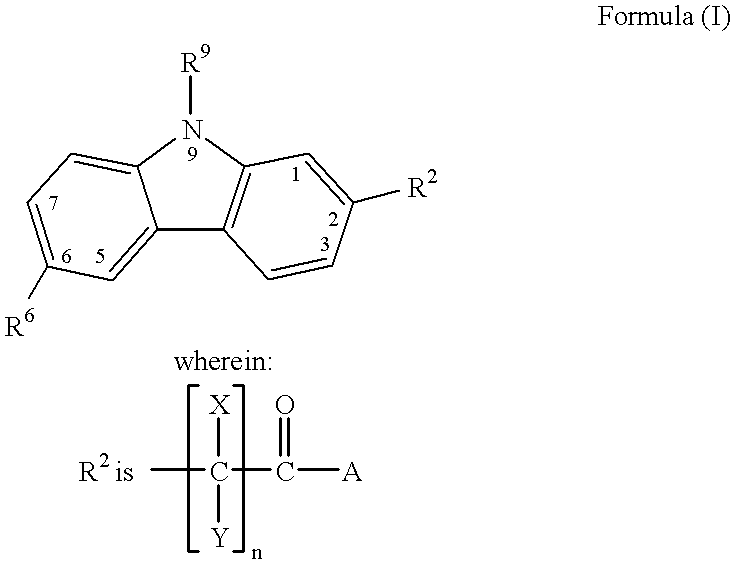
The invention provides a compound of the formula (I) for use in the prophylaxis or treatment of a disease state or condition mediated by a cyclin dependent kinase: wherein A is a group R2 or CH2—R2 where R2 is a carbocyclic or heterocyclic group having from 3 to 12 ring members; B is a bond or an acyclic linker group having a linking chain length of up to 3 atoms selected from C, N, S and O; R1 is hydrogen or a group selected from SO2Rb, SO2NR7R8, CONR7R8, NR7R9 and carbocyclic and heterocyclic groups having from 3 to 7 ring members; R3, R4, R5 and R6 are the same or different and are each selected from hydrogen, halogen, hydroxy, trifluoromethyl, cyano, nitro, carboxy, amino, carbocyclic and heterocyclic groups having from 3 to 12 ring members; a group Ra—Rb wherein Ra is a bond, O, CO, X1C(X2), C(X2)X1, X1C(X2)X1, S, SO, SO2, NRc, SO2NRc or NRcSO2; and Rb is selected from hydrogen, carbocyclic and heterocyclic groups having from 3 to 12 ring members, and a C1-8 hydrocarbyl group optionally substituted by one or more substituents selected from hydroxy, oxo, halogen, cyano, nitro, amino, mono- or di-C1-4 hydrocarbylamino, carbocyclic and heterocyclic groups having from 3 to 12 ring members and wherein one or more carbon atoms of the C1-8 hydrocarbyl group may optionally be replaced by O, S, SO, SO2, NRc, X1C(X2), C(X2)X1 or X1C(X2)X1; Rc is hydrogen or C1-4 hydrocarbyl; X1 is O, S or NRc and X2 is ═O, ═S or ═NRc; R7 is selected from hydrogen and a C1-8 hydrocarbyl group optionally substituted by one or more substituents selected from hydroxy, oxo, halogen, cyano, nitro, amino, mono- or di-C1-4 hydrocarbylamino, carbocyclic and heterocyclic groups having from 3 to 12 ring members and wherein one or more carbon atoms of the C1-8 hydrocarbyl group may optionally be replaced by O, S, SO, SO2, NRc, X1C(X2), C(X2)X1 or X1C(X2)X1; R8 is selected from R7 and carbocyclic and heterocyclic groups having from 3 to 12 ring members; R9 is selected from R8, COR8 and SO2R8; or NR7R8 or NR7R9 may each form a heterocyclic group having from 5 to 12 ring members; but excluding the compounds N-[(morpholin-4-yl)phenyl-1H-indazole-3-carboxamide and N-[4-(acetylaminosulphonyl)phenyl-1H-indazole-3-carboxamide.
Owner:ASTEX THERAPEUTICS LTD
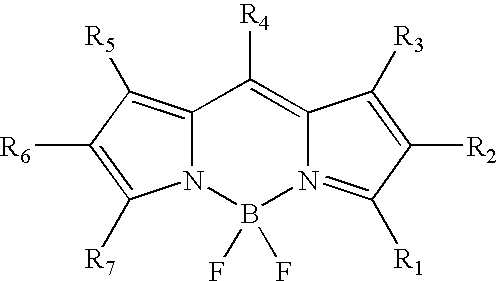
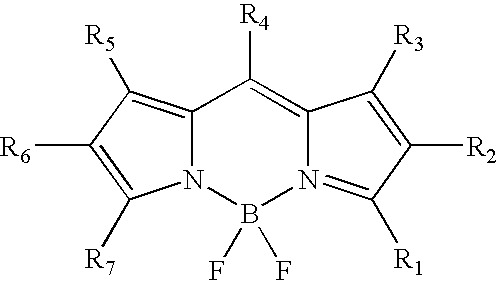
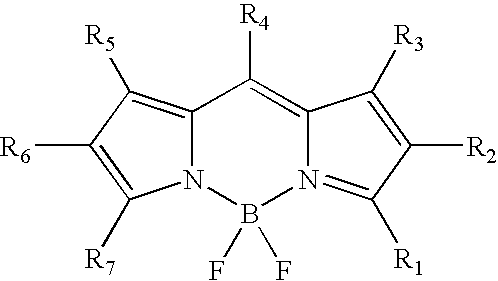
Labeling of immobilized proteins using dipyrrometheneboron difluoride dyes
InactiveUS6972326B2Hybrid immunoglobulinsChemiluminescene/bioluminescenceFluorescenceImmobilized protein
The invention describes methods for labeling or detecting of immobilized poly(amino acids), including peptides, polypeptides and proteins, on membranes and other solid supports, using fluorescent dipyrrometheneboron difluoride dyes. Such immobilized poly(amino acids) are labeled or detected on blots or on arrays of poly(amino acids), or are attached to immobilized aptamers.
Owner:MOLECULAR PROBES
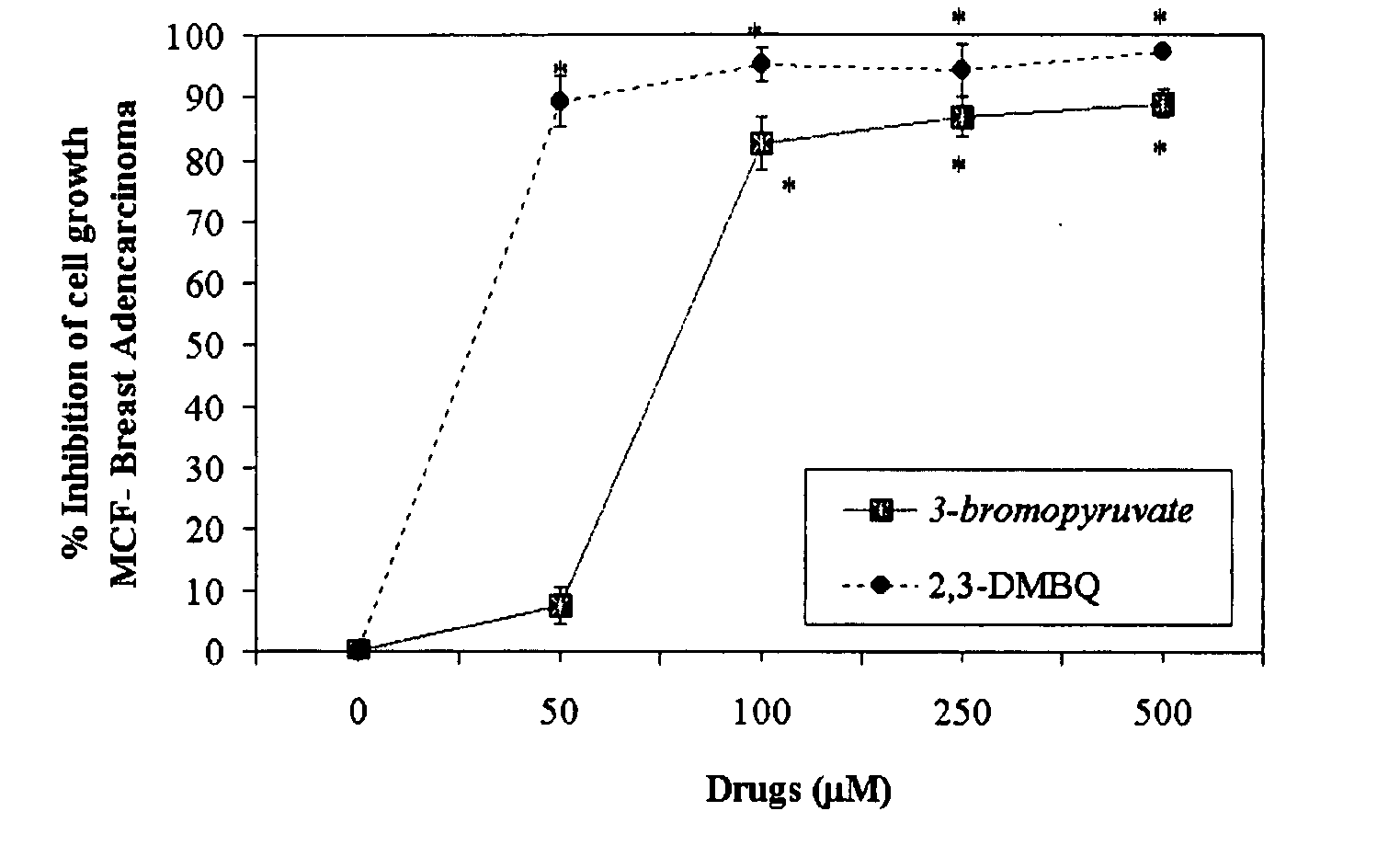
Nutraceutical composition and method of use for treatment / prevention of cancer
InactiveUS20070248693A1Function increaseAbility to createBiocideAlgae medical ingredients1,4-BenzoquinonePantothenic acid
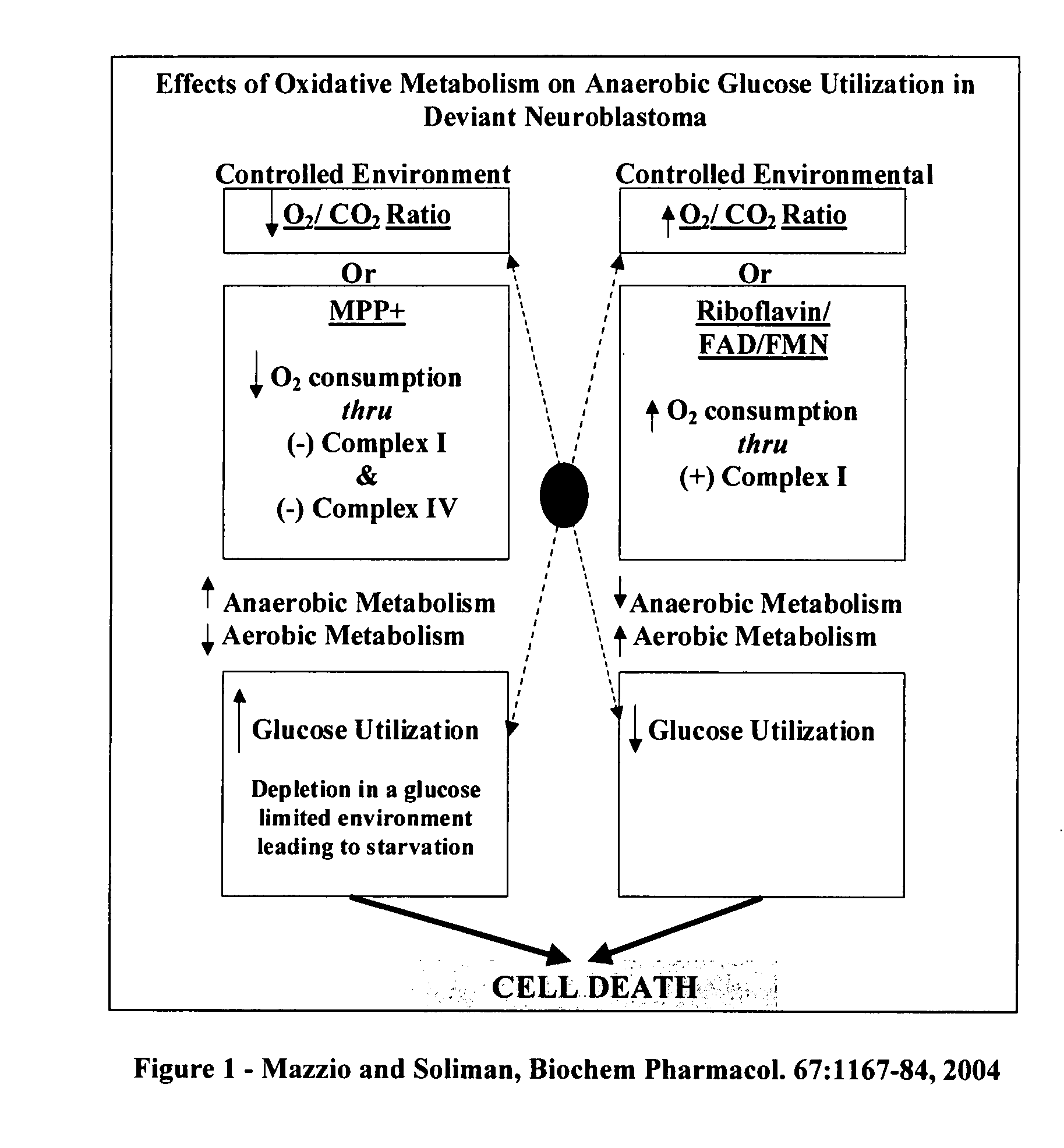
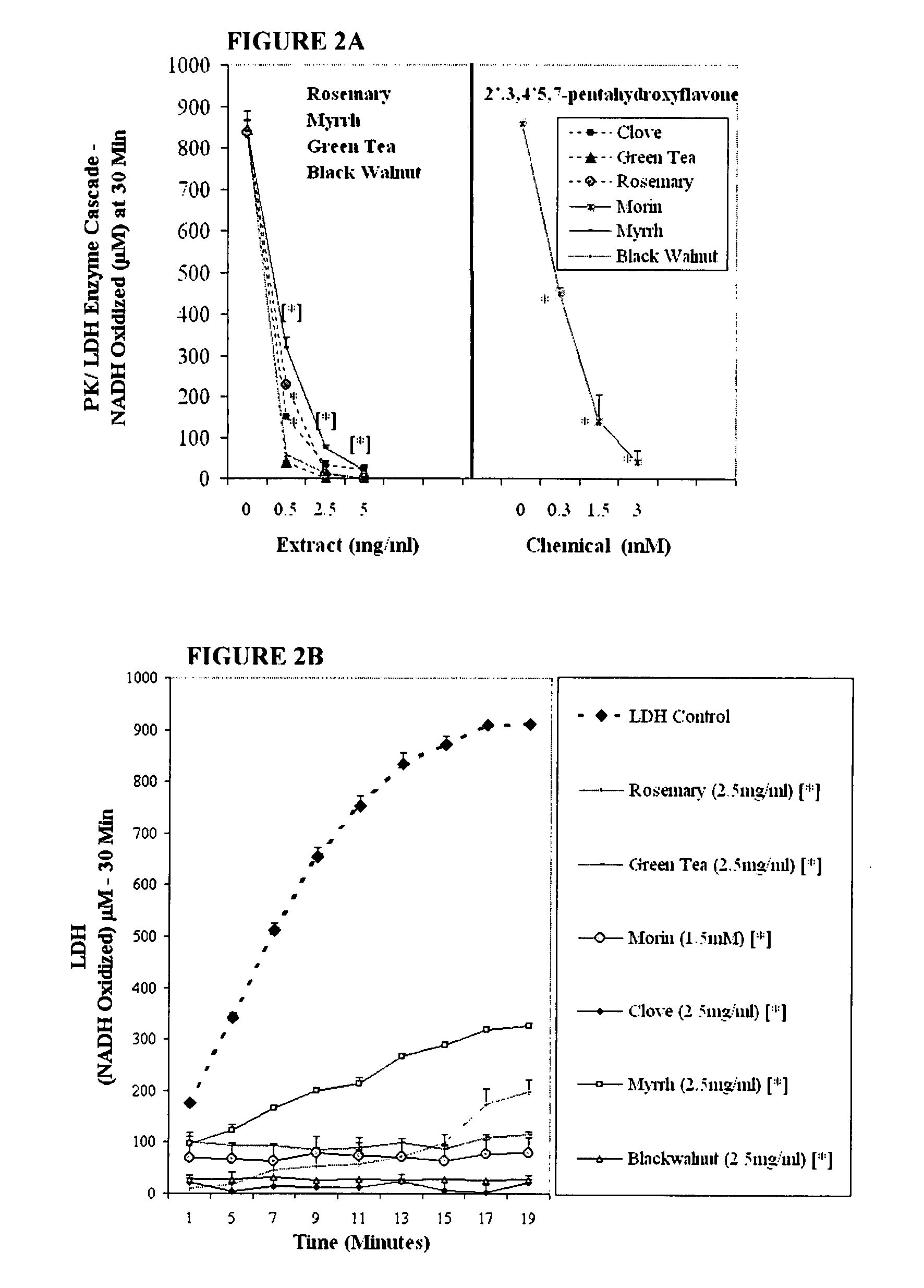
The invention describes a pharmaceutical composition and method for treating cancer comprised of A) 2,3-dimethoxy-5-methyl-1,4-benzoquinone and / or B) at least one of wild yam root, teasel root, balm of gilead bud, bakuchi seed, dichroa root, kochia seed, kanta kari, bushy knotweed rhizome, arjun, babul chall bark, opopanax and bhumy amalaki; optionally one or more of frankincense, garcinia fruit, vitex, dragons blood, mace, sage and red sandalwood with at least c) one compound capable of maximizing oxidative mitochondrial function preferably riboflavin or vitamin B2 derivatives, FAD, FMN, 5-amino-6-(5′-phosphoribitylamino)uracil, 6,7-Dimethyl-8-(1-D-ribityl)lumazine, ribitol, 5,6-dimethylbenzimidazole, tetrahydrobiopterin, vitamin B1, lipoic acid, biotin, vitamin B6, vitamin B12, folate, niacin, vitamin C and pantothenate and / or d) at least one lactic acid dehydrogenase inhibitor (preferably 2′,3,4′5,7-pentahydroxyflavone) and optionally f) an alkalizing agent (aloe vera, chlorella, wheat grass, sodium or potassium bicarbonate, potassium) g) an antiproliferative herb (speranskia or goldenseal) and h) a pharmaceutically acceptable carrier.
Owner:MAZZIO ELIZABETH +1
Therapeutic combinations comprising poly (ADP-ribose) polymerases inhibitor
This invention generally relates to use of 8-fluoro-2{4-[(methylamino)methyl]phenyl}-1,3,4,5-tetrahydro-6H-azepino[5,4,3-cd]indol-6-one represented by formula 1 as a chemosensitizer that enhances the efficacy of cytotoxic drugs or radiotherapy. This invention provides pharmaceutical combinations of 8-fluoro-2{4-[(methylamino)methyl]phenyl}-1,3,4,5-tetrahydro-6H-azepino[5,4,3-cd]indol-6-one, or a pharmaceutically acceptable salt thereof and at least one additional therapeutic agent, kits containing such combinations and methods of using such combinations to treat subjects suffering from diseases such as cancer.
Owner:AGOURON PHARMA INC +1
Propanoic acid polyhexamethylene guanide and preparation method thereof
InactiveCN101037503AOvercome strong hygroscopicityImprove the bactericidal effectBiocideFungicidesPolyhexamethylene guanidinePropynoic acid
The invention discloses polyhexamethylene guanidine propionic acid and preparing method thereof. The preparing method employs stepwise synthesis, which includes steps of: sufficiently mixing dicyandiamide and alanine under high temperature to synthesize aminoguanidine propionic acid, mixing the aminoguanidine propionic acid, triethylidenepropyldiamine and initiator under high temperature to synthesize the polyhexamethylene guanidine propionic acid. Advantages of the invention are: sterilization effect is excellent, toxity is actually nontoxic level, effect of sterilization is better than existent technique, due to overcoming strong hygroscopicity in existence, the polyhexamethylene guanidine propionic acid can be produced to stable powder, thereby being widely used in fields such as weave, plastic, daily chemicals and water treatment.
Owner:SHANGHAI HIPOLY IND
Composition and method for reducing the risk or progression of cardiovascular, glaucoma, tardive dyskinesia and other diseases
InactiveUS20040087479A1Reduce riskShorten the progressBiocideOrganic active ingredientsBeta-CaroteneAdditive ingredient
Elevated levels of homocysteine have been implicated as an important risk factor for cardiovascular and other diseases. A composition for decreasing levels of plasma homocysteine and a method for administering the composition are provided, the composition containing dextromethorphan (DM), folic acid and vitamins B6 and B12. The composition provides a synergistic therapeutic effect so that lower amounts of the above ingredients may be employed to minimize any undesirable side effects caused by the use of high levels of a component such as DM. Preferred compositions for cardiovascular diseases further include lecithin, vitamin E, betacarotene, procyanidins / flavonoids, trimethylglycine, garlic oil and minerals. Other compositions for treating glaucoma include bilberry, bioflavonoids and beta-carotene and for treating tardive dyskinesia include an antioxidant such a grape seed extract and pine bark extract, lecithin and oligomeric proanthocyanidins. The compositions may be administered using any suitable means such as orally or intravenous.
Owner:SOSNOWSKI ROBERT E +1
Monomethylvaline compounds capable of conjugation to ligands
ActiveUS20080248053A1Improve bioavailabilityImprove compoundAntibacterial agentsTripeptide ingredientsD-norephedrineBiochemistry
Owner:SEAGEN INC
Polynucleotides encoding polypeptides involved in the stress response to environmental changes in Methylophilus methylotrophus
Owner:AJINOMOTO CO INC
Salts and crystalline forms of an apoptosis-inducing agent
Salts and crystalline forms of 4-(4-{[2-(4-chlorophenyl)-4,4-dimethylcyclohex-1-en-1-yl]methyl}piperazin-1-yl)-N-({3-nitro-4-[(tetrahydro-2H-pyran-4-ylmethyl)amino]phenyl}-sulfonyl)-2-(1H-pyrrolo[2,3-b]pyridin-5-yloxy)benzamide are suitable active pharmaceutical ingredients for pharmaceutical compositions useful in treatment of a disease characterized by overexpression of one or more anti-apoptotic Bcl-2 family proteins, for example cancer.
Owner:ABBVIE INC
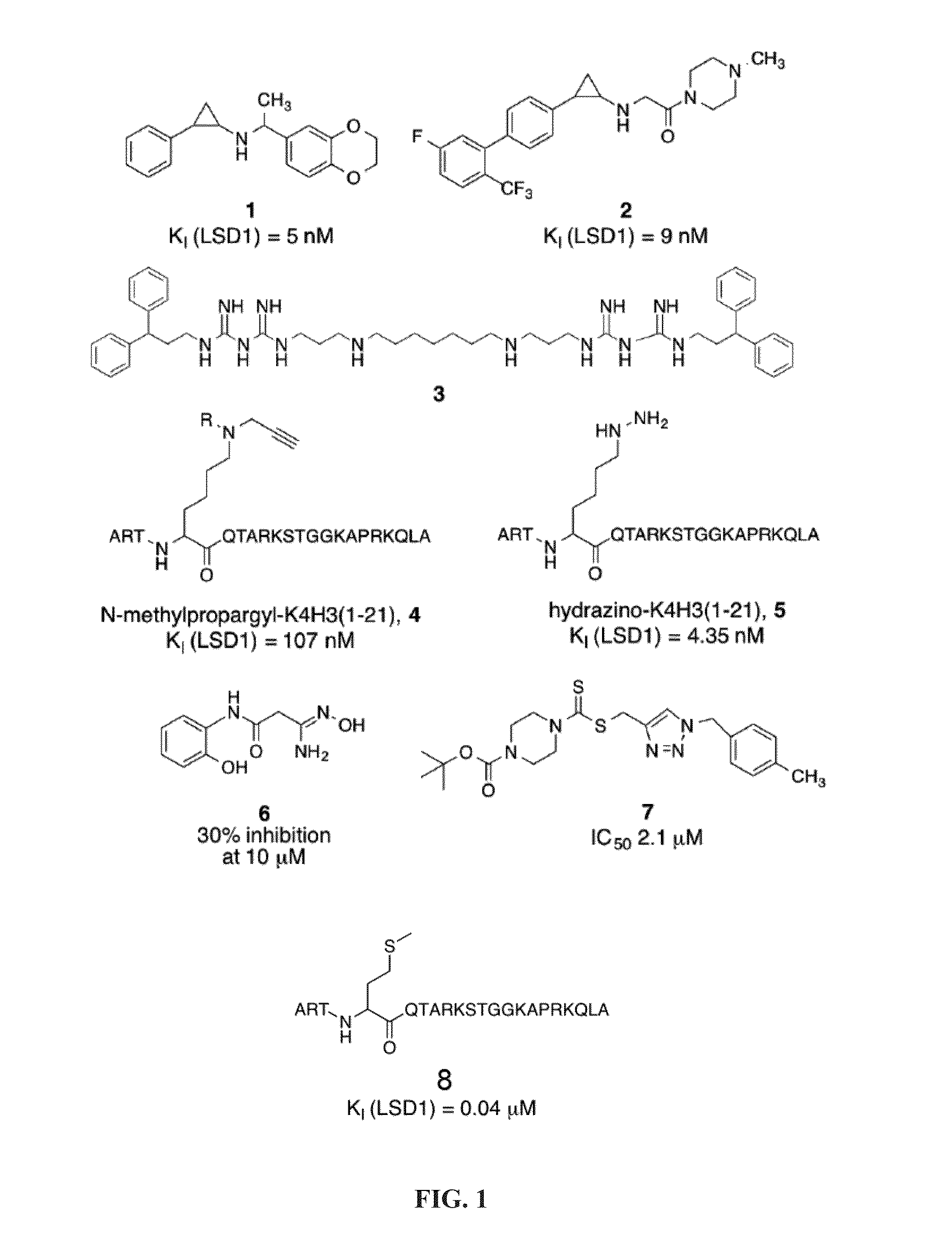
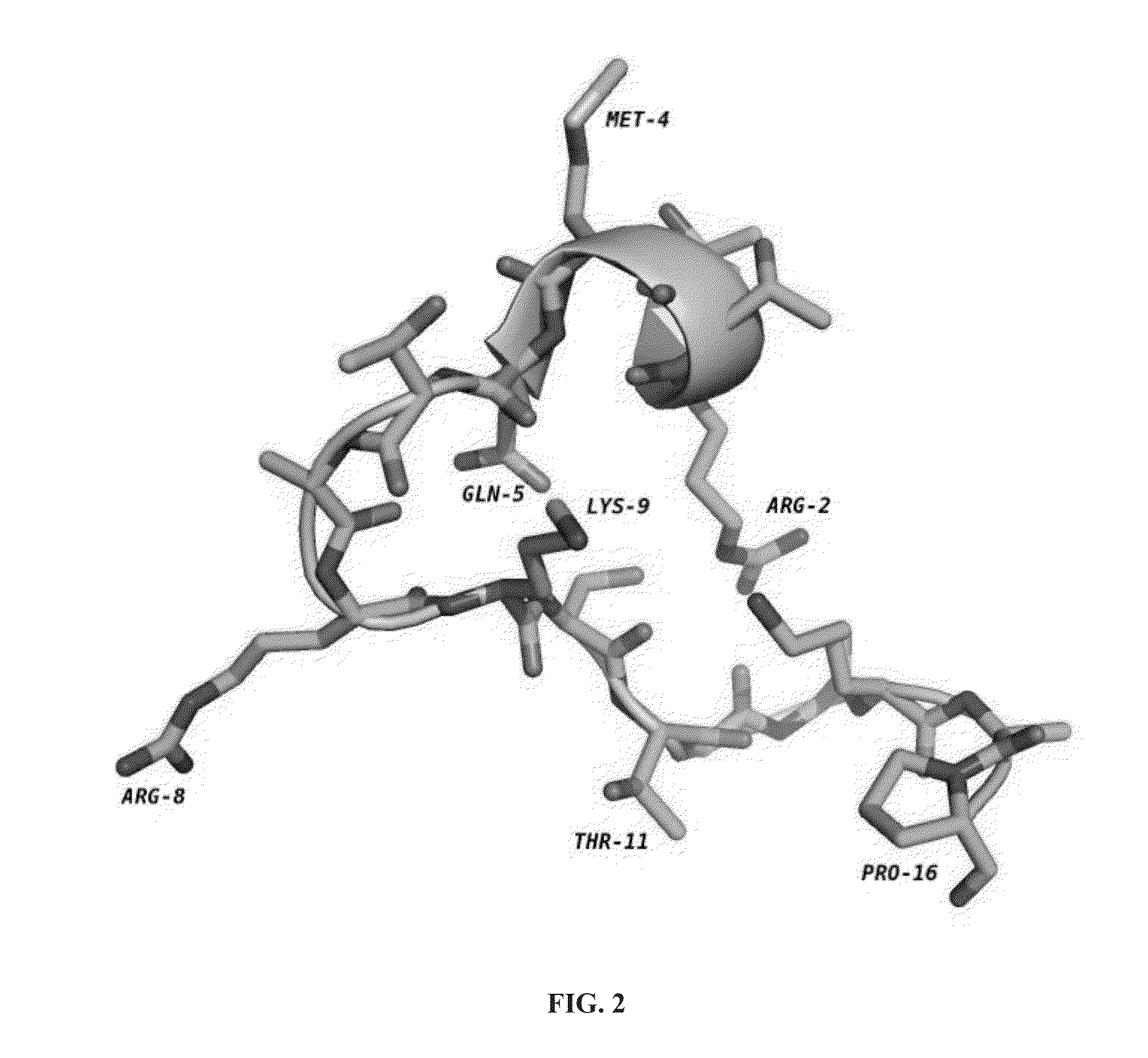
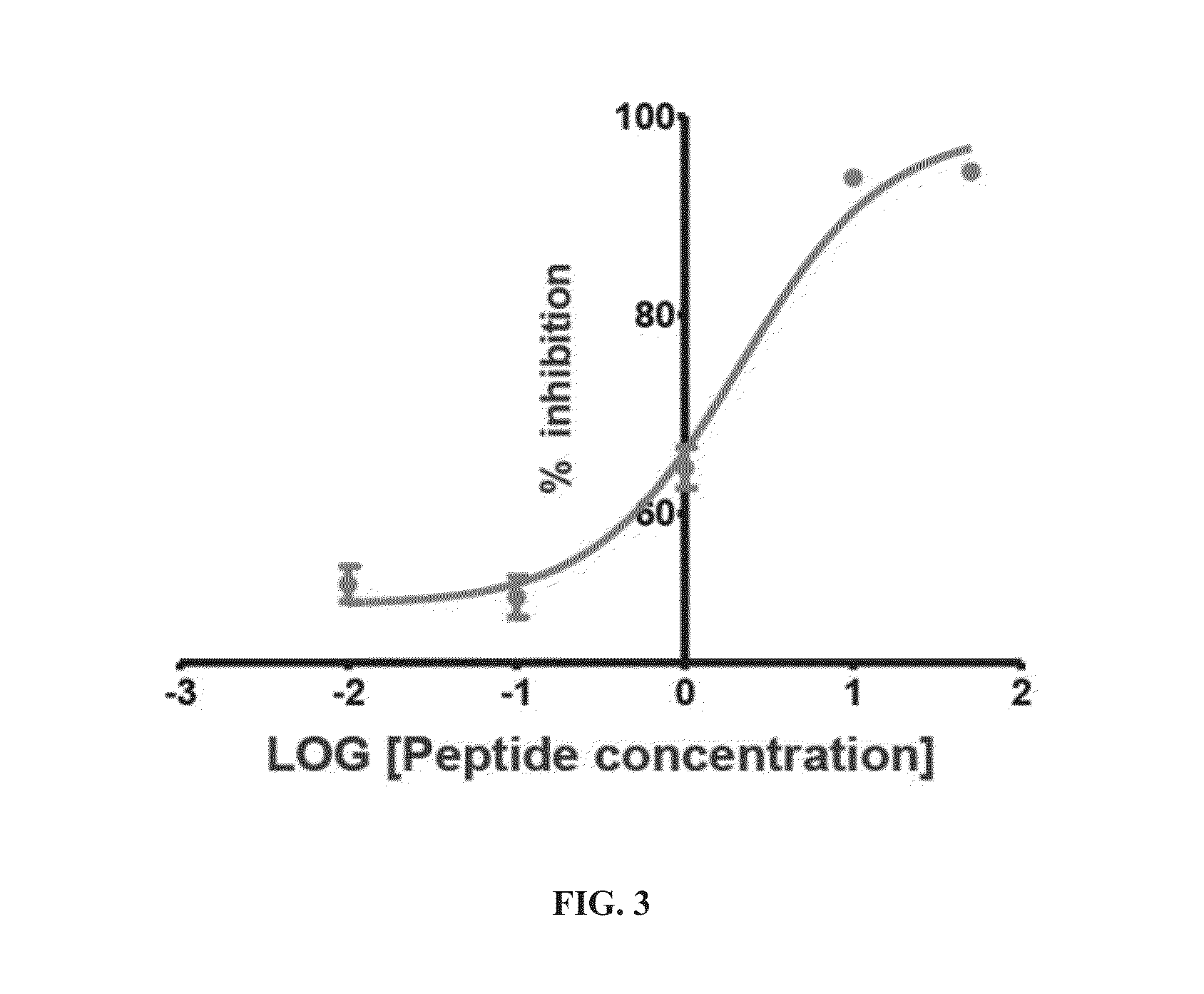
Cyclic peptide inhibitors of lysine-specific demethylase 1
ActiveUS20150065434A1Inhibit cell growthInhibit cell proliferationPeptide-nucleic acidsDepsipeptidesCyclic peptideIsrapafant
Provided herein are cyclic peptide inhibitors of lysine-specific demethylase 1. These cyclic peptides have the potential to treat cancer, diabetes, cardiovascular disease, and neurological disorders.
Owner:MUSC FOUND FOR RES DEV
Water-resistant vegetable protein adhesive compositions
InactiveUS20050222358A1Low costGood exterior durabilityProtein adhesivesWood working apparatusAdhesiveWater resistant
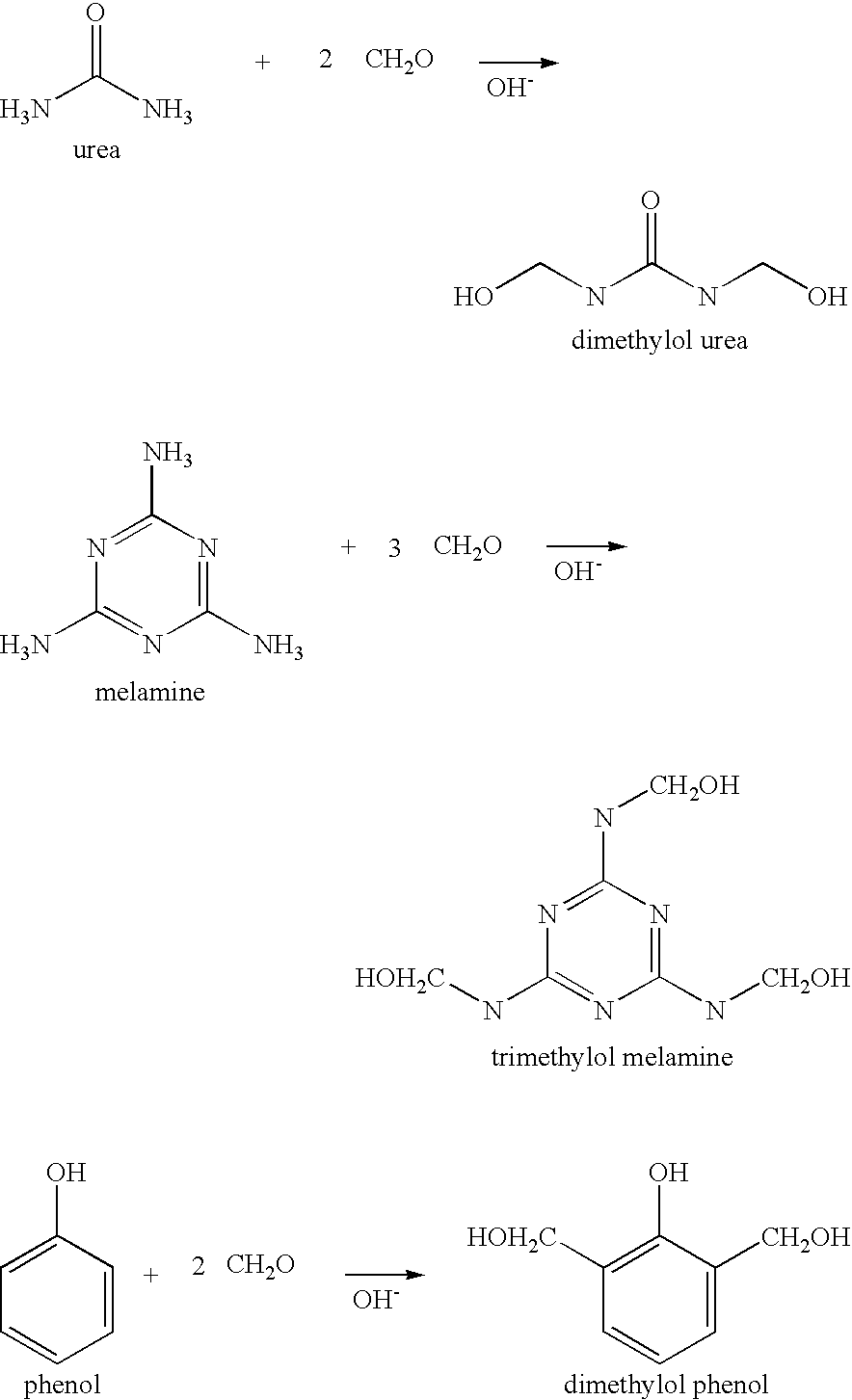
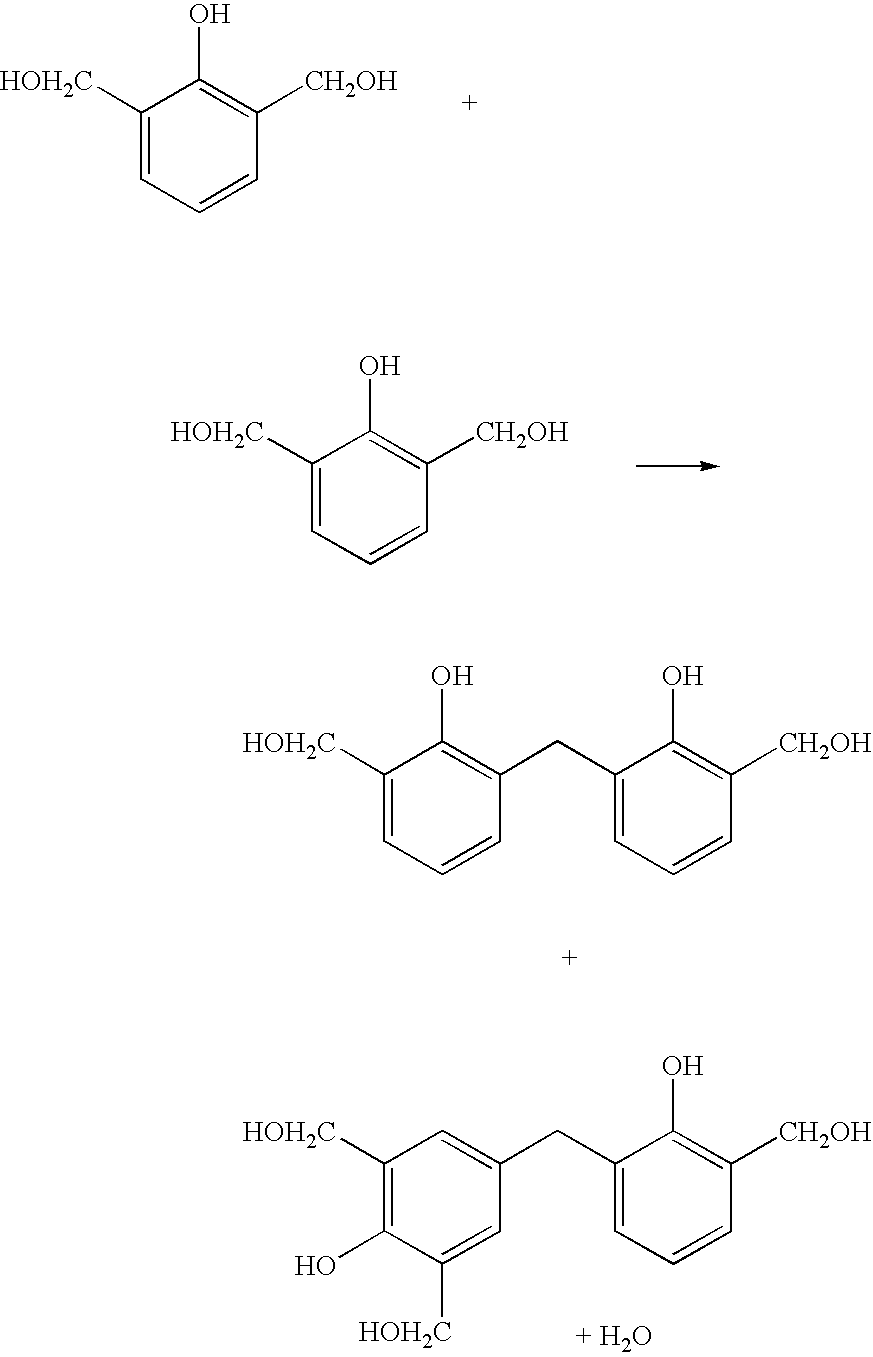
Water-resistant, protein-based adhesive compositions and methods for preparing them are provided. The adhesives are prepared by copolymerizing a denatured vegetable protein, such as soy flour, that has been functionalized with methylol groups with one or more reactive comonomers. The adhesives exhibit superior water resistance, and can be used to bond wood substrates, such as panels or laminate, or in the preparation of composite materials.
Owner:UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF AGRI THE +2
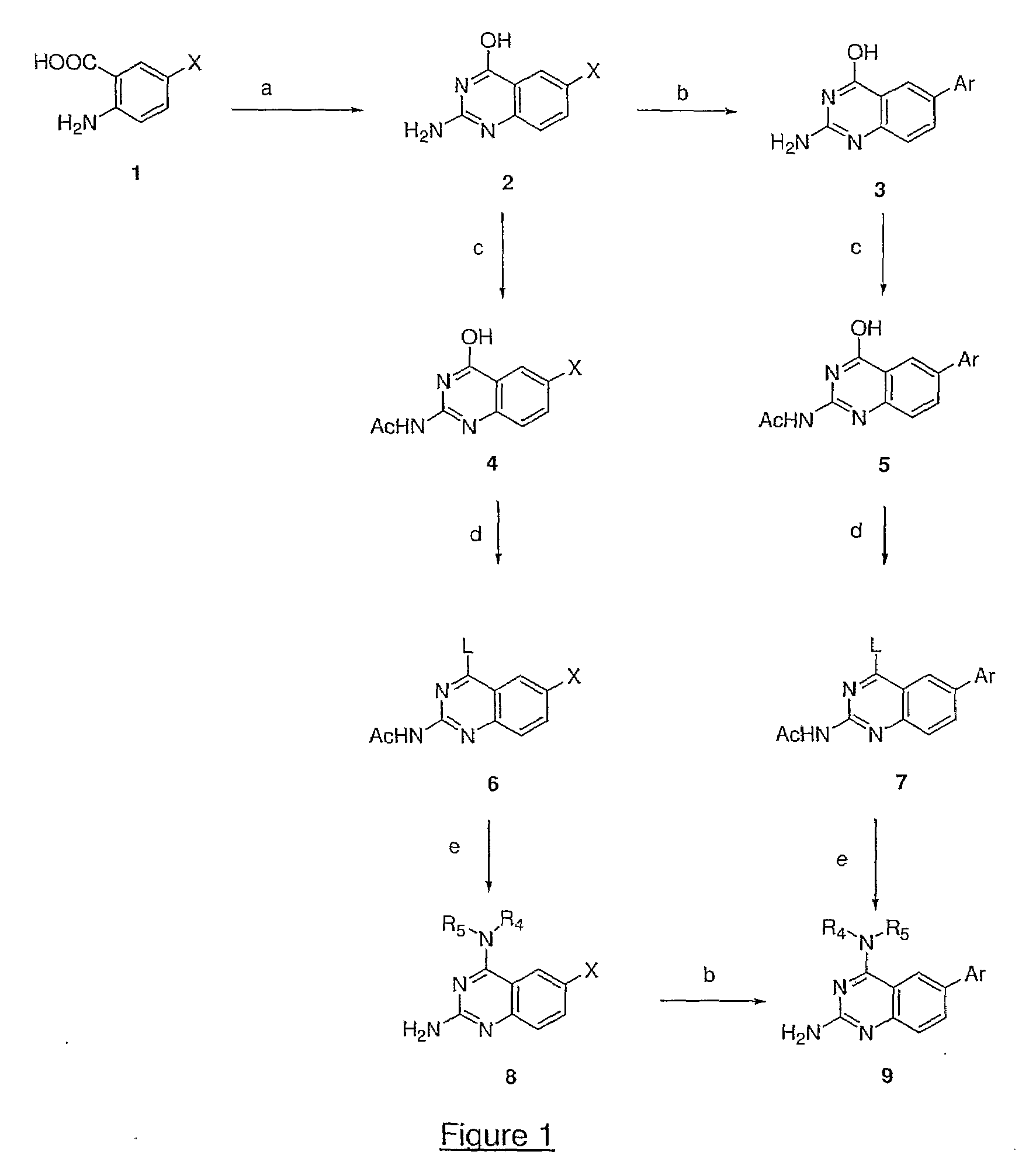
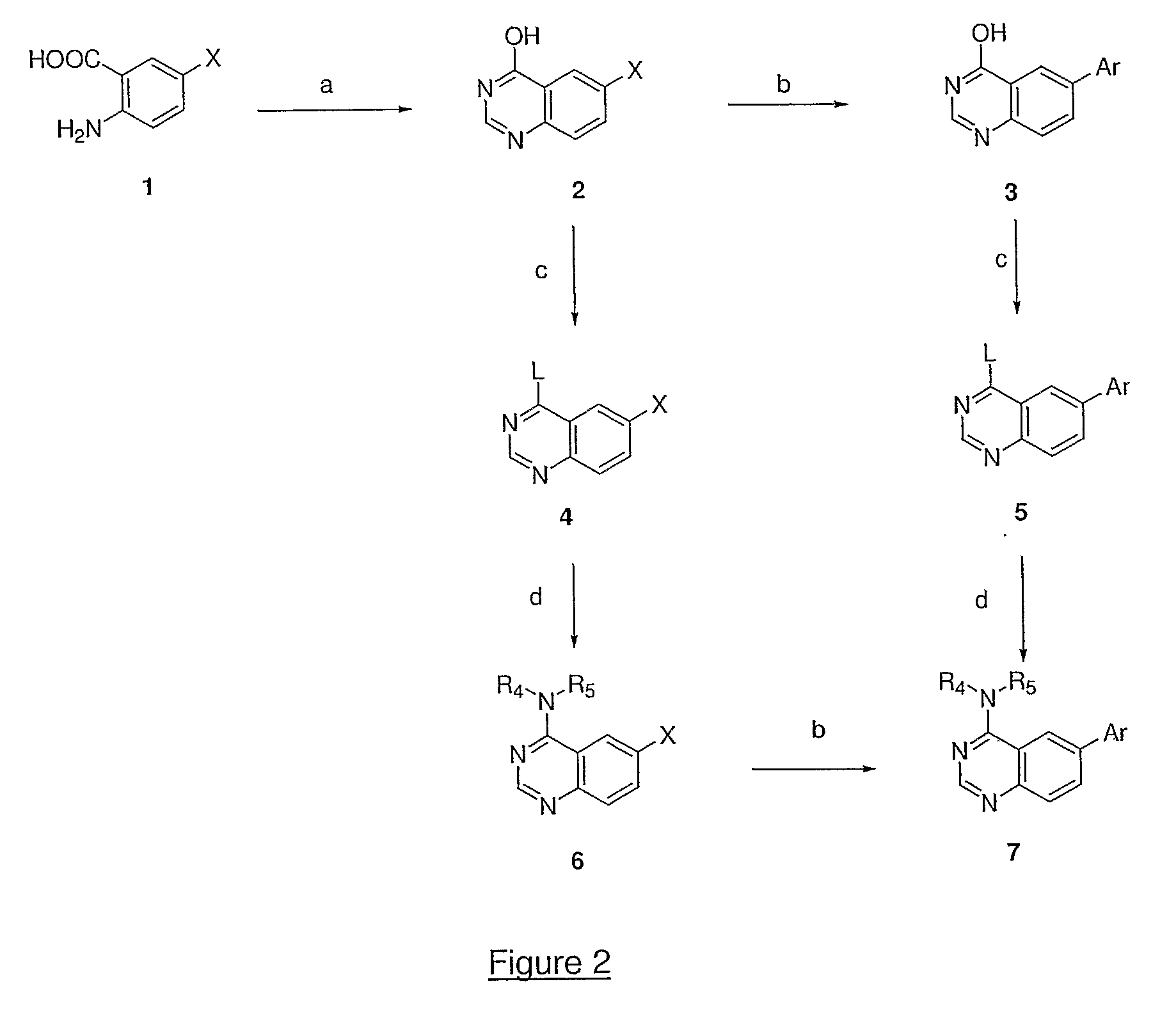
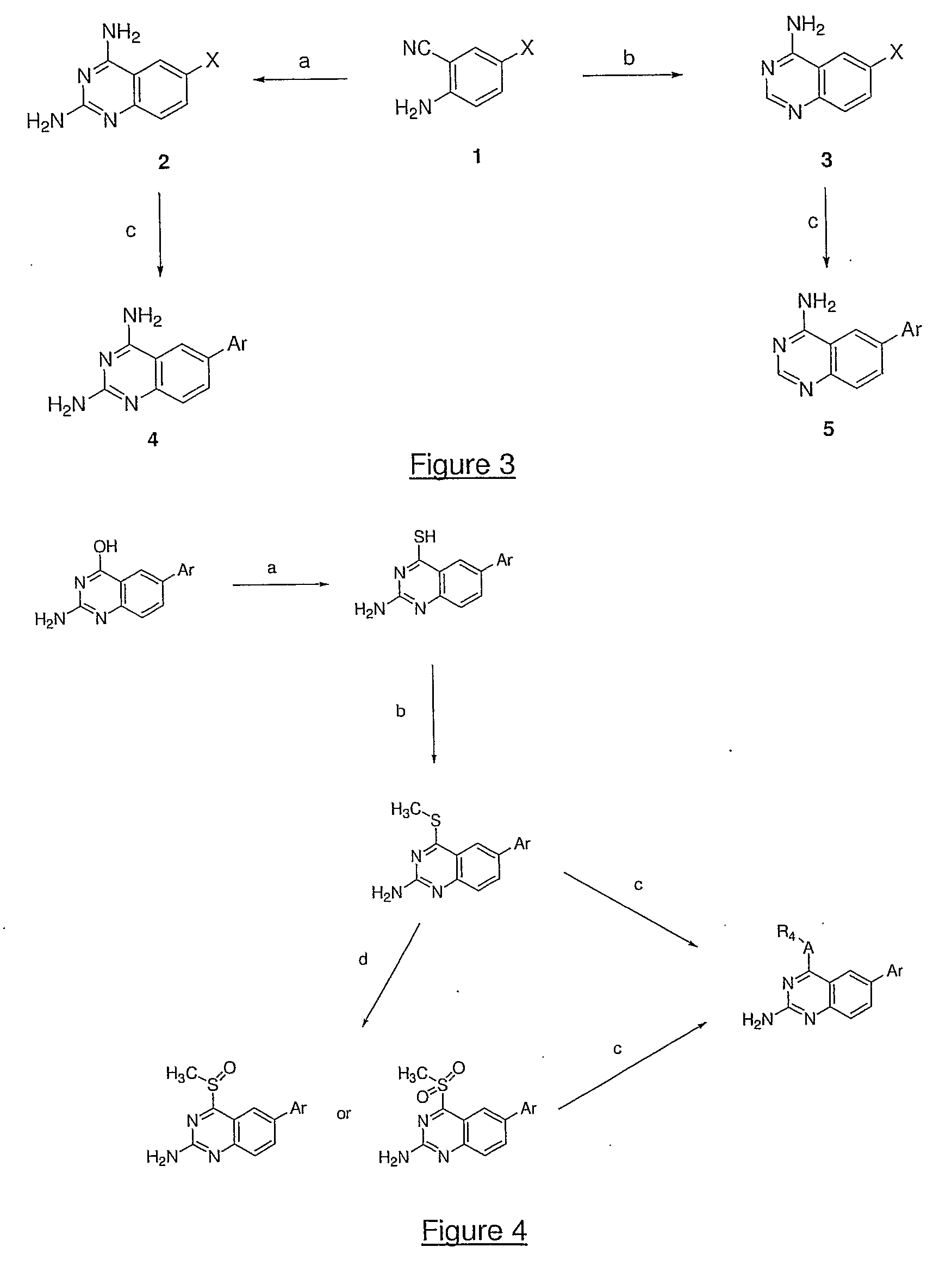
4,6-di- and 2,4,6-trisubstituted quinazoline derivatives useful for treating viral infections
This invention provides quinazoline derivatives represented by the structural formula: (I); wherein: R2 is hydrogen, NR′R″, C1-7 alkyl, arylC1-7 alkyl or C3-10 cycloalkyl; R4 is amino, C1-7 alkyl, C2-7 alkenyl, C3-10 cycloalkyl, C3-10 cycloalkenyl, aryl, heterocyclic, arylalkyl, heterocyclic-substituted C1-7 alkyl or C3-10 cycloalkyl-C1-7 alkyl; R5 is hydrogen or C1-7 alkyl, or R5 and R4 together with the nitrogen atom to which they are attached form a heterocyclic ring; Y is a single bond, C1-7alkylene, C2-7 alkenylene or C2-7 alkynylene; R6 is halogen, heteroaryl or aryl; R′ and R″ are each independently hydrogen, C1-7 alkyl-carbonyl or C1-7 alkyl; provided that R4 is not phenyl substituted with morpholino when R2 is H and R5 is H, and provided that when NR4R5 is piperazinyl, said NR4R5 is either non-substituted or substituted with methyl or acetyl; a pharmaceutically acceptable addition salt, a stereoisomer, a mono- or a di-N-oxide, a solvate or a pro-drug thereof, for the treatment of viral infections.
Owner:GILEAD SCI INC
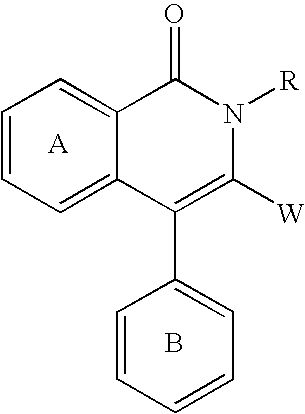
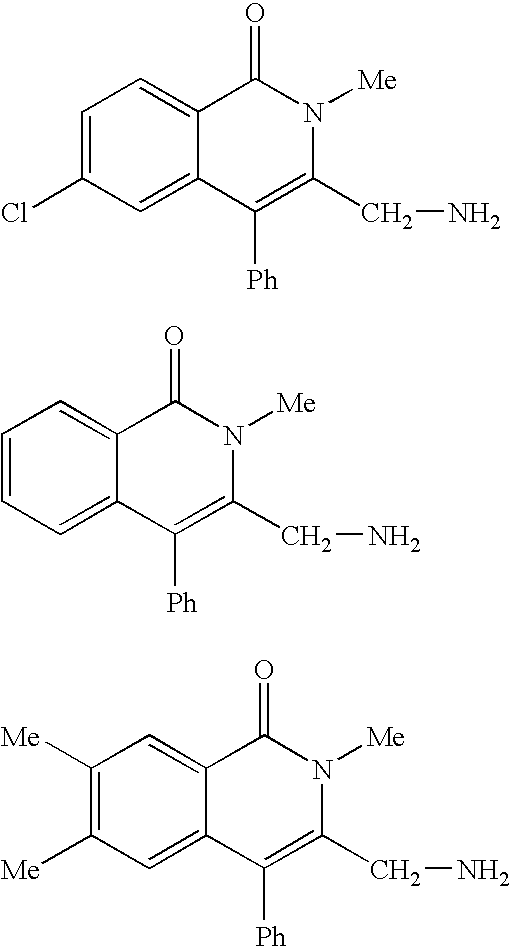
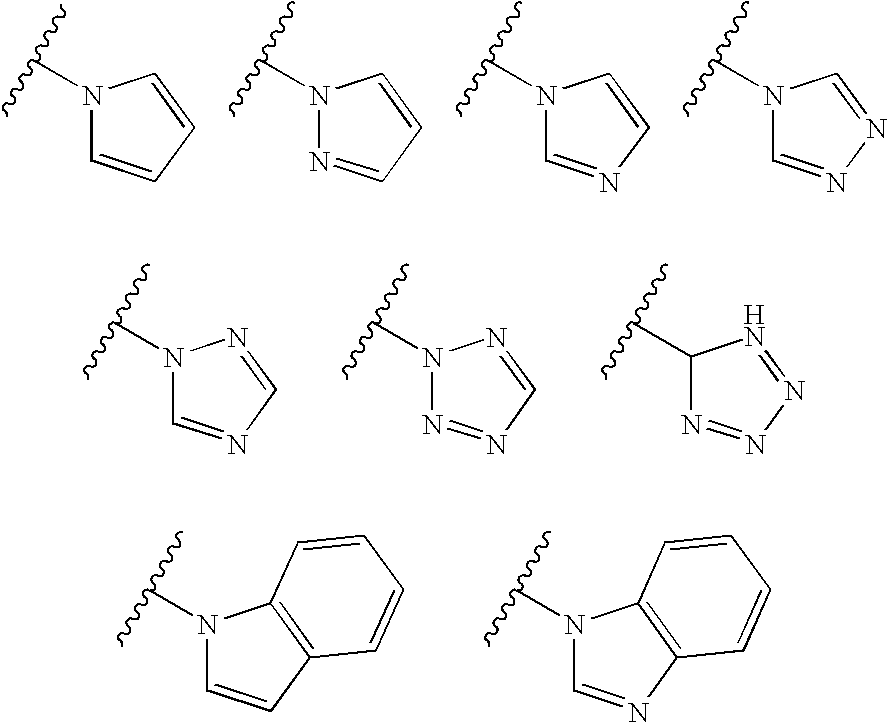
Fused heterocyclic compounds
The present invention provides a compound of the formula: wherein ring A is an optionally substituted 5 to 10-membered aromatic ring; R1 and R2 are the same or different and each is an optionally substituted hydrocarbon group or an optionally substituted heterocyclic group; X is a bond and the like; and L is a divalent hydrocarbon group, and a salt thereof, except 3-(aminomethyl)-2,6,7-trimethyl-4-phenyl-1(2H)-isoquinolinone, 3-(aminomethyl)-2-methyl-4-phenyl-1(2H)-isoquinolinone, 3-(aminomethyl)-6-chloro-2-methyl-4-phenyl-1(2H)-isoquinolinone and 3-(aminomethyl)-2-isopropyl-4-phenyl-1(2H)-isoquinolinone. The compound shows a superior peptidase-inhibitory activity and is useful as an agent for the prophylaxis or treatment of diabetes and the like.
Owner:TAKEDA PHARMA CO LTD
Functionally assembled antigen-specific intact recombinant antibody and a method for production thereof
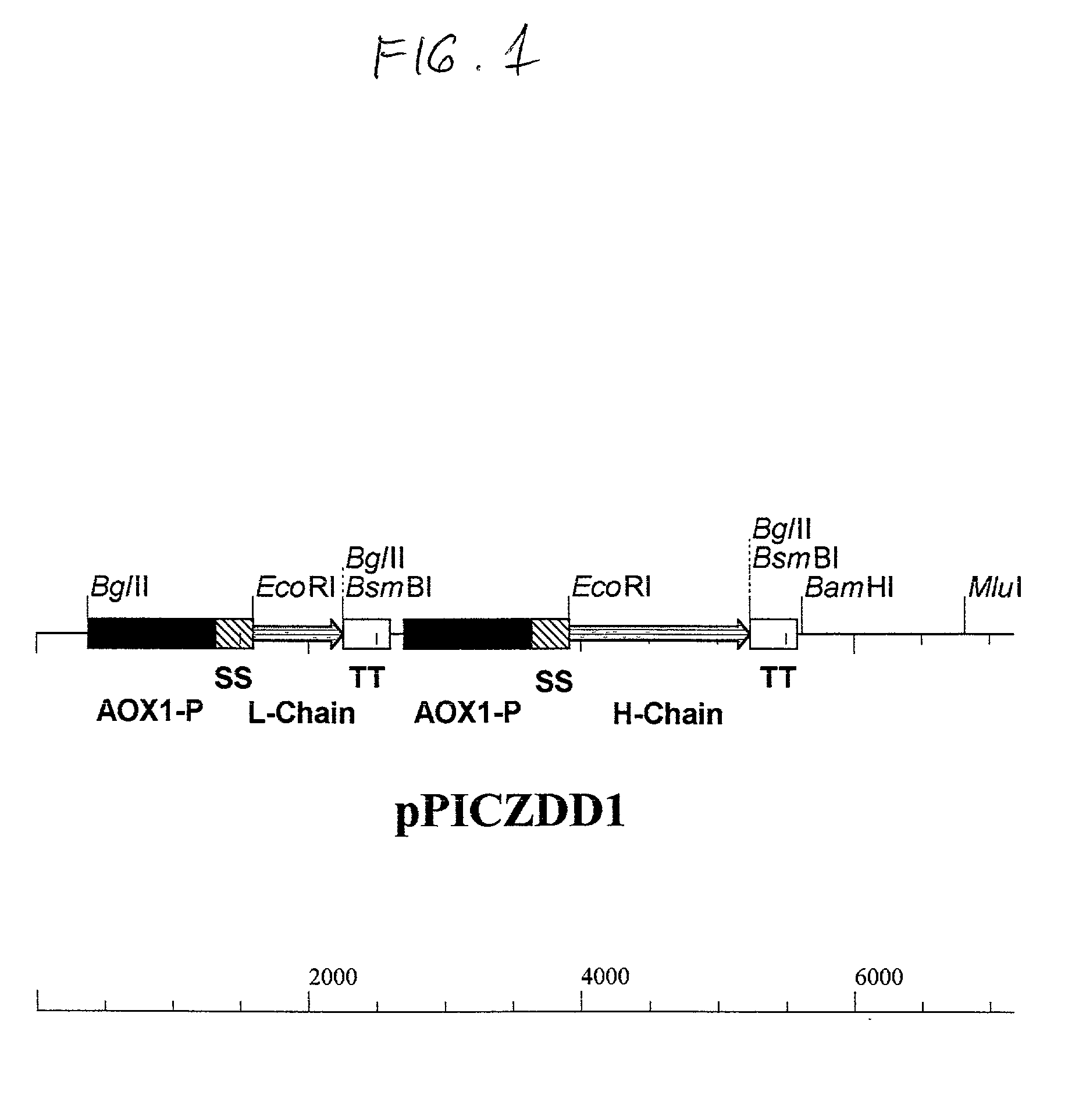
Functionally assembled antigen-specific intact recombinant monoclonal antibody produced by transformation of the methylotropic yeast, P. pastoris with mouse / human immunoglobulin genes encoding heavy and light chains. A method for production of the intact monoclonal antibodies, a recombinant yeast expression vector and the antibody-specific MRNA synthesis. A process for a large-scale production of the functionally assembled intact recombinant antibody.
Owner:RGT UNIV OF CALIFORNIA
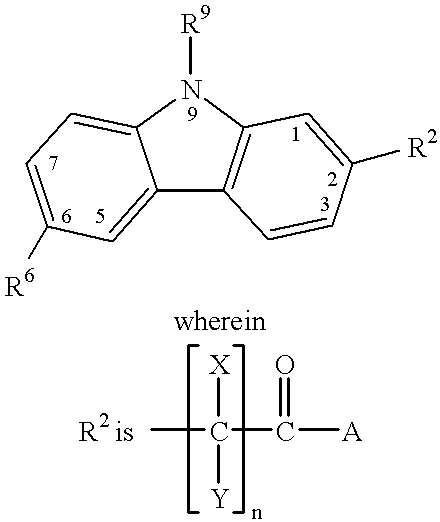
Treating or preventing the early stages of degeneration of articular cartilage or subchondral bone in mammals using carprofen and derivatives
Treating or preventing the early stages of degeneration of articular cartilage or subchondral bone in the affected joint of a mammal is accomplished by administering a chondroprotective compound of Formula (I):where A is hydroxy, (C1-C4)alkoxy, amino, hydroxy-amino, mono-(C1-C2)alkylamino, di-(C1-C2)alkylamino; X and Y are independently H or (C1-C2)alkyl; and n is 1 or 2; R6 is halogen, (C1-C3)alkyl, trifluoromethyl, or nitro; R9 is H; (C1-C2)alkyl; phenyl or phenyl-(C1-C2)alkyl, where phenyl is optionally mono-substituted by fluoro or chloro; -C(=O)-R, where R is (C1-C2)alkyl or phenyl, optionally mono-substituted by fluoro or chloro; or -C(=O)-O-R', where R1 is (C1-C2)alkyl.This treatment ameliorates, diminishes, actively treats, reverses or prevents any injury, damage or loss of articular cartilage or subchondral bone subsequent to said early stage of said degeneration. Whether or not a mammal needs such treatment is determined by whether or not it exhibits a statistically significant deviation from normal standard values in synovial fluid or membrane from the affected joint, with respect to at least five of the following substances: increased interleukin-1 beta (IL-1beta); increased tumor necrosis factor alpha (TNFalpha); increased ratio of IL-1beta to IL-1 receptor antagonist protein (IRAP); increased expression of p55 TNF receptors (p55 TNF-R); increased interleukin-6 (IL-6); increased leukemia inhibitory factor (LIF); decreased insulin-like growth factor-1 (IGF-1); decreased transforming growth factor beta (TGFbeta); decreased platelet-derived growth factor (PDGF); decreased basic fibroblast growth factor (b-FGF); increased keratan sulfate; increased stromelysin; increased ratio of stromelysin to tissue inhibitor of metalloproteases (TIMP); increased osteocalcin; increased alkaline phosphatase; increased cAMP responsive to hormone challenge; increased urokinase plasminogen activator (uPA); increased cartilage oligomeric matrix protein; and increased collagenase.
Owner:PFIZER INC +1
Pharmaceutical compositions
This invention relates to combinations comprising 3-[(R)-2-(N,N-dimethylamino)ethylthio-Sar]-4-(gammahydroxymethylleucine)cyclosporine, or a pharmaceutically acceptable salt, solvate or hydrate thereof; and certain nucleoside analogues, and their use in the treatment of hepatitis C virus.
Owner:SCYNEXIS INC
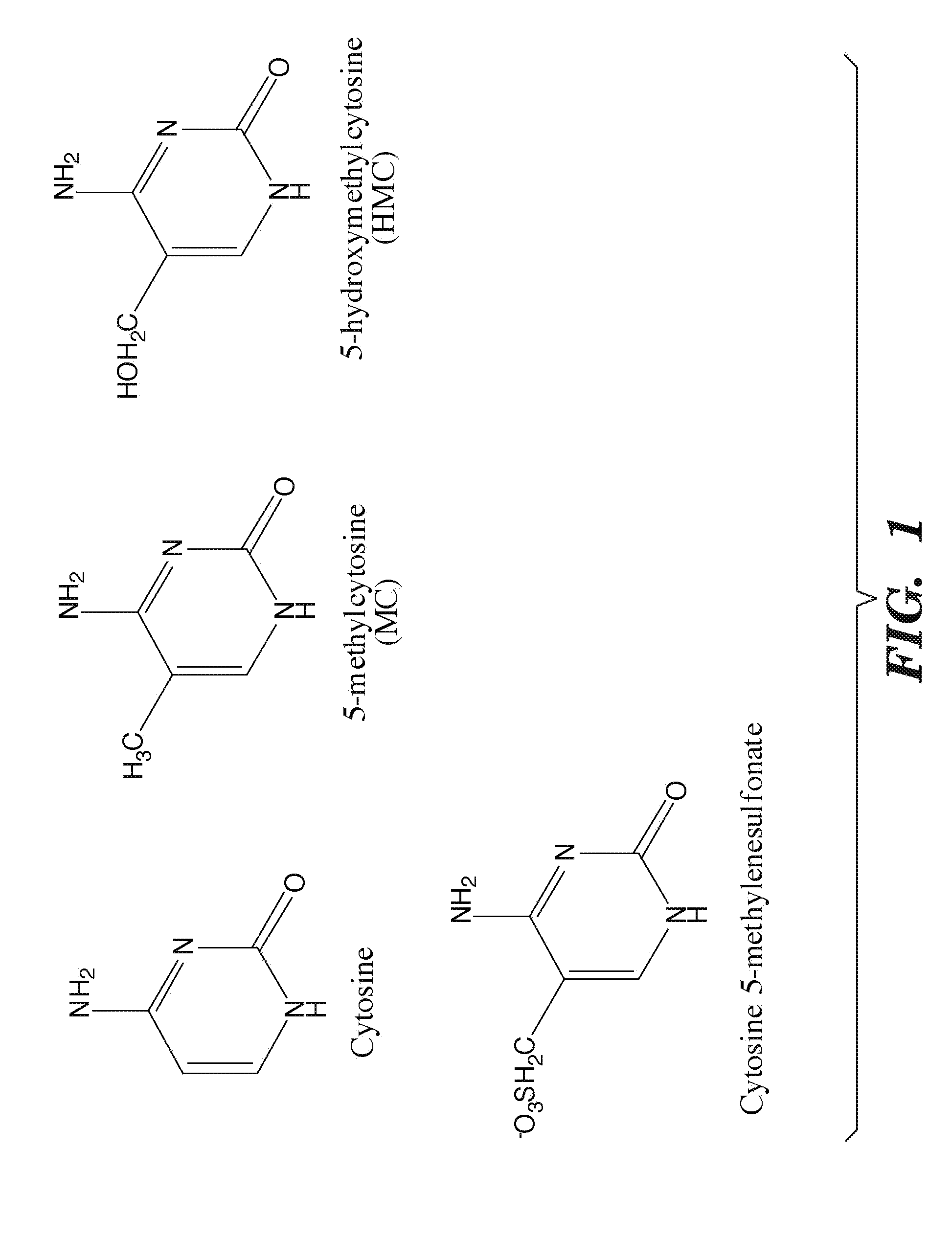
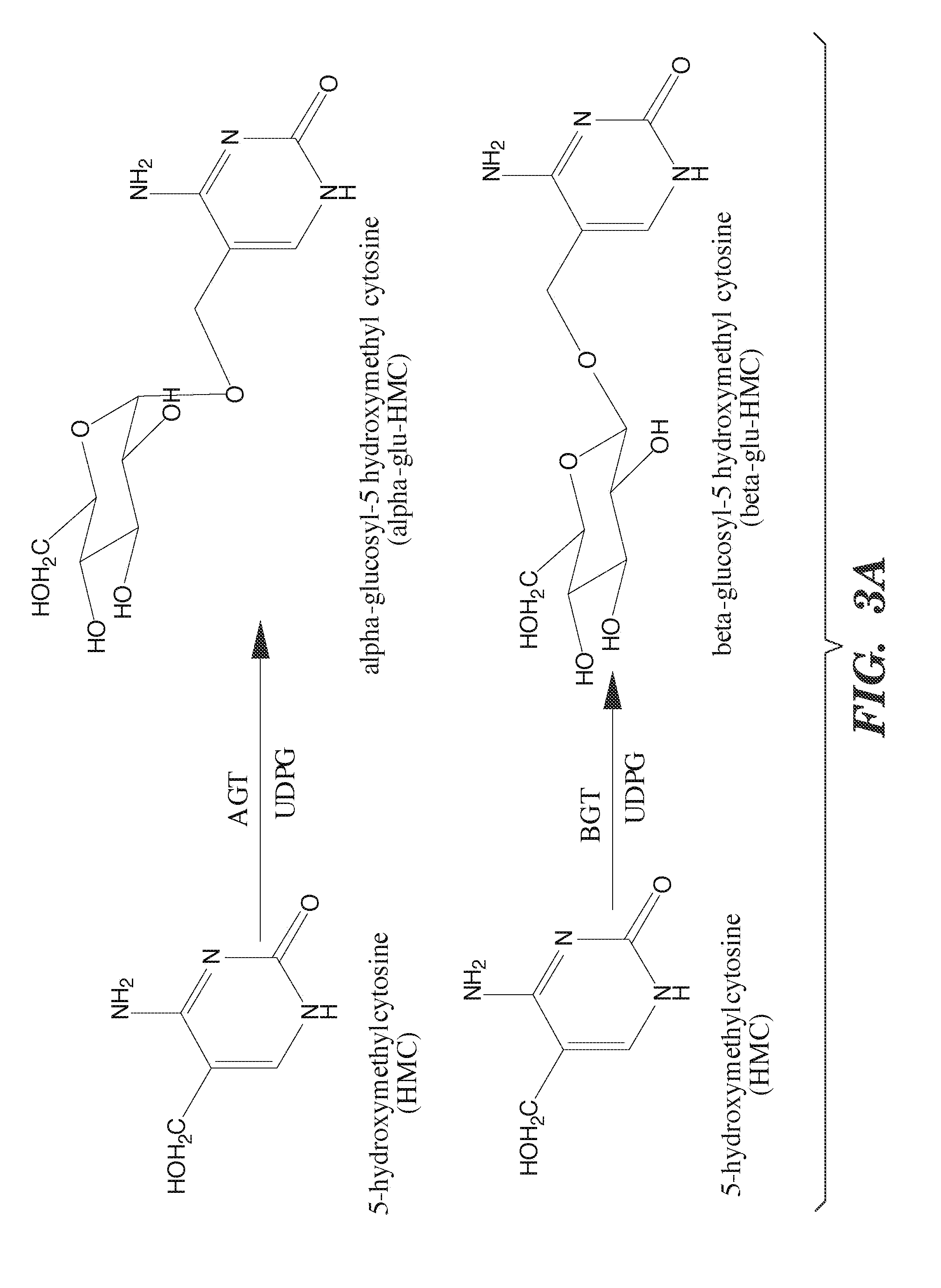
Selective oxidation of 5-methylcytosine by TET-family proteins
ActiveUS9115386B2Promotes reprogrammingImprove efficiencyCompound screeningApoptosis detectionHydroxymethylMethyl cytosine
Owner:UNITED STATES OF AMERICA +1
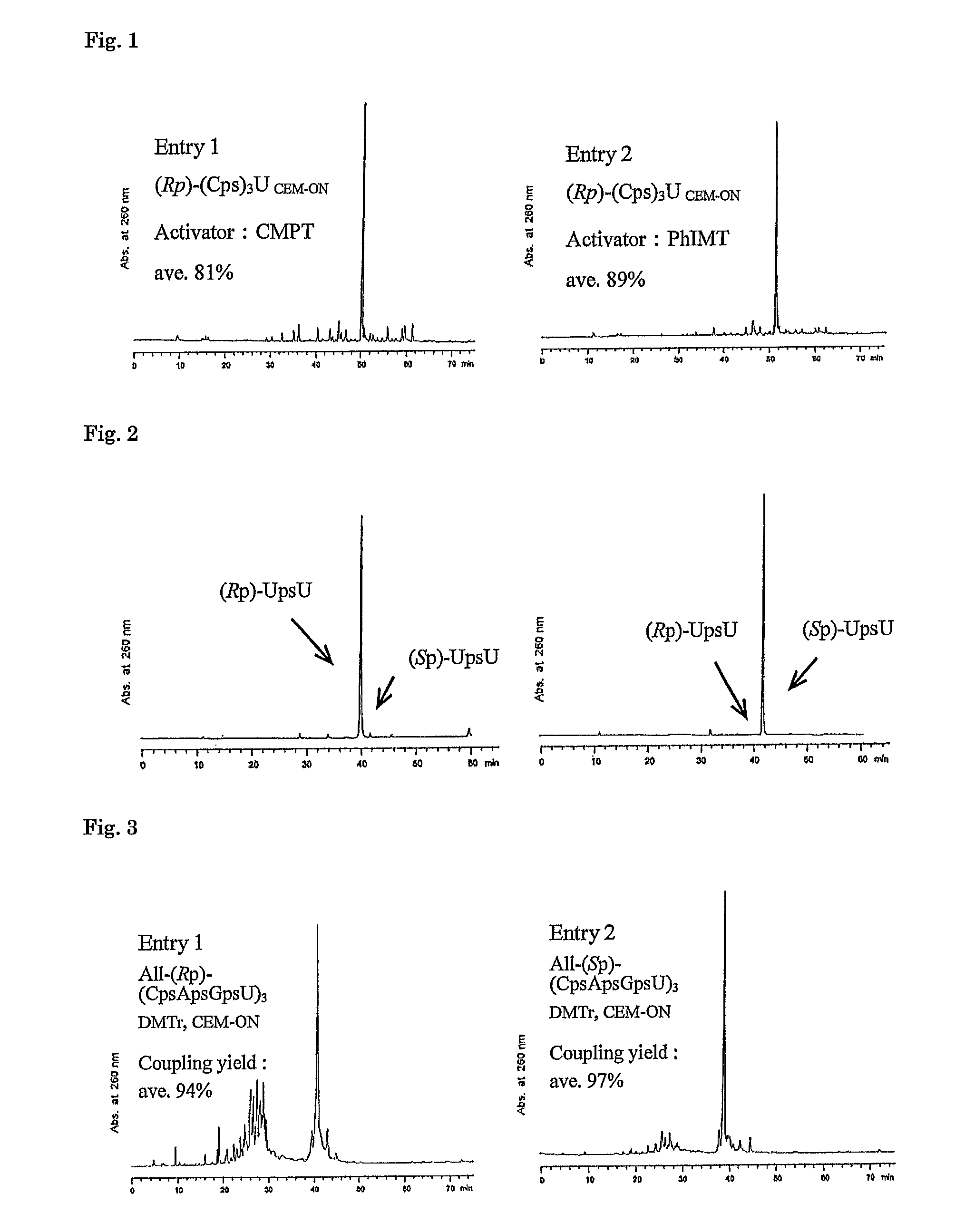
Method for preparing ribonucleoside phosphorothioate
ActiveUS8859755B2Efficient synthesisImprove efficiencySugar derivativesBulk chemical productionPhospholipinRibonucleoside
Owner:WAVE LIFE SCI LTD
Regulation of heterologous recombinant protein expression in methylotrophic and methanotrophic bacteria
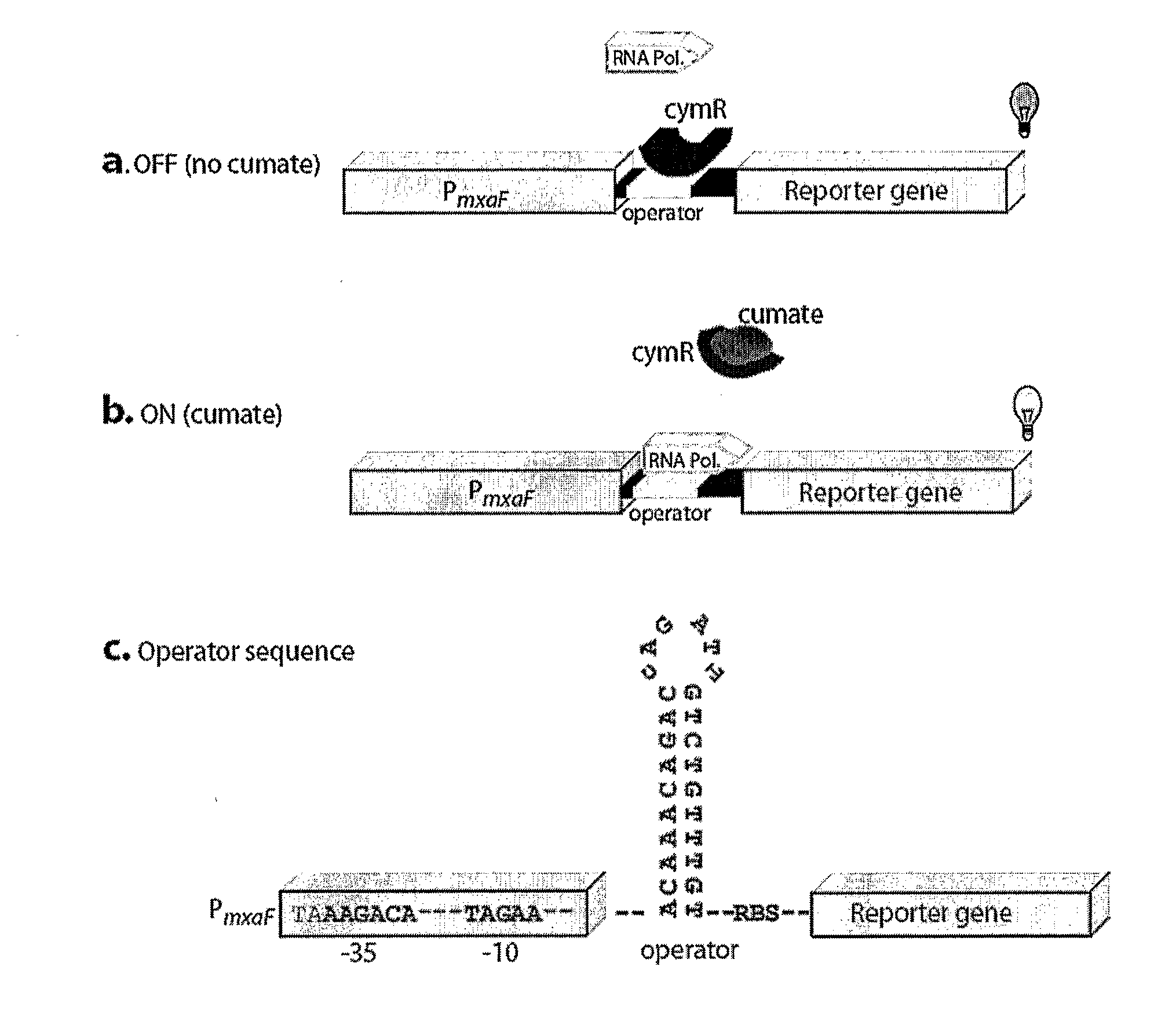
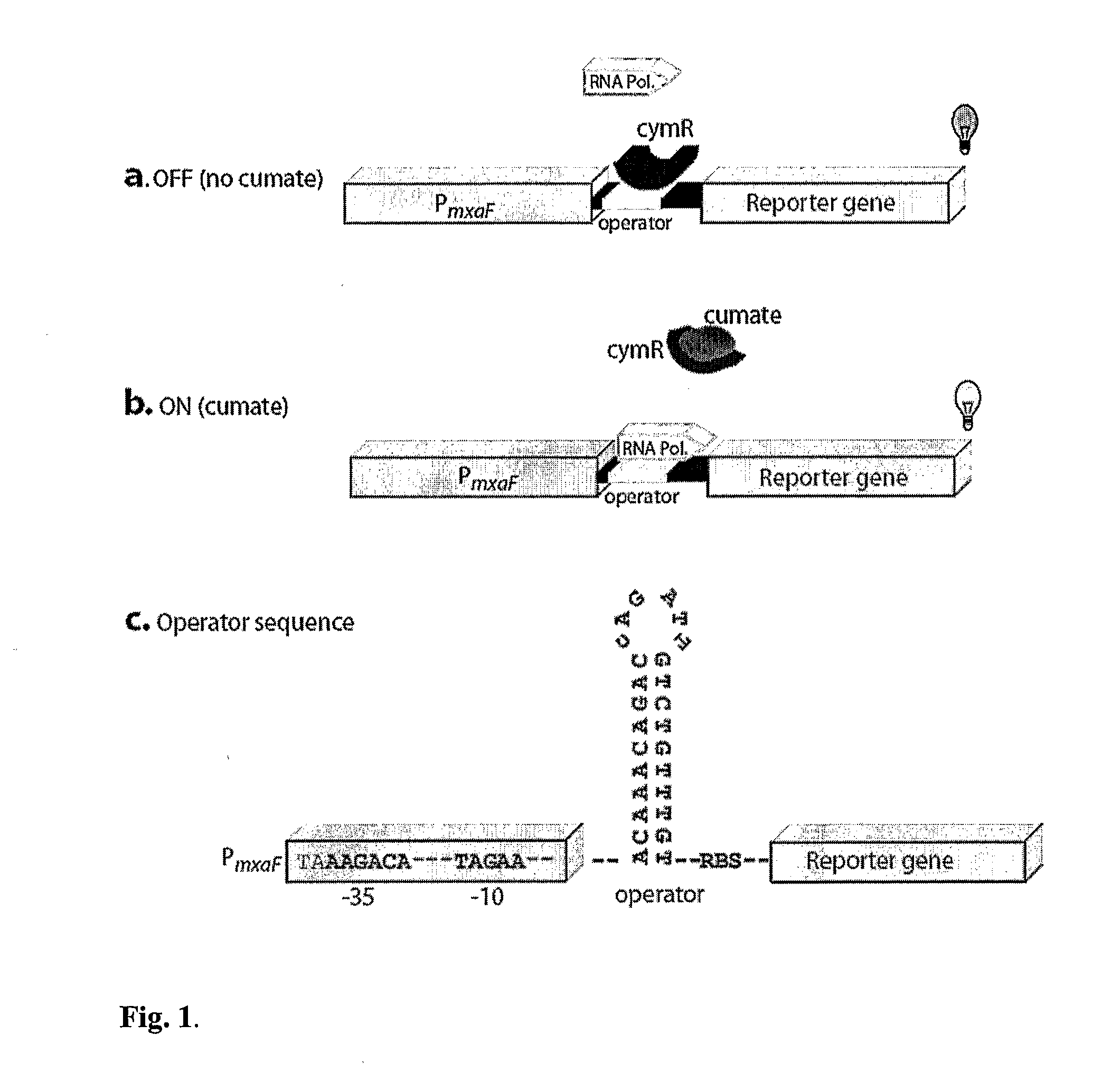
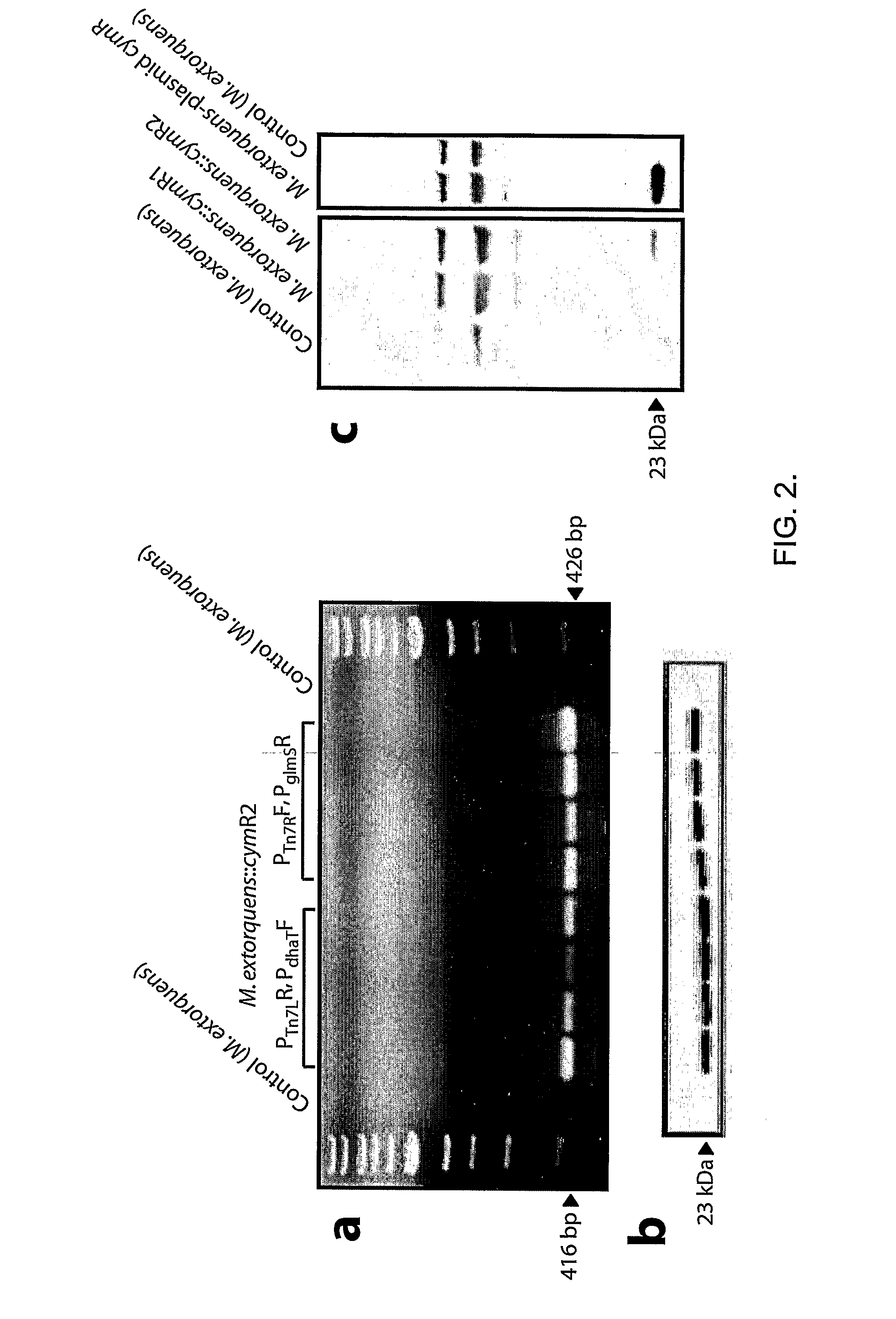
Methylotrophic or methanotrophic bacteria such as Methylobacterium are transformed with a gene of interest, and expression of the gene is regulated by means of a cumate repressor protein and an operator sequence which is operatively linked to the gene of interest, and the addition of an external agent. Specifically, the cymR repressor and cmt operator from Pseudomonas putida may serve to regulate gene expression in methylotrophic or methanotrophic bacteria with the addition of cumate.
Owner:NAT RES COUNCIL OF CANADA
Thyroid receptor ligands and method II
New thyroid receptor ligands are provided which have general formula (I) in which: n is an integer from 0 to 4; R1 is halogen, trifluoromethyl, or alkyl of 1 to 6 carbons or cycloalkyl of 3 to 7 carbons; R2 and R3 are the same or different and are hydrogen, halogen, alkyl of 1 to 4 carbons or cycloalkyl of 3 to 5 carbons, at least one of R2 and R3 being other than hydrogen; R4 is a carboxylic acid amide (CONR′R″) or an acylsulphonamide (CONHSO2R′) derivative, or a pharmaceutically acceptable salt thereof, and all stereoisomers thereof; or when n is equal to or greater than one, R4 may be a heteroaromatic moiety which may be substituted or unsubstituted, or an amine (NR′R″). R5 is hydrogen or an acyl (such as acetyl or benzoyl) or other group capable of bioconversion to generate the free phenol structure (wherein R5=H). In addition, a method is provided for preventing, inhibiting or treating a disease associated with metabolism dysfunction or which is dependent upon the expression of a T3 regulated gene, wherein a compound as described above is administered in a therapeutically effective amount. Examples of such diseases associated with metabolism dysfunction or are dependent upon the expression of a T3 regulated gene include obesity, hypercholesterolemia, atherosclerosis, cardiac arrhythmias, depression, osteoporosis, hypothyroidism, goiter, thyroid cancer as well as glaucoma, congestive heart failure and skin disorders.
Owner:KARO BIO AB
Compositions for treating CNS disorders
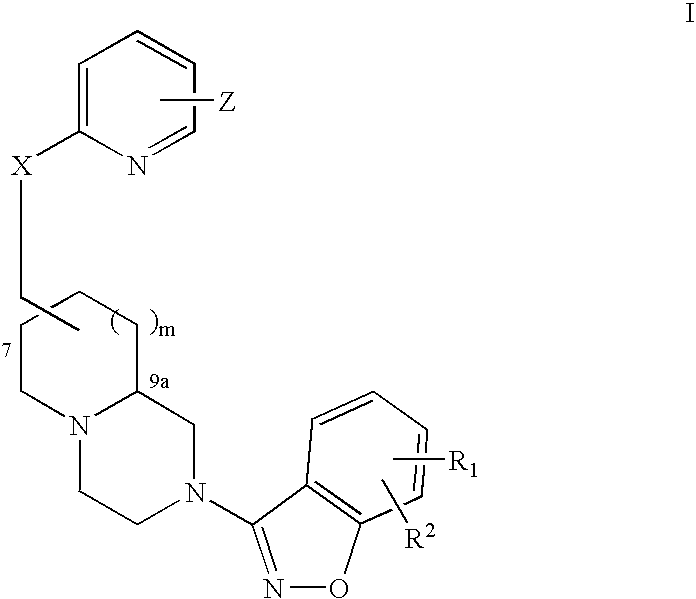
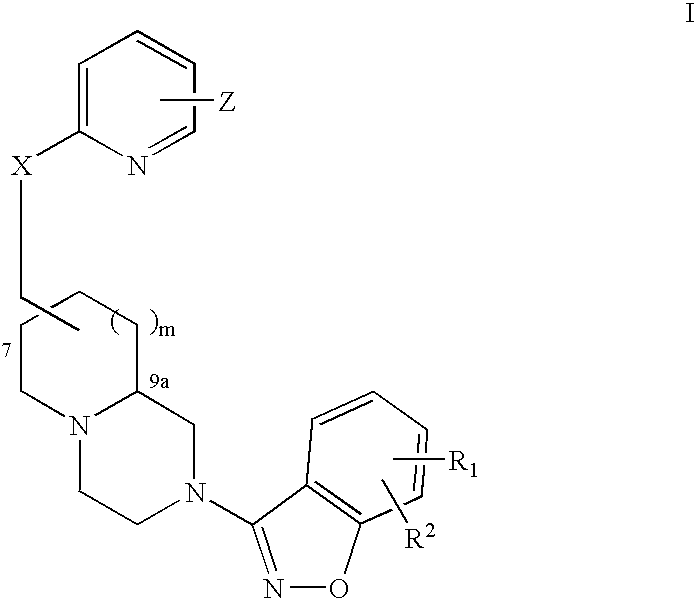
An aminomethylpyridyloxymethyl / benzisoxazole substituted azabicyclic compound, a pharmaceutical composition comprising same, and a method of treating one or more CNS or other disorders, including concurrent treatment of disorders such as schizophrenia and depression.
Owner:PFIZER INC
Encapsulation system
InactiveUS20090269313A1Function increaseReduces host 's immune responseBiocideMetabolism disorderDelivery vehicleIslet cells
An encapsulation system for use in the treatment of diabetes (Types 1 or 2, and LADA) are provided. The system has (1) a delivery vehicle comprising a selectively permeable membrane that allows passage of glucose, insulin and other nutrients through the membrane, but prevents large molecules such as antibodies or inflammatory cells from passing through the membrane; (2) a population of islet cells or insulin producing cells encapsulated by said membrane; and (3) a biological response modifier that may be in contact with the membrane or encapsulated by the membrane. Generally, the biological response modifier is a compound, including resolved enantiomers, diastereomers, tautomers, salts and solvates thereof, having the following formula:wherein:X, Y and Z are independently selected from a member of the group consisting of C(R3), N, N(R3) and S;R1 is selected from a member of the group consisting of hydrogen, methyl, C(5-9)alkyl, C(5-9)alkenyl, C(5-9)alkynyl, C(5-9)hydroxyalkyl, C(3-8)alkoxyl, C(5-9)alkoxyalkyl, the R1 being optionally substituted;R2 and R3 are independently selected from a member of the group consisting of hydrogen, halo, oxo, C(1-20)alkyl, C(1-20)hydroxyalkyl, C(1-20)thioalkyl, C(1-20)alkylamino, C(1-20)alkylaminoalkyl, C(1-20)aminoalkyl, C(1-20)aminoalkoxyalkenyl, C(1-20)aminoalkoxyalkynyl, C(1-20)diaminoalkyl, C(1-20)triaminoalkyl, C(1-20)tetraaminoalkyl, C(5-15)aminotrialkoxyamino, C(1-20)alkylamido, C(1-20)alkylamidoalkyl, C(1-20)amidoalkyl, C(1-20)acetamidoalkyl, C(1-20)alkenyl, C(1-20)alkynyl, C(3-8)alkoxyl, C(1-11)alkoxyalkyl, and C(1-20)dialkoxyalkyl.
Owner:DIAKINE THERAPEUTICS
Method for preparing ribonucleoside phosphorothioate
ActiveUS20130184450A1Improve efficiencyEfficient synthesisSugar derivativesBulk chemical productionRibonucleosideCombinatorial chemistry
Owner:WAVE LIFE SCI LTD
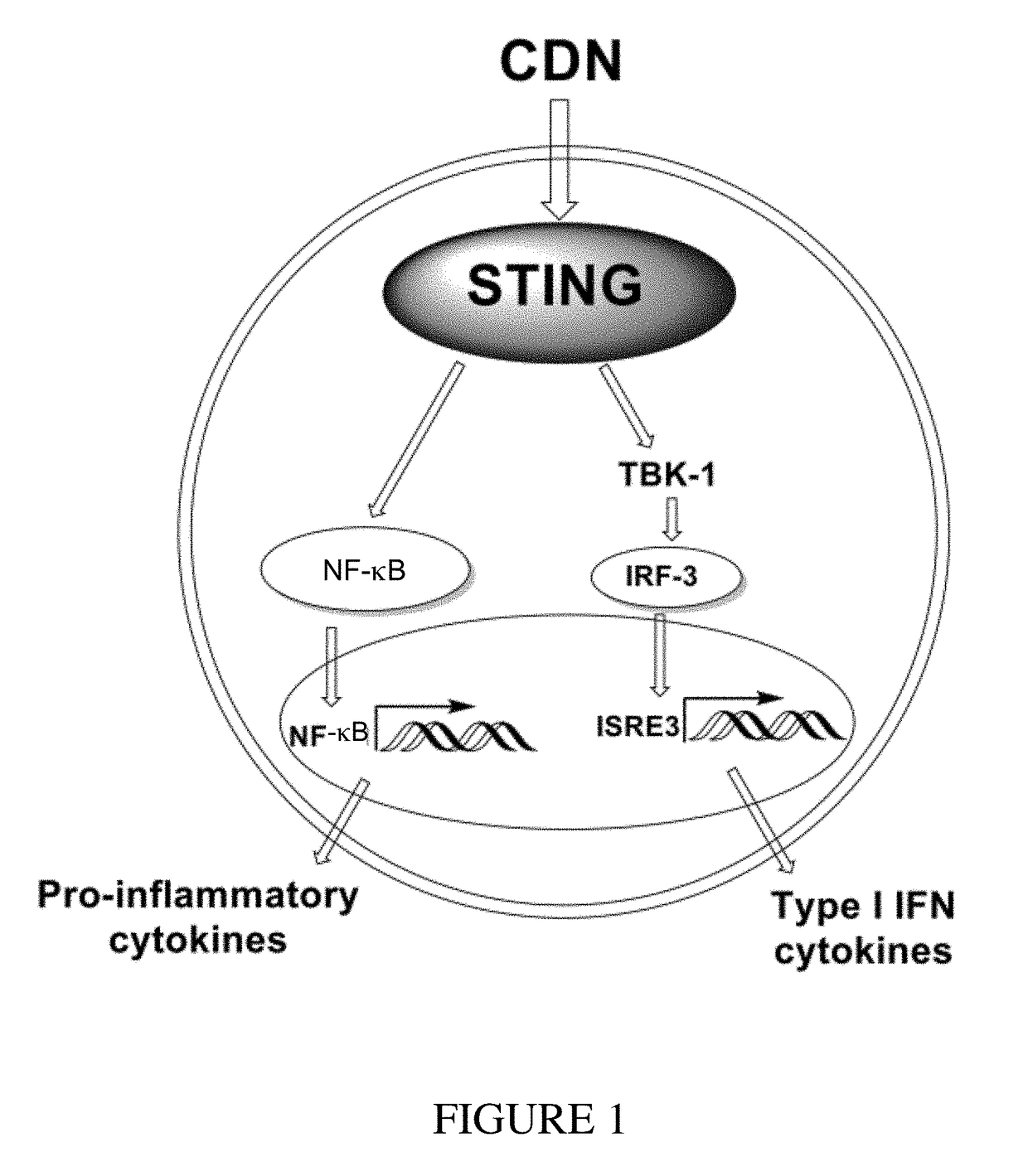
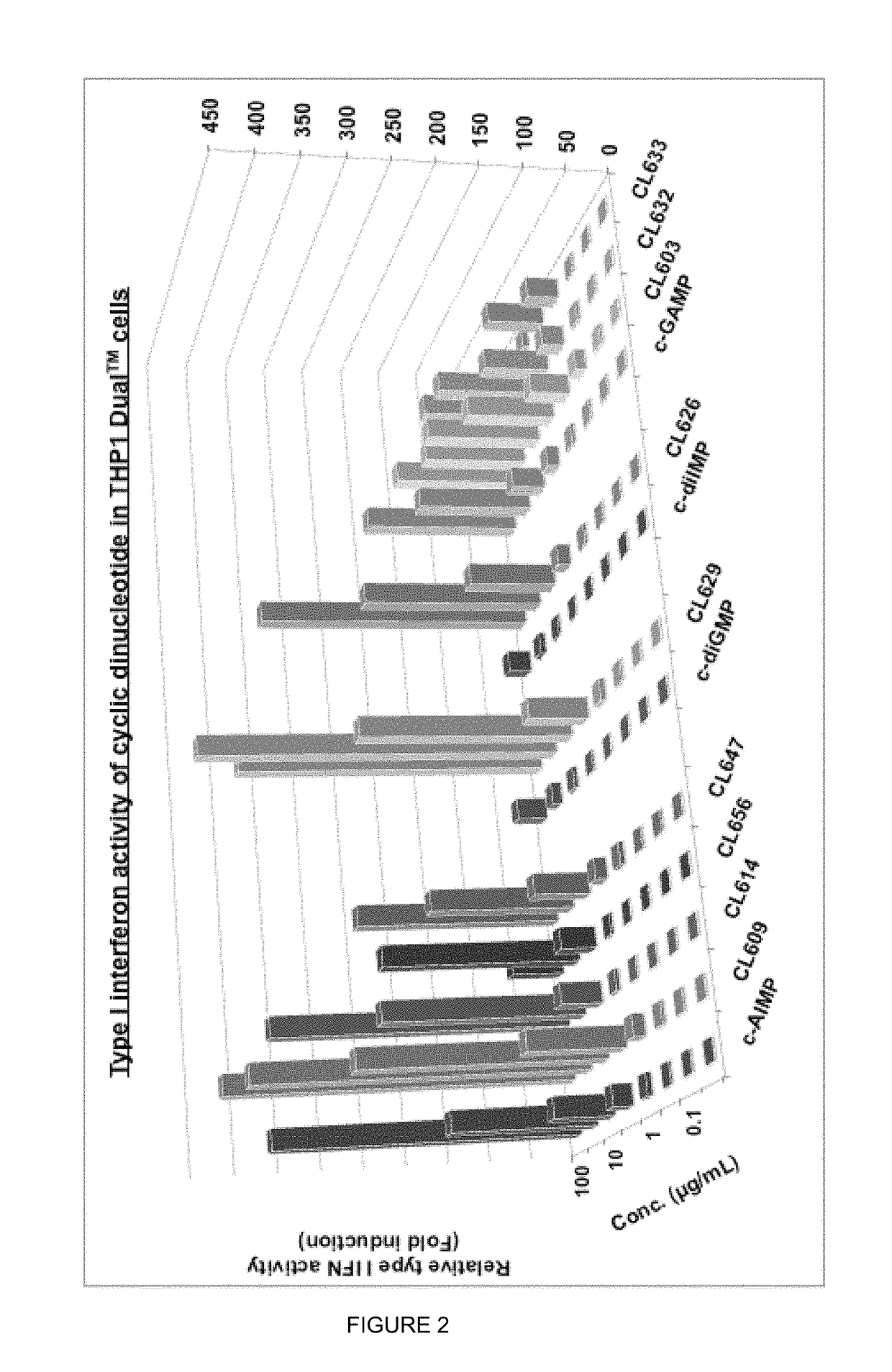
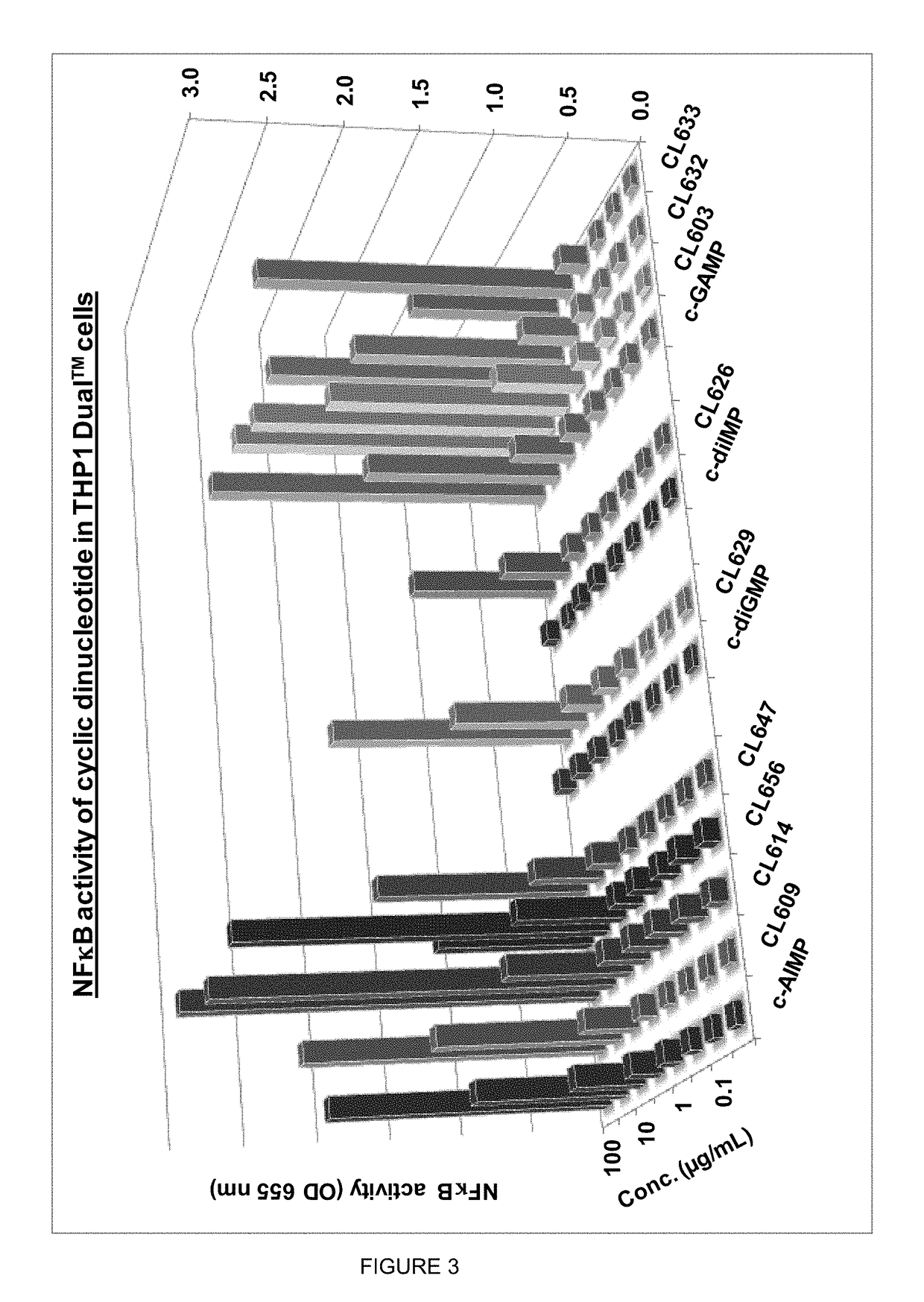
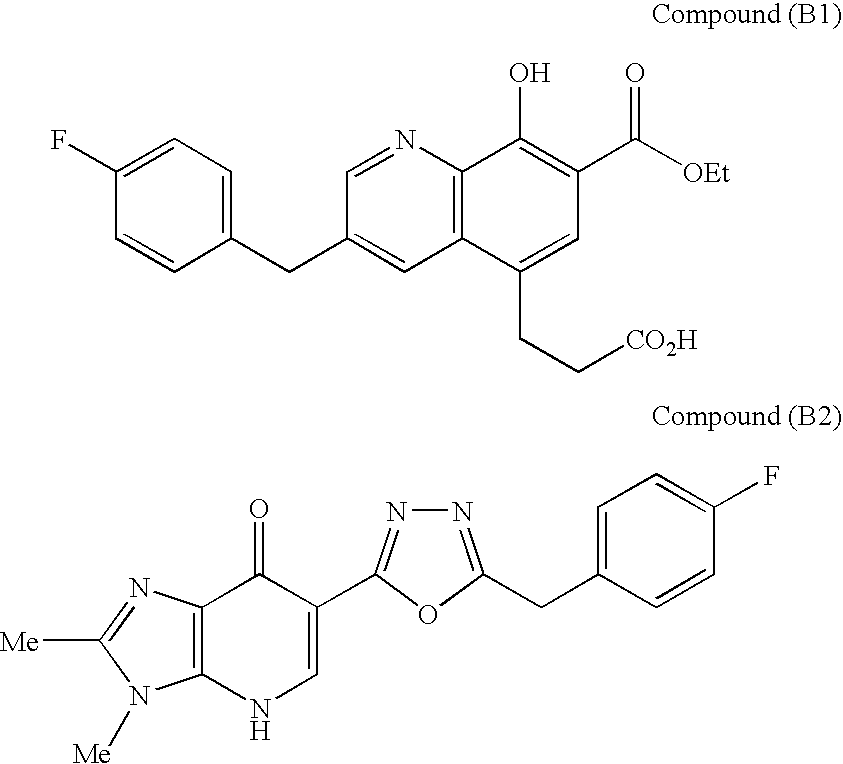
Cyclic dinucleotides for cytokine induction
ActiveUS10011630B2High activityImprove bioavailabilityAntibacterial agentsOrganic active ingredientsPurineIn vivo
A cyclic dinucleotide compound of Formula (I):wherein X1 is H or F; X2 is H or F; at least one among X1 and X2 is a fluorine atom; Z is OH, OR1, SH or SR1, wherein: R1 is Na or NH4, or R1 is an enzyme-labile group which provides OH or SH in vivo such as pivaloyloxymethyl; B1 and B2 are bases chosen from Adenine, Hypoxanthine or Guanine, and B1 is a different base than B2 and a pharmaceutically acceptable salt thereof. Pharmaceutical compositions including the cyclic dinucleotide, as well as their use in the treatment of a bacterial infection, a viral infection or a cancer are also described.
Owner:KAYLA THERAPEUTICS
Combination therapy
ActiveUS20050288326A1Low effective doseLow cytotoxicityBiocideAntiviralsSide effectReverse transcriptase
The present invention relates to a combination therapy for treating an HIV infection or inhibiting integrase comprising (S)-6-(3-Chloro-2-fluorobenzyl)-1-(1-hydroxymethyl-2-methylpropyl)-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (“Compound A”) or a pharmaceutically acceptable solvate or salt thereof in combination with at least one other anti-HIV agent. In some embodiments of the present invention, the other anti-HIV agents are chosen from reverse transcriptase inhibitors and protease inhibitors. In certain embodiments of the present invention, the other anti-HIV agents are chosen from AZT, 3TC, PMPA, efavirenz, indinavir, nelfinavir, a combination of AZT / 3TC, and a combination of PMPA / 3TC. Since Compound A has a high inhibitory activity specific for integrases, when used in combinations with other anti-HIV agents it can provide a combination therapy with fewer side effects for humans.
Owner:JAPAN TOBACCO INC
Novel acetyloxymethyl esters and methods for using the same
Novel acetyloxymethyl esters are disclosed. Methods of treating an illness, including cancer, hemological disorders and inherited metabolic disorders, and treating or ameliorating other conditions using these compounds are also disclosed. The compounds are effective in the inhibition of histone deacetylase.
Owner:ERRANT GENE THERAPEUTICS
Polynucleotides encoding polypeptides involved in intermediates metabolism of the central metabolic pathway in Methylophilus methylotrophus
The present invention provides polypeptides and polynucleotides involved in central intermediates metabolism in Methylophilus methylotrophus and methods of producing amino acids in microorganisms having enhanced or attenuated expression of these polypeptides and / or polynucleotides.
Owner:AJINOMOTO CO INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com