Preparation method for flunarizine hydrochloride crystal
A crystallization technology of flunarizine hydrochloride, which is applied in the field of preparation of crystallization of flunarizine hydrochloride to achieve the effects of complete crystal form, low labor intensity and uniform particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
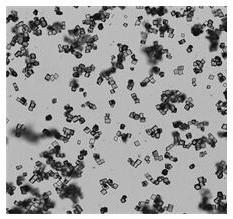
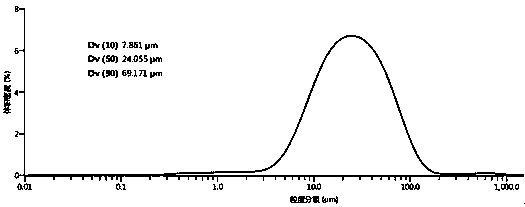
[0029] Add 20 g of flunarizine hydrochloride into a three-necked flask filled with 200 mL of a mixed solvent of methanol and water (the volume ratio of methanol to water is 90:10), raise the temperature to 50°C and stir to dissolve. Then add activated carbon for decolorization and filter; transfer the filtrate to a crystallizer at 50°C, start to evaporate and crystallize, control the vacuum degree of the system at 0.03MPa, and the evaporation rate is 30mL / hour. After growing the crystal for 30 minutes, continue to evaporate and crystallize, and stop the evaporation when the total amount of solvent evaporated reaches 60 mL. The crystal was grown with heat preservation and stirring for 30 minutes, and then the temperature was lowered to 5° C. with stirring at a cooling rate of 5° C. / hour, and the temperature was kept and stirred for 1 hour. Suction filtration, and rinse the filter cake with methanol, and dry at 40°C under normal pressure for 8 hours to obtain 18.06g block-shaped...
Embodiment 2
[0031] Add 20g of flunarizine hydrochloride into a three-necked flask filled with 300mL of ethanol and water mixed solvent (the volume ratio of ethanol to water is 95:5), raise the temperature to 65°C and stir to dissolve. Then add activated carbon for decolorization and filter; transfer the filtrate to a crystallizer at 65°C, start to evaporate and crystallize, control the vacuum degree of the system at 0.05MPa, and the evaporation rate is 50mL / hour. After growing the crystal for 45 minutes, continue to evaporate and crystallize, and stop evaporation when the total amount of solvent evaporated reaches 195 mL. The crystal was grown with heat preservation and stirring for 120 minutes, and then the temperature was lowered to 8° C. with stirring at a cooling rate of 6° C. / hour, and the heat preservation and stirring were carried out for 45 minutes. Suction filtration, rinse the filter cake with ethanol, and dry under normal pressure at 60°C for 10 hours to obtain 18.27g of block-...
Embodiment 3
[0033] Add 20g of flunarizine hydrochloride into a three-necked flask filled with 400mL of ethanol and water mixed solvent (the volume ratio of ethanol to water is 85:15), raise the temperature to 50°C and stir to dissolve. Then add activated carbon to decolorize and filter; transfer the filtrate to a crystallizer at 65°C, start to evaporate and crystallize, control the vacuum degree of the system at 0.04MPa, and the evaporation rate is 60mL / hour. After growing the crystal for 45 minutes, continue to evaporate and crystallize, and stop evaporation when the total amount of solvent evaporated reaches 200mL. The crystal was grown with heat preservation and stirring for 100 minutes, and then the temperature was lowered to 5° C. with stirring at a cooling rate of 5° C. / hour, and the heat preservation and stirring were carried out for 45 minutes. Suction filtration, rinse the filter cake with ethanol, and dry under normal pressure at 60°C for 12 hours to obtain 18.06 g of block-shap...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Master granularity | aaaaa | aaaaa |
| Master granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com