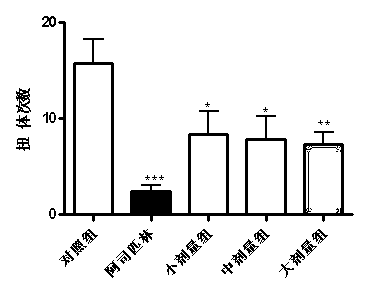
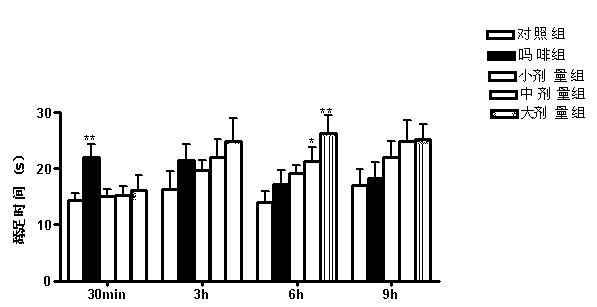
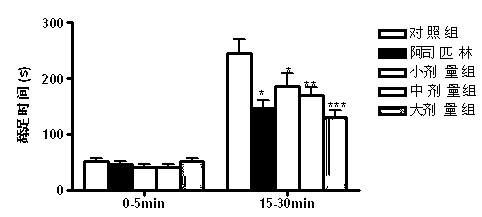
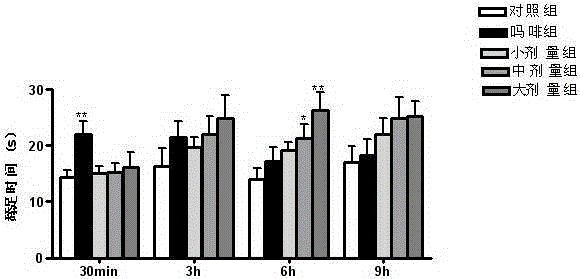
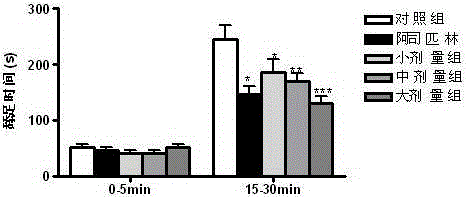
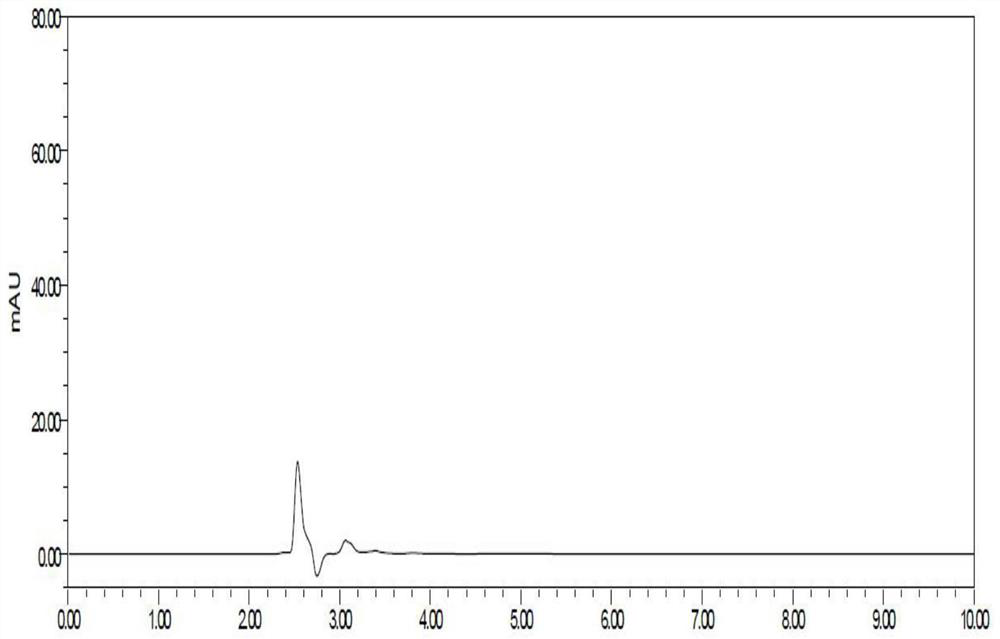
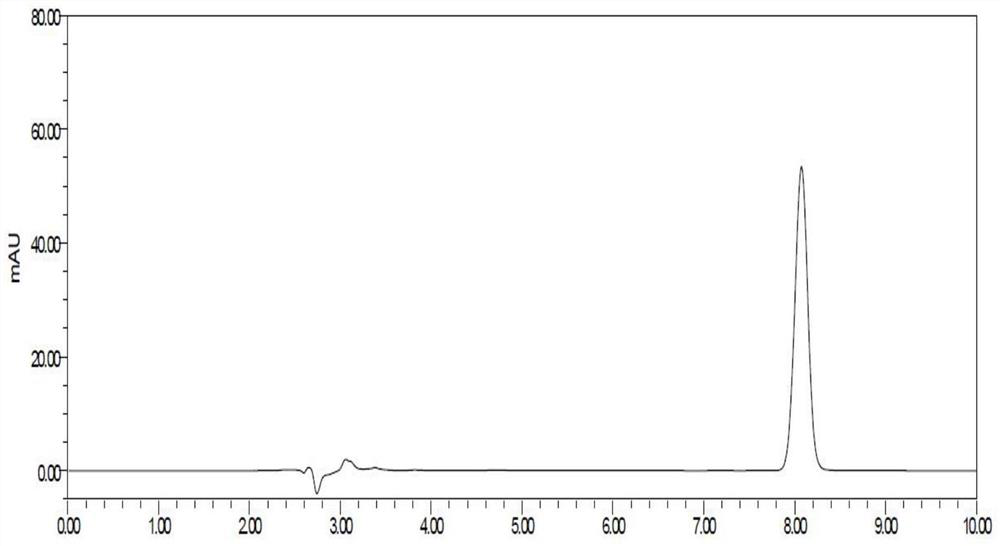
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38 results about "Flunarizine Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Film agent quickly dissolved in oral cavity and preparation method thereof
ActiveCN103211801ALess pain timeAvoid reducing efficacyNervous disorderAntipyreticCobra venomTreatment effect
The invention discloses a film agent quickly dissolved in the oral cavity and a preparation method thereof. The film agent consists of the following components in percentage by weight: 0.1 to 70 percent of medicinal active ingredients, 30 to 90 percent of water-soluble film forming material, 2 to 20 percent of plasticizer, 0 to 5 percent of disintegrating agent, and 1 to 20 percent of water, wherein the medicinal active ingredients are selected from modified or non-modified cobra venom, sumatriptan succinate, ergotamine dihydrogen tartrate and lomerizine hydrochloride or flunarizine hydrochloride. The film agent is quick in response, and avoids decomposition in gastrointestinal tracts due to oral administration to reduce the treatment effect. The preparation method is simple, economic and practical, and facilitates industrialized batch production.
Owner:SUZHOU RENBEN PHARMA
Flunarizine hydrochloride capsules and preparation method thereof
InactiveCN104382878AQuality improvementImprove stabilityOrganic active ingredientsCapsule deliveryMedicineFlunarizine Hydrochloride
The invention relates to an oral capsule preparation of flunarizine hydrochloride and a preparation method thereof, belonging to the technical field of medicines. The capsule comprises the following components in parts by weight: 58-60 parts of flunarizine hydrochloride, 114-115 parts of starch, 212.6-213 parts of 63 percent ethanol, 35.3-35.5 parts of sodium carboxymethyl starch and 11.6-12 parts of magnesium stearate. The flunarizine hydrochloride disclosed by the invention has high quality stability, the quality of the flunarizine hydrochloride capsules is stable and controllable, and safety and effectiveness of clinical application are ensured.
Owner:SHIJIAZHUANG HUAXIN PHARMA
Flunarizine hydrochloride matrix sustained-release tablets and preparing method thereof
ActiveCN105395504AOvercoming the relatively short half-lifeOvercomes the drawback of requiring multiple dosesOrganic active ingredientsSenses disorderExtended release tabletsBlood concentration
Flunarizine hydrochloride matrix sustained-release tablets are prepared from, by weight, 25-55 parts of flunarizine hydrochloride, 35-55 parts of a matrix material, 5-10 parts of diluent, 3-5 parts of a binding agent and 2-5 parts of a lubricating agent. A preparing method of the flunarizine hydrochloride matrix sustained-release tablets comprises the following steps that flunarizine hydrochloride, the matrix material, the diluent the lubricating agent and the binding agent are mixed evenly and them directly pressed into the tablets in a powder mode or flunarizine hydrochloride, the matrix material, the diluent and the binding agent are mixed to be prepared into granules, the granules are then mixed with the lubricating agent evenly, and the tablets are obtained through tabletting. The flunarizine hydrochloride matrix sustained-release tablets overcome the defect that in the prior art, multiple times of administration needs to be conducted due to the short relative half-life period of flunarizine hydrochloride, and it can be avoided that to maintain the blood concentration with the effective amount to reach a treatment effect, initial over-doses in the initial administration period occur, and consequently many side effects are caused; the problem that due to insufficient doses, the pain of a patient cannot be relieved, and the treatment effect cannot be achieved is also solved.
Owner:ZHENGZHOU BARY ANIMAL PHARMA
Flunarizine-hydrochloride pharmaceutical composition
ActiveCN105998024ALong-term storage stabilitySolve the splinter problemOrganic active ingredientsNervous disorderFlunarizine HydrochlorideMagnesium stearate
The invention relates to a flunarizine-hydrochloride pharmaceutical composition and a preparing method thereof, and belongs to the technical field of medicine making. The flunarizine-hydrochloride pharmaceutical composition in the technical scheme is characterized in that every one thousand compositions comprise 5.9 g of flunarizine hydrochloride, 62.5 g-82.5 g of lactose, 70 g-90 g of microcrystalline cellulose, 3.2-12.8 g of sodium carboxymethylcellulose, 0.8 g of silicon dioxide, 0.8 g of magnesium stearate and 1-1.6 g of docusate sodium. According to the flunarizine-hydrochloride pharmaceutical composition and the preparing method thereof, a tablet with the small resolving degree RSD is provided through reasonable compatibility, the prepared tablet is stable in long-term storage, and high-quality medicine is provided for a clinic.
Owner:DISHA PHARMA GRP
Flunarizine hydrochloride capsule and its preparation method
InactiveCN1839838AImprove absorption rateImprove bioavailabilityOrganic active ingredientsCapsule deliveryMagnesium stearateLactose
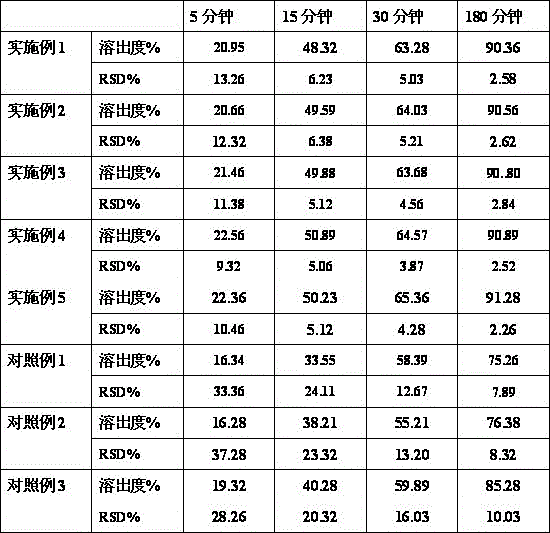
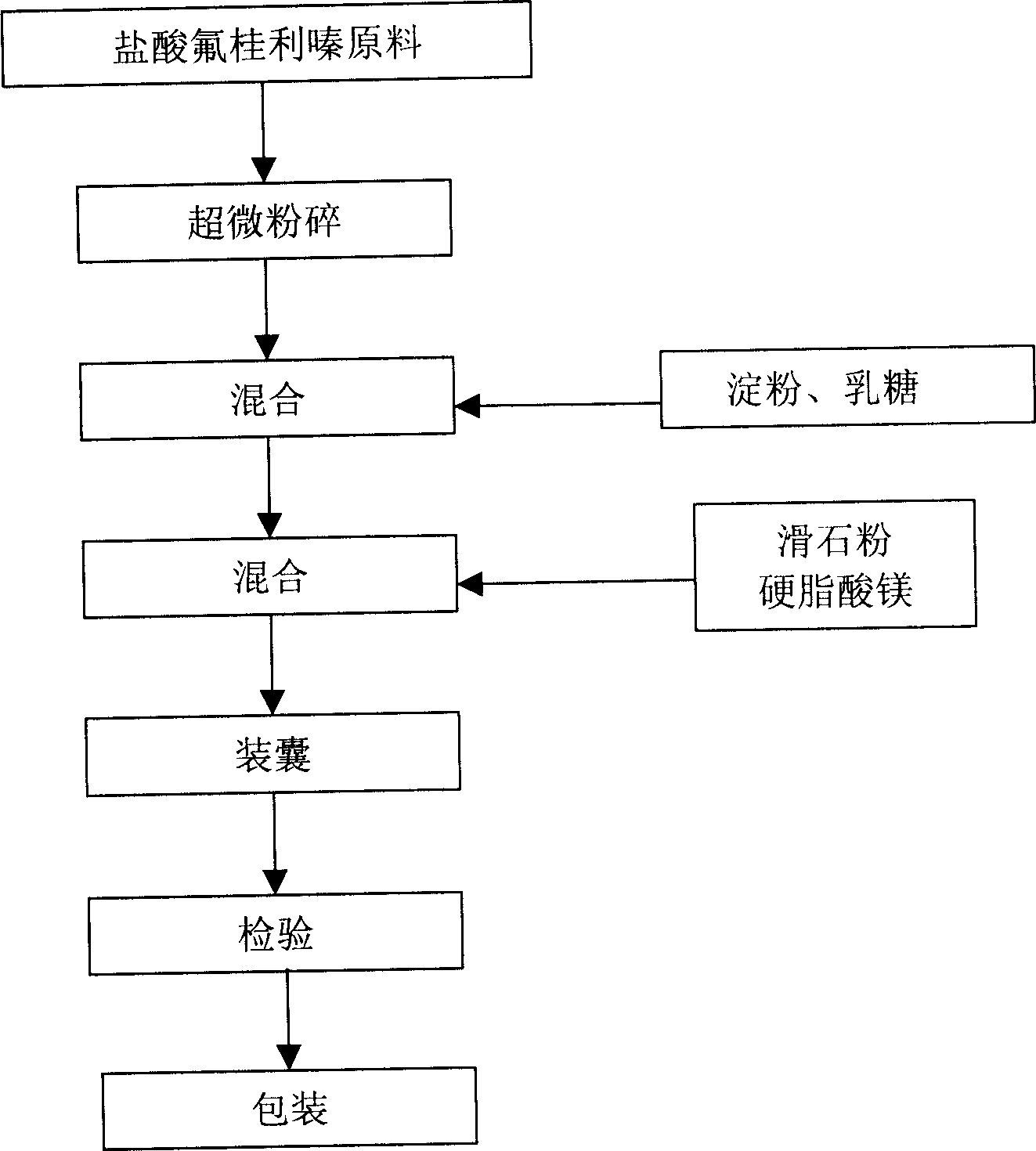
The invention relates to a Flunarizine hydrochloride capsule and its preparing process, wherein the capsule contains ultramicro-powders of flunarizine hydrochloride with internal diameter less than 10 mum, and the preparing process comprises disintegrating Flunarizine hydrochloride raw material, mixing the obtained ultramicro-powder with starch and lactose, then mixing with talcum powder and magnesium stearate, finally loading into capsule and packaging.
Owner:周有财
Flunarizine hydrochloride composition capsule and preparation method thereof
InactiveCN104069086AHigh dissolution rateImprove liquidityOrganic active ingredientsNervous disorderMolten statePolyethylene glycol
The invention relates to the field of medical technology, and discloses a flunarizine hydrochloride composition capsule and a preparation method thereof. The flunarizine hydrochloride composition capsule comprises flunarizine hydrochloride, polyethyleneglycol, talcum powder, magnesium stearate, lactose and starch. As auxiliary material composition is changed, that is, flunarizine hydrochloride is added into polyethyleneglycol which is in a molten state, and then uniformly mixed with other auxiliary materials in an equivalently progressive increasing manner, the dissolution rate and the flowability of the end product are improved remarkably.
Owner:周有财
Flunarizine hydrochloride injection and its prepn process
InactiveCN1383825AAct quicklySignificant effectOrganic active ingredientsImmunological disordersAlcoholCurative effect
Flunarizine hydrochloride injection is compounded with Flunarizine hydrochloride, benzoic alcohol solution, propylene glycol solution, active carbon and water for injection in certain proprtion. It is used for salving critical patient and has quick and obvious curative effect. It can be used alone.
Owner:曹长城
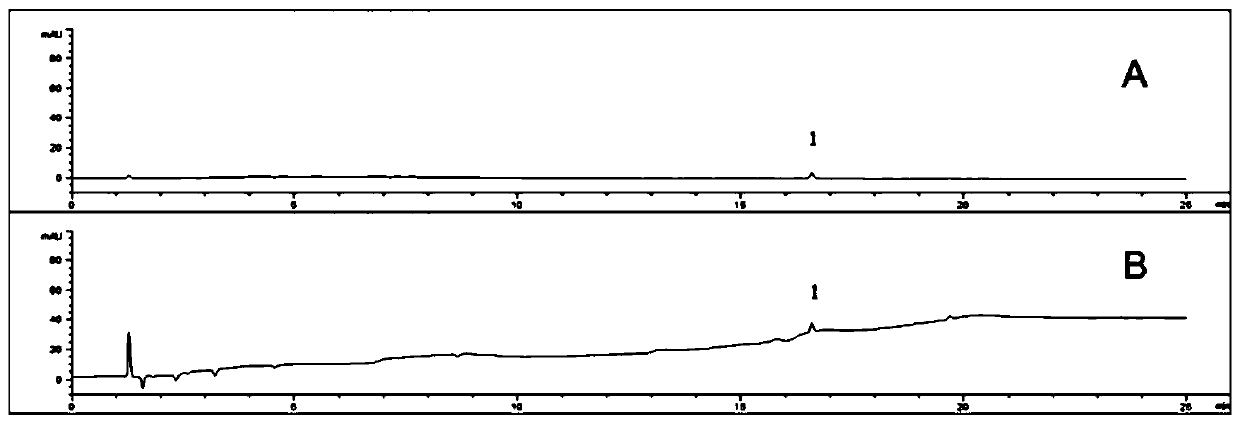
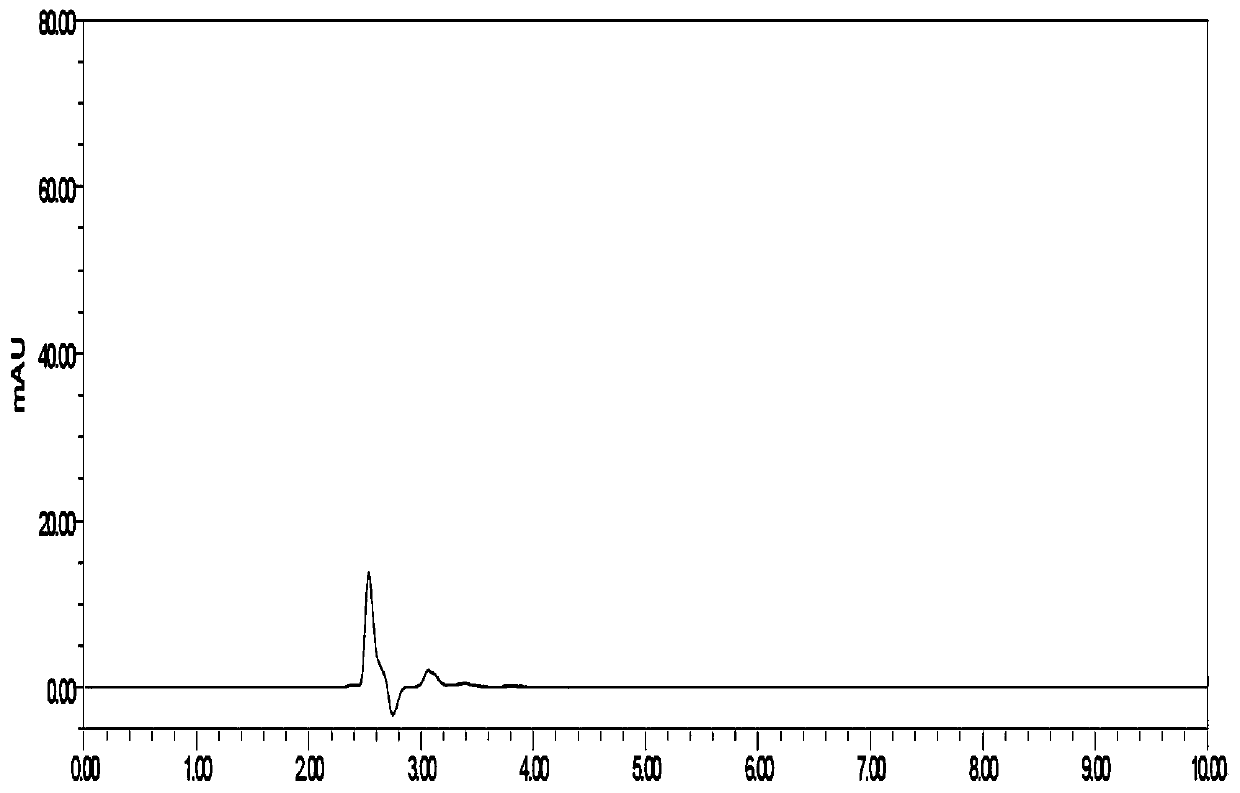
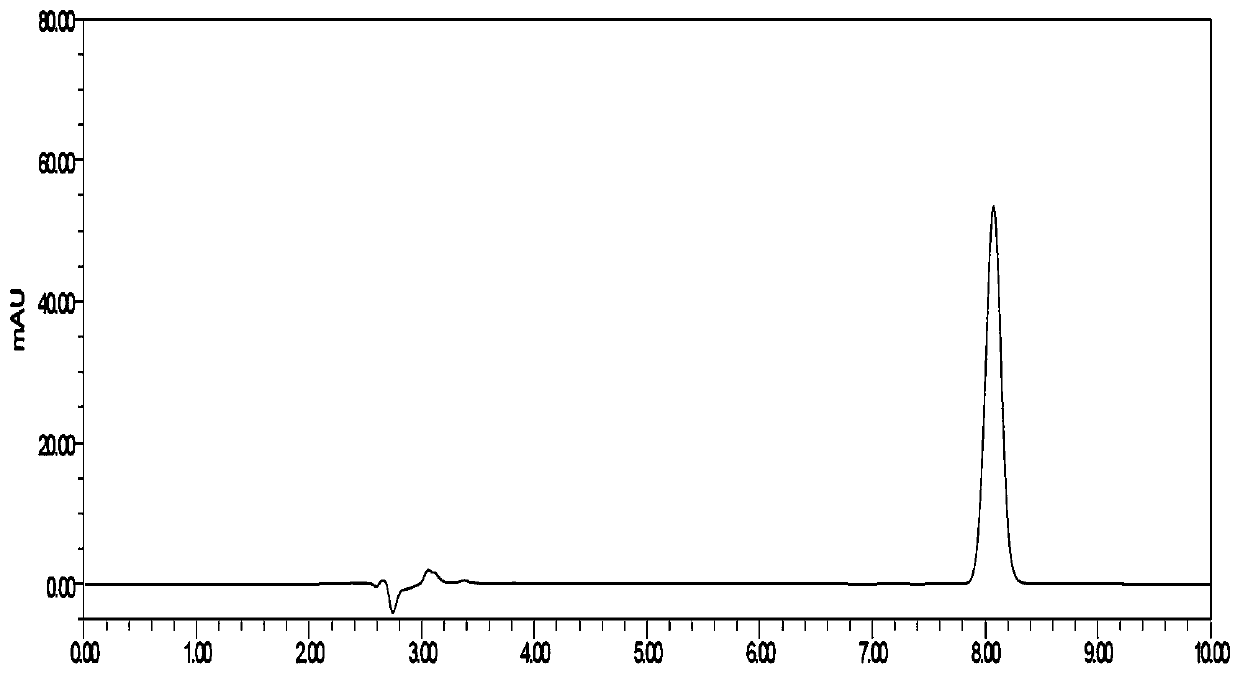
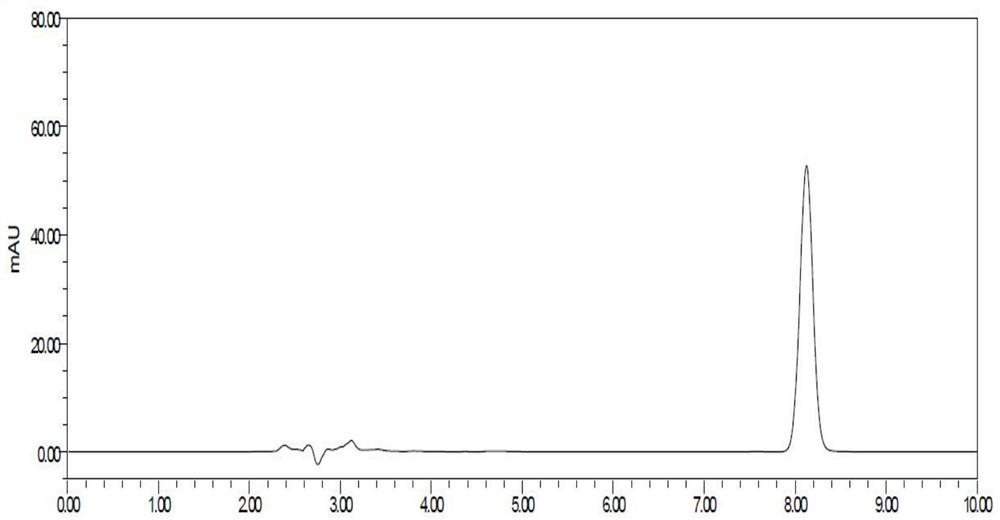
Method for detecting related substances of flunarizine hydrochloride preparation
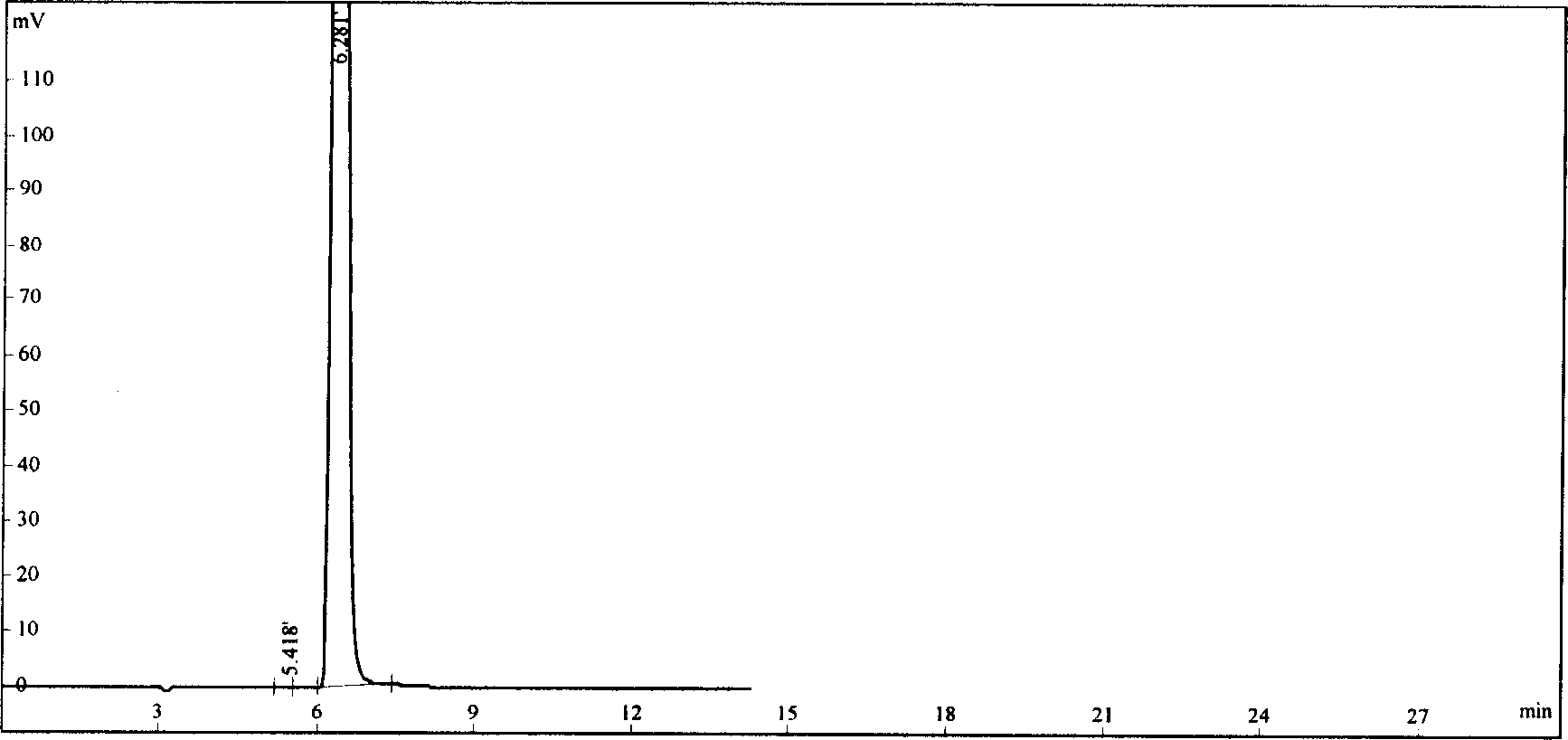
ActiveCN110967421AEfficient separationRealize monitoringComponent separationFluid phasePhysical chemistry
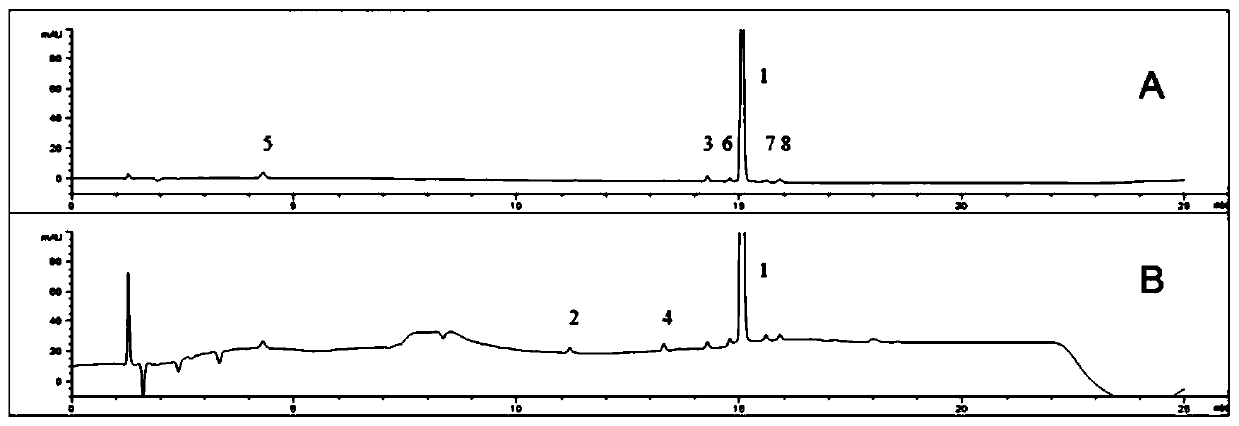
The invention discloses a method for detecting related substances of a flunarizine hydrochloride preparation, which comprises the following steps: respectively taking a test solution, a contrast solution and an impurity mixed solution for high performance liquid chromatography detection, and respectively detecting the content of each impurity in the test solution at 210nm and 253nm, wherein peaksof the impurity A and 4, 4-difluorobenzhydrol are detected at 210 nm, and the impurity content is calculated according to an external standard method; and detecting other impurity peaks at 253nm, andcalculating the impurity content according to a self-contrast method. The method is easy to operate, short in analysis time, high in specificity and high in sensitivity, seven impurities can be effectively separated, the separation degree of two process impurities and five degradation impurities, the most difficult-to-separate impurity B and a main peak reaches 1.2 or above, and the separation degree of other impurities and adjacent peaks can all reach 1.5 or above.
Owner:CHIATAI QINGCHUNBAO PHARMA
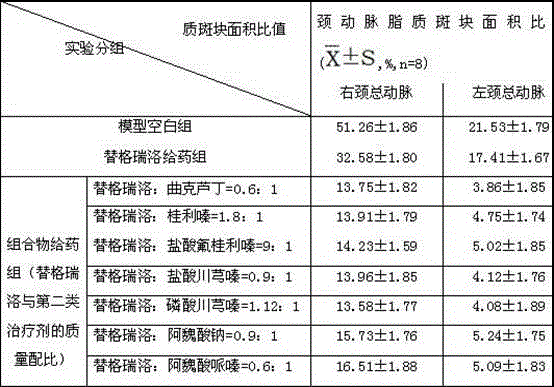
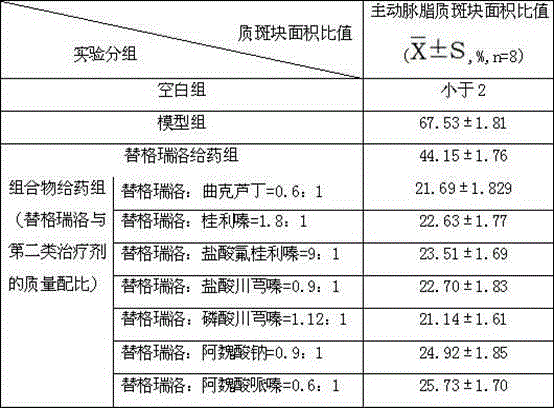
Pharmaceutical composition for heart and cerebral vessels as well as preparation method and application of pharmaceutical composition
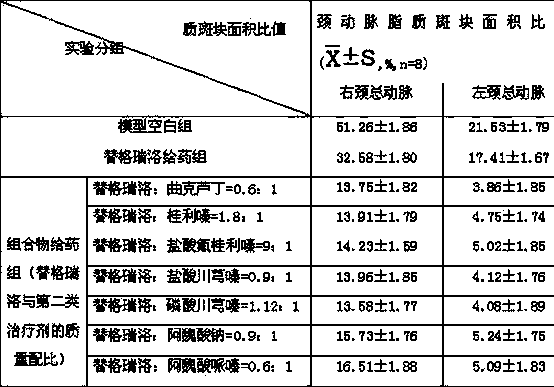
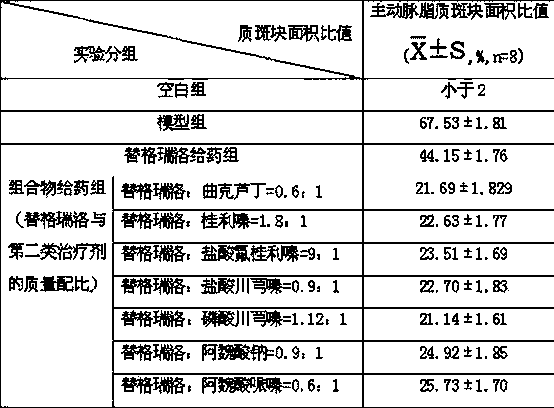
The invention relates to a pharmaceutical composition of ticagrelor and a type II therapeutic agent and a preparation method of the pharmaceutical composition. The pharmaceutical composition is characterized in that the type II therapeutic agent is selected from one or more of troxerutin, cinnarizine, flunarizine hydrochloride, ligustrazine, ligustrazine phosphate, sodium ferulate or piperazine ferulate. The invention also relates to application of the pharmaceutical composition in drugs, in particular the application in inhibiting or reversing of atherosclerosis lipid plaques of artery blood vessel.
Owner:江苏海悦康医药科技有限公司
Flunarizine hydro chloride high-capacity injecta and preparing process thereof
InactiveCN1221261CGood curative effectQuality improvementOrganic active ingredientsPharmaceutical delivery mechanismFlunarizine HydrochlorideSolvent
The present invention provides a flunarizine hydrochloride bulk injection and its production process. Its preparation range, every ampule contains 2mg-50mg of flunarizine hydrochloride and 0.1 ml-10 ml of 1.2-propylene glycol, the solvent respectively can be glucose, sodium chloride and glucose sodium chloride, and their contents respectively are 1g-50g of glucose and 0.3g-10g of sodium chloride. Its production process includes the following steps: placing 20ml of 1.2-propylene glycol in a container, then adding flunarizine hydrochloride, in said container, heating, stirring and dissolving; using injection water to dissolve glucose, stirring, adding it into container, diluting and dissolving, adding injection water to full dose, adding active carbon, heating to 80-90 deg.C, heat-insulating for 15 min., filtering and removing carbon, secondary filtering, adding flurnarizine hydrochloride and 1.2-propylene glycol mixed liquor, stirring them uniformly, regulating pH to 3.5-5.0 so as to obtain the invented product.
Owner:河南福森药业有限公司
Detection method for dissolution determination of flunarizine hydrochloride capsules
ActiveCN110542737ASolve the adsorption problemExtended service lifeComponent separationDissolutionFlunarizine Hydrochloride
The invention discloses a detection method for the dissolution determination of flunarizine hydrochloride capsules. The method is characterized in that a dissolution method is a paddle method in whichthe speed is 100 revolutions per minute; a medium of lauryl sodium sulfate is added to serve as a dissolution medium; a dissolved matter is detected by high performance liquid chromatography; octadecyl silane bonded silica gel is taken as a filling agent; the detection wavelength of an ultraviolet detector is 251 to 255nm; the column temperature is 25 to 45 DEG C; the flow rate is 0.9 to 1.1ml / min; and a flowing phase is prepared from methane and a phosphate Buffer solution. Through adoption of the method, the problem concerned with flunarizine hydrochloride capsule filter membrane adsorptionis solved; an obtained chromatographic peak has a good peak pattern; high column efficiency and a more accurate result can be achieved; the service life of a chromatographic column is prolonged; andthe test cost is lowered.
Owner:贵州缔谊健康制药有限公司 +1
Preparation method for flunarizine hydrochloride crystal
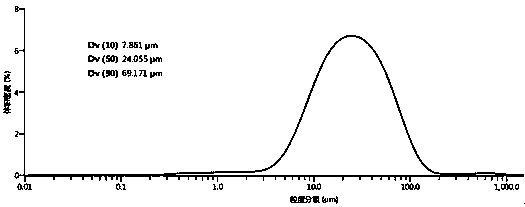
The invention relates to a preparation method for a flunarizine hydrochloride crystal. The invention relates to a preparation method for a flunarizine hydrochloride crystal with a particle size of 20-30 microns. The preparation method comprises the following steps: firstly, adding flunarizine hydrochloride into an aqueous solution of a low-boiling-point organic solvent; carrying out reduced-pressure evaporation at a temperature of 50-80 DEG C and a vacuum degree of 0.03-0.08 MPa, evaporating out a part of the solvent at an evaporation rate of 10-25% / h of the total amount of the solvent, performing crystal growing for 30-60 min after the crystal is separated out, then continuously carrying out reduced-pressure evaporation, and stopping evaporation and performing crystal growing when the volume of a distillate is 30-65% of the volume of the solvent; carrying out cooling to 5-10 DEG C at a cooling speed of 5-10 DEG C / h, and maintaining the temperature for crystal growing for 30-60 min; and carrying out filtering, and washing a filter cake with a solvent. The invention provides the preparation method for the flucinarizine hydrochloride crystal with controllable granularity.
Owner:迪嘉药业集团股份有限公司
Flunarizine hydrochloride and preparation process thereof
ActiveCN102579989BHigh yieldThe group is rational and orderlyOrganic active ingredientsNervous disorderCelluloseGastrodia
The invention relates to compound flunarizine hydrochloride and a preparation process thereof. The compound flunarizine hydrochloride is prepared from the following raw materials in part by weight: 0.7 part of flunarizine hydrochloride, 25 parts of root of red-rooted salvia, 20 parts of common burreed rhizome, 18 parts of zedoary, 15 parts of tall gastrodia tuber and 10 parts of glossy privet fruit, starch live edible cellulose serving as a pharmaceutic adjuvant are added according to the conventional pharmaceutic method to prepare formulations such as capsules, tablets, granules and the like. The compound flunarizine hydrochloride medicinal composition addresses both root causes and symptoms on epilepsy, is non-toxic and harmless to human bodies, does not have side effect, is low in cost and easy and convenient to prepare, and has a bright application prospect.
Owner:仁和堂药业有限公司
Hydrochloric acid flunarizine dropping pills
InactiveCN101134025AReduce dosageIncrease contact areaOrganic active ingredientsPill deliveryActive componentPolyethylene glycol
The present invention relates to a kind of flunarizine hydrochloride dripping pills. It is made up by using flunarizine hydrochloride as active component, using polyethylene glycol as matrix and adopting a diameter-limited cylindrical concave orifice dripping-head dripping preparation process to produce the invented flunarizine hydrochloride dripping pills. The weight of every pill is 5mg-100mg.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Flunarizine hydrochloride pills and preparation method thereof
InactiveCN101716152APromote dissolutionRoundness controllableOrganic active ingredientsSenses disorderIn vivo absorptionFlunarizine Hydrochloride
The invention discloses flunarizine hydrochloride pills which are prepared through the following steps: after evenly mixing 1-10 parts of flunarizine hydrochloride and 10-100 parts of matrices by weight, heating and melting the mixture, mixing up, using a diameter-limited cylindrical concave type pipe dripper for making drops under the heat-retaining condition of 70-95 DEG C, using a pill forming machine to form pills and using a coolant at temperature of 50-minus 10 DEG C for gradient cooling. Compared with the flunarizine hydrochloride capsules, the invention is characterized by fast in vitro dissolution, high dissolution and rapid in vivo absorption and distribution. Compared with the current pill production technology, the invention is characterized in that the prepared flunarizine hydrochloride pills have small pill weight different and good pill sphericity, high yield and more stable quality.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Western medicine composition for treating schizophrenia
InactiveCN106390102AImprove immunityEasy to use for a long timeOrganic active ingredientsNervous disorderPhosphodiesteraseCarbenoxolone sodium
The invention discloses a western medicine composition for treating schizophrenia. The western medicine combination is prepared from the following raw materials in parts by weight: 0.5 to 2 parts of placental hormone, 2 to 5 parts of sodium carboxymethyl cellulose, 1 to 8 parts of clavulanic acid, 1 to 5 parts of phosphodiesterase, 2 to 5 parts of naglina, 2 to 8 parts of flunarizine hydrochloride oral liquid, 2 to 5 parts of glucurolactone slices, 2 to 5 parts of carbenoxolone sodium, 2 to 5 parts of guadisu, 0.5 to 2 parts of risperidone, 0.5 to 1 part of perphenazine, 0.5 to 1 part of vitamin B and 50 to 100 parts of distilled water. The western medicine composition has the advantages that raw materials are compounded to achieve the synergic action; the curative effect is good; the treatment period is short; the toxic and side effects are small; no complication occurs; the preparation method is simple; the production cost is low; the human body immunity can be effectively enhanced; the western medicine composition belongs to medicine for effectively treating schizophrenia; the mouthfeel is good; the western medicine composition is favorable for the long-time persistent administration by people.
Owner:ZHENGZHOU RENHONG PHARMA CO LTD
Flunarizine hydro chloride high-capacity injecta and preparing process thereof
ActiveCN1528308AGood curative effectQuality improvementOrganic active ingredientsPharmaceutical delivery mechanismFlunarizine HydrochlorideSolvent
The present invention provides a flunarizine hydrochloride bulk injection and its production process. Its preparation range, every ampule contains 2mg-50mg of flunarizine hydrochloride and 0.1 ml-10 ml of 1.2-propylene glycol, the solvent respectively can be glucose, sodium chloride and glucose sodium chloride, and their contents respectively are 1g-50g of glucose and 0.3g-10g of sodium chloride. Its production process includes the following steps: placing 20ml of 1.2-propylene glycol in a container, then adding flunarizine hydrochloride, in said container, heating, stirring and dissolving; using injection water to dissolve glucose, stirring, adding it into container, diluting and dissolving, adding injection water to full dose, adding active carbon, heating to 80-90 deg.C, heat-insulating for 15 min., filtering and removing carbon, secondary filtering, adding flurnarizine hydrochloride and 1.2-propylene glycol mixed liquor, stirring them uniformly, regulating pH to 3.5-5.0 so as to obtain the invented product.
Owner:河南福森药业有限公司
Application of histamine receptor inhibitor and derivatives thereof in preparation of anti-Zika virus drugs
ActiveCN110694065AClear anti-Zika virus effectEnhanced inhibitory effectOrganic active ingredientsAntiviralsVirus ProteinBrompheniramine Maleate
The invention provides a histamine receptor inhibitor and derivatives thereof in the preparation of anti-Zika virus drugs. The histamine receptor inhibitor is preferably one or more of mebhydrolin napadisylate, ketotifen fumarate, flunarizine hydrochloride, cyproheptadinehydrochloride, JNJ-7777120, desloratadine, brompheniramine maleate, brompheniramine maleate, chlorpheniramine maleate and cetirizine hydrochloride. The invention brings forward for the first time that the histamine receptor inhibitor and its derivatives have clear anti-Zika virus effect and can significantly inhibit the expression level of Zika virus protein and Zika virus RNA. The invention also screens out histamine receptor inhibitors with better inhibitory effect on Zika virus, including mebhydrolin napadisylate, cyproheptadinehydrochloride, JNJ-7777120, desloratadine, etc., whose inhibition effect on Zika virus at a concentration of 10 uM is equivalent to inhibition effect of ribavirin at a concentration of 40 uM.The histamine receptor inhibitor and the derivatives can be used as good candidate drugs for anti-Zika virus drugs.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
Flunarizine hydrochloride and preparation process thereof
ActiveCN102579989AHigh yieldThe group is rational and orderlyOrganic active ingredientsNervous disorderCelluloseSide effect
The invention relates to compound flunarizine hydrochloride and a preparation process thereof. The compound flunarizine hydrochloride is prepared from the following raw materials in part by weight: 0.7 part of flunarizine hydrochloride, 25 parts of root of red-rooted salvia, 20 parts of common burreed rhizome, 18 parts of zedoary, 15 parts of tall gastrodia tuber and 10 parts of glossy privet fruit, starch live edible cellulose serving as a pharmaceutic adjuvant are added according to the conventional pharmaceutic method to prepare formulations such as capsules, tablets, granules and the like. The compound flunarizine hydrochloride medicinal composition addresses both root causes and symptoms on epilepsy, is non-toxic and harmless to human bodies, does not have side effect, is low in costand easy and convenient to prepare, and has a bright application prospect.
Owner:仁和堂药业有限公司
Flunarizine hydrochloride capsule preparation and preparation method thereof
ActiveCN113262207AReduce granulationReduce drynessOrganic active ingredientsNervous disorderFlunarizine HydrochlorideLactose
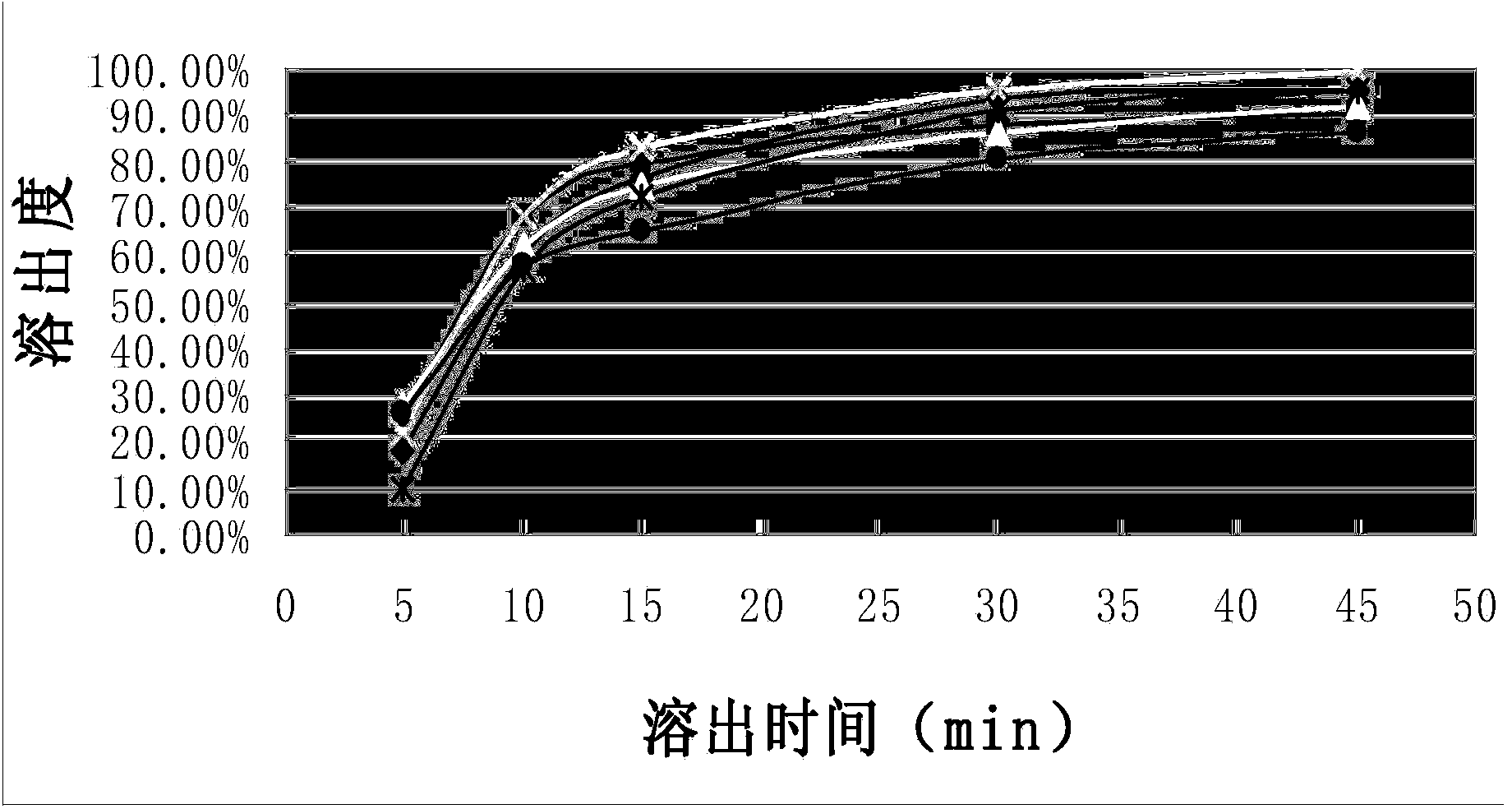
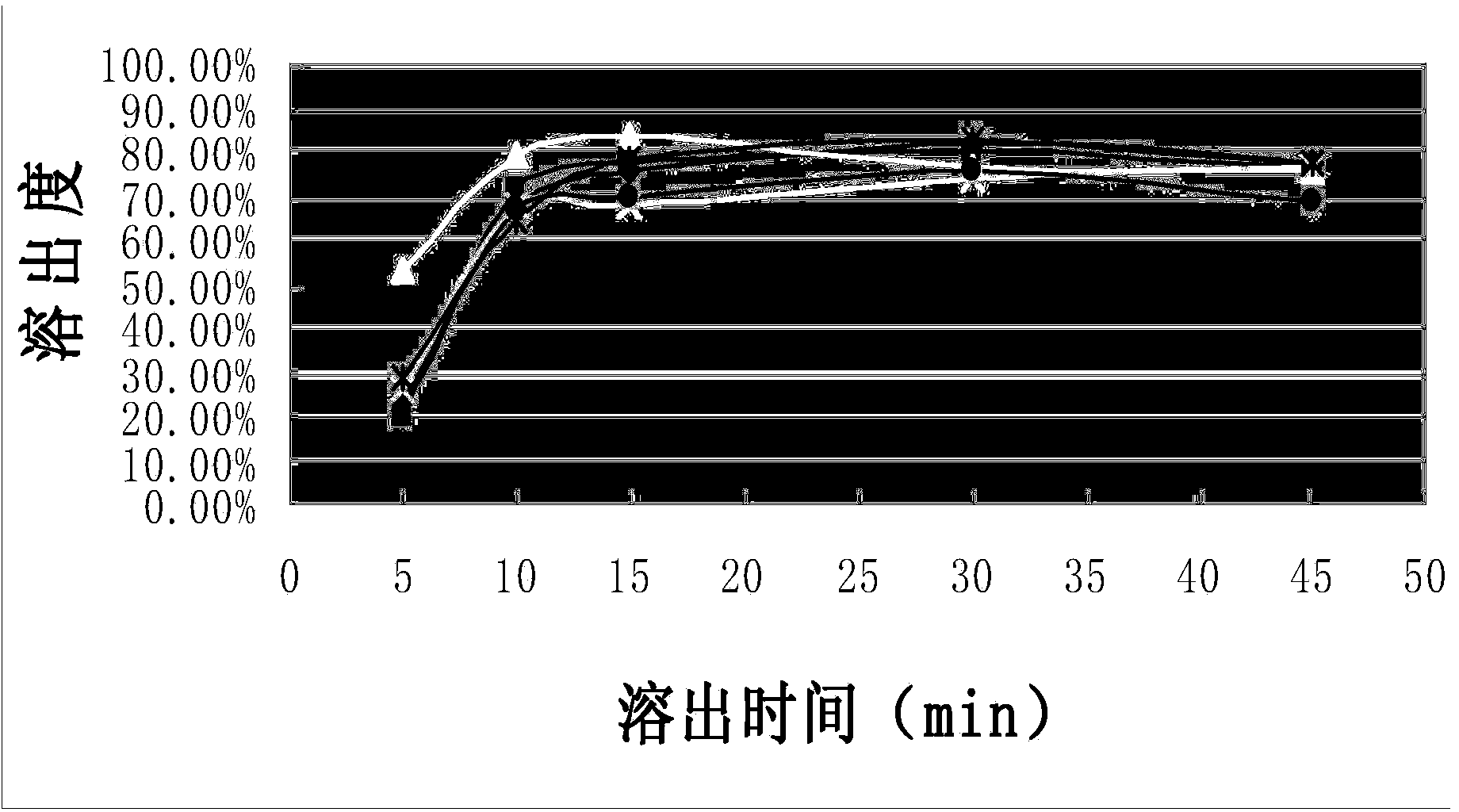
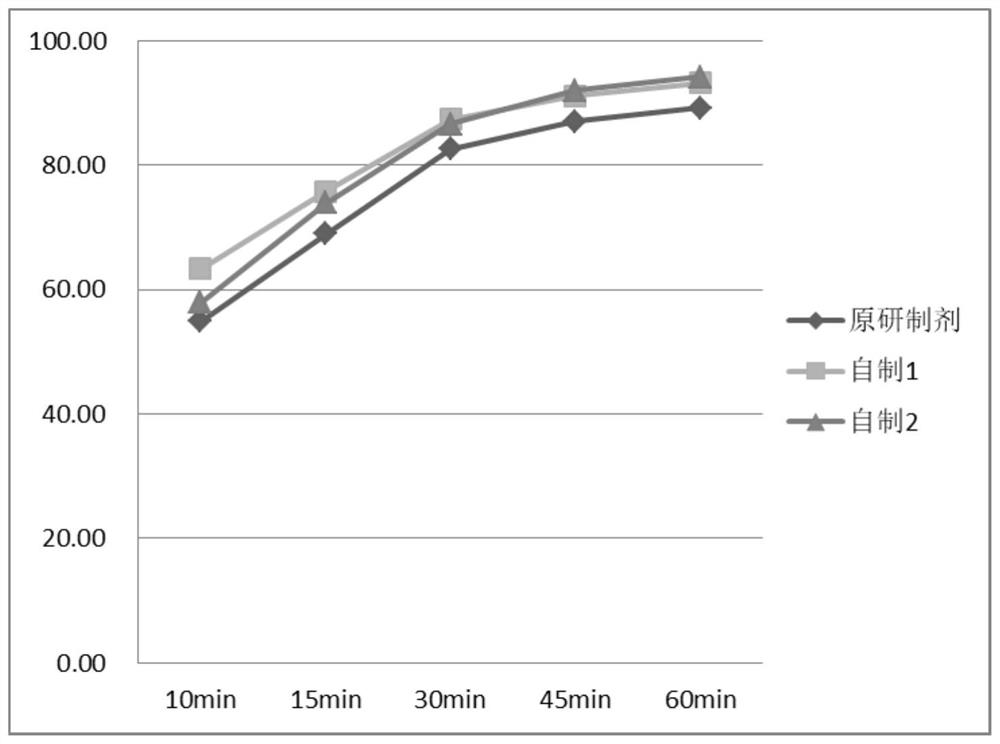
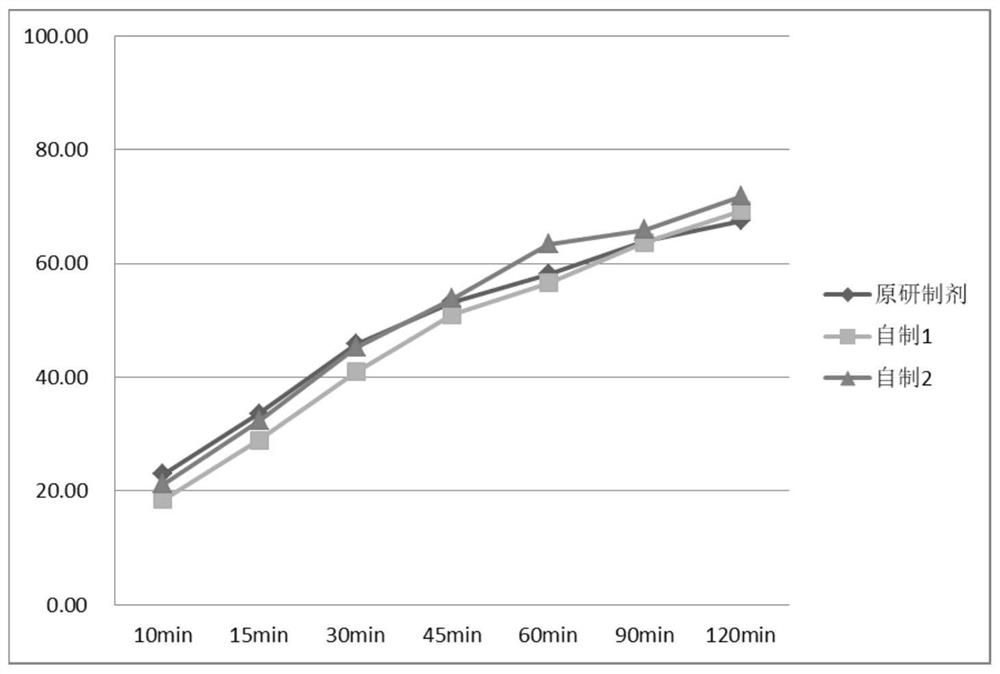
The invention discloses a flunarizine hydrochloride capsule preparation and a preparation method thereof. The flunarizine hydrochloride capsule preparation is prepared from the following raw materials in parts by weight: 2-10 parts of flunarizine hydrochloride, 100-150 parts of lactose, 10-40 parts of starch, 0.1-1 part of a flow aid and 2-20 parts of a lubricant. The flunarizine hydrochloride capsule prepared by the invention is placed for 3 months under a high-temperature experimental condition, the dissolution curve of the flunarizine hydrochloride capsule in a medium with a pH value of 3.0 has no obvious change, and an f2 value is greater than 50; and the dissolution curve of an original developed preparation is obviously reduced, and the f2 value is 12.7. The in-vitro dissolution curve stability of the flunarizine hydrochloride capsule prepared by the invention is superior to the in-vitro dissolution curve stability of the original preparation.
Owner:CHIATAI QINGCHUNBAO PHARMA
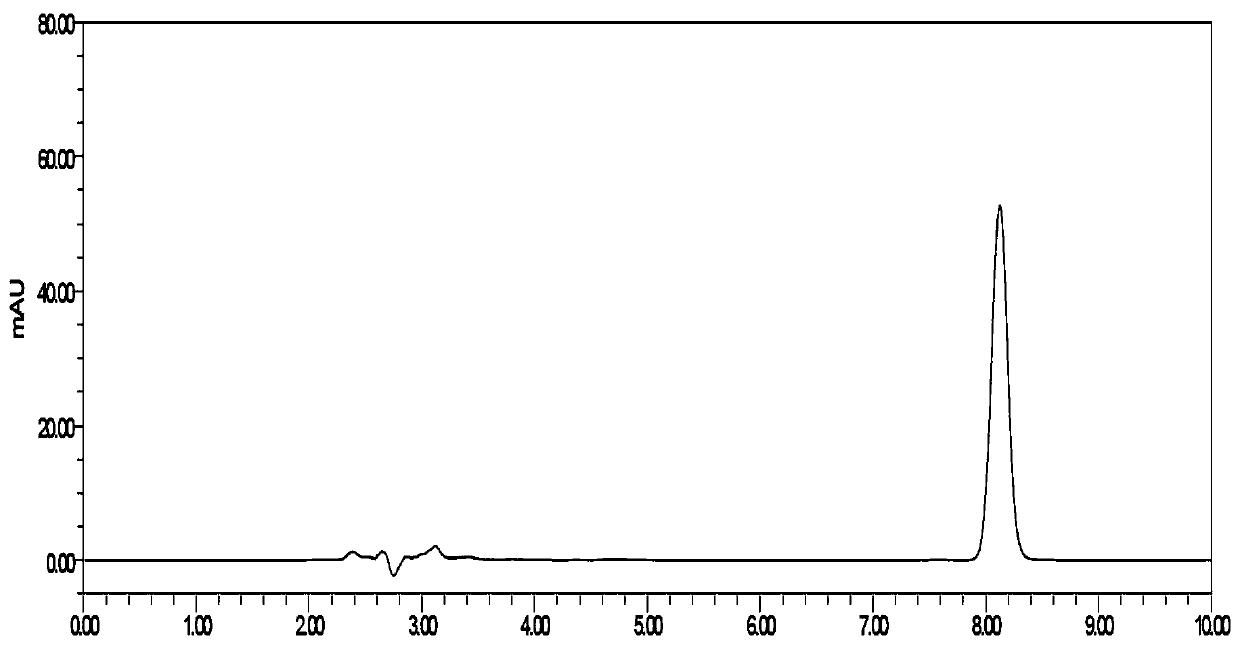
A method for detecting related substances in flunarizine hydrochloride preparations
ActiveCN110967421BEfficient separationRealize monitoringComponent separationFlunarizine HydrochlorideImpurity
The invention discloses a method for detecting related substances in flunarizine hydrochloride preparations. The test solution, control solution and impurity mixed solution are respectively taken for high-performance liquid chromatography detection, and each substance in the test product is detected at 210nm and 253nm respectively. Impurity content; where impurity A and 4,4-difluorobenzyl alcohol peaks were detected at 210nm, and the impurity content was calculated by the external standard method; other impurity peaks were detected at 253nm, and the impurity content was calculated by the self-control method. The method of the invention is easy to operate, has short analysis time, strong specificity and high sensitivity, and can effectively separate 7 impurities, including 2 process impurities and 5 degraded impurities. The separation between impurities and adjacent peaks can reach above 1.5.
Owner:CHIATAI QINGCHUNBAO PHARMA
Flunarizine hydrochloride lyophilized agent for injection and its preparing method
InactiveCN1850055AImprove product qualityEasy to useOrganic active ingredientsPowder deliveryMANNITOL/SORBITOLFreeze-drying
The present invention discloses a flunaizine hydrochloride freeze-dried preparation for injection. Its composition includes (by wt%) 1-50% of flunarizine hydrochloride and 50-99% of auxiliary material, said auxiliary material is mannitol or mannitol composite. Its preparation includes the following steps: a), uniformly mixing flunarizine hydrochloride and medicinal auxiliary material, using acid or alkali to regulate pH value to 2.0-3.5; b), adding 0.01%-0.1% of active carbon, stirring at room temperature, filtering, removing heat source, filtering and sterilizing; c), quickly cooling to-30-50 deg.C, retaining for 2-6 hr.; and d), vacuum freezing, drying and sealing.
Owner:南京虹桥医药技术研究所有限公司
Cardiovascular pharmaceutical composition and its preparation method and application
Owner:江苏海悦康医药科技有限公司
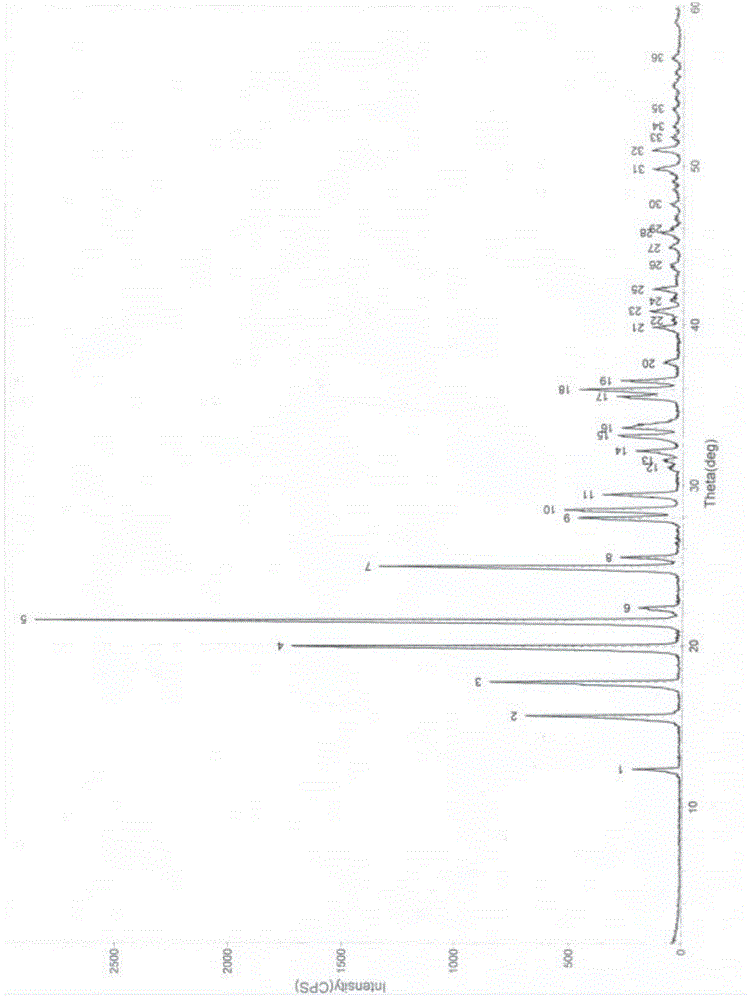
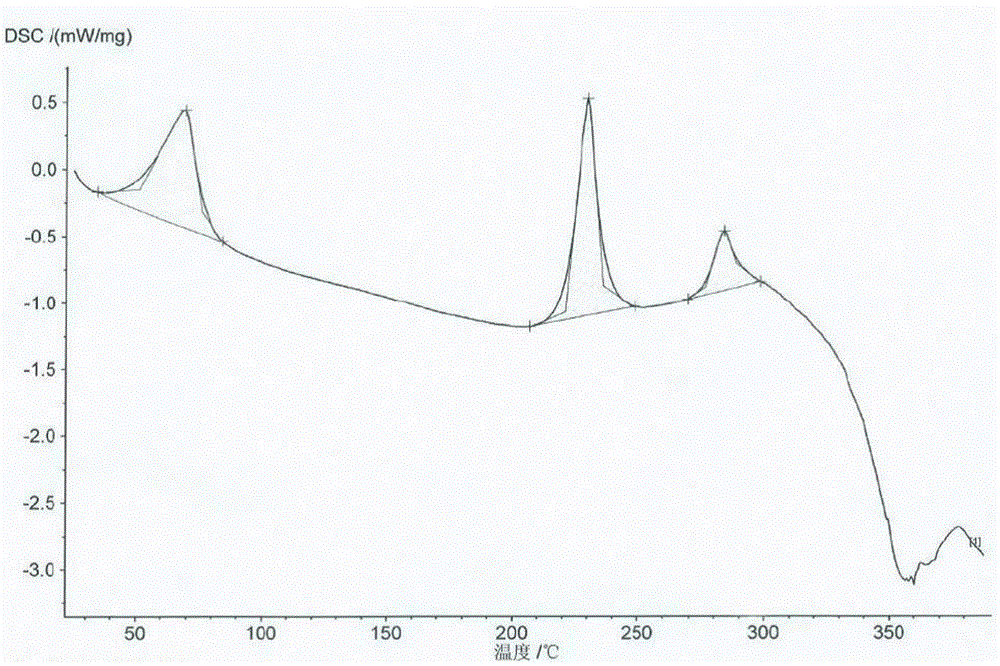
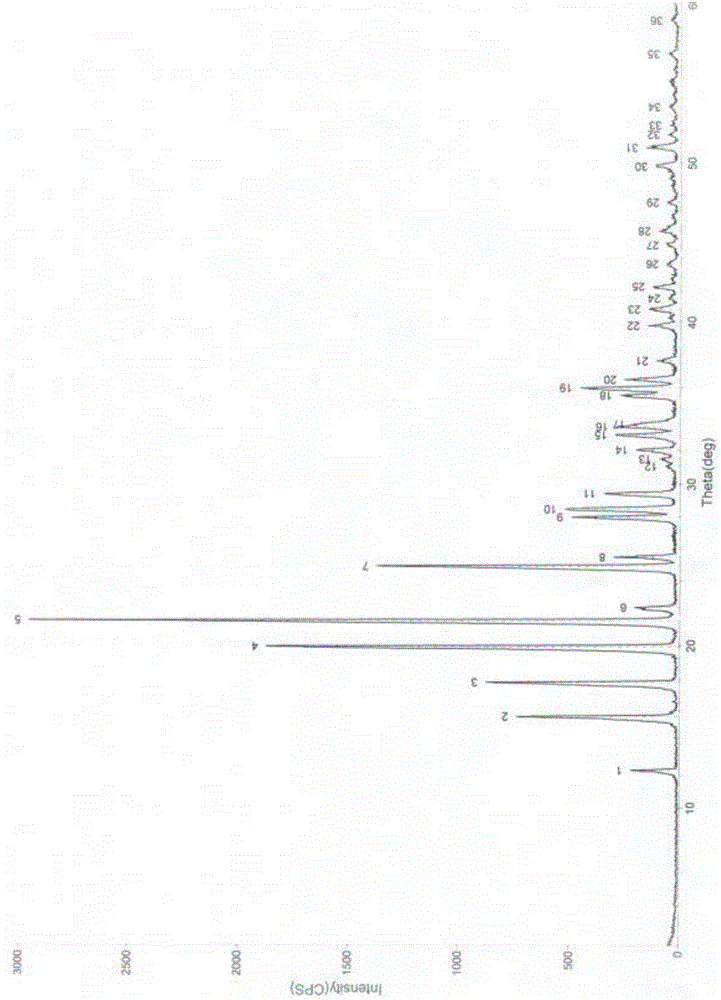
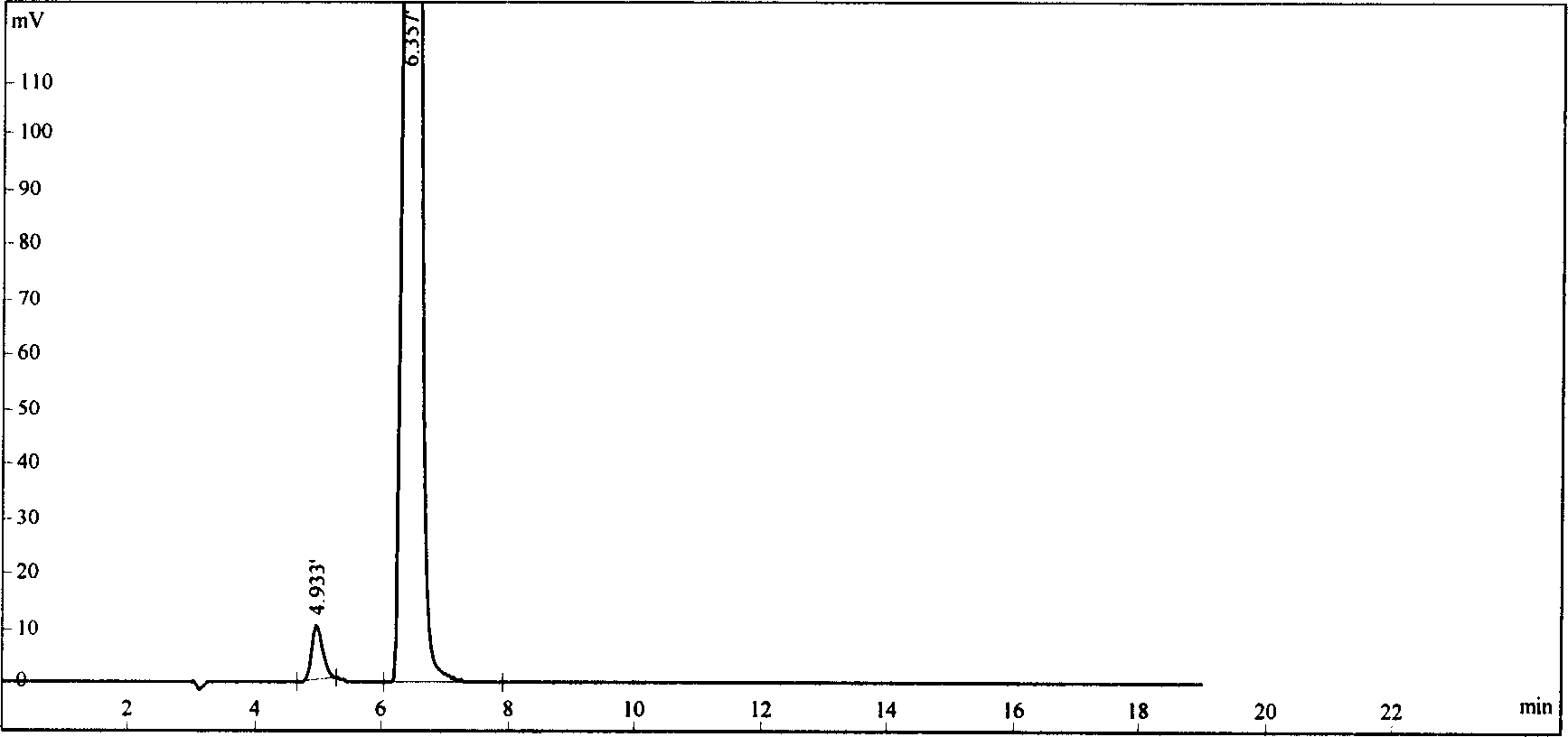
Crystal form B of flunarizine hydrochloride and preparation method thereof
ActiveCN110713470AEasy to prepareReduce humidityOrganic chemistry methodsPharmaceutical drugFlunarizine Hydrochloride
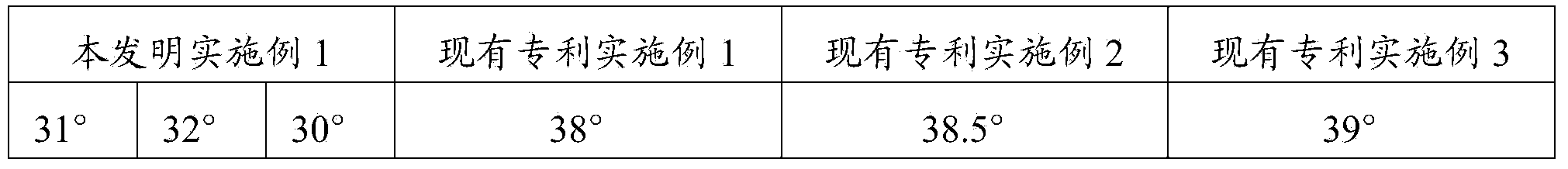
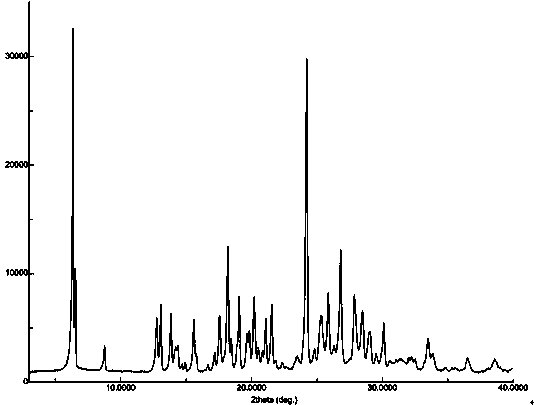
The invention relates to a novel crystal form of flunarizine hydrochloride and a preparation method thereof, and belongs to the technical field of medicine crystallization. According to a technical scheme, the X-ray powder diffraction pattern of the crystal form B of the flunarizine hydrochloride has the following characteristic peaks at 2[theta]+ / -0.2 degrees: 6.35, 6.55, 8.76, 12.76, 13.07, 13.83, 14.17, 14.36, 14.67, 14.93, 15.60, 15.78, 16.64, 17.18, 17.56, 18.19, 18.45, 18.90, 19.05, 19.65 and 19.84. The invention provides the novel crystal form with stable performance of the flunarizinehydrochloride and a preparation method thereof.
Owner:迪嘉药业集团股份有限公司
A kind of flunarizine hydrochloride matrix sustained-release tablet and preparation method thereof
ActiveCN105395504BMaintain steady-state effective plasma concentrationFully playOrganic active ingredientsSenses disorderSustained Release TabletSide effect
Flunarizine hydrochloride matrix sustained-release tablets are prepared from, by weight, 25-55 parts of flunarizine hydrochloride, 35-55 parts of a matrix material, 5-10 parts of diluent, 3-5 parts of a binding agent and 2-5 parts of a lubricating agent. A preparing method of the flunarizine hydrochloride matrix sustained-release tablets comprises the following steps that flunarizine hydrochloride, the matrix material, the diluent the lubricating agent and the binding agent are mixed evenly and them directly pressed into the tablets in a powder mode or flunarizine hydrochloride, the matrix material, the diluent and the binding agent are mixed to be prepared into granules, the granules are then mixed with the lubricating agent evenly, and the tablets are obtained through tabletting. The flunarizine hydrochloride matrix sustained-release tablets overcome the defect that in the prior art, multiple times of administration needs to be conducted due to the short relative half-life period of flunarizine hydrochloride, and it can be avoided that to maintain the blood concentration with the effective amount to reach a treatment effect, initial over-doses in the initial administration period occur, and consequently many side effects are caused; the problem that due to insufficient doses, the pain of a patient cannot be relieved, and the treatment effect cannot be achieved is also solved.
Owner:ZHENGZHOU BARY ANIMAL PHARMA
Western medicine composition for treating lame impediment
InactiveCN106668021AReduce dosageHeal fastOrganic active ingredientsAntipyreticWestern medicineSide effect
The invention discloses a western medicine composition for treating lame impediment. The western medicine composition for treating lame impediment is prepared from the following raw materials in parts by weight: 1-5 parts of catechin, 5-13 parts of North America eriodictin, 11-19 parts of flunarizine hydrochloride and 3-7 parts of benzophenone. A preparation method comprises the following steps: mixing catechin with North America eriodictin and grinding, adding deionized water, and carrying out stirring treatment for 38-40 minutes at the temperature of 73 DEG C, so that a mixture A is obtained; and mixing the prepared benzophenone solution with the mixture A, carrying out stirring treatment for 12 minutes at the temperature of 68 DEG C, adding catechin, then carrying out ultrasonic treatment for 25 minutes at the temperature of 50 DEG C, then stirring until dry at the temperature of 60 DEG C, and granulating, so that the western medicine composition for treating lame impediment is obtained. The western medicine composition can treat both symptoms and root causes of lame impediment and has the characteristics of definite effect and quick healing, near-term and long-term curative effects are respectively better than those of a non-steroidal anti-inflammatory western medicine, and the western medicine composition disclosed by the invention is low in dosage, has no toxic or side effect and no allergy reaction and is high in curative ratio, low in recurrence rate, low in price and easily acceptable to patients.
Owner:郑州欧印数字科技有限公司
Drug to reduce side effects of flunarizine hydrochloride to treat angioneurotic headache
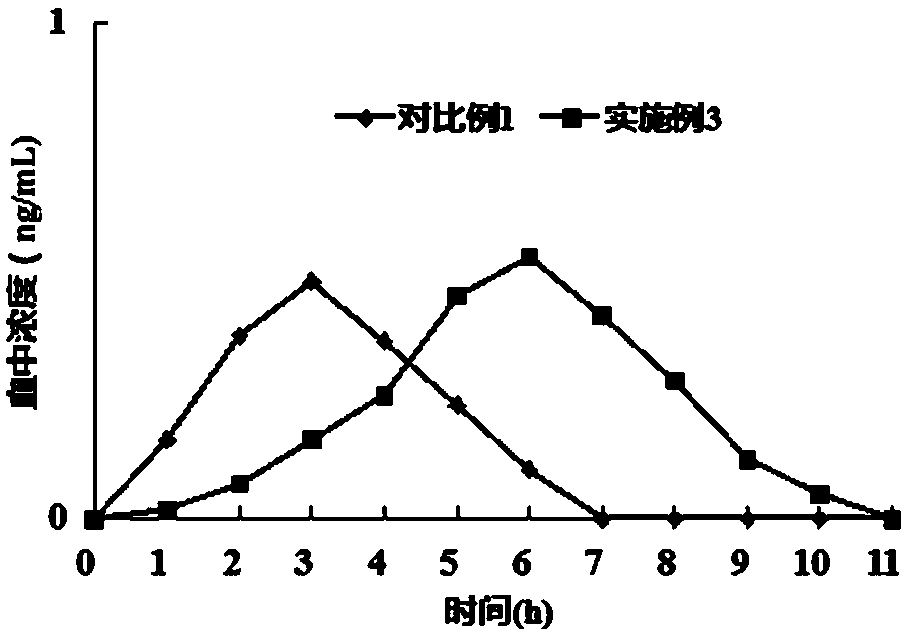
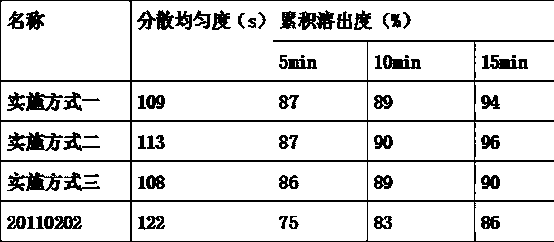
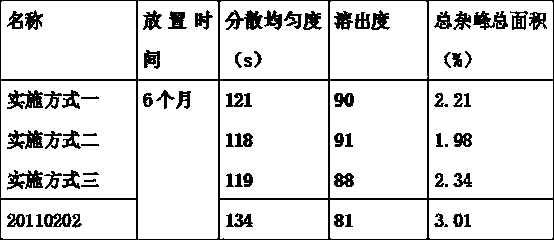
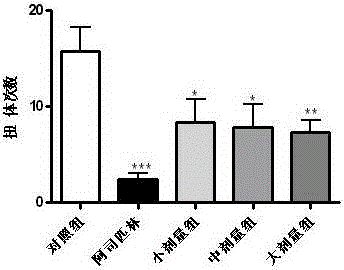
The invention discloses a drug to reduce side effects of flunarizine hydrochloride to treat angioneurotic headache and belongs to the technical field of drug application. Tests prove that the drug herein has stable and qualified indexes, such as content, dissolution rate, dispersion uniformity, total area of impure peaks, and appearance. Flunarizine hydrochloride and Ophiorrhiza japonica extract have significant synergistic effect; the drug has little toxic and side effects and has total cure rate of up to 97.5%.
Owner:LINYI DELIKANG MEDICAL REHABILITATION EQUIP CO LTD
A film that dissolves rapidly in the oral cavity and its preparation method
ActiveCN103211801BLess pain timeAvoid reducing efficacyNervous disorderAntipyreticCobra venomDecomposition
Owner:SUZHOU RENBEN PHARMA
A kind of detection method for dissolution determination of flunarizine hydrochloride capsules
ActiveCN110542737BSolve the adsorption problemExtended service lifeComponent separationPhosphateFlunarizine Hydrochloride
The invention discloses a detection method for the dissolution determination of flunarizine hydrochloride capsules. The method is as follows: the dissolution method is the paddle method, 100 revolutions per minute, and the medium with sodium lauryl sulfate is added as the dissolution medium; high-efficiency liquid phase Chromatographic detection of dissolved matter, with octadecylsilane bonded silica gel as filler; UV detector, detection wavelength is 251-255nm; column temperature is 25-45°C; flow rate is 0.9-1.1ml / min; mobile phase is Methanol and Phosphate Buffers. The method of the invention solves the adsorption problem of the flunarizine hydrochloride capsule filter membrane, and the obtained chromatographic peak shape is good, the column efficiency is high, the obtained result can be made more accurate, the service life of the chromatographic column is prolonged, and the test cost is reduced .
Owner:贵州缔谊健康制药有限公司 +1
A kind of flunarizine hydrochloride pharmaceutical composition
ActiveCN105998024BLong-term storage stabilitySolve the splinter problemOrganic active ingredientsNervous disorderDocusate SodiumFlunarizine Hydrochloride
The invention relates to a flunarizine-hydrochloride pharmaceutical composition and a preparing method thereof, and belongs to the technical field of medicine making. The flunarizine-hydrochloride pharmaceutical composition in the technical scheme is characterized in that every one thousand compositions comprise 5.9 g of flunarizine hydrochloride, 62.5 g-82.5 g of lactose, 70 g-90 g of microcrystalline cellulose, 3.2-12.8 g of sodium carboxymethylcellulose, 0.8 g of silicon dioxide, 0.8 g of magnesium stearate and 1-1.6 g of docusate sodium. According to the flunarizine-hydrochloride pharmaceutical composition and the preparing method thereof, a tablet with the small resolving degree RSD is provided through reasonable compatibility, the prepared tablet is stable in long-term storage, and high-quality medicine is provided for a clinic.
Owner:DISHA PHARMA GRP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com