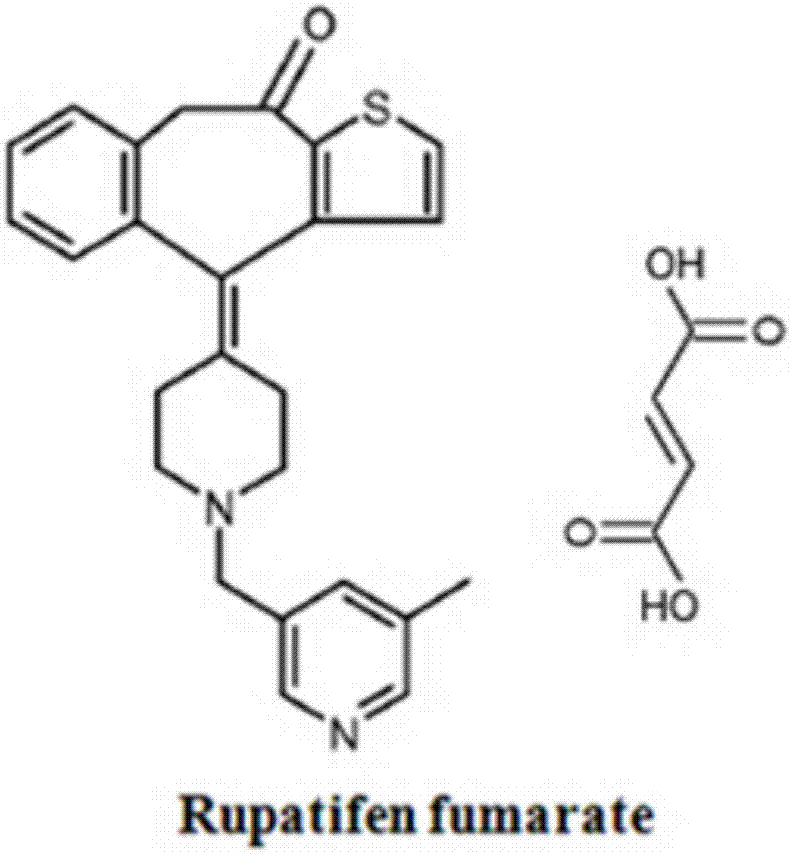
A kind of rupatifene fumarate mixed crystal and preparation method thereof
A fumaric acid, mixed crystal technology, applied in organic chemistry and other directions, to achieve good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
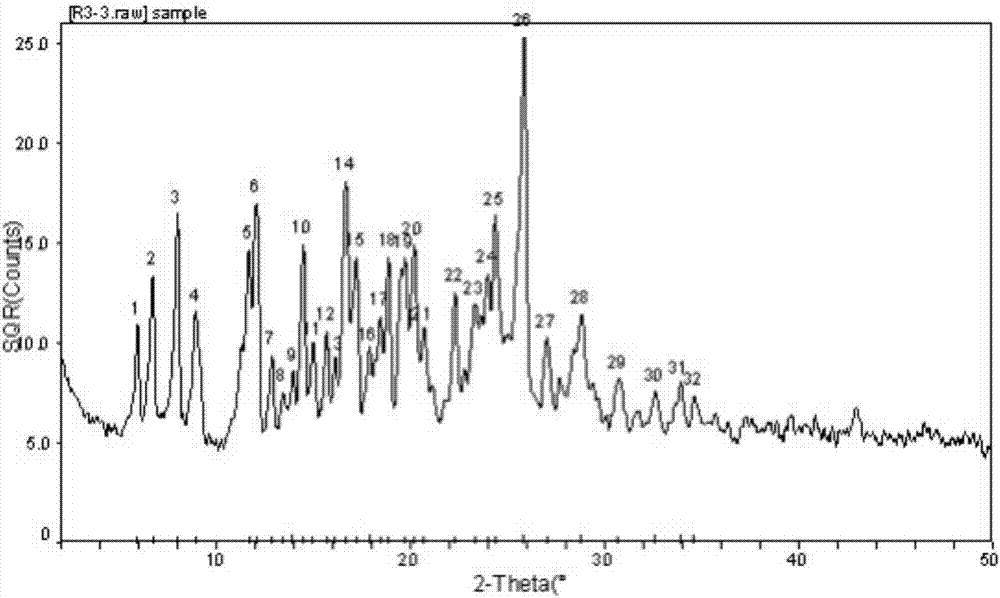
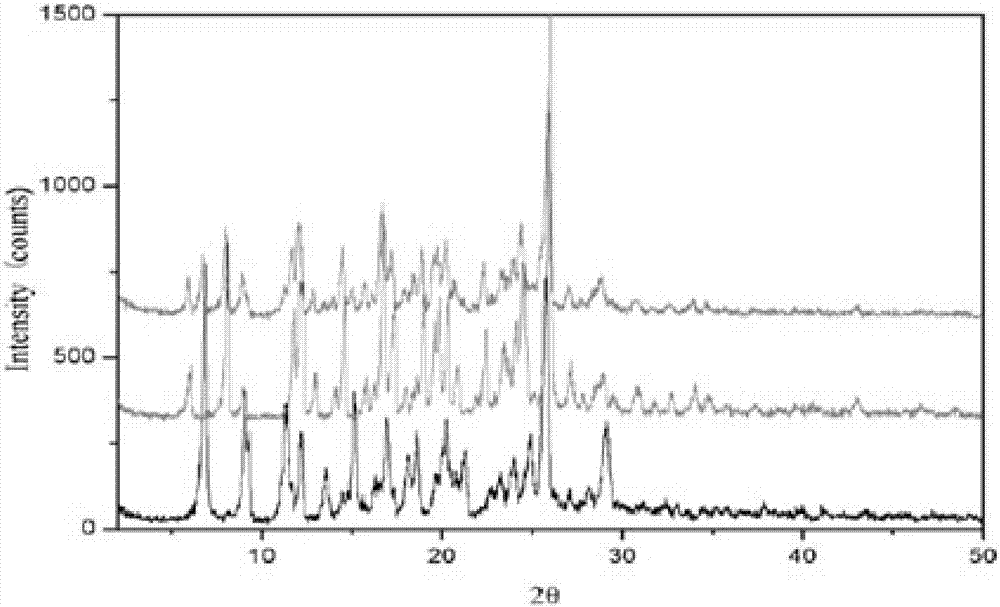
Image
Examples
Embodiment 1
[0020] Dissolve 1.0g rupatifene free base and 1.0g fumaric acid in an appropriate amount of 20ml 95% ethanol solution, stir and react at room temperature for 1hr, concentrate under reduced pressure to 25-30% volume, and cool the solution naturally to room temperature, then in Static crystallization at 2-8°C, rod-shaped crystals precipitated, filtered, washed with 95% ethanol solution at 2-8°C, dried under reduced pressure at 45°C, and obtained.
Embodiment 2
[0022] Weigh 2.0g rupatifene free base and 0.5g fumaric acid and dissolve in an appropriate amount of 50ml 95% ethanol solution, stir and react at room temperature for 2hr, concentrate under reduced pressure to 25-30% volume, and cool the solution naturally to room temperature, then Static crystallization at 2-8°C, rod-shaped crystals precipitated, filtered, washed with 95% ethanol solution at 2-8°C, dried under reduced pressure at 45°C, and obtained.
Embodiment 3
[0024] Dissolve 1.0g rupatifene free base and 2.0g fumaric acid in an appropriate amount of 20ml 95% ethanol solution, stir and react at room temperature for 1hr, concentrate under reduced pressure to 25-30% volume, and cool the solution naturally to room temperature, then Static crystallization at 2-8°C, rod-shaped crystals precipitated, filtered, washed with 95% ethanol solution at 2-8°C, dried under reduced pressure at 45°C, and obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com