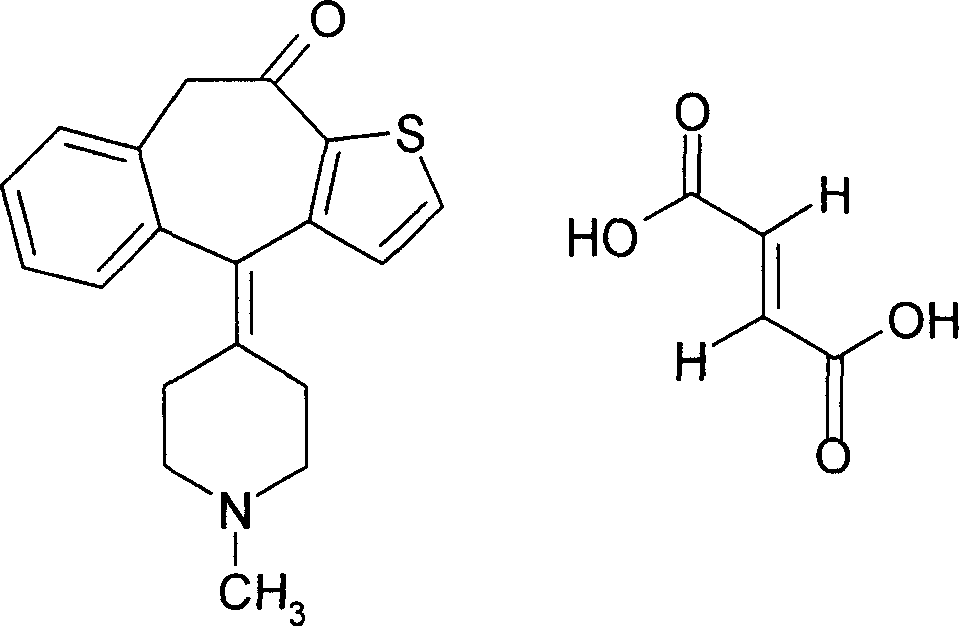
Boletic acid ketotifen eye drops containing sodium hyaluronate and its preparing process
A technology of ketotifen fumarate and sodium hyaluronate, applied to medical preparations containing active ingredients, pharmaceutical formulas, organic active ingredients, etc., can solve problems such as inconvenient use and poor reducibility, and achieve the effect of preventing spillage and loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The preparation method of the ketotifen fumarate eye drops of the present invention is very simple, and can be produced by adopting a conventional eye drop production process, and simple physical mixing is enough.
[0034] The using method of ketotifen fumarate eye drops of the present invention is as follows:
[0035] Eye drops, 1-2 drops at a time, 3 times a day.
[0036] Because the ketotifen fumarate eye drop of the present invention is added with sodium hyaluronate, its antiallergic effect is more durable, and the ocular surface is moistened and comfortable at the same time.
[0037] The present invention also provides a method for preparing the eye drops, which comprises mixing 0.25-1200 parts by weight of ketotifen fumarate, 1-1200 parts by weight of sodium hyaluronate, 100-3000 parts by weight of isotonic The regulator and the pharmaceutically acceptable carrier adjust the pH value of the ketotifen fumarate eye drop to 4.0-7.5.
[0038] The present invention als...
Embodiment 1
[0049] 100ml water for injection contains:
[0050] Ketotifen 50mg
[0051] Sodium Hyaluronate 150mg
[0053] Benzalkonium Chloride 10mg
[0054] Preparation Process:
[0055] 1. Add sodium hyaluronate to 25ml of water for injection, and swell at room temperature to obtain a fully swollen sodium hyaluronate solution.
[0056] 2. Sodium chloride and benzalkonium chloride were dissolved in 50ml of water for injection, then added into the fully swollen sodium hyaluronate solution, stirred evenly to obtain a mixed solution, and left to stand for use.
[0057] 3. Add ketotifen fumarate to the mixed solution obtained in step 2, and stir evenly to obtain a solution.
[0058] 4. Adjust the pH of the solution obtained in step 3 to 5.2 with hydrochloric acid.
[0059] 5. Add 25ml of water for injection to the solution obtained in step 4, stir evenly and filter and fill.
Embodiment 2
[0061] 100ml water for injection contains:
[0062] Ketotifen Fumarate 80mg
[0063] Sodium Hyaluronate 300mg
[0064] Boric acid 1500mg
[0065] Borax 300mg
[0066] Methylparaben 30mg
[0067] Preparation Process:
[0068] 1. Add sodium hyaluronate to 25ml of water for injection, and swell at room temperature to obtain a fully swollen sodium hyaluronate solution.
[0069] 2. Dissolve boric acid, borax, and benzalkonium chloride in 50ml of water for injection, then add it into the fully swollen sodium hyaluronate solution, stir evenly to obtain a mixed solution, and set aside.
[0070] 3. Add ketotifen fumarate to the mixed solution obtained in step 2 to dissolve, and stir evenly to obtain a solution.
[0071] 4. Add 25ml of water for injection to the solution obtained in step 3, stir evenly and filter and fill. The resulting eye drops had a pH of 4.5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com