A kind of ketotifen fumarate tablet and preparation method thereof
A technology of ketotifen fumarate and ketone fumarate, which can be used in pharmaceutical formulations, medical preparations containing active ingredients, allergic diseases, etc., and can solve the problem of low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
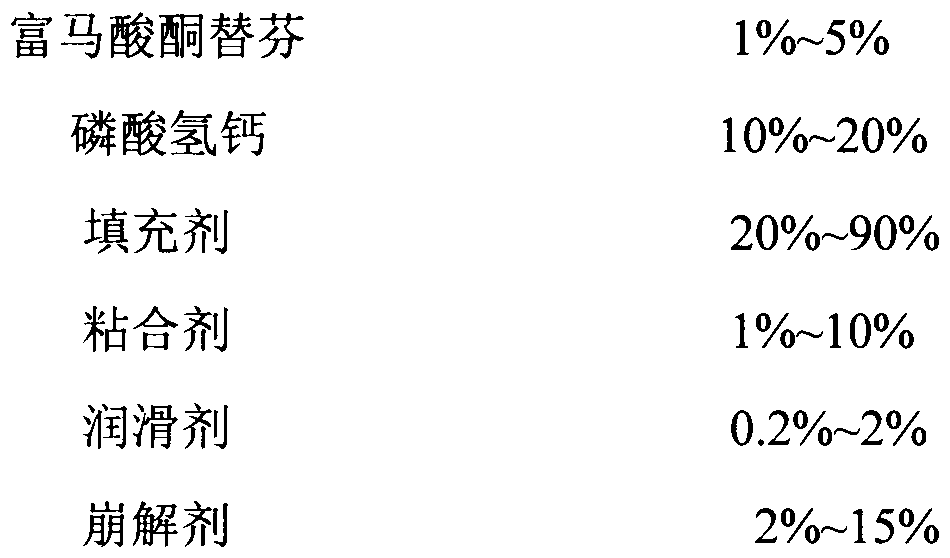
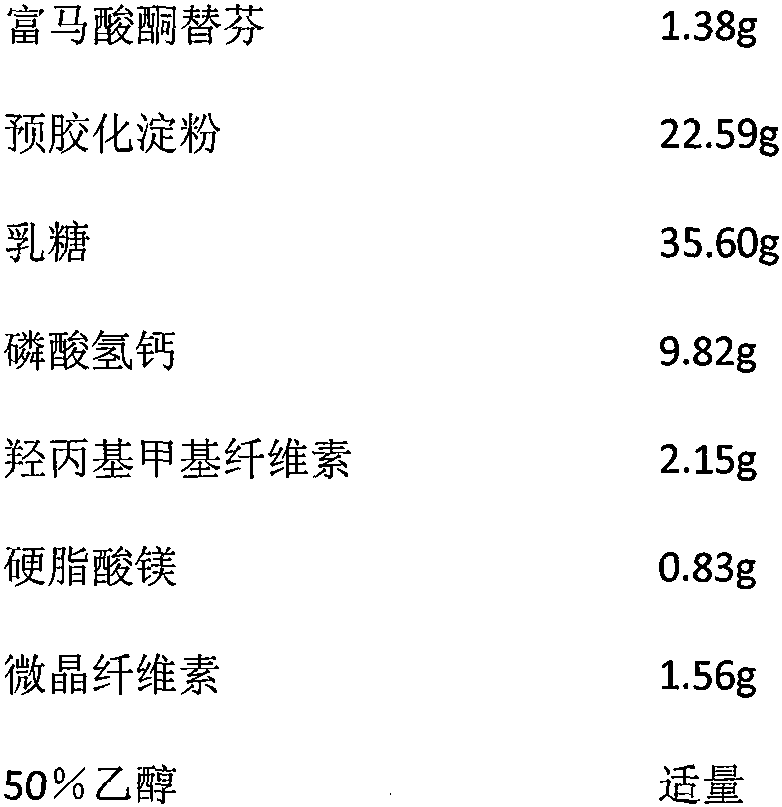
[0015] Ketotifen fumarate tablet prescription and preparation method thereof (calculated in 1000 tablets)
[0016] Element quality Ketotifen fumarate 1.38g pregelatinized starch 22.59g lactose 35.60g Calcium hydrogen phosphate 9.82g Hydroxypropylmethylcellulose 2.15g Magnesium stearate 0.83g microcrystalline cellulose 1.56g 50% ethanol Appropriate amount
[0017] According to the above prescription, weigh the ketotifen fumarate fine powder, pregelatinized starch, lactose, calcium hydrogen phosphate, and microcrystalline cellulose that have been ultrafinely pulverized and passed through a 200-mesh sieve, mix thoroughly for 60 minutes, and add hydroxypropyl methylcellulose Vegetables, appropriate amount of 50% ethanol soft material, sieved and granulated. Then dry the wet granules at 65-75°C, sieve the dried granules for granulation, add magnesium stearate, mix for 30 minutes, and press into tablets to obtain the pr...
Embodiment 2
[0019] Ketotifen fumarate tablet prescription and preparation method thereof (calculated in 1000 tablets)
[0020] Element quality Ketotifen fumarate 1.38g starch 45.50g dextrin 18.98g Calcium hydrogen phosphate 10.25g Hydroxypropylmethylcellulose 2.43g talcum powder 0.89g microcrystalline cellulose 1.50g 50% ethanol Appropriate amount
[0021] According to the above prescription, take the ketotifen fumarate fine powder, starch, dextrin, calcium hydrogen phosphate, and microcrystalline cellulose that have been ultrafinely pulverized and passed through a 200-mesh sieve, mix thoroughly for 60 minutes, and add hydroxypropyl methylcellulose fine powder. Powder, appropriate amount of 50% ethanol soft material, sieved and granulated. Then dry the wet granules at 65-75°C, sieve the dried granules for granulation, add talc powder, mix for 30 minutes, and press into tablets to obtain the product.
Embodiment 3
[0023] Ketotifen fumarate tablet prescription and preparation method thereof (calculated in 1000 tablets)
[0024] Element quality Ketotifen fumarate 1.38g starch 40.88g lactose 18.47g Calcium hydrogen phosphate 10.65g Hydroxypropylmethylcellulose 2.50g Magnesium stearate 1.00g Sodium carboxymethyl starch 2.86g 50% ethanol Appropriate amount
[0025] According to the above prescription, take the ketotifen fumarate fine powder, starch, lactose, calcium hydrogen phosphate, and sodium carboxymethyl starch that have been ultrafinely pulverized and passed through a 200-mesh sieve, mix thoroughly for 60 minutes, add hydroxypropyl methylcellulose, Appropriate amount of 50% ethanol to make soft material, sieve and granulate. Then dry the wet granules at 65-75°C, sieve the dried granules for granulation, add magnesium stearate, mix for 30 minutes, and press into tablets to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com