Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Ketotifen Fumarate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
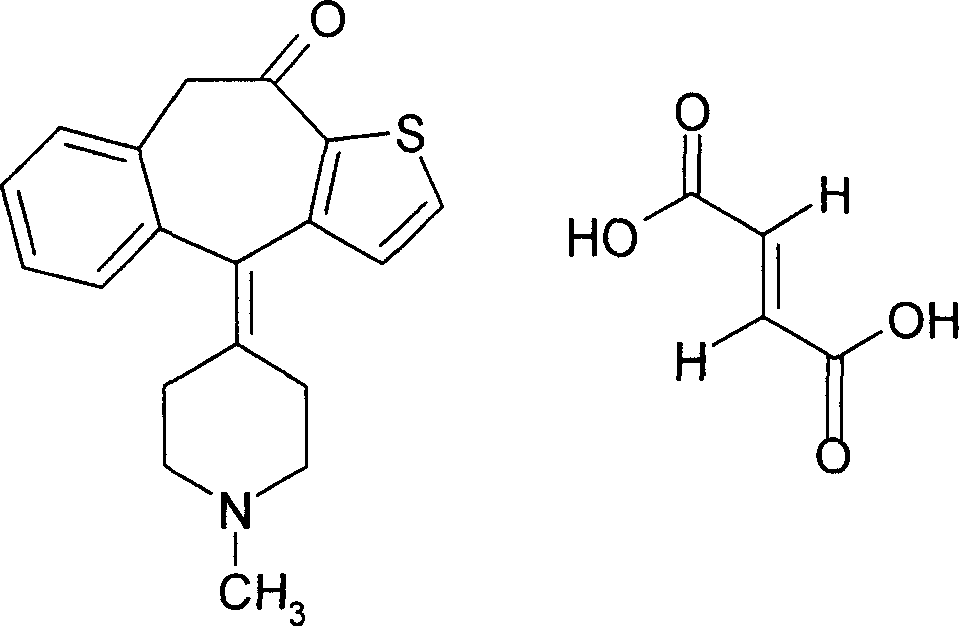
The fumarate salt of ketotifen, a cycloheptathiophene derivative with anti-allergic activity. Ketotifen selectively blocks histamine (H1) receptors and prevents the typical symptoms caused by histamine release. This agent also interferes with the release of inflammatory mediators from mast cells involved in hypersensitivity reactions, thereby decreasing chemotaxis and activation of eosinophils.
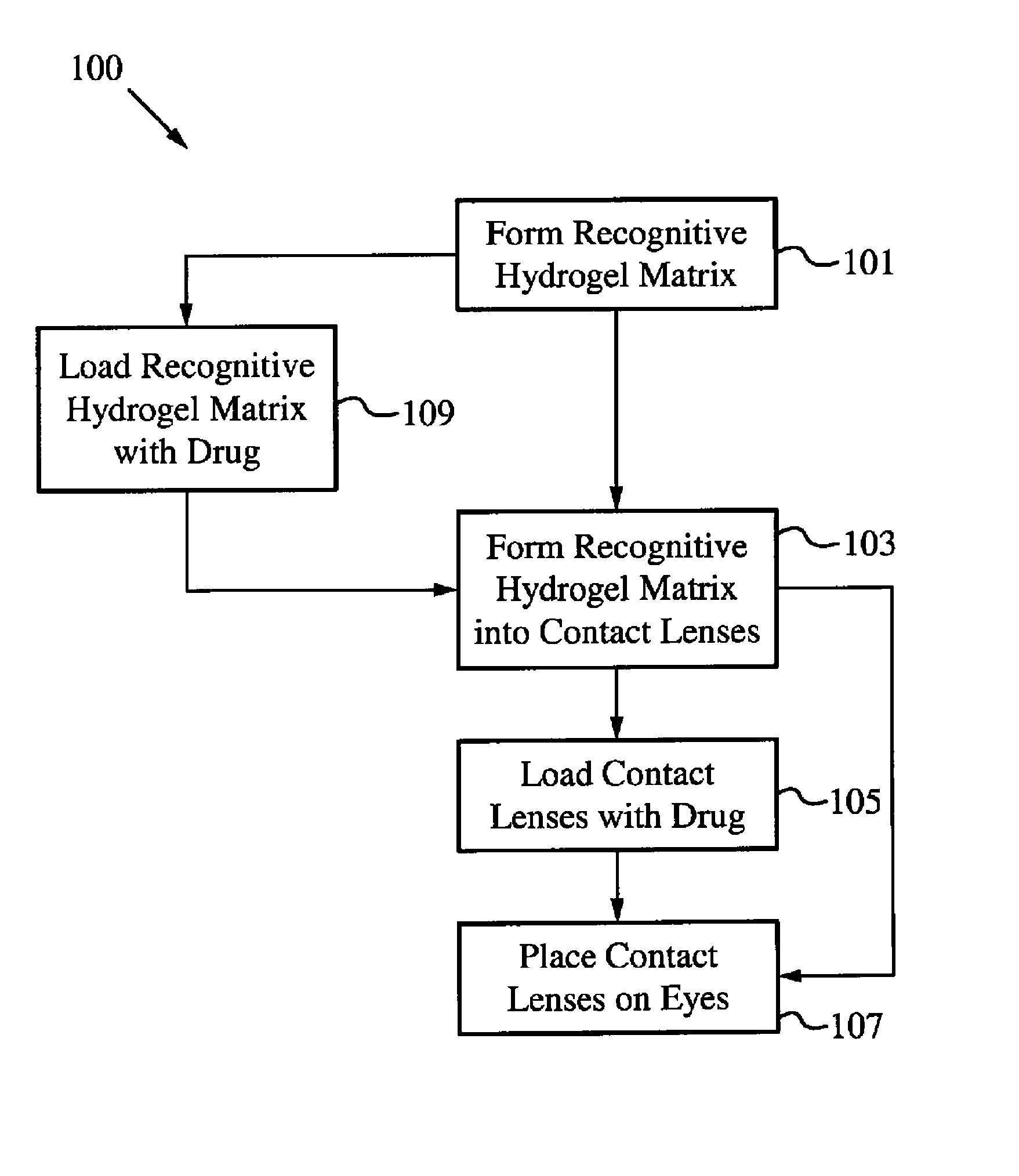
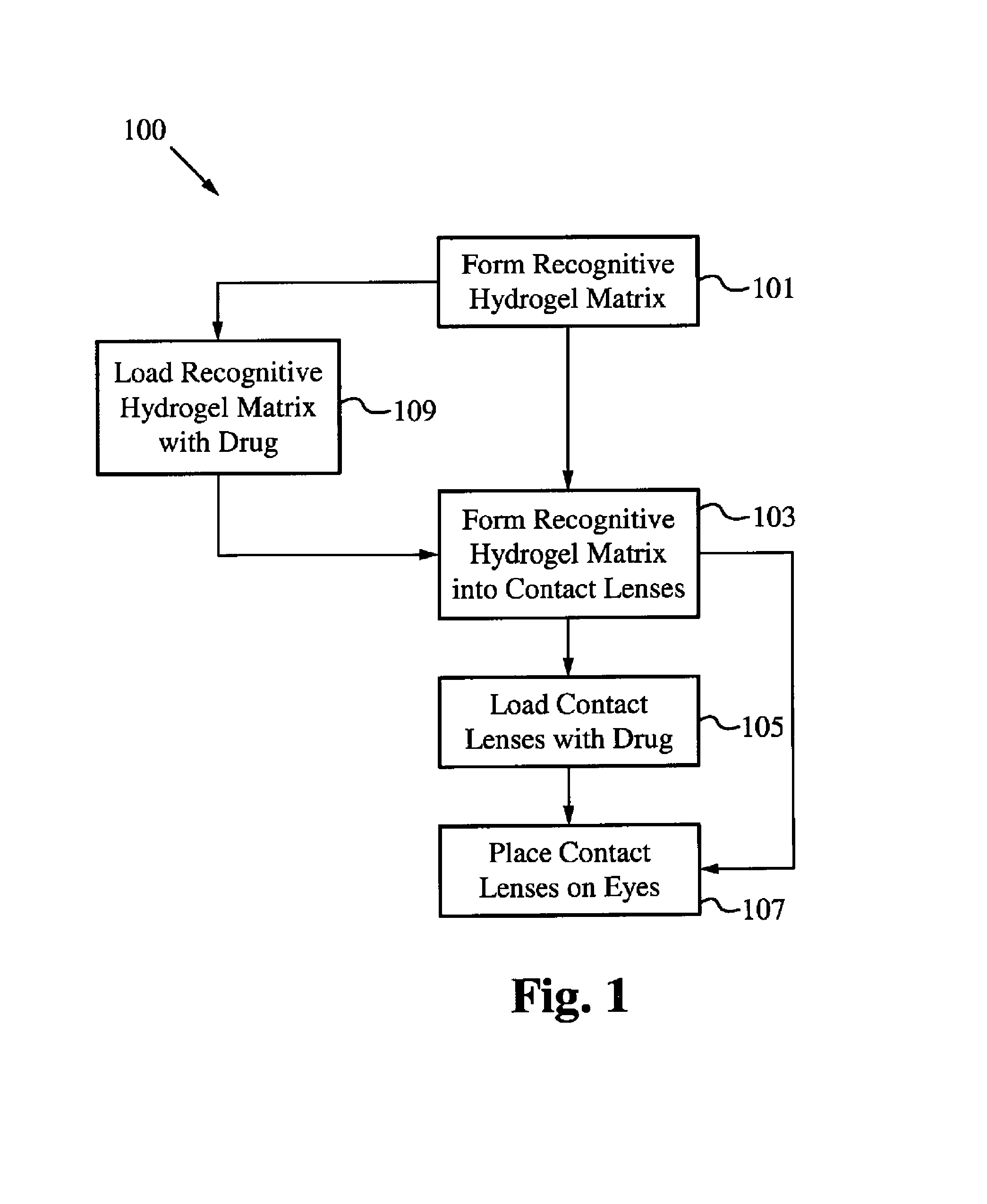
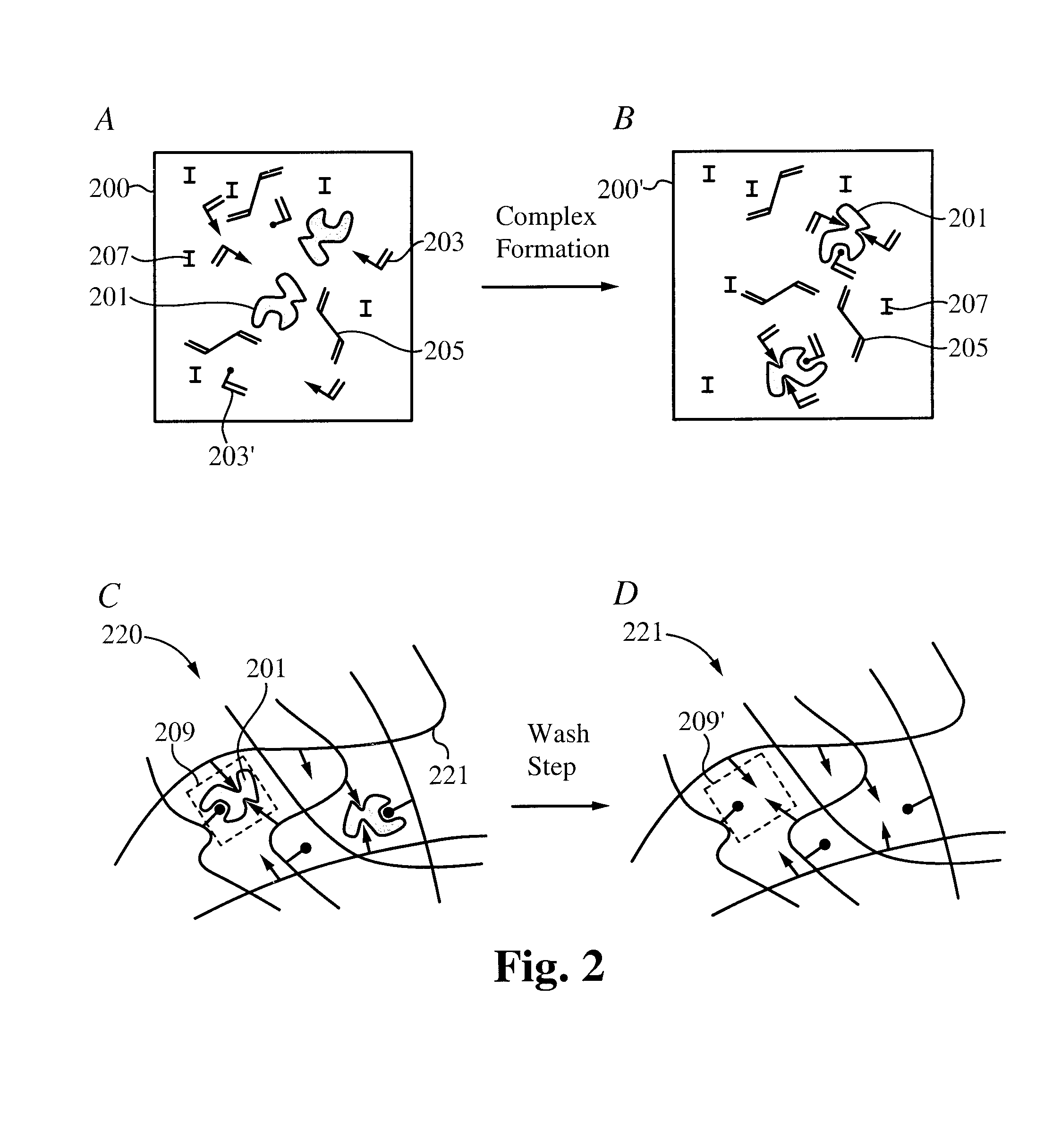
Controlled and extended delivery of hyaluronic acid and comfort molecules via a contact lens platform
ActiveUS8388995B1Avoid effectivenessImprove bioavailabilityPharmaceutical delivery mechanismPharmaceutical non-active ingredientsCross-linkDiclofenac Sodium
A drug delivery system is disclosed. The drug delivery system includes a recognitive polymeric hydrogel through which a drug is delivered by contacting biological tissue. The recognitive polymeric hydrogel is formed using a bio-template, which is a drug or is structurally similar to the drug, functionalized monomers, preferably having complexing sites, and cross-linking monomers, which are copolymerized using a suitable initiator. The complexing sites of the recognitive polymeric hydrogel that is formed mimic receptor sites of a target biological tissue, biological recognition, or biological mechanism of action. A system in accordance with some embodiments is a contact lens for delivering a drug through contact with an eye. In some embodiments, the drug is an anti-microbial, such as an anti-fungal agent for treatment of large animals. In some embodiments, a comfort molecule hyaluronic acid (HA) is delivered. In some embodiments, ketotifen fumarate (anti-histamine) and / or diclofenac sodium (anti-inflammatory) are delivered.
Owner:AUBURN UNIV
Methods and treatment for allergies and inflammation associated with gastrointestinal diseases
InactiveUS20110206659A1Avoid developmentInhibit inflammationBiocideSalicyclic acid active ingredientsAntigenProphylactic treatment
Methods of the prophylaxis of the development of allergy in a patient at risk of sensitization to an antigen(s) or allergen(s) due to impaired gastrointestinal functions include administering a mast cell inhibitor, e.g., ketotifen, e.g., ketotifen fumarate. Methods for prophylactically treating, reducing, delaying or controlling gastrointestinal disorders include administering a mast cell stabilizer, e.g., ketotifen to a patient in need thereof. Pharmaceutical preparation, composition for use in methods described, are also disclosed. Also disclosed are methods of prophylaxis or treating gastrointestinal and esophageal inflammation, and methods for the prophylaxis of the development of additional allergies to a newly introduced substance in a patient with a preexisting allergy. Such methods include delivery of a mast cell stabilizer, e.g., ketotifen. Oral and topical administration are contemplated within the scope of the methods.
Owner:MASTCELL PHARMA
Percutaneous Absorption Formulation
InactiveUS20090220580A1Good effectSuppression problemBiocideSenses disorderPercutaneous absorptionPack material
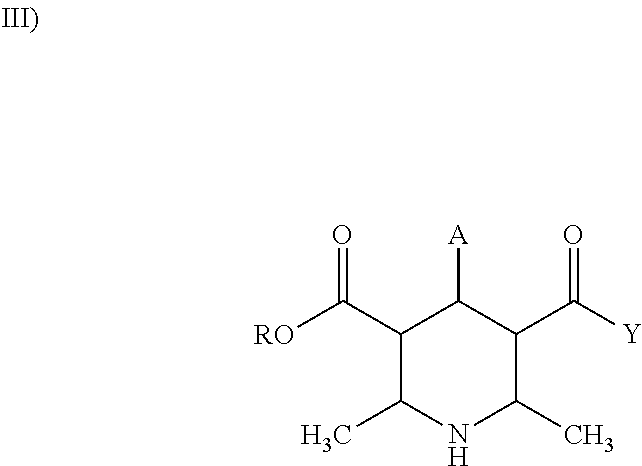
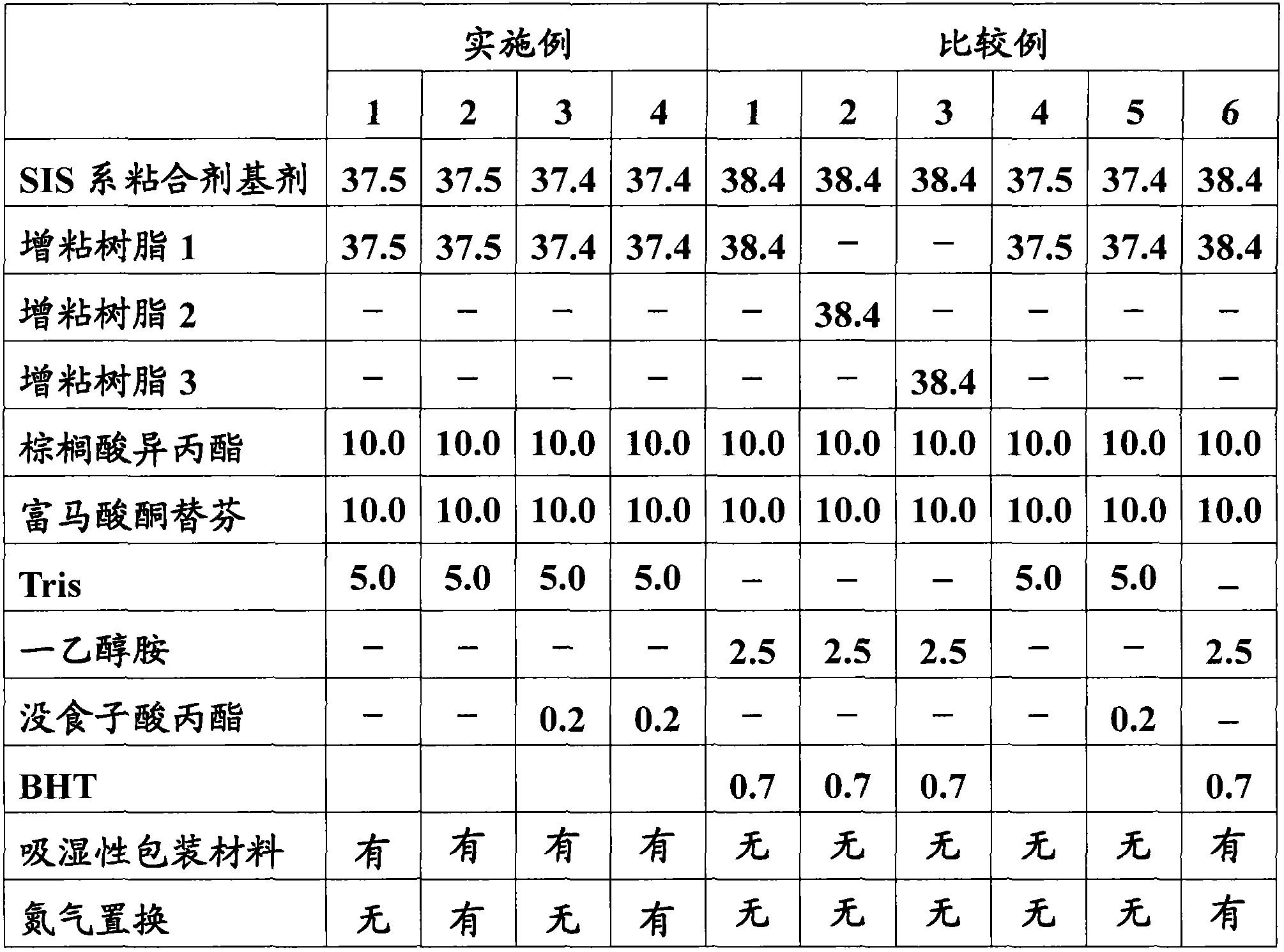
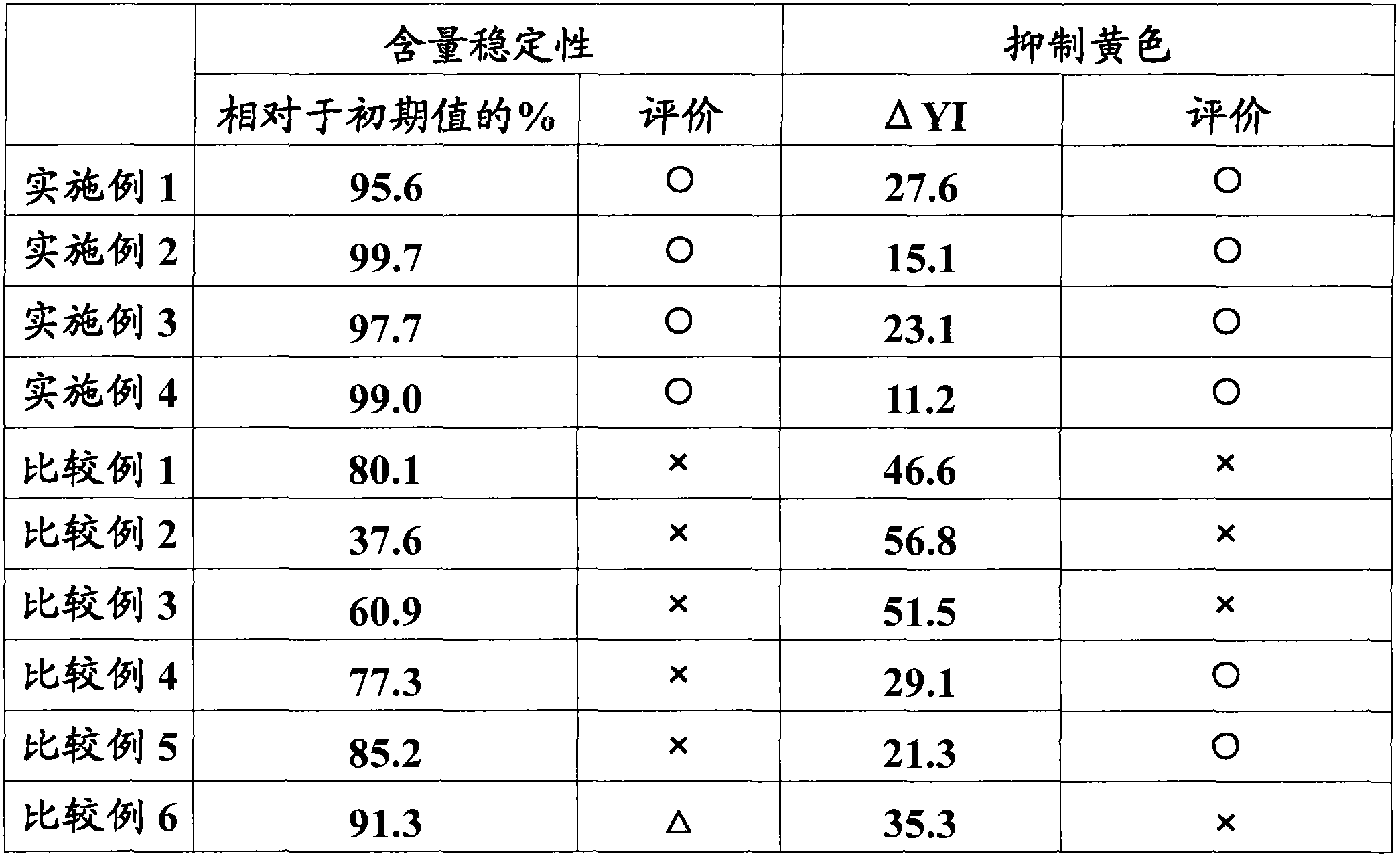
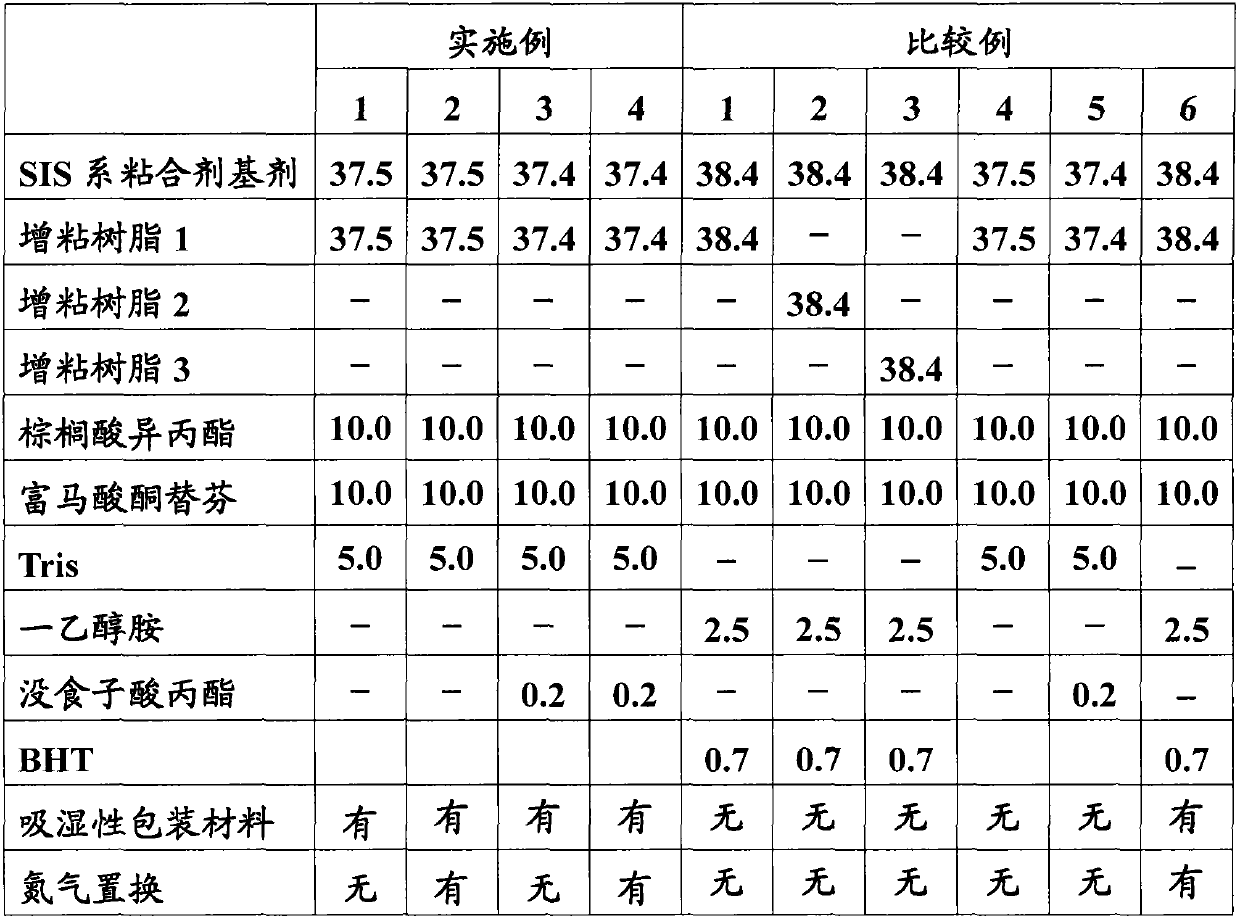
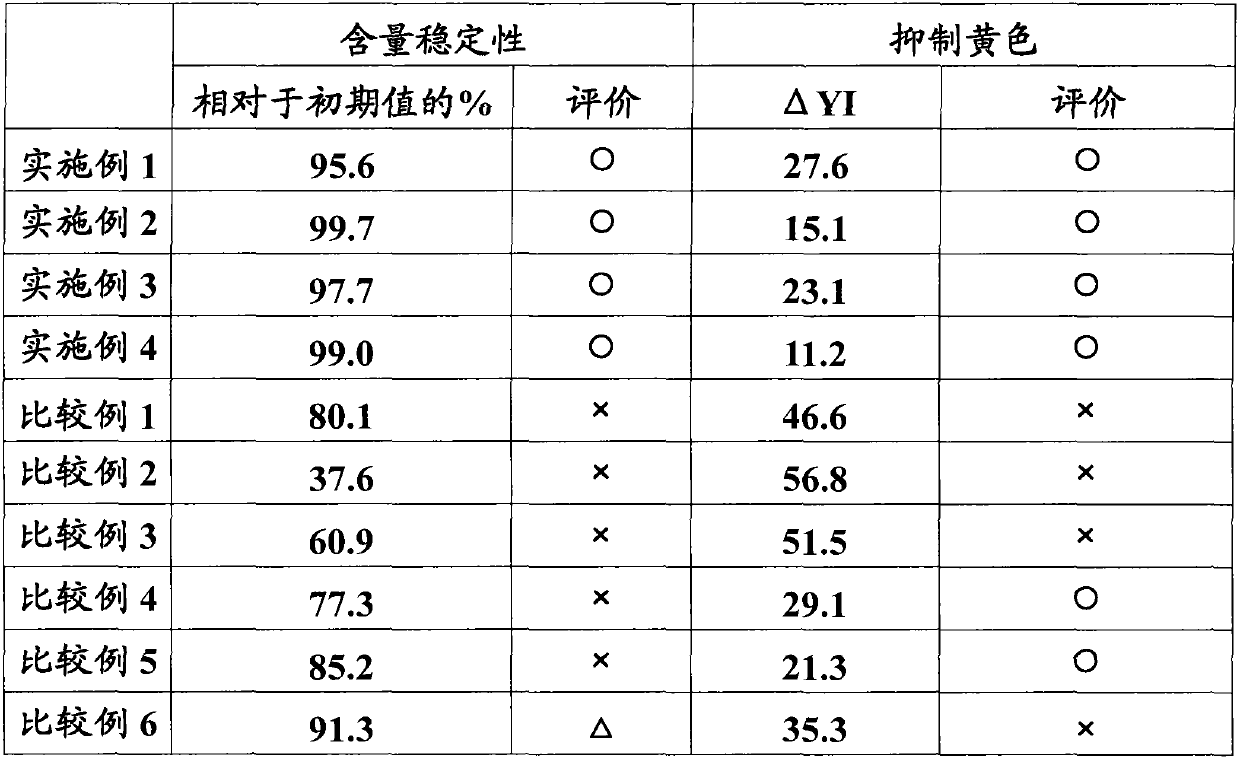
The present invention provides a percutaneous absorption formulation including a patch having an adhesive layer disposed on a substrate and the adhesive layer contains ketotifen fumarate and tris(hydroxymethyl)aminomethane, and the patch is packaged in a hygroscopic packaging material. In the percutaneous absorption formulation, tris(hydroxymethyl)aminomethane particularly selected from various basic substances is incorporated, and by packaging the patch in a hygroscopic packaging material, the percutaneous absorptivity and content stability of a drug can be simultaneously improved and the yellowing of the drug can be suppressed. These effects can be further improved by the incorporation of propyl gallate, the use of an adhesive layer including an SIS-based adhesive base and a rosin ester-based adhesion imparting resin, and / or the removal of oxygen from the atmosphere in the inside of the packaging material.
Owner:SENJU PHARMA CO LTD
Formula of compound medicine preparation for relieving cough and preventing asthma and preparation method thereof
InactiveCN101982174ARespiratory disorderHeterocyclic compound active ingredientsSedative EffectsDisease
The invention relates to a formula of a compound medicine preparation for relieving cough and preventing asthma and a preparation method thereof. The formula mainly comprises the following components in parts by weight: 5-25 parts of methoxyphenamine hydrochloride, 3-15 parts of narcotine, 10-50 parts of aminophylline, 0.1-2 parts of ketotifen fumarate, and an appropriate amount of pharmaceutically acceptable auxiliary materials. In the invention, the methoxyphenamine hydrochloride, the narcotine, the aminophylline and the ketotifen fumarate are prepared into the compound medicine preparation for the first time; and the obtained compound medicine preparation has more obvious treatment effect on diseases such as cough, asthma, bronchial asthma, asthmatic bronchitis and the like; combination of the ketotifen fumarate and the aminophylline in the formula can enhance the bronchiectasis action of the aminophylline; and the mild sedation of the ketotifen fumarate can simultaneously counteract the central nervous system stimulation action caused by the aminophylline.
Owner:岳阳新华达制药有限公司
Medicinal preparation for percutaneous absorption
InactiveCN101166531APromote absorptionImprove stabilityOrganic active ingredientsSenses disorderDrug contentPack material
The invention provides a medicinal preparation for percutaneous absorption characterized by comprising a hygroscopic packaging material and, packed therein, a pressure-sensitive adhesive patch which comprises a substrate and formed thereon a pressure-sensitive adhesive layer and in which the pressure-sensitive adhesive layer contains ketotifen fumarate and tris(hydroxymethyl)aminomethane. The medicinal preparation for percutaneous absorption contains tris(hydroxymethyl)aminomethane, which was especially selected among many basic substances, and this substance has been packed in a hygroscopic packaging material. Due to this, percutaneous drug absorbability and drug content stability can be simultaneously improved and yellowing can be inhibited. These effects can be further improved by adding propyl gallate, by using a pressure-sensitive adhesive layer comprising an SIS type pressure-sensitive adhesive base and a rosin ester tackifier resin, and / or by removing oxygen from the atmosphere in the packing material.
Owner:SENJU PHARMA CO LTD
Ketotifen fumarate tablets and preparation method thereof
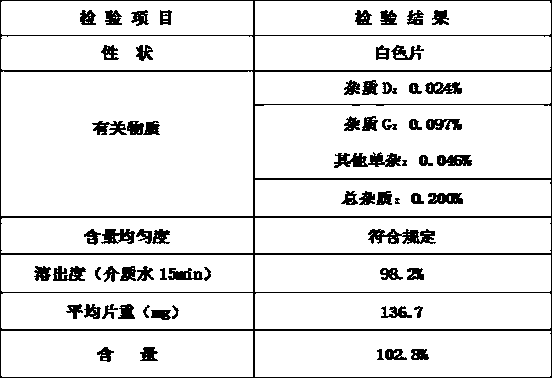
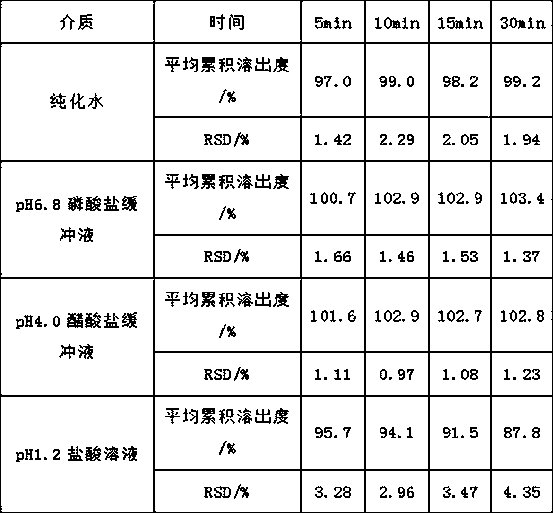
The invention provides ketotifen fumarate tablets. The ketotifen fumarate tablets are prepared by adopting superfine grinding technology and tablet process technology and are high in dissolution rate, lasting in action, few in administration times, stable in blood-drug concentration and higher in safety, and treatment effect of the ketotifen fumarate tablets is brought into full play.
Owner:CHANGZHOU PHARMA FACTORY
Transdermal therapeutic preparation
InactiveCN102078619APromote absorptionImprove stabilityOrganic active ingredientsSenses disorderMoistureHydroxymethyl
The invention provides an application of tri(hydroxymethyl) methyl amine and a moisture absorbing packing material. In a patch of ketotifen fumarate provided with an adhering agent layer on a supporting body, the tri(hydroxymethyl) methyl amine and the moisture absorbing packing material are used for improving the content stability of the ketotifen fumarate and inhibiting the xanthochromia.
Owner:SENJU PHARMA CO LTD
Methods and treatment for allergies and inflammation associated with gastrointestinal diseases
ActiveUS20140302153A1Increase topical resident timeLonger local effectBiocideAntipyreticAntigenProphylactic treatment
Methods of the prophylaxis of the development of allergy in a patient at risk of sensitization to an antigen(s) or allergen(s) due to impaired gastrointestinal functions include administering a mast cell inhibitor, e.g., ketotifen, e.g., ketotifen fumarate. Methods for prophylactically treating, reducing, delaying or controlling gastrointestinal disorders include administering a mast cell stabilizer, e.g., ketotifen to a patient in need thereof. Pharmaceutical preparation, composition for use in methods described, are also disclosed. Also disclosed are methods of prophylaxis or treating gastrointestinal and esophageal inflammation, and methods for the prophylaxis of the development of additional allergies to a newly introduced substance in a patient with a preexisting allergy. Such methods include delivery of a mast cell stabilizer, e.g., ketotifen. Oral and topical administration are contemplated within the scope of the methods.
Owner:MASTCELL PHARMA
Dripping pills of ketotifen fumarate and its preparation method
InactiveCN1602868ADisintegration and dissolution fastHigh dissolution rateOrganic active ingredientsPill deliveryTraditional medicineDysphagia
The invention ketotifen fumarate pill, prepared by superfine crushing and pill producing techniques, can achieve the purposes of increasing disintegration and dispersion speeds, and dissolving-out speed and degree, quickly taking effect, improving drug stability, reducing auxiliary use, reducing production cost, being convenient to carry and take. Its compliance is good and it is especially suitable for children, the aged, patients in bed and patients difficult in swallowing to take.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Ketotifen fumarate tablet and preparation method thereof
InactiveCN108567756AReduce heat source riskOrganic active ingredientsPharmaceutical non-active ingredientsCelluloseMagnesium stearate
The invention discloses a ketotifen fumarate tablet, which is prepared from the following components in parts by mass: 50 to 80 parts of ketotifen fumarate, 5 to 10 parts of hydroxy propyl cellulose,1 to 5 parts of magnesium stearate and 1 to 2 parts of starch. Meanwhile, the invention further discloses a preparation method of the ketotifen fumarate tablet. By pretreating the ketotifen fumarate in a low temperature environment, the heat source risk in the ketotifen fumarate raw material is lowered, in a subsequent preparation process, low temperature preparation is continued, and the possibility of existence of the heat source is further avoided.
Owner:JIANGSU PENGYAO PHARMA
Ketotifen fumarate sterile nasal spray and preparation method thereof
InactiveCN107753426ASignificant effectAvoid harmOrganic active ingredientsAerosol deliveryPreservative freeEthylene diamine
The invention belongs to the field of pharmaceutical preparations and in particular relates to ketotifen fumarate sterile nasal spray and a preparation method thereof. The ketotifen fumarate sterile nasal spray is prepared from the following main components: ketotifen fumarate, EDTA (Ethylene Diamine Tetraacetic Acid), glycerol, sodium hydroxide and water for injection; the obtained pharmaceuticalpreparation does not contain a preservative and the toxin, caused by the preservative, on nose cilium can be reduced; damages, caused by the fact that a medicine containing the preservative is used for long time, to bodies are reduced; meanwhile, the form of the pharmaceutical preparation of the nasal spray can be accurately metered; the ketotifen fumarate sterile nasal spray is relatively convenient to use and a preparation technology is simple.
Owner:YANGTAI PHARMA SHANDONG
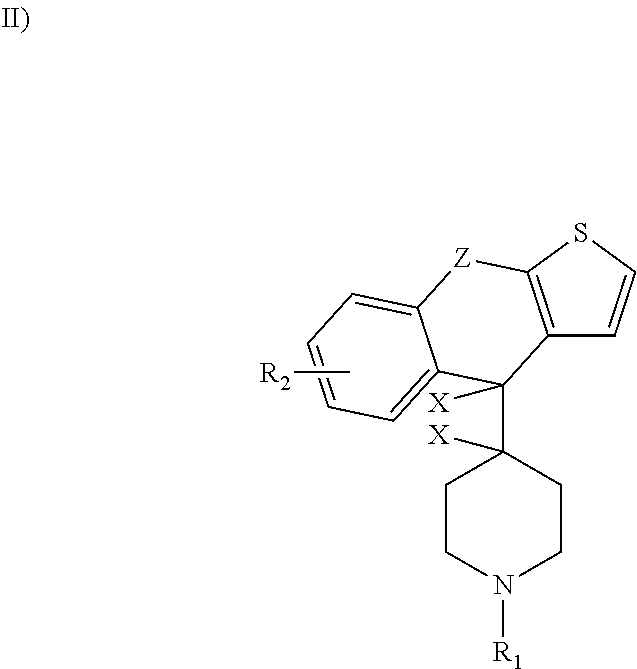
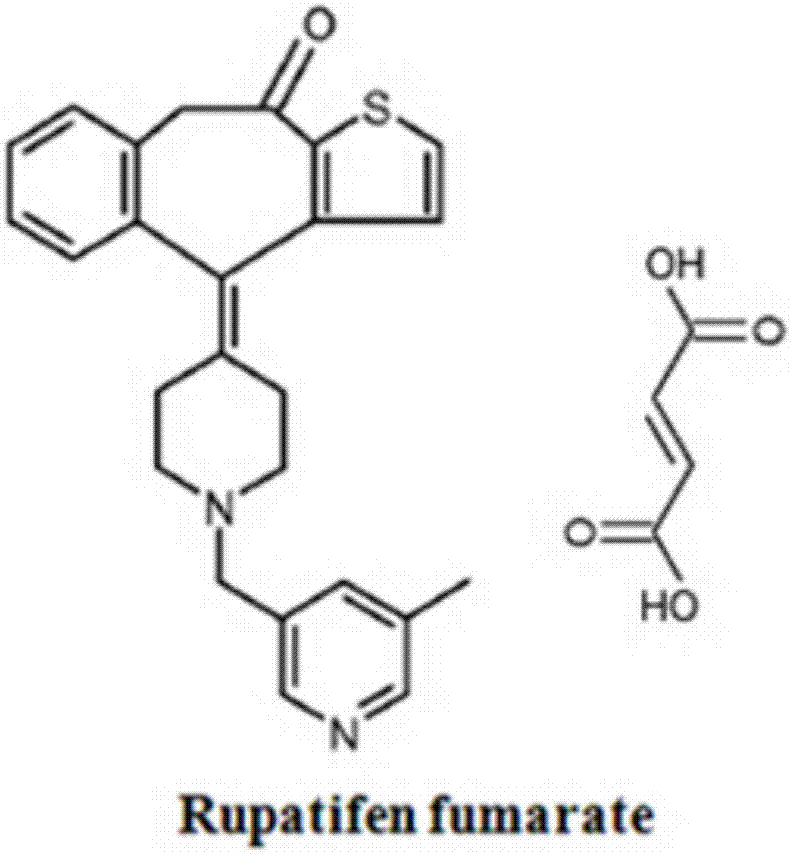
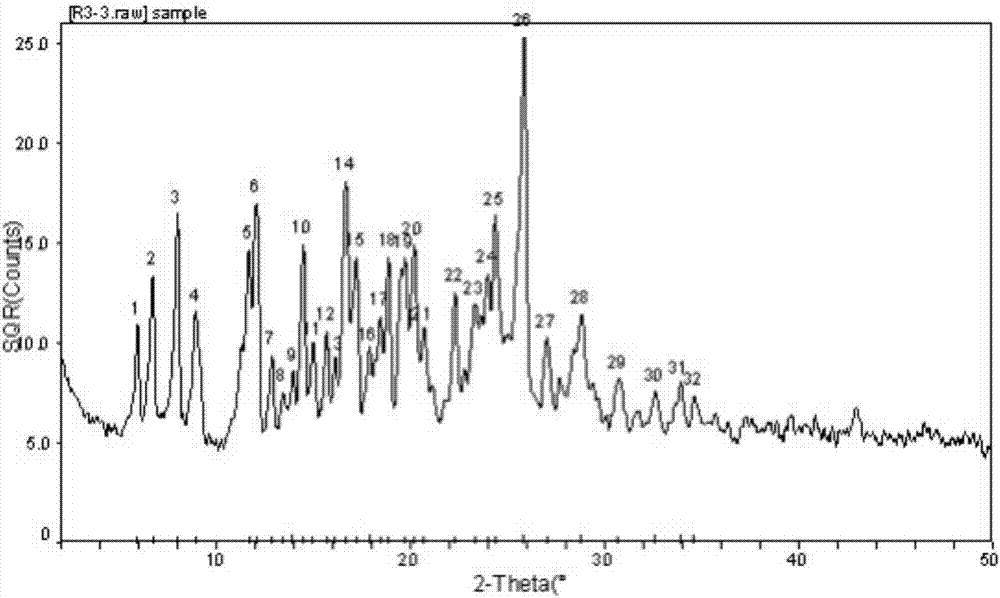
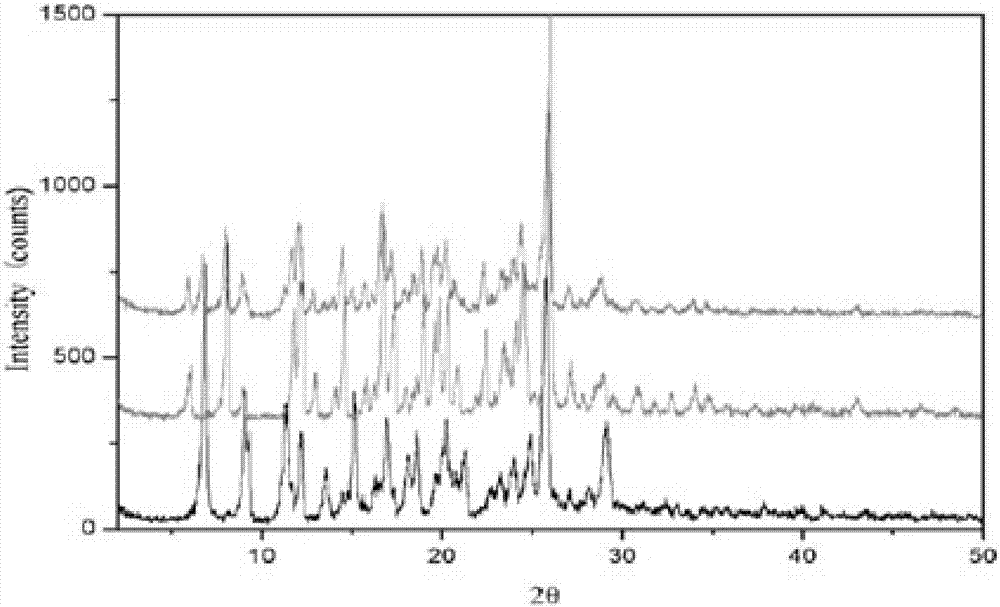
A kind of rupatifene fumarate mixed crystal and preparation method thereof
The invention relates to a method for preparing a mixed crystal form of A and B of rupatifen fumarate. The invention relates to a method for preparing a mixed crystal form of 4-[1-(5-methyl-pyridin-3-ylmethyl)-piperidin-4-ylidene]-4,9-dihydro-1-thio-one fumarate shown in the formula (1). The mixed crystal form comprises special characteristic peaks of a crystal form A and a crystal form B; the melting point is 154 + / - 2 DEG C, and the decomposition point is 186 + / - 2 DEG C. The method for preparing the crystal form comprises the following steps of dissolving the free base of rupatifen and fumaric acid in ethanol solution, stirring and reacting for 1 hour at room temperature, concentrating under a reduced pressure to 25-30vol%, or dissolving the rupatifen fumarate crude product in an appropriate amount of ethanol, refluxing in a heating and stirring manner to obtain a saturated solution, naturally cooling the solution prepared in the above method to room temperature, and then standing at 2-8 DEG C to separate out rod-like crystals, filtering, washing with an appropriate amount of ethanol solution of which the temperature is 2-8 DEG C and drying under reduced pressure at 45 DEG C.
Owner:FUJIAN MINDONG REJUVENATION PHARMA
Medicament for treating bronchitis
InactiveCN104840719ACompatibility is reasonableEasy to take medicineTetracycline active ingredientsRespiratory disorderChlorobenzeneEthylic acid
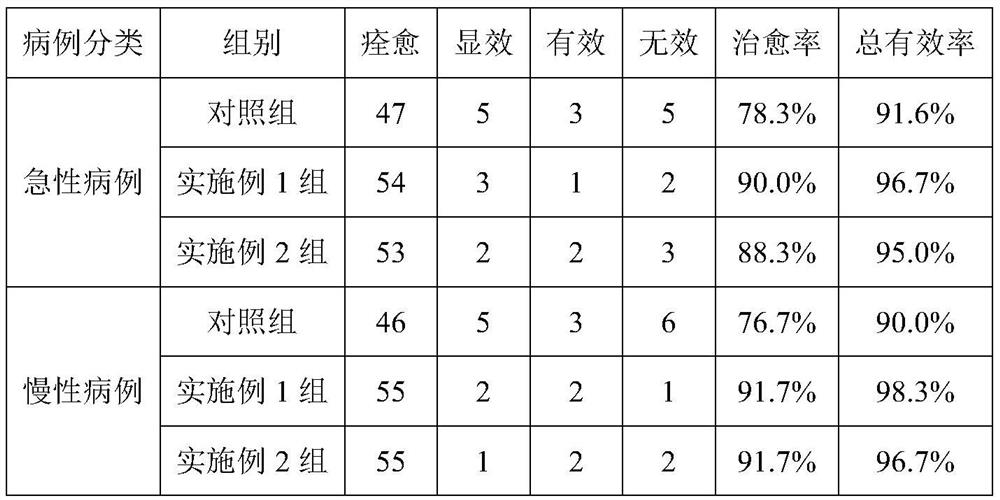
The invention relates to a medicament for treating bronchitis. The medicament comprises the following raw materials in parts by weight: 8 to 12 parts of liquoric root extract powder, 0.1 to 0.3 parts of chlorpheniramine maleate, 0.068 to 0.1 parts of ketotifen fumarate, 0.18 to 0.32 parts of prednisone acetate, 2.5 to 4.1 parts of aminophylline, 6.5 to 8.6 parts of oxytetracycline, 0.2 to 0.4 parts of dioxopromethazine hydrochloride and 25 to 35 parts of unibract fritillary bulb. The medicament for treating bronchitis has the advantages of reasonable compatibility, convenience in taking, prevention of relapse, short treatment period, quick response, achievement of remarkable effect on the administration day, curative rate of up to 89.5 percent, total effective rate of up to 99 percent, simple preparation method, low cost and particularly remarkable effects on emphysema and lasting chronic bronchitis.
Owner:孟智琴
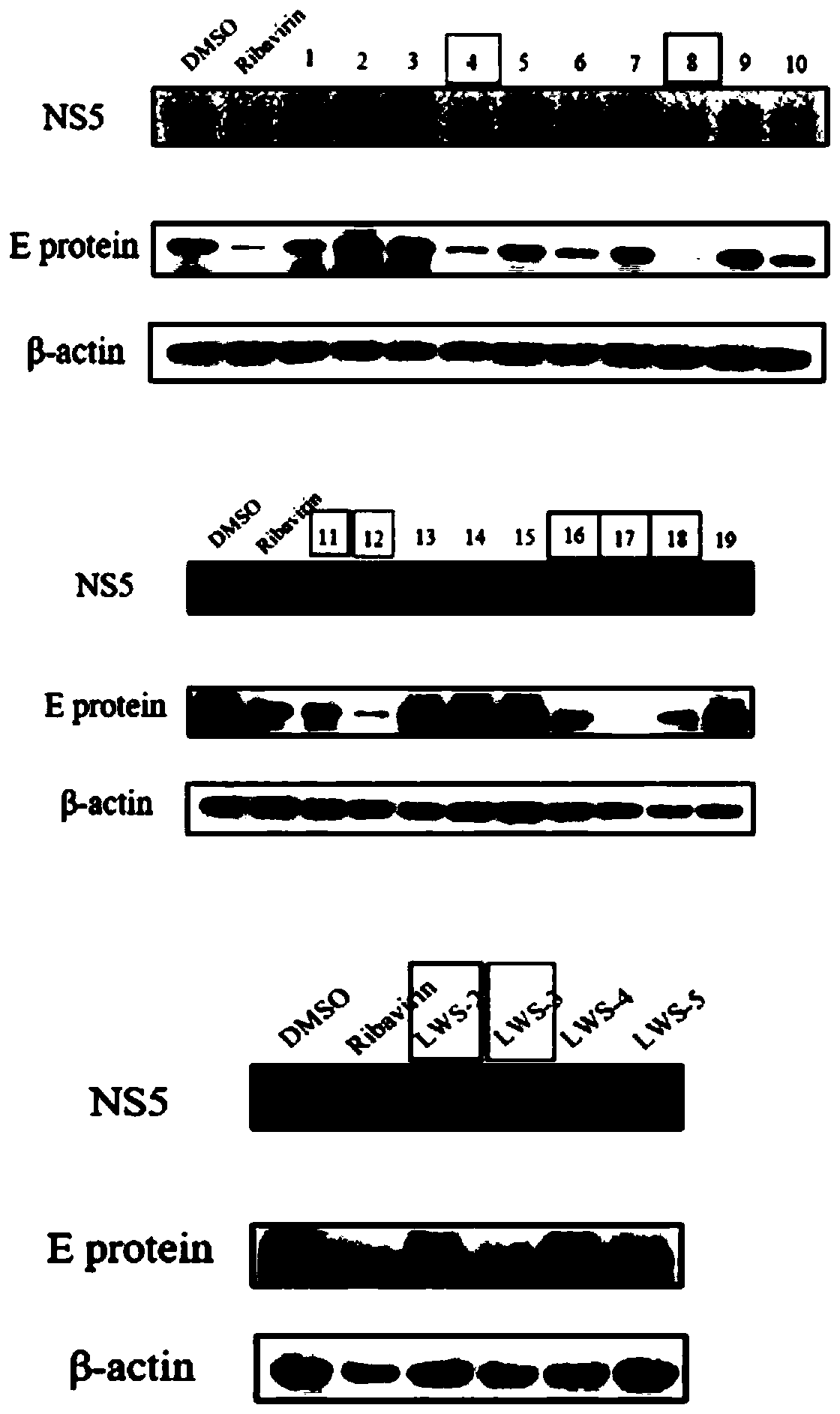
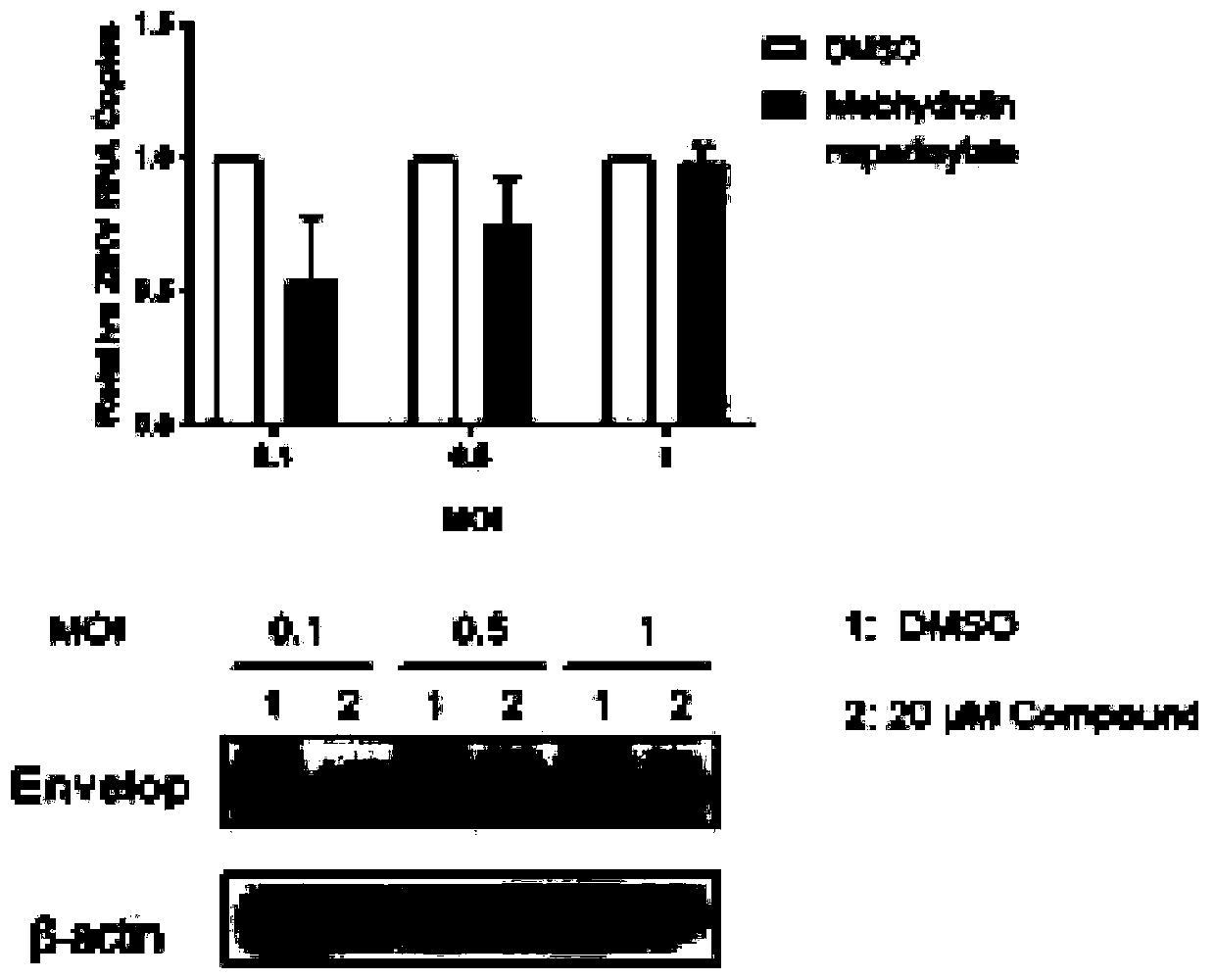
Application of histamine receptor inhibitor and derivatives thereof in preparation of anti-Zika virus drugs
ActiveCN110694065AClear anti-Zika virus effectEnhanced inhibitory effectOrganic active ingredientsAntiviralsVirus ProteinBrompheniramine Maleate
The invention provides a histamine receptor inhibitor and derivatives thereof in the preparation of anti-Zika virus drugs. The histamine receptor inhibitor is preferably one or more of mebhydrolin napadisylate, ketotifen fumarate, flunarizine hydrochloride, cyproheptadinehydrochloride, JNJ-7777120, desloratadine, brompheniramine maleate, brompheniramine maleate, chlorpheniramine maleate and cetirizine hydrochloride. The invention brings forward for the first time that the histamine receptor inhibitor and its derivatives have clear anti-Zika virus effect and can significantly inhibit the expression level of Zika virus protein and Zika virus RNA. The invention also screens out histamine receptor inhibitors with better inhibitory effect on Zika virus, including mebhydrolin napadisylate, cyproheptadinehydrochloride, JNJ-7777120, desloratadine, etc., whose inhibition effect on Zika virus at a concentration of 10 uM is equivalent to inhibition effect of ribavirin at a concentration of 40 uM.The histamine receptor inhibitor and the derivatives can be used as good candidate drugs for anti-Zika virus drugs.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
Boletic acid ketotifen eye drops containing sodium hyaluronate and its preparing process
InactiveCN1923203APrevent spillageMoisturizes the corneaOrganic active ingredientsSenses disorderMedicineKetotifen fumarate ophthalmic solution
The invention relates to a gutta which comprises boletic acid at 0.25-1200 deals, hyaluronic acid soda at 1-1200 deals, osmotic regulation adjuster at 100-3000 deals, and acceptable carrier. Wherein, the pH value of said invention is 4.0-7.5. The invention can avoid overflow to keep drug at needed part. And it can humidify cornea.
Owner:SHANGHAI SINE PHARMA LAB
Medicine composite for treating B type diabetes
InactiveCN101698103AAvoid allergiesImprove blood sugar controlOrganic active ingredientsPeptide/protein ingredientsMicrosphereAllergy
The invention discloses a medicine composite for treating B type diabetes, which comprises a trypsin class medicine and an antiallergic medicine, wherein the weight ratio of the trypsin class medicine to the antiallergic medicine is 1:(1.5-1700). When being taken, the medicine composite can be prepared into various medicament forms, such as an oral microsphere preparation or injection and the like. The trypsin class medicine is any one of recombinant human trypsin, pig trypsin and dulcin trypsin. The antiallergic medicine is sodium cromoglyate or / and ketotifen fumarate. Animal experiments prove that the trypsin class medicine and the antiallergic medicine are combined according to a certain proportion, which not only can prevent the generation of trypsin allergy but also can enhance the blood sugar control action of trypsin and lighten the obesity degree. The action mechanism of the medicine composite is to regulate the excessive mast cells of the fat tissues of patients with obesity and diabetes by two antiallergic medicines of the sodium cromoglyate and the ketotifen fumarate, thereby exerting a synergistic action with the trypsin and preventing and relieving the anaphylactic reaction of the trypsin.
Owner:SHANDONG UNIV
Ketotifen fumarate tablets and preparation technology thereof
The invention discloses ketotifen fumarate tablets. The ketotifen fumarate tablets comprise components in parts by mass as follows: 30-50 parts of ketotifen fumarate, 5-12 parts of hydroxypropyl cellulose, 1-3 parts of magnesium stearate and 1-2 parts of starch. Meanwhile, the invention further discloses a preparation method of the ketotifen fumarate tablets. According to the ketotifen fumarate tablets and the preparation method thereof, the ketotifen fumarate is pretreated in a low-temperature environment, heat source risks of ketotifen fumarate raw materials are reduced, low-temperature preparation is adopted continuously in a later preparation process, and therefore, the probability of existence of a heat source is further eliminated.
Owner:仁和堂药业有限公司
Method for preparing salbutamol compound inhalation aerosol
InactiveCN105078982AQuick effectSmall toxicityOrganic active ingredientsAerosol deliverySolventLevosalbutamol
The invention discloses compound inhalation aerosol and a method for preparing the same. The compound inhalation aerosol comprises sulfuric acid levalbuterol which is an active constituent, ketotifen fumarate which is another active constituent, surfactants, diluents / complex solubilizers and propellants. The method includes micronizing crude medicines, and to be more specific, grinding the sulfuric acid levalbuterol and the ketotifen fumarate by the aid of an airflow grinder; weighing formulated amounts of the sulfuric acid levalbuterol, the ketotifen fumarate, the surfactants, anhydrous ethanol and anhydrous sodium sulfate; dissolving the surfactants; adding the surfactants into the anhydrous ethanol, slowly adding the formulated amounts of the sulfuric acid levalbuterol and the ketotifen fumarate into the anhydrous ethanol, and continuously stirring the anhydrous ethanol until clear transparent liquor is formed; drying the liquor, to be more specific, adding a formulated amount of drying agents into the liquor, stirring and drying the liquor for 30min, and filtering the drying agents by the aid of titanium rods; filling tying valves with the liquor, filling the valves with tetrafluoroethane of the propellants, detecting leakage and carrying out weighing; packaging the compound inhalation aerosol, and to be more specific, mounting a mobility aid (case).
Owner:SICHUAN XUSHENG PHARMA CO LTD
Transdermal therapeutic preparation
InactiveCN102078619BPromote absorptionImprove stabilityOrganic active ingredientsSenses disorderMoistureHydroxymethyl
The invention provides an application of tri(hydroxymethyl) methyl amine and a moisture absorbing packing material. In a patch of ketotifen fumarate provided with an adhering agent layer on a supporting body, the tri(hydroxymethyl) methyl amine and the moisture absorbing packing material are used for improving the content stability of the ketotifen fumarate and inhibiting the xanthochromia.
Owner:SENJU PHARMA CO LTD
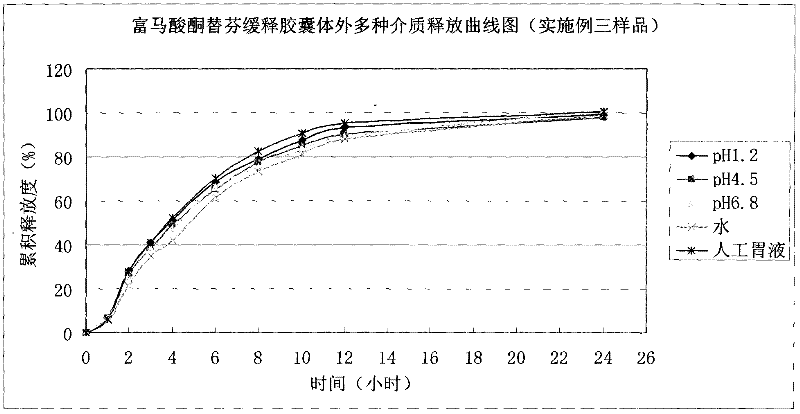
Ketotifen fumarate sustained-release capsules and preparation method thereof
InactiveCN102525999AUniform particle sizeStable drug releaseOrganic active ingredientsDigestive systemSustained release pelletsSustained Release Tablet
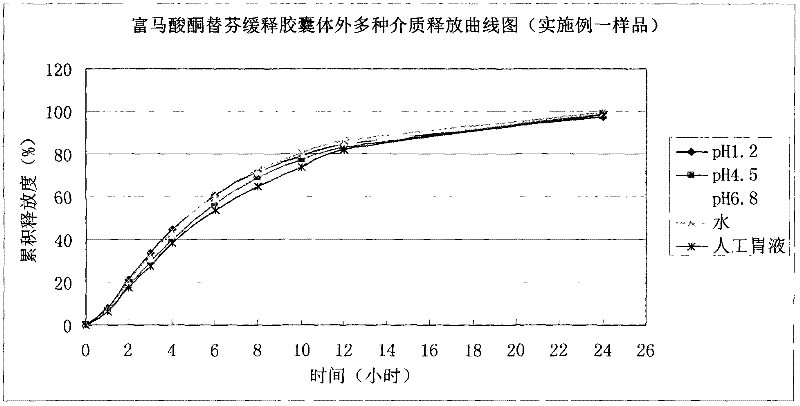
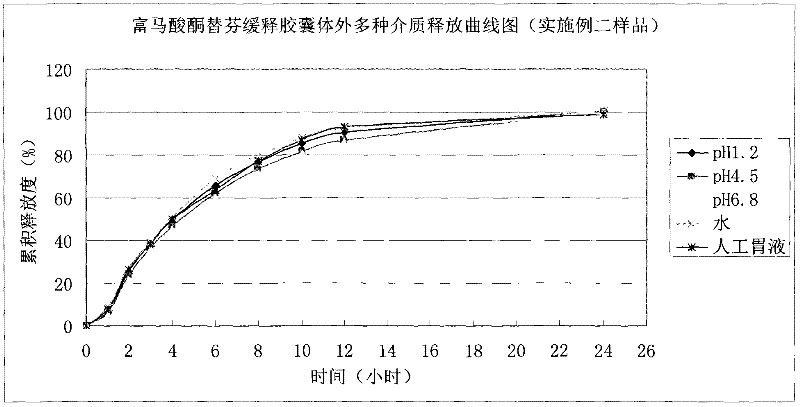
The invention discloses ketotifen fumarate sustained-release capsules and a preparation method thereof. The ketotifen fumarate sustained- release capsules comprise sustained-release pellets and hollow capsules, and each sustained-release pellet comprises 75-77% (by weight) of ketotifen fumarate-containing pellet core and 3-35% (by weight) of sustained-release layer. The ketotifen fumarate sustained-release capsules of the invention have uniform pellet diameter and stable drug release, can be released in a sustained manner within 24 hours, and are administered once every day. As the sustained-release pellets in the capsules comprise a plurality of pellets of uniform size, the errors or defects in preparation of individual pellets do not have a strong impact on drug release of the entire preparation, the ketotifen fumarate sustained-release capsules are safer than sustained-release tablets, and have smaller irritation to gastrointestinal tract, more stable blood concentration and higher bioavailability. The preparation method of the invention adopts an extrusion rolling method and a drug coating method to prepare the drug-containing pellet cores, and adopts a fluidized bed to coat the sustained-release coat layers; and has simple process, and is easy for industrial production.
Owner:广州科的信医药技术有限公司
Method for detecting ketotifen fumarate nasal drop content with UPLC (ultra-performance liquid chromatography)
InactiveCN105675767AAvoid interferenceThe test result is accurateComponent separationMedicineFiltration
The invention provides a method for detecting ketotifen fumarate nasal drop content with UPLC (ultra-performance liquid chromatography), and aims at providing a method for detecting ketotifen fumarate nasal drop content with UPLC, which is capable of preventing interferences from other impurities, efficient and time-saving, and objective in identification result. The method sequentially comprises the following steps: precisely taking 1ml of a ketotifen fumarate nasal drop sample solution, and placing the solution in a 1000ml measuring flask; adding methanol to dilute the solution to a scale; performing filtration by adopting a filtering membrane, and injecting 2 microliters of filtrate into an ultra-performance liquid chromatograph for detection. The method belongs to the technical field of chemical detection.
Owner:WUZHOU INST FOR FOOD & DRUG CONTROL
A kind of ketotifen fumarate tablet and preparation method thereof
The invention provides ketotifen fumarate tablets. The ketotifen fumarate tablets are prepared by adopting superfine grinding technology and tablet process technology and are high in dissolution rate, lasting in action, few in administration times, stable in blood-drug concentration and higher in safety, and treatment effect of the ketotifen fumarate tablets is brought into full play.
Owner:CHANGZHOU PHARMA FACTORY
Ketotifen fumarate tablet and application thereof
InactiveCN110664767AImprove various indicatorsDissolution stabilityOrganic active ingredientsPill deliveryCelluloseStarch corn
The invention belongs to the technical field of pharmaceutical preparations, and discloses a ketotifen fumarate tablet. The ketotifen fumarate tablet is prepared from the following formulation compositions: ketotifen fumarate, lactose monohydrate, pregelatinized starch, corn starch, hydroxy propyl cellulose, water and magnesium stearate. The ketotifen fumarate tablet has stable performance, controllable quality and high dissolution rate.
Owner:仁和堂药业有限公司
Medicine for treating bronchitis and preparation method thereof
InactiveCN113288969AReduce the burden onLow costAnthropod material medical ingredientsAntipyreticMedicinal herbsFritillaria cirrhosa
The invention provides a medicine for treating bronchitis and a preparation method thereof. The medicine is prepared from the following raw medicines in parts by weight: 8 to 12 parts of liquorice root extract powder, 0.1 to 0.3 part of chlorpheniramine maleate, 0.068 to 0.1 part of ketotifen fumarate, 0.18 to 0.32 part of prednisone acetate, 2.5 to 4.1 parts of aminophylline, 6.5 to 8.6 parts of oxytetracycline, 0.2 to 0.4 part of dioxopromethazine hydrochloride, 25 to 35 parts of bulbus fritillariae cirrhosae, 24 to 31 parts of rhizoma anemarrhenae, 9 to 19 parts of rhizoma pinelliae, 19 to 26 parts of Chinese gall leaven and 14 to 26 parts of radix asteris. The preparation method comprises the following steps: decocting and concentrating the traditional Chinese medicines in corresponding parts by weight, adding the western medicine powder, and preparing into tablets, granules, pills or capsules and other dosage forms according to a conventional pharmaceutical processing method, thereby obtaining the medicine for treating bronchitis. The traditional Chinese medicines and the western medicines are creatively matched for use, so that the effects of freeing lung, relieving exterior syndrome, relieving cough, reducing sputum and relieving asthma are achieved. The adopted medicinal materials are easy to obtain, low in cost and free of toxic and side effects, and the burden of patients is relieved.
Owner:河南省恺展养生保健服务有限公司
Ketotifen fumarate tablets and preparation method thereof
InactiveCN110917155AReduce heat source riskOrganic active ingredientsPharmaceutical non-active ingredientsCelluloseMedicine
The invention relates to ketotifen fumarate tablets and a preparation method thereof. The ketotifen fumarate tablets disclosed by the invention comprise the following components in parts by mass of 40-90 parts of ketotifen fumarate, 7-18 parts of hydroxypropyl cellulose, 1-5 parts of magnesium stearate and 1-2 parts of starch. Besides, the invention further discloses a preparation method of the ketotifen fumarate tablets. The ketotifen fumarate is preprocessed in low-temperature environment, so that the risk of heat sources in ketotifen fumarate raw materials can be reduced; and in the later preparation process, low-temperature preparation is continuously performed, so that the possibility that the heat sources exist is further stopped.
Owner:仁和堂药业有限公司
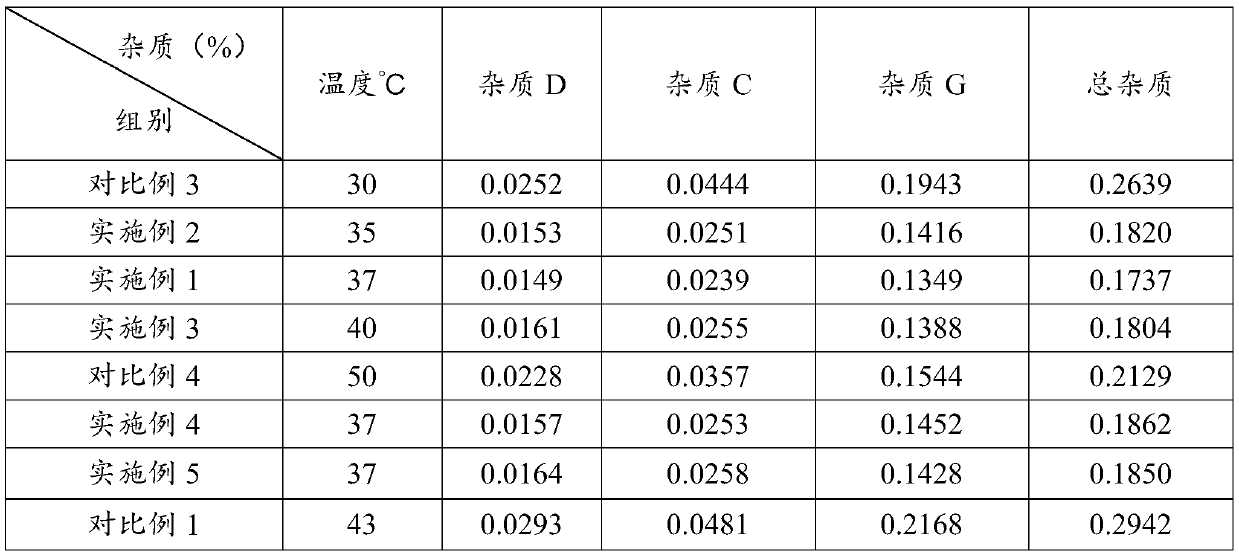
Method for effectively reducing impurity content in oral solution for treating bronchial asthma
PendingCN114537898ALow impurity contentControl contentPackaging under vacuum/special atmosphereBronchial epitheliumOral solutions
Owner:辽宁大熊制药有限公司
A kind of compound tea ketone sustained-release tablet and preparation method thereof
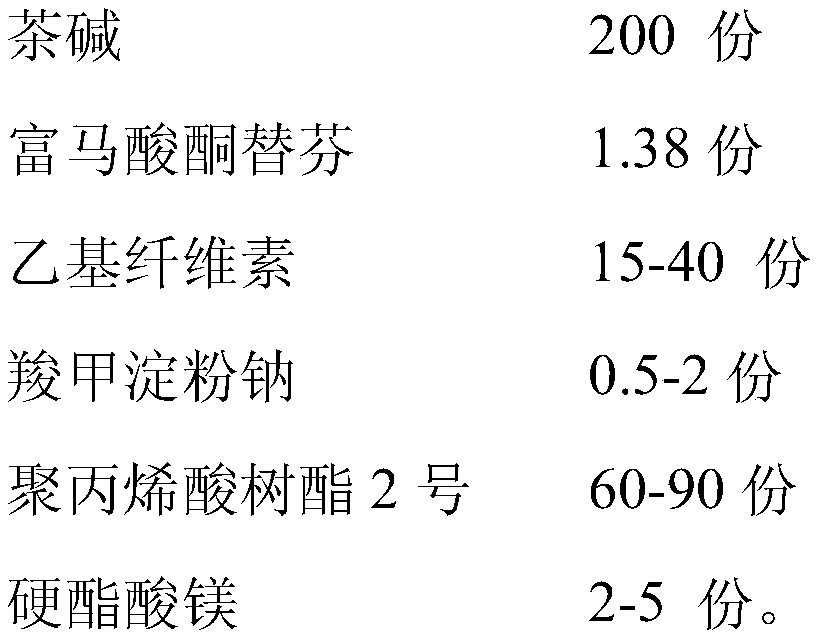
ActiveCN106913572BImprove stabilityGood compatibilityPharmaceutical non-active ingredientsRespiratory disorderSide effectKetone
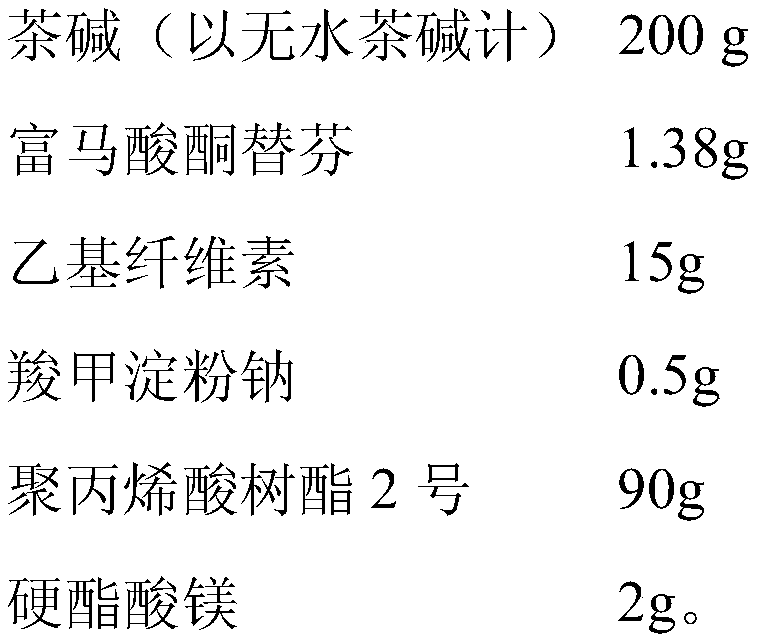
The invention discloses a compound theophlline ketotifen sustained released tablet and a preparation method of the compound theophlline ketotifen sustained released tablet. The compound theophlline ketotifen sustained released tablet contains active ingredients and pharmaceutic adjuvants, wherein the active ingredients comprise theophylline and ketotifen fumarate, and the pharmaceutic adjuvants comprise ethyecellulose, carboxymethyl starch sodium, polyacrylic resin II, magnesium stearate and alcohol. The method provided by the invention is simple and feasible, the obtained product can permanently release the active ingredients, the plasma concentration is enabled to be stable, the variation range of the peak concentration and valley concentration is small, the taking dosage and times are reduced, and the side effects are small.
Owner:DIAO GRP CHENGDU PHARMA
Compound theophlline ketotifen sustained released tablet and preparation method thereof
ActiveCN106913572AImprove stabilityGood compatibilityPharmaceutical non-active ingredientsRespiratory disorderCarboxymethyl starchAlcohol
The invention discloses a compound theophlline ketotifen sustained released tablet and a preparation method of the compound theophlline ketotifen sustained released tablet. The compound theophlline ketotifen sustained released tablet contains active ingredients and pharmaceutic adjuvants, wherein the active ingredients comprise theophylline and ketotifen fumarate, and the pharmaceutic adjuvants comprise ethyecellulose, carboxymethyl starch sodium, polyacrylic resin II, magnesium stearate and alcohol. The method provided by the invention is simple and feasible, the obtained product can permanently release the active ingredients, the plasma concentration is enabled to be stable, the variation range of the peak concentration and valley concentration is small, the taking dosage and times are reduced, and the side effects are small.
Owner:DIAO GRP CHENGDU PHARMA
Preparation method of ketotifen fumarate oral solution
ActiveCN111450051ALow impurity contentImprove uniformityOrganic active ingredientsDispersion deliveryBenzoic acidSucrose
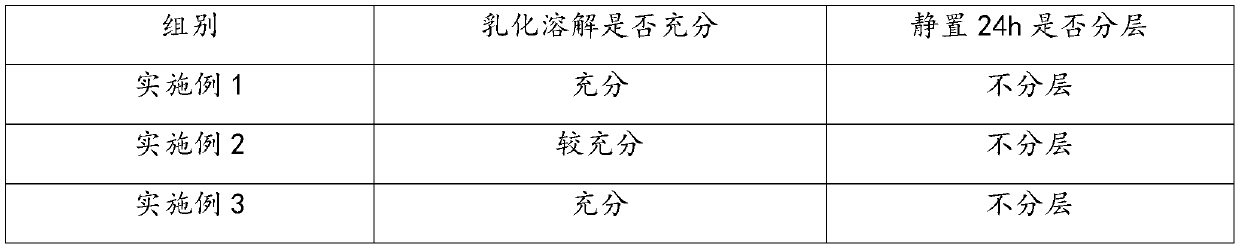
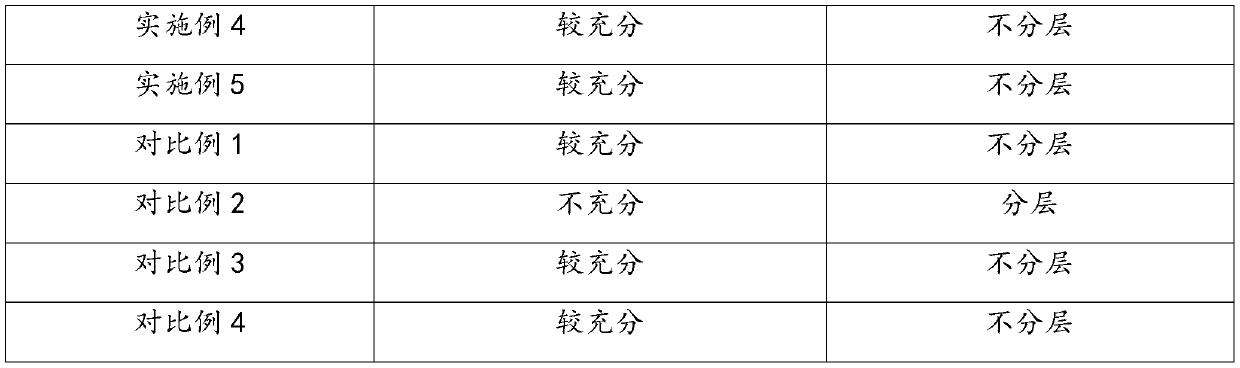
The present invention discloses a preparation method of a ketotifen fumarate oral solution. The method comprises the following steps: adding water into a closed container at a pressure of 0.10 MPa-0.15 MPa and a temperature of 35-40 DEG C, adding citric acid, disodium hydrogen phosphate and ketotifen fumarate under stirring condition, arranging an ultrasonic instrument in the closed container, andturning on the ultrasonic instrument to perform ultrasound at an ultrasonic frequency of 12 KHz-15 KHz and ultrasonic time of 6-10 min to obtain a solution I; adding a co-solvent to another container, adding methyl p-hydroxybenzoate and propyl p-hydroxybenzoate under stirring conditions, and conducting stirring and dissolving to obtain a solution II; adding the solution II to the solution I to bestirred evenly; adding sucrose and sorbitol while stirring until the solution is completely dissolved to obtain a solution III; and adding essence to the solution III, adding water to a constant volume, and conducting stirring and still putting until no bubbles exists. The obtained ketotifen fumarate oral solution prepared by the method has low impurity content.
Owner:武汉贝参药业股份有限公司
Method for rapidly measuring content of ketotifen fumarate in ketotifen fumarate naristillae
InactiveCN105954391AThe test result is accurateHigh detection sensitivityComponent separationAlcoholAcetonitrile
The invention provides a method for rapidly measuring content of ketotifen fumarate in ketotifen fumarate naristillae, and aims at providing the method which is high-efficiency and time-saving and has good repeatability and high detection sensitivity for measuring content of ketotifen fumarate in ketotifen fumarate naristillae. The method includes the following steps in order: taking 1ml sample solution of ketotifen fumarate naristillae accurately, putting the sample solution into a 100ml measuring flask, adding acetonitrile to dilute to the scale, filtering the solution with a 0.22 micron filter membrane, and injecting 2 mul solution to the liquid chromatography. The chromatographic conditions are as follow: chromatographic column is Thermo Syncronis Dim. mmAQ1.7 mum 50*2.1 mm; column temperature is 30 DEG C; flow velocity is 0.8 ml / min; volume ratio of methyl alcohol-water-acetonitrile in the mobile phase is 30:40:30; and measurement wavelength is 287nm. The invention belongs to the technical field of chemical detection.
Owner:WUZHOU INST FOR FOOD & DRUG CONTROL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com