Therapeutic combinations comprising poly (ADP-ribose) polymerases inhibitor
a polymerases and inhibitor technology, applied in the field of 8fluoro2 4(methylamino) methylphenyl 1, 3, 4, 5tetrahydro6hazepino5, 4, 3cdindol6one, can solve the problems of inability to meet the needs of patients, inability to respond well to cytotoxic chemotherapy agents or regimens, and significant toxicity in conjunction with suboptimal efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Inhibition of Poly-ADP-Ribose Polymerase
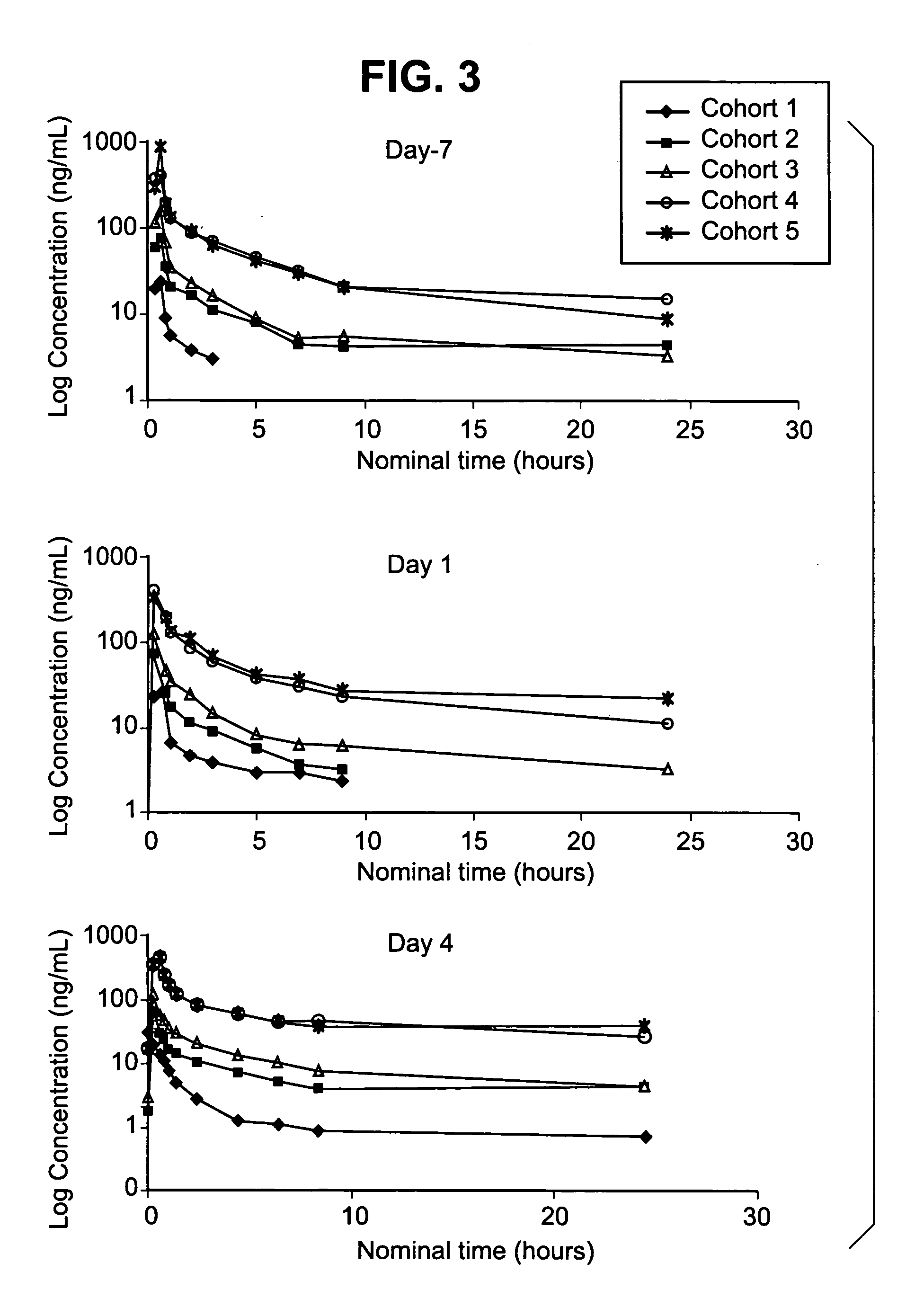
[0228] Crystallographic analysis of the compound of formula 1 bound to the inhibited target enzyme revealed that the drug binds to the active site of PARP-1, forming 3 hydrogen bonds. The PARP enzyme inhibiting activity of the compound of formula 1 was assayed as described in U.S. Pat. No. 6,495,541. The Ki determined using 32P-NAD+ incorporation into polymer by purified full-length human PARP-1, is 1.4 nM (Table 3). The compound of formula 1 is also a potent inhibitor of PARP-2 (Ki=0.17 nM) and, based on strong structural similarities in the amino acid sequences among the various PARP family enzymes (tankyrase, V-PARP), the phosphate salt of the compound of formula 1 (Compound I) will likely bind with high affinity to these enzymes as well.
TABLE 3Kinetic Constants for the Interaction of the Compound ofFormula 1 with PARPPARP-1 KiPARP-2 KiCompound(nM ± SD*)(nM ± SD*)compound of1.4 ± 0.20.17 ± 0.05formula 1
*SD = standard deviation.
example 2
Inhibition of Cell Growth
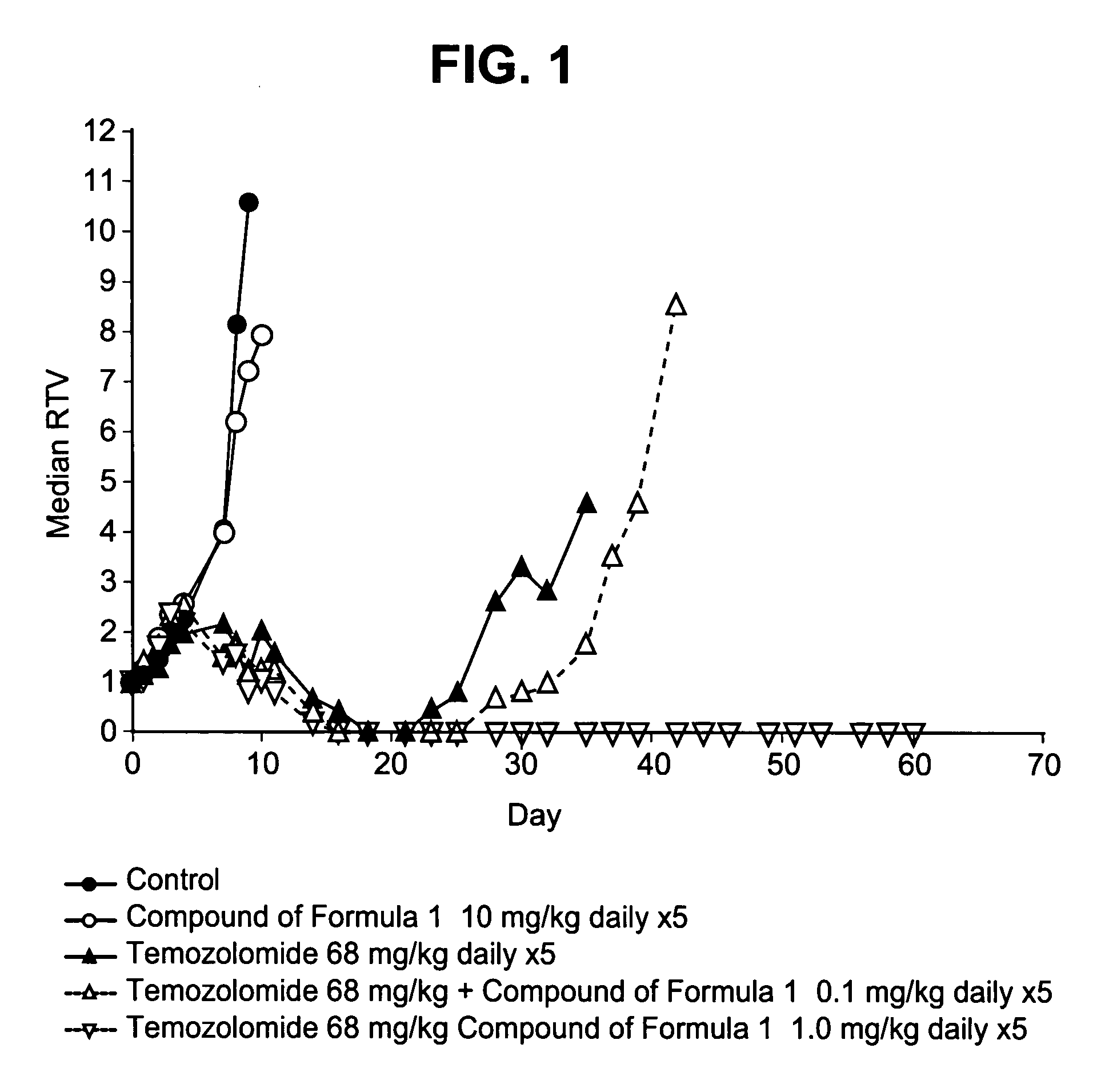
[0229] The intrinsic growth inhibitory activity of the compound of formula 1 following 5-day continuous exposure (Table 4) was determined in A549, LoVo and SW620 cell lines as described in U.S. Pat. No. 6,495,541. GI50 values (the concentration required to inhibit growth by 50%) ranged from 7 to 12 μM. Similarly, the ability of 0.4 μM the compound of formula 1 (ie, 50) to increase the growth inhibitory potency of temozolomide and topotecan was determined (Table 2). The potentiation factor at the IC50 concentration; PF50, is calculated as: GI50 temozolomide or topotecan alone / GI50 temozolomide or topotecan+0.4 μM the compound of formula 1. There was an 8-fold decrease in the GI50 of temozolomide in the LoVo cells and a 3.5-fold decrease in the GI50 of temozolomide in A549 cells upon addition of 0.4 μM the compound of formula 1. There was a 1.6-fold decrease in the GI50 of topotecan in the LoVo cells and a 2.6-fold decrease in the IC50 of topotecan in both A5...
example 3
Chemosensitization of Standard Chemotherapeutic Agents by Compound I
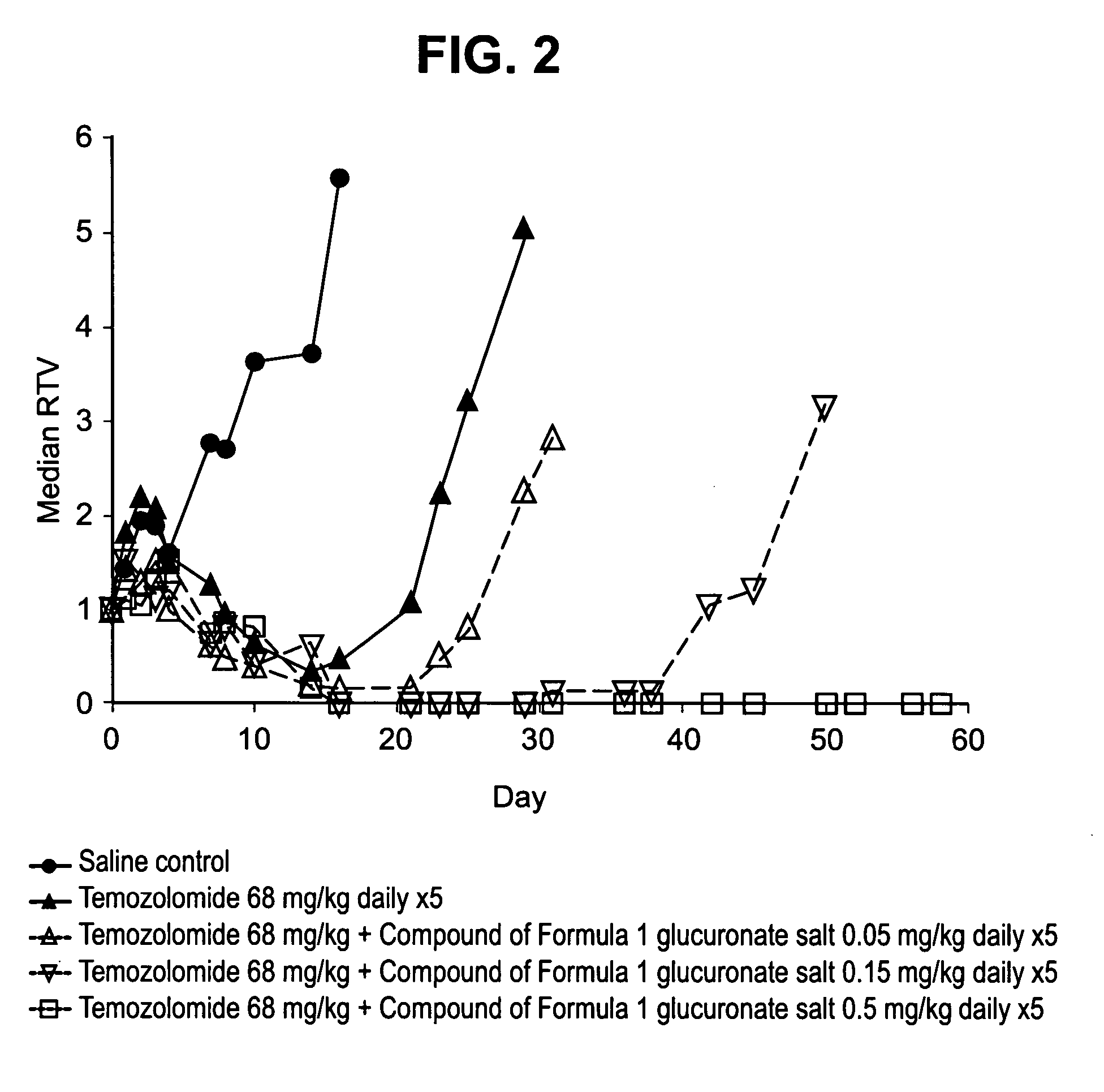
[0230] In vitro studies of human tumor cells lines carried out according to the procedure described in U.S. Pat. No. 6,495,541 have shown that at sub-micromolar concentrations the compound of formula 1 enhances the sensitivity of cells to temozolomide and the type-1-topoisomerase inhibitors, topotecan and SN-38 (the active metabolite of irinotecan) against human H460 non-small cell lung cancer (NSCLC) cells (Table 5).
TABLE 5Effect of the compound of formula 1 as a Glucuronate Salt onthe In Vitro Potency of Standard Chemotherapeutic Agents inHuman H460 NSCLC CellsChemotherapeuticAgentTypesPF50 (H460)aPaclitaxelMicrotubule antagonist0.775-FluorouracilPyrimidine antagonist0.92GemcitabinePyrimidine antagonist1.26-thioguaninePurine antagonist1.1DoxorubicinAnthracycline antibiotic1.1OxaliplatinPlatinum compound0.98bCisplatinPlatinum compound1.2EtoposideTopoisomerase II inhibitor0.75bTopotecanTopoisomerase I inhibitor1....
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com