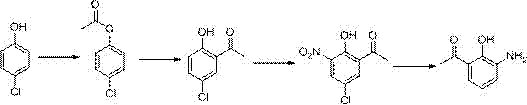
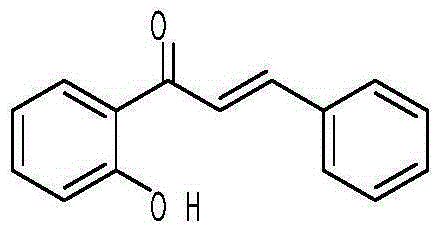
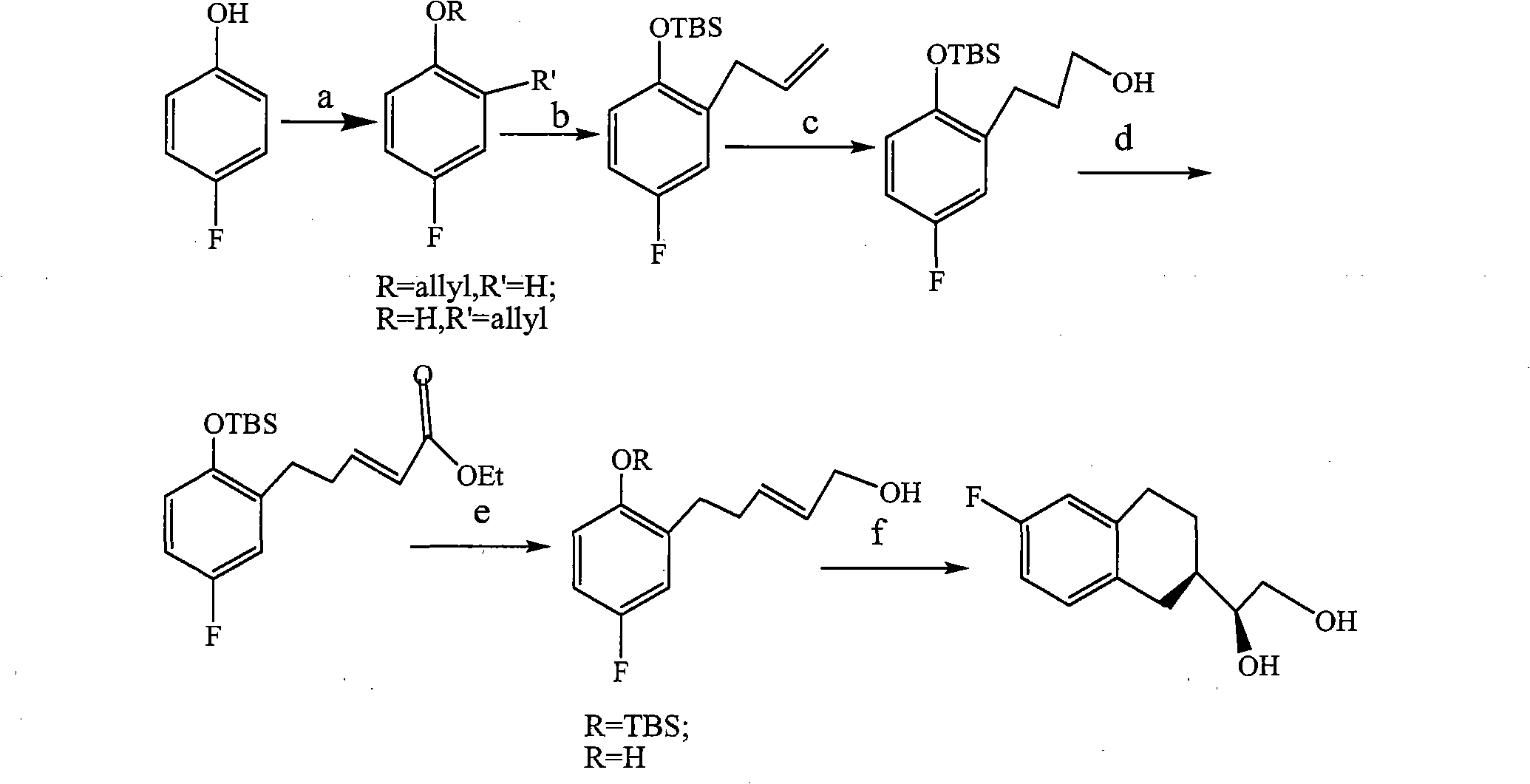
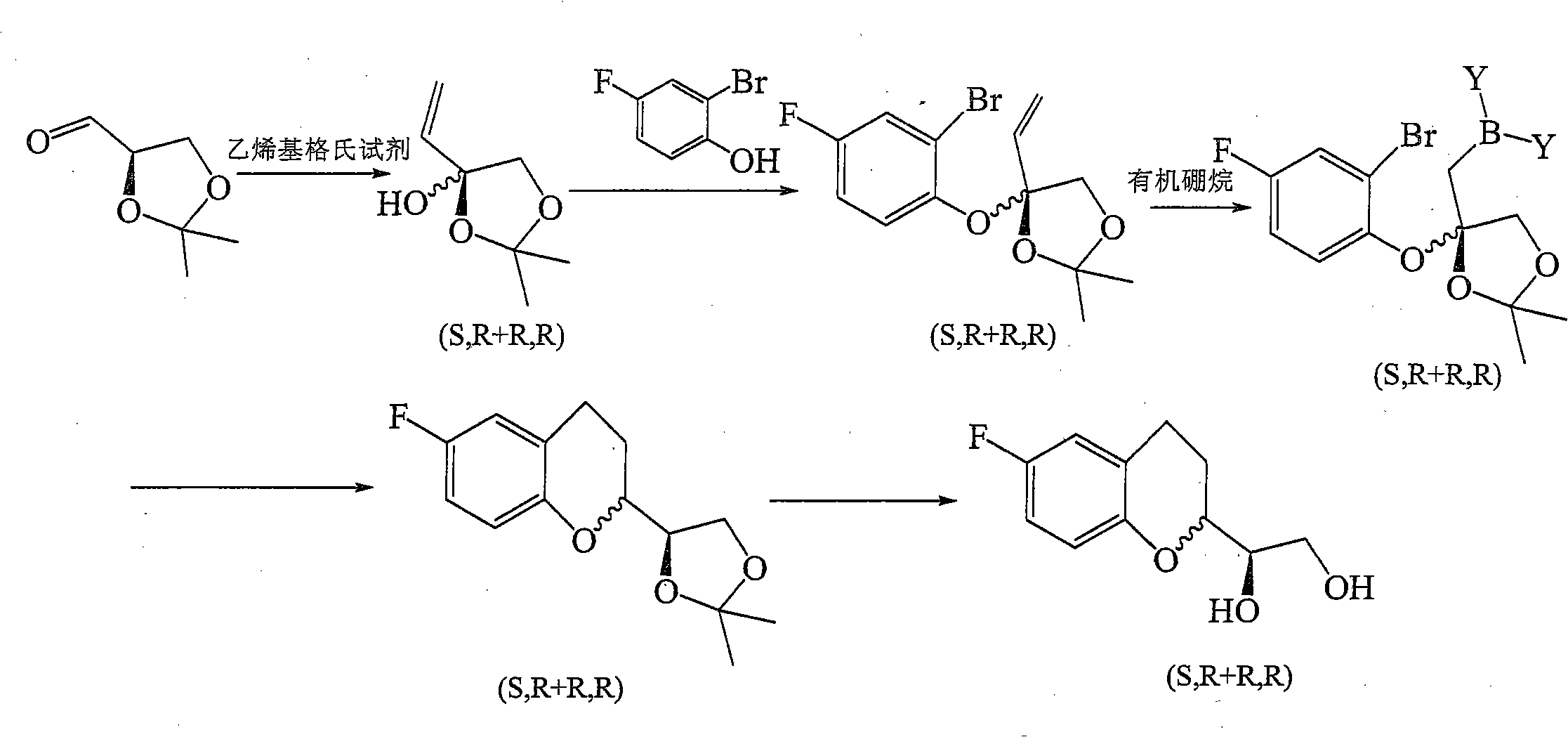
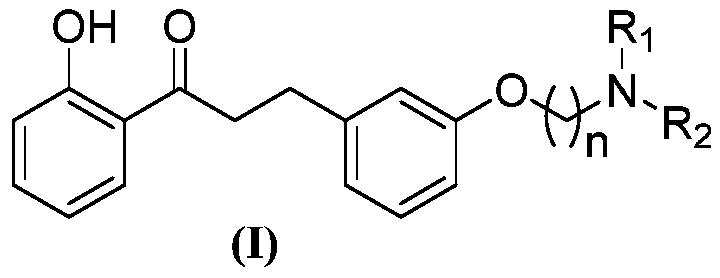
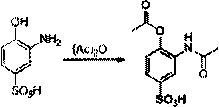Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
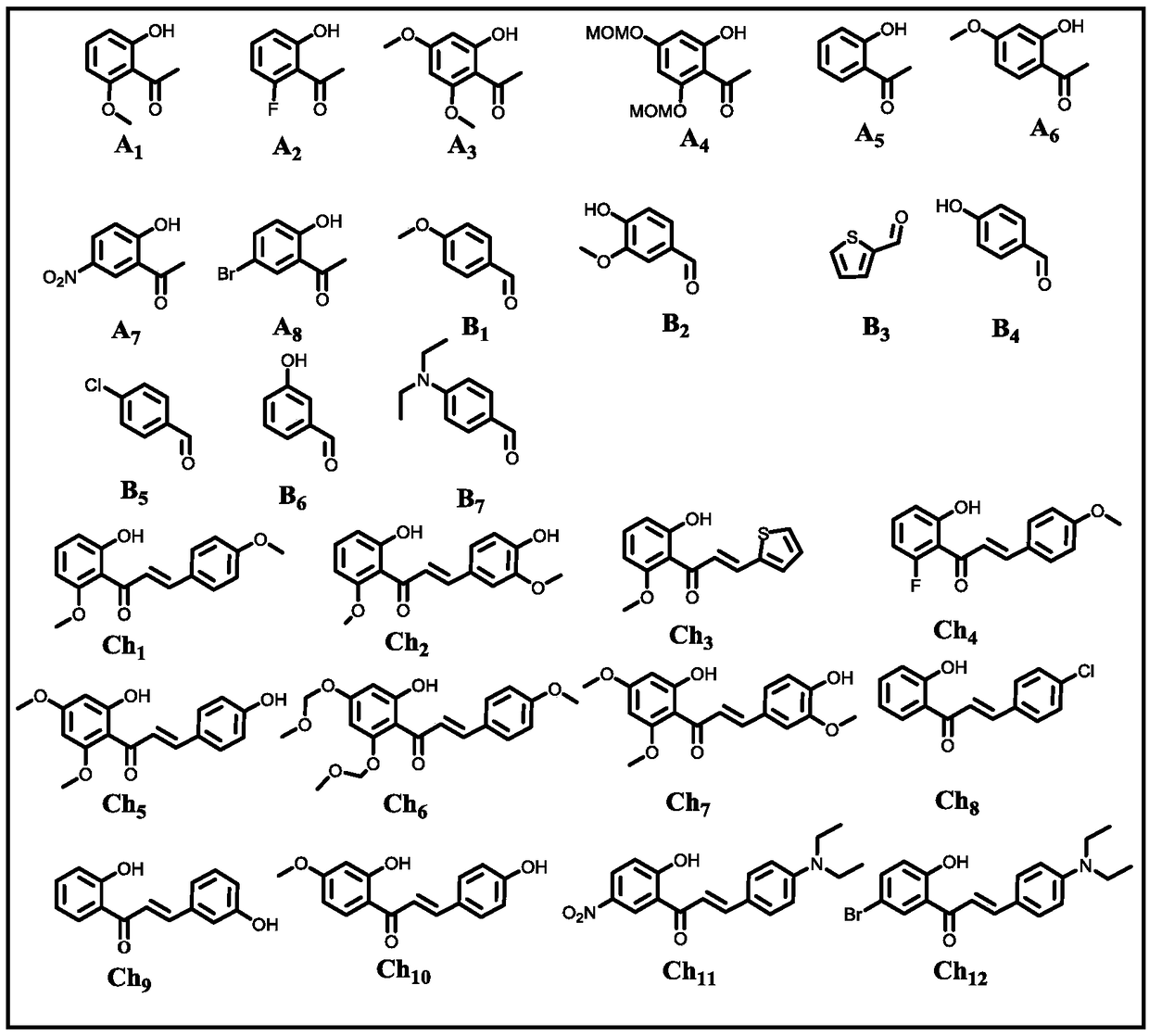
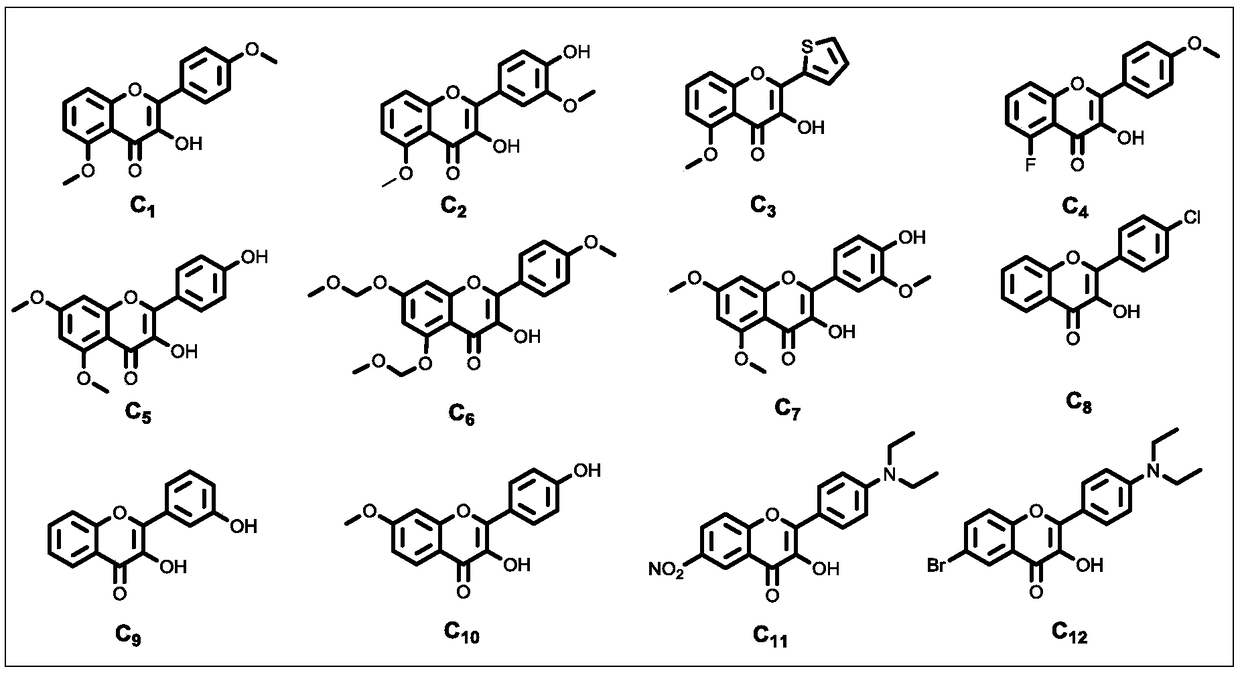
33 results about "2-HYDROXYACETOPHENONE" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
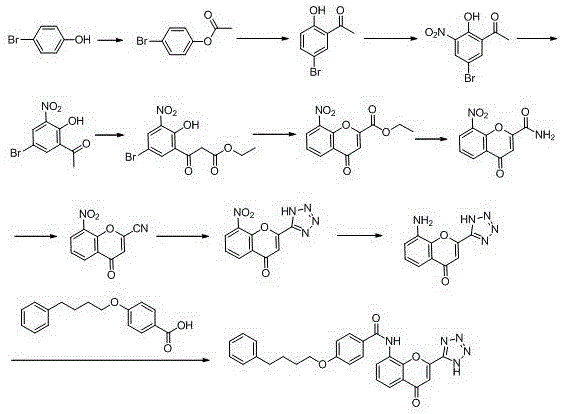
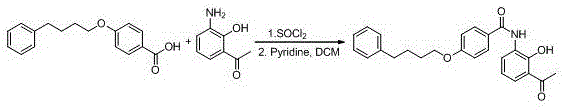
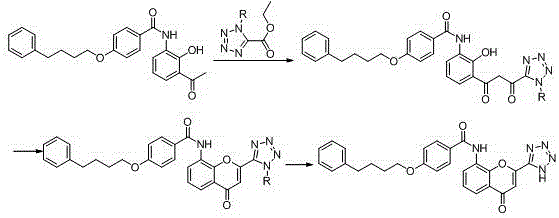
New preparation method of Pranlukast
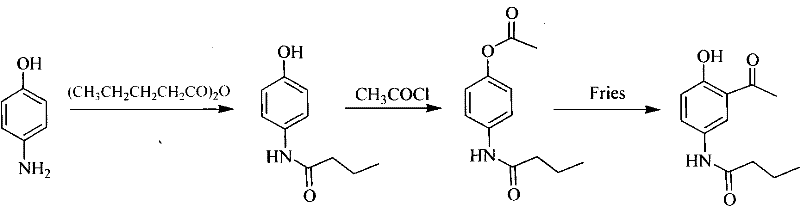
The invention provides a new preparation method of drug Pranlukast for treating asthma. The new preparation method includes the specific steps that with 2-aminophenol-4-sulfonic acid as a starting material, a key intermediate 3-amino-2-hydroxyacetophenone is prepared by means of acylation, Fries rearrangement and deprotection, then reacts with 4-(phenylbutoxy)benzoic acid, and then is subjected to condensation with ethyl 1H-tetrazole-5-acetate, and finally preparation is achieved through ring closing under the acidic condition. Compared with the prior art, the raw material used for the new preparation method is low in price and easy to obtain, industrialization of a process can be achieved easily, and the obtained final product is high in purity; and no dangerous process exists, equipment is simple, and the route is novel.
Owner:上海微巨实业有限公司
Method for preparing (R)-phenylglycol from SD-AS sequence coupled (R)-carbonyl reductase and glucose dehydrogenase
InactiveCN104830744AHigh optical purityHigh yieldBacteriaMicroorganism based processesEscherichia coliRecombinant escherichia coli
The invention discloses a method for preparing (R)-phenylglycol from SD-AS sequence coupled (R)-carbonyl reductase and glucose dehydrogenase and belongs to the technical field of biological catalytic asymmetric transformation. The invention provides recombinant escherichia coli E. coli RIL / pET-R-SD-AS-G. The recombinant escherichia coli E. coli RIL / pET-R-SD-AS-G is preserved in the China center for type culture collection (CCTCC) and has a preservation number of CCTCC NO: M2015170. R-carbonyl reductase and glucose dehydrogenase are coupled by a coexpression way, and the coupled enzyme, a cultured recombinant strain and 2-hydroxyacetophenone as a substrate undergo a catalytic asymmetric transformation reaction under optimized biotransformation reaction conditions to produce (R)-phenylglycol with optical purity of 100% and a yield of 99.9%. The method utilizes a SD-AS sequence high efficiency coupling double-enzyme technology, solves the problem of limitation of chiral catalytic reaction coenzyme regeneration cycle and provides an effective approach for high efficiency bio-preparation of the (R)-phenylglycol.
Owner:JIANGNAN UNIV
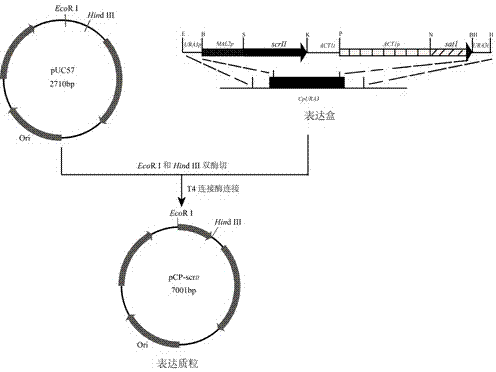
Method for using recombinant candida parapsilosis strain to efficiently prepare (S)-phenyl glycol
InactiveCN104774778AHelps understand the effect of stereoselectivityFungiMicroorganism based processesPhenyl glycolDrug biotransformation
The invention provides a method for using a recombinant candida parapsilosis strain to efficiently prepare (S)-phenyl glycol and belongs to the technical field of biological catalytic asymmetric transformation. The invention provides the recombinant candida parapsilosis strain Candida parapsilosis pCP-scrII preserved at the typical culture preservation center in China and marked with a preservation CCTCC NO: M2015107. According to the method, an expression plasmid of exogenous protein in candida parapsilosis is established, S-carbonyl reductase II is integrated to a candida parapsilosis genome to obtain the pCP-scrII, the candida parapsilosis is converted through electric shock to obtain the recombinant candida parapsilosis strain. By optimizing biotransformation reaction conditions, catalytic conversion is conducted on 2-hydroxyacetophenone through the recombinant strain to obtain the product S-phenyl glycol, the optical purity and yield of the product are up to 99.9%, an effective way is provided for efficient and low-cost preparation of the S-phenyl glycol, and a solid foundation is laid for industrial application of biological catalysis of chiral alcohol.
Owner:JIANGNAN UNIV
Synthesis of (R)-styrene glycol by coupling acceleration of (R)-carbonyl reduction enzyme and formic dehydrogenase
ActiveCN101469318AHigh optical purityHigh yieldBacteriaMicroorganism based processesEscherichia coliHigh concentration
The invention relates to promotion of synthesis of (R)-styrene glycol by coupling (R)-carbonyl reductase and formic dehydrogenase, which belongs to the technical field of asymmetrical transformation of biological catalysis. The invention provides a recombinant Escherichia coli, namely E.coli Rosetta / pETDuet-rcr-fdh, with a preservation number of CCTCC NO: M208123, wherein the (R)-special carbonyl reductasei and the formic dehydrogenase are coupled by a co-expression mode; asymmetrical transformation reaction is catalyzed by utilizing a cultured recombinant strain and taking 2-hydroxyacetophenone as a substrate; and the optical purity of the (R)-styrene glycol reaches 100 percent e.e., and the yield of the (R)-styrene glycol reaches 85.9 percent by optimizing the biotransformation reaction conditions. The invention utilizes double-enzyme coupling technology to solve the problem of limitation of regeneration cycle of coenzyme under the condition of high-concentration substrate, provides an effective path for high-efficiency and low-cost preparation of the (R)-styrene glycol, and lays a foundation for industrial application of the biosynthesized (R)-styrene glycol.
Owner:JIANGNAN UNIV
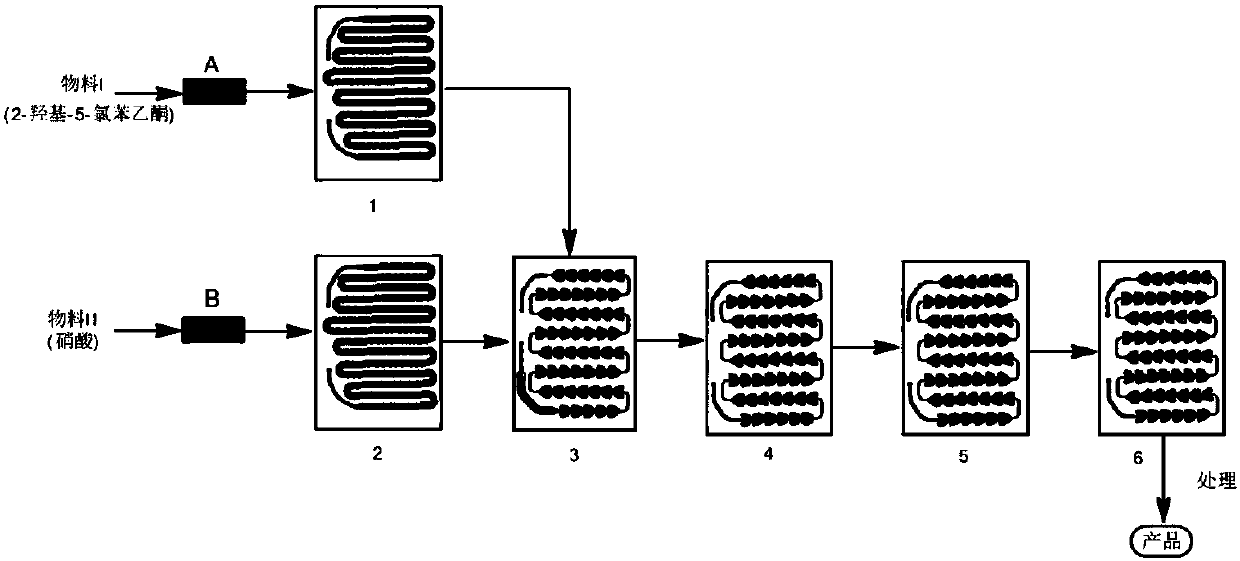
Preparation method for 5-chlorine-2-hydroxyl-3-nitroacetophenone
InactiveCN104402728AReduce usageReduce manufacturing costNitro compound preparationDistillationSolvent free
The invention belongs to the technical field of preparation of medical intermediates, and particularly relates to a preparation method for 5-chlorine-2-hydroxyl-3-nitroacetophenone. The preparation method comprises the steps: under the existence of a catalyst, reacting parachlorophenol with an acetylation reagent in a solvent-free condition, and performing decompression distillation to obtain acetic acid parachlorophenol and glacial acetic acid serving as a byproduct; adding lewis acid into acetic acid parachlorophenol to perform Fries rearrangement reaction, adding water into a system after the reaction is completed, stirring the mixture to obtain solid sediments, performing recrystallization by using methyl alcohol to obtain 5-chlorine-2-hydroxyacetophenone, adding glacial acetic acid for dissolving, then dropping a nitration reagent, and stirring and filtering to obtain 5-chlorine-2-hydroxyl-3-nitroacetophenone. According to the preparation method, the production cost is lowered, the reaction period is short, the purity is high, the yield is high, the operation is simple and convenient, and industrialization is easily realized.
Owner:SHANDONG JINCHENG PHARMA & CHEM
Carbonyl reductase mutant mut-AcCR(l147V/G152L) and application and coding genes thereof
ActiveCN109355265AOvercoming activityOvercome the disadvantage of poor substrate toleranceOxidoreductasesGenetic engineeringSpecific enzymeMutant
The invention provides a carbonyl reductase mutant mut-AcCR(l147V / G152L) and application and coding genes thereof. A carbonyl reductase AcCR can catalyze various prochiral carbonyl compounds to be subjected to asymmetric reduction, however, the carbonyl reductase AcCR has the low tolerance for activities and substrates of aromatic compounds, the enzyme molecule modification means is adopted for mutating the glycosylation reductase AcCR, the mutant mut-AcCR(l147V / G152L) is obtained, the specific enzyme activity of the mutant for 2-hydroxyacetophenone can reach 6.4 U / mg, and the specific activity of the non-mutated carbonyl reductase is improved by 17.4 times. The substrate tolerance concentration is increased to 200 mmol / L from 50 mmol / L. The carbonyl reductase mutant is widely applied to carbonyl compound asymmetric reduction.
Owner:SOUTH CHINA UNIV OF TECH
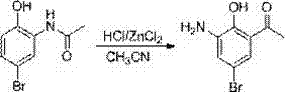
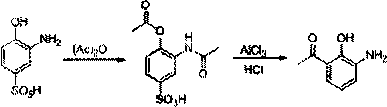
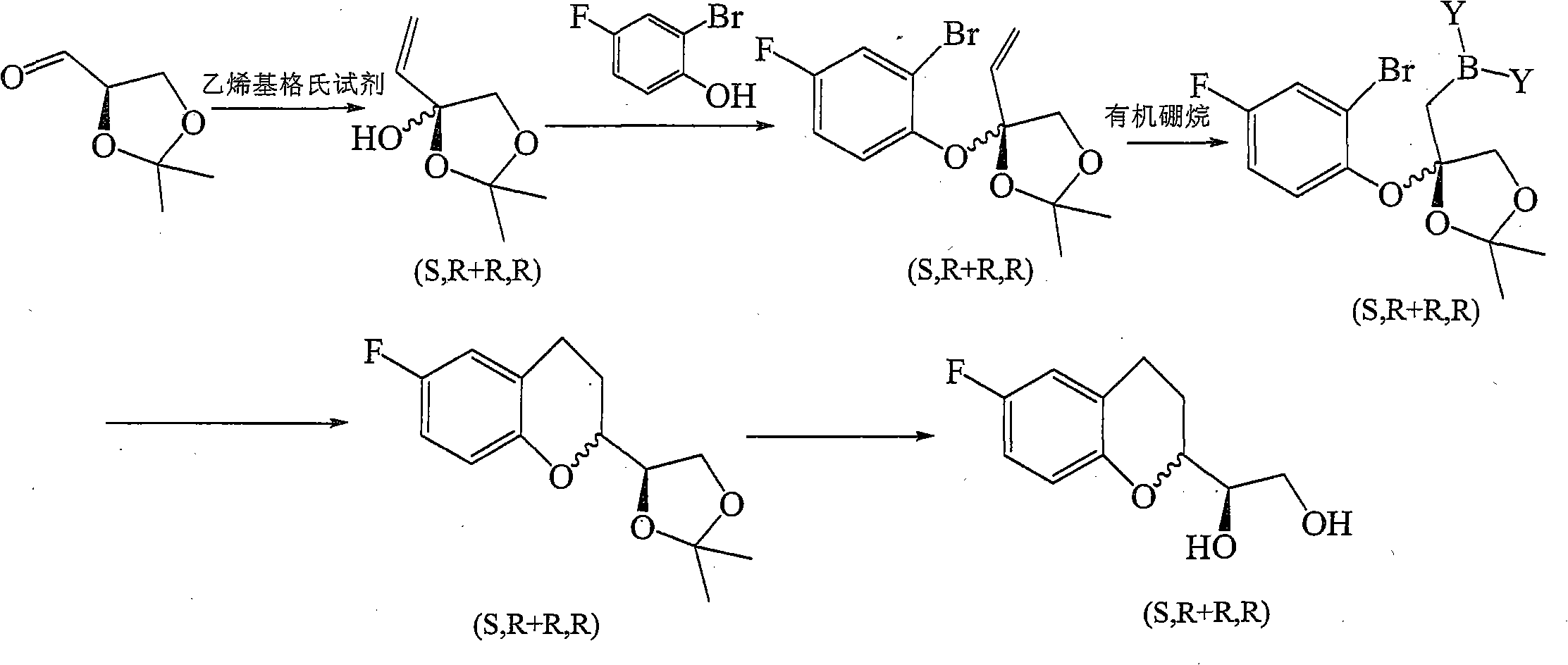
Method for preparing pranlukast key intermediate 3-amino-2-hydroxyacetophenone
ActiveCN107098822ACheap and easy to getRich choiceOrganic compound preparationCarboxylic acid amides preparationAcetic anhydrideBromine
The invention provides a method for preparing a pranlukast key intermediate 3-amino-2-hydroxyacetophenone. The method takes 2-aminophenol as an initial raw material, 2-aminophenol and acetic anhydride are subjected to a synthesis reaction in water to obtain 2-acetaminophenol, then 2-acetamido-4-bromophenol is prepared by 2-ace-taminophenol and NBS under room temperature, 2-hydroxy-3-amino-5-bromoacetophenone is prepared by a Hoesch reaction, finally 2-hydroxy-3-amino-5-bromoacetophenone is dissolved in ethanol, and a Pd / C is added for catalytic hydrogenation and bromine removal to obtain the 3-amino-2-hydroxyacetophenone. The raw materials have the advantages of low cost, easy acquisition, diversified raw material selection, easy realization of production technology, and easy control, the purity of the final product is high, no dangerous process is generated, the equipment is simple, the synthesis route is novel, the synthesis route is short, the production power is increased, and production processing cost is reduced.
Owner:上海微巨实业有限公司
Preparation method for 5-fluorin-2-hydroxyacetophenone
InactiveCN102557909AOrganic compound preparationCarbonyl compound preparationFries rearrangementClaisen rearrangement
The invention provides a preparation method for 5-fluorin-2-hydroxyacetophenone. The preparation method comprises the following steps of: performing double esterification on amino groups and phenolic hydroxy in one step by taking amino-phenol as a raw material; performing Fries rearrangement under the condition of aluminum chloride / sodium chloride; heating a hydrolyzate after performing fluorine diazotization to obtain finished 5-fluorin-2-hydroxyacetophenone. The preparation method has the advantages of low prices of raw materials, relatively mild reaction conditions, total yield of up to 54.5 percent and industrial production application value.
Owner:SHANGHAI SINOFLUORO SCI
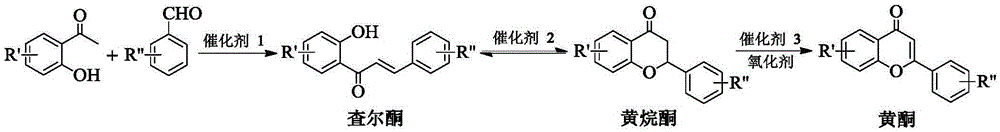
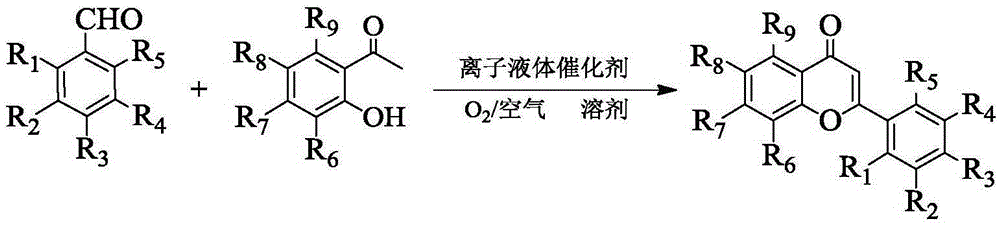
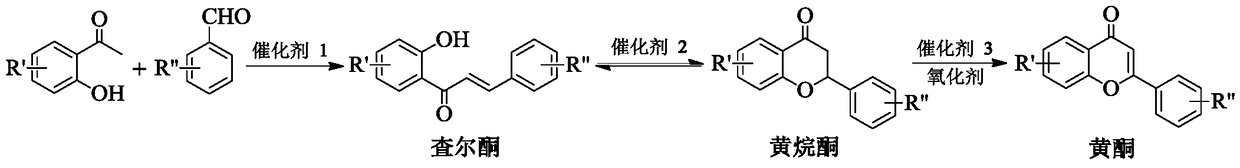
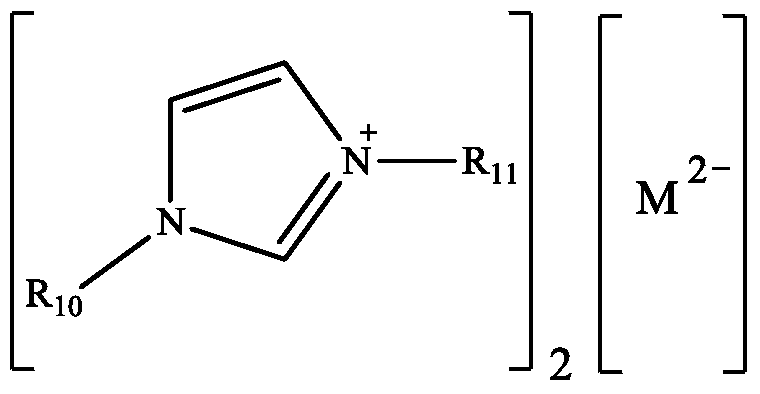
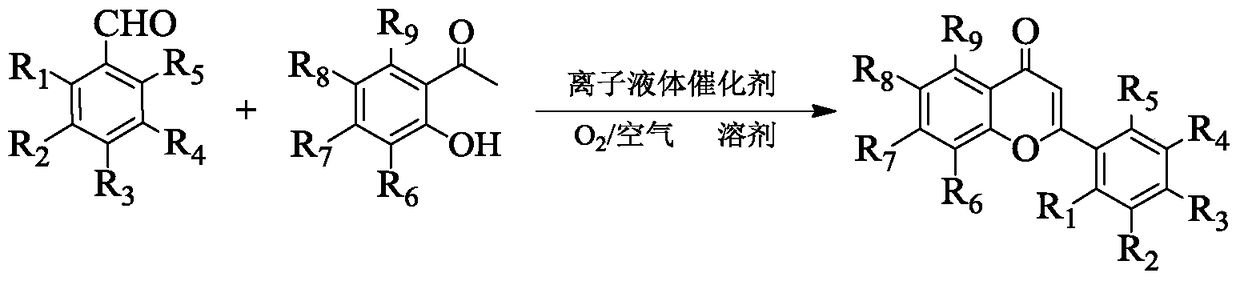
Method for synthesizing flavonoids compound in one step by virtue of catalysis of 1,3-dialkylimidazolium oxometallate
ActiveCN105294627AHigh yieldHigh productivityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsOrganic solventBenzaldehyde
The invention discloses a method for synthesizing a flavonoids compound in one step by virtue of catalysis of 1,3-dialkylimidazolium oxometallate. The method is characterized by comprising the following steps: sequentially adding raw material benzaldehyde or benzaldehyde derivatives, raw material 2-hydroxyacetophenone or 2-hydroxyacetophenone derivatives, an ion liquid catalyst and an organic solvent into a reactor, stirring, heating to 50 to 90 DEG C, reacting for 2 to 7 hours at a constant temperature by taking oxygen or air as an oxidant, cooling, distilling under reduced pressure, carrying out the column chromatography, and re-crystallizing and separating to obtain the target product flavonoids compound. The method has the characteristics that the ion liquid is used as the catalyst, the yield of the flavonoids compound synthesized in one step reaches 85 percent or more, therefore, compared with the traditional synthetic method, the reaction flow is shortened, and the synthetic efficiency of the flavonoids compound is remarkably improved. The method has advantages of high product yield, low production cost, simple operation procedures, moderate reaction conditions and the like and is proved to be a novel method for high-efficiently synthesizing the flavonoids compound.
Owner:JIANGXI NORMAL UNIV
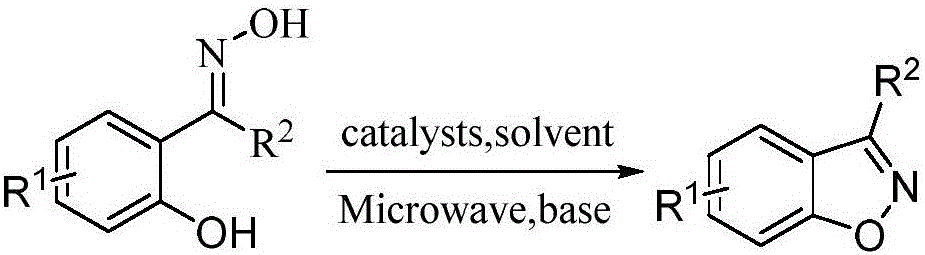
Method for preparing benzisoxazole in one pot based on 2-hydroxyacetophenone oxime and derivatives thereof
ActiveCN106543095AAbundant and easy to obtainShort synthetic stepsOrganic chemistryOrganic solventMicrowave
The invention relates to the technical field of medicinal chemistry and in particular discloses a method for preparing benzisoxazole in one pot based on 2-hydroxyacetophenone oxime and derivatives thereof. The preparation method is as follows: under microwave or oil bath heating conditions, in an organic solvent, one-pot inversion is carried out on 2-hydroxyacetophenone oxime and derivatives thereof to form benzisoxazole compounds by utilizing a fluorine-containing accelerator and alkali. The method for synthesizing benzisoxazole has the advantages that raw materials are rich in source and easy to get, synthetic steps are short, the operation is simple and convenient, the product yield is high, and the highest yield is 93%. A benzisoxazole structure exists in multiple drug molecules, so that the novel one-pot synthesis method disclosed by the invention has potential practical values.
Owner:HUAWEI TEHCHNOLOGIES CO LTD
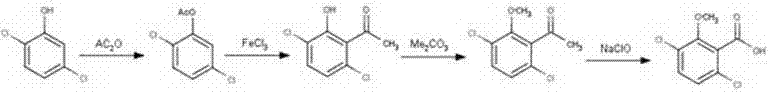
Synthesis method for raw material drugs of dicamba
InactiveCN107501089AReduce pollutionSimple processOrganic compound preparationCarboxylic acid esters preparationSynthesis methods2-methoxybenzoic acid
The invention relates to a synthesis method for raw material drugs of dicamba. The synthesis method for the raw material drugs of dicamba comprises the following steps: taking 2,5-dichlorophenol as a starting material, carrying out esterification under the actions of an organic solvent and an organic esterification reagent to generate 2,5-dichlorophenol acetate; dissolving 2,5-dichlorophenol acetate in the organic solvent and adding a catalyst to generate Fries rearrangement, thus generating 3,6-dichloro-2-hydroxyacetophenone; making 3,6-dichloro-2-hydroxyacetophenone react with a methylating reagent under the action of an acid-binding agent to obtain 3,6-dichloro-2-methylacetophenone; synthesizing dicamba (3,6-dichloro-2-methoxybenzoic acid) under the action of 3,6-dichloro-2-methylacetophenone. The synthesis method for the raw material drugs of dicamba disclosed by the invention is simple in process, highly available in raw materials and the catalyst, low in cost, simple in post-treatment operation, low in environmental pollution, high in safety of reaction operation, high in reaction yield, good in product quality and favorable for industrialization.
Owner:HENAN HDF CHEM CO LTD
Water-phase one-pot synthesis method of 3-flavonol and 3-flavonol derivative
InactiveCN109320488AStrong substrate adaptabilityGood substrate adaptabilityOrganic chemistryChemical synthesisSynthesis methods
The invention belongs to the field of chemical synthesis, and particularly relates to a water-phase one-pot synthesis method of 3-flavonol and a 3-flavonol derivative. According to the method, 2-hydroxyacetophenone, 2-hydroxyacetophenone derivatives, benzaldehyde and benzaldehyde derivatives are used as reaction substrates, or 2-hydroxy-chalcone and hydroxy-chalcone derivatives are used as reaction substrates; water or ethanol water solution is used as a solvent; under the aerobic condition at 20 to 100 DEG C, reaction is performed to obtain the 3-flavonol and the 3-flavonol derivative. The invention provides a fire-new reaction mechanism, and develops a novel 3-flavonol synthesis method with the advantages of high efficiency, convenience, high speed and wide substrate adaptability. The invention also provides a fire-new 3-flavonol derivative synthesized by the novel method; important application values are realized in the field of medical care sanitation.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
Flavonone compound synthesized through one-pot process and preparation method thereof
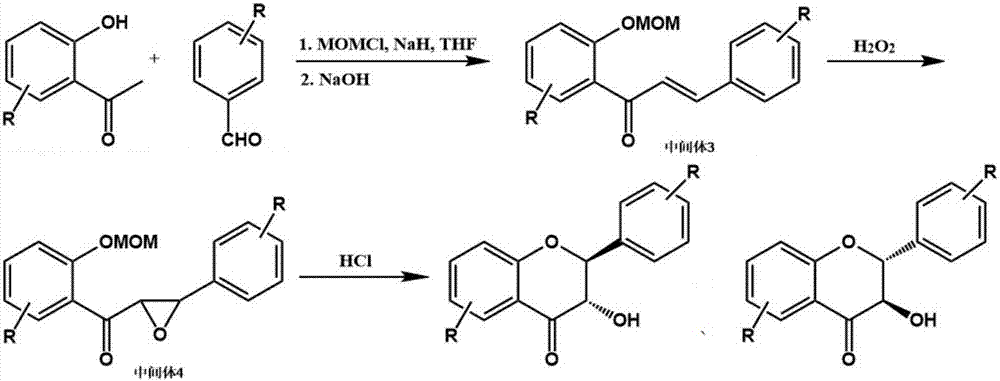
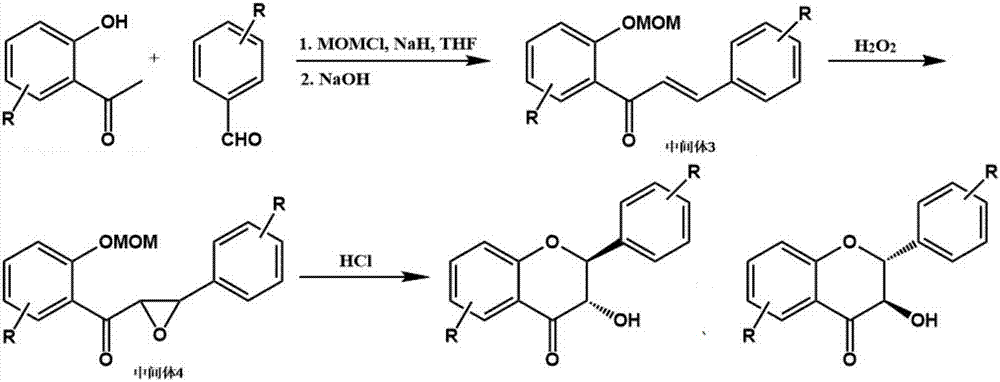
The invention discloses a flavonone compound synthesized through one-pot process and a preparation method thereof. The preparation method includes following steps: (1), using 2-hydroxyacetophenone compound and benzaldehyde compound as initial raw materials to prepare an intermediate 3 under joint action of chloromethyl methyl ether, sodium hydride and sodium hydroxide water solution; (2), adding hydrogen peroxide of 30% to obtain an intermediate 4; (3), feeding hydrogen chloride gas into reaction liquid, adding water and ethyl acetate for extraction after reaction is finished, drying and concentrating an organic phase, and passing a silica gel column for purifying to obtain the flavonone compound. The preparation method is mild in synthesis condition, unitary in reagent and solvent and simple in operation, treatment and purification of each reaction step are omitted, solvent consumption is lowered, and labor cost and time cost are reduced.
Owner:无锡紫杉药业股份有限公司
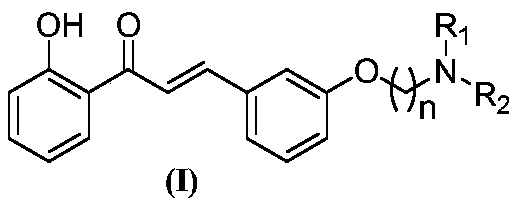
Amine alkoxy chalcone compound and preparation method and application thereof
ActiveCN110240549AGood inhibitory effectModerate or weak inhibitory activitySenses disorderNervous disorderDisease3-Hydroxybenzaldehyde
The invention discloses an amine alkoxy chalcone compound, and a preparation method and application thereof. The preparation method comprises the following steps: (1) 3-hydroxybenzaldehyde is used as a starting material and reacts with a dibromide compound under a first solvent and a first alkaline condition to obtain bromoalkoxybenzaldehyde; (2) the bromoalkoxybenzaldehyde reacts with secondary amine in a second solvent under a second alkaline condition to obtain amine alkoxy benzaldehyde; and (3) the amine alkoxy benzaldehyde and 2-hydroxyacetophenone are subjected to a third solvent and a third alkaline condition to obtain the target compound, i.e. amine alkoxy chalcone compound. The amine alkoxy chalcone compound has selective butyrylcholinesterase inhibitory activity, selective monoamine oxidase B inhibitory activity, selective metal ion chelating activity and Abeta aggregation inhibitory activity in vitro, and can be used for treating and / or preventing neurodegenerative related diseases.
Owner:NANYANG NORMAL UNIV
Asymmetrical synthetic method of R-/S-acebutolol
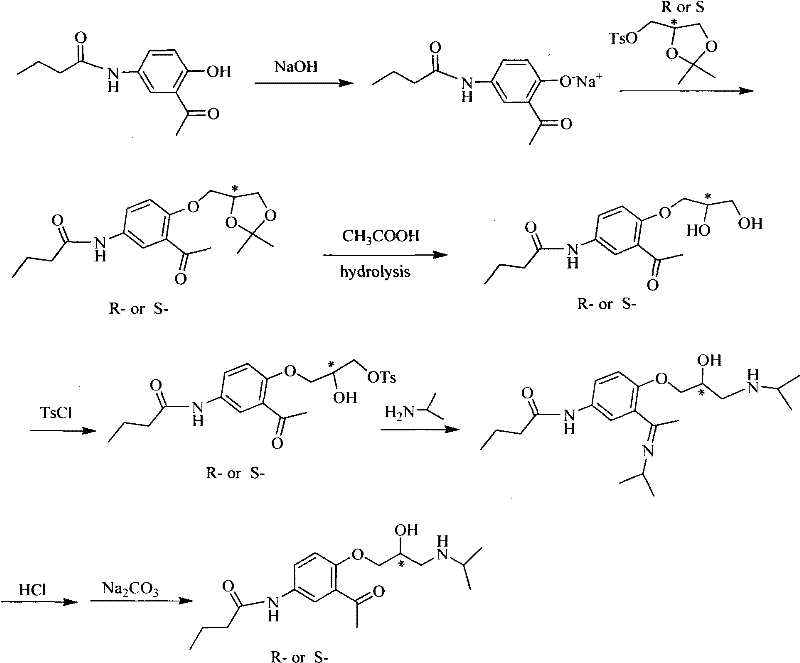
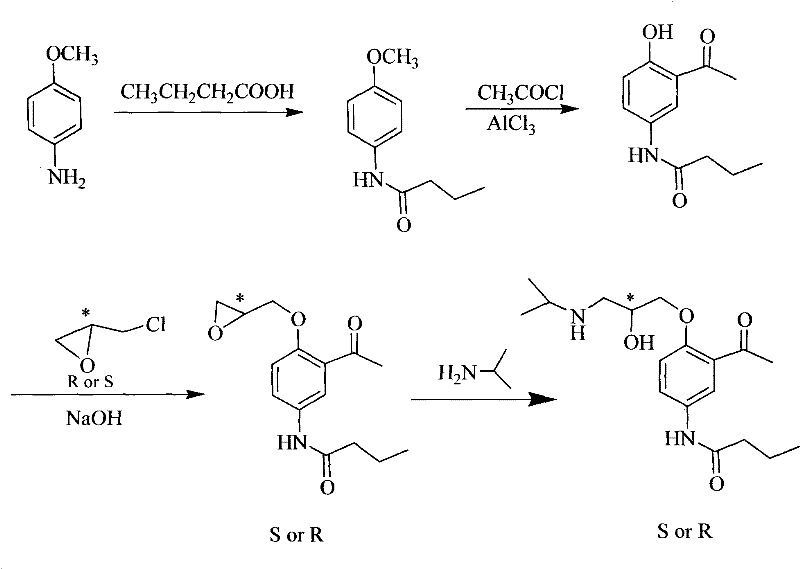
InactiveCN102241603AMild reaction conditionsSimple and fast operationCarboxylic acid amides optical isomer preparationAcebutololAcetophenone
The invention relates to an asymmetrical synthetic method of R- / S-acebutolol. The method comprises the following steps of: making a p-aminoanisole solution react with ethyl acetic acid to obtain N-(4-methoxyphenyl)-butyrylamide; mixing a solution of the N-(4-methoxyphenyl)-butyrylamide with Lewis acid, dropwise adding an acylation reagent to obtain a 5-butyramide-2-hydroxyacetophenone solid product, and dissolving the solid product in an inorganic alkali solution; and dropwise adding R- / S-epoxy chloropropane in the presence of a phase transfer catalyst to obtain R- / S-5-butyramide-2-(2,3-glycidoxy)-acetophenone, and making the R- / S-5-butyramide-2-(2,3-glycidoxy)-acetophenone react with isopropylamine in the presence of water to obtain R- / S-acebutolol with high optical purity. According to the method, a small number of steps are performed, the yield of each step is over 74 percent, the total yield is over 48 percent, and proved by OD-H chiral column HPLC (High Performance Liquid Chromatography) analysis, the e.e. value of a product is up to 94 percent in maximum.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
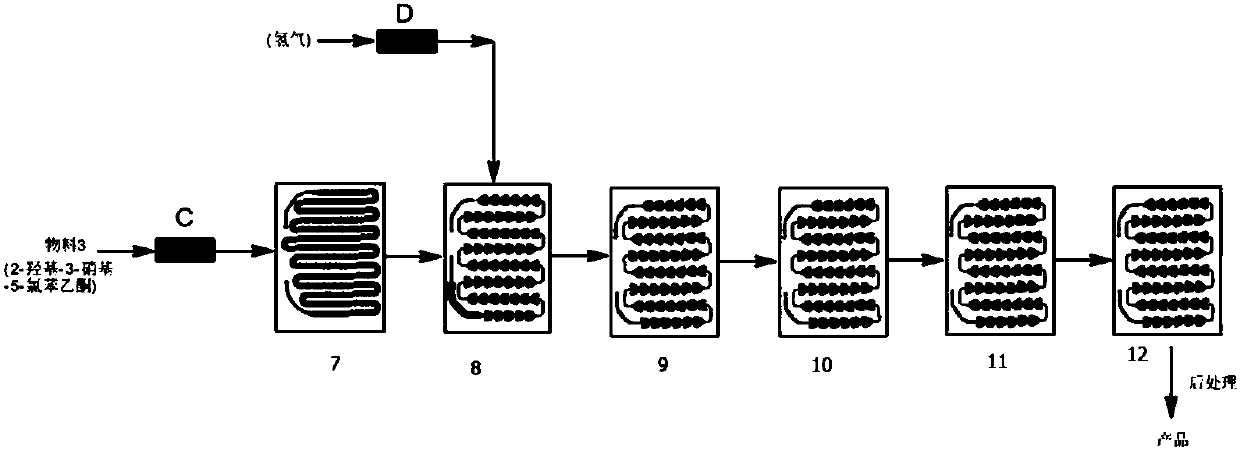
Synthesis method of 3-amino-2-hydroxyacetophenone
ActiveCN107698452AReduce production safety hazardsHigh mass and heat transfer efficiencyOrganic compound preparationChemical/physical/physico-chemical microreactorsM-aminoacetophenoneAcetic acid
Owner:HEILONGJIANG XINCHUANG BIOLOGICAL TECH DEV CO LTD
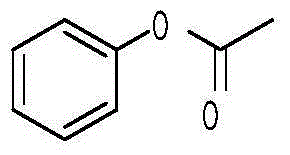
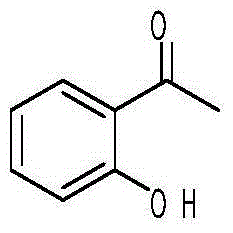
Preparation method of 2-hydroxyacetophenone
InactiveCN105130781ALow priceSignificant technological progressPreparation from carboxylic acid halidesOrganic compound preparationReaction temperatureOrganic layer
The invention discloses a preparation method of 2-hydroxyacetophenone. The preparation method comprises the steps: weighing phenol and acetyl chloride with the molar ratio of phenol to acetyl chloride of 1 to (1.0-1.5), adding phenol and acetyl chloride to a first organic solvent for reaction, carrying out a reaction at the temperature of 20-30 DEG C, then washing with a weak base, and drying and spin-drying to obtain phenyl acetate; adding Lewis acid and an ionic liquid in phenol acetate, wherein the mass of the ionic liquid is 3-10 times that of phenyl acetate, and the reaction temperature is 120-160 DEG C; hydrolyzing the obtained product with a hydrochloric acid solution, and extracting with a second organic solvent; and concentrating an organic layer, then adding a third organic solvent with the volume 1-2 times that of the organic layer, freezing the organic layer, filtering, repeating the operations for 2-3 times, and distilling the obtained filter cake to obtain the product 2-hydroxyacetophenone. The adopted raw materials are low in price, the experimental conditions are mild, the yield is high, the ortho-para ratio can reach 3.55:1, and the preparation method has a great application value in production.
Owner:SHANGHAI INST OF TECH
Synthesis method of (R)-2-(1-aminoethyl)-4-fluoroaniline
ActiveCN107586796AEasy to degradeHigh reaction conversion rateOrganic compound preparationFermentationSynthesis methodsSolvent
The invention provides a synthesis method of (R)-2-(1-aminoethyl)-4-fluoroaniline. The method comprises the following steps: uniformly stirring an amino donor, a coenzyme, a cosolvent, transaminase dry powder and a buffer solution, adding a substrate 5-fluoro-2-hydroxyacetophenone, heating the above obtained mixture in a -0.03 to -0.06 Mpa vacuum system to 25-35 DEG C, and carrying out a reaction;and carrying out post-treatment and purification after the reaction is finished in order to obtain the target product. A biocatalyst omega-transaminase is adopted, and has a high-efficiency selectivity, so the chiral purity of the reaction is greatly improved. The qualified product can be obtained through simple acid-alkali extraction, concentration and poor solvent addition crystallization in post-treatment of the method, and the optical purity and the yield of the product produced through the method are greatly improved.
Owner:暨明医药科技(苏州)有限公司
A kind of preparation method of 3-amino-2-hydroxyacetophenone
ActiveCN106831457BCheap and easy to getHigh purityOrganic compound preparationSulfonic acid preparationFries rearrangementHydrolysis
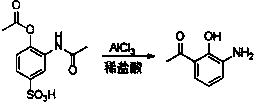
The invention discloses a new preparation method of a key intermediate, namely 3-amino-2-hydroxyphenylacetone, for preparation of Pranlukast. The new preparation method comprises the following main steps: taking 2-aminophenol-4-sulfonic acid as a starting raw material, and carrying out acylation, Fries rearrangement, hydrolysis and deprotection, so that 3-amino-2-hydroxyphenylacetone is obtained. Compared with the prior art, the new preparation method disclosed by the invention has the advantages that the used raw materials are cheap and easily available, technology can easily realize industrialization, and the obtained final product is high in purity; no danger technology is adopted, and equipment is simple; and route is novel, and synthesis route is short.
Owner:上海微巨实业有限公司
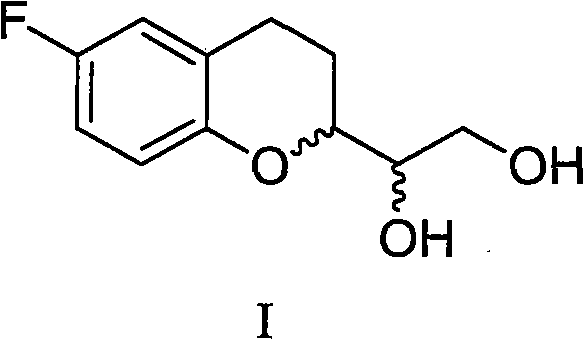
Method for preparing benzodihydropyran compound
ActiveCN102311417BShort reaction stepsMild reaction conditionsOrganic chemistryBulk chemical productionBenzeneAfter treatment
Owner:四川弘远药业有限公司
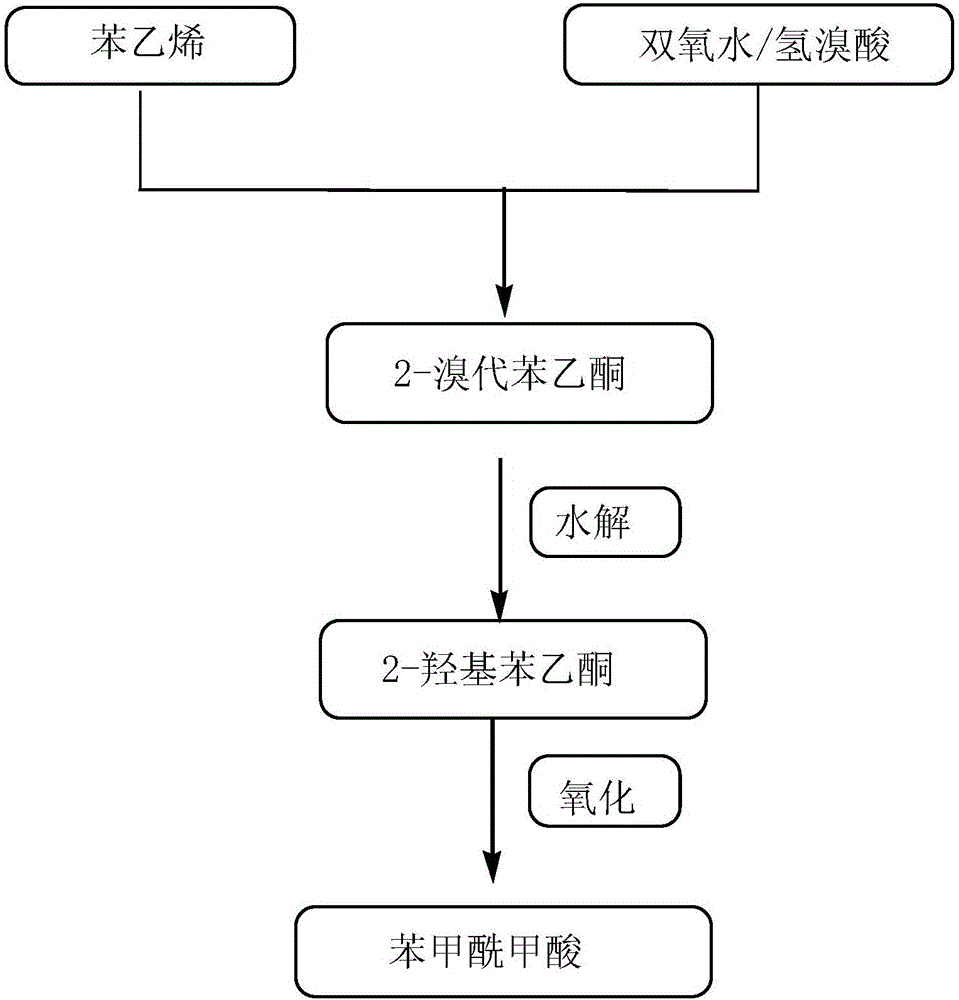
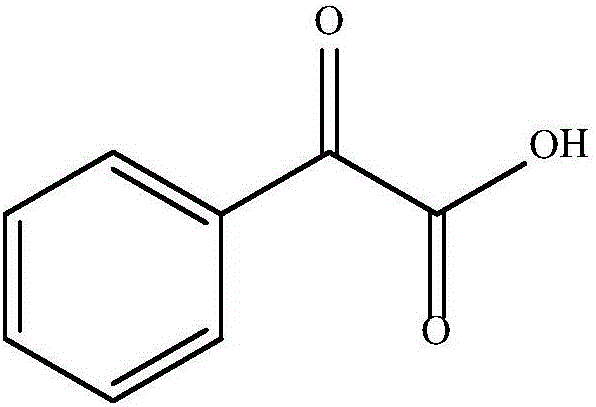
Clean method for synthesis of phenylglyoxylic acid
InactiveCN106187749AImprove pollutionHigh reaction yieldOrganic compound preparationCarbonyl compound preparationOrganic solventCleaning methods
The invention discloses a clean method for synthesis of phenylglyoxylic acid. The clean method is an environmental economic method for high selectivity synthesis of phenylglyoxylic acid from styrene and hydrogen peroxide as base raw materials and water as a solvent. Under mild reaction conditions, styrene and hydrogen peroxide undergo a reaction under hydrobromic acid catalysis to produce 2-bromoacetophenone, under microwave action, the 2-bromoacetophenone is hydrolyzed to form 2-hydroxyacetophenone and the 2-hydroxyacetophenone is oxidized to form a desired compound. The clean method utilizes styrene as a reaction substrate, utilizes water as a reaction medium to replace an organic solvent in the whole reaction process, has a high reaction yield, good reaction selectivity, a low cost, simple processes, mild reaction conditions and high product purity, prevents severe environmental pollution and a high cost of the traditional method and is a method with environmental economy and an industrial application prospect.
Owner:CHINA PETROLEUM & CHEM CORP +1
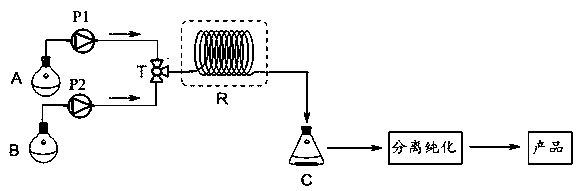
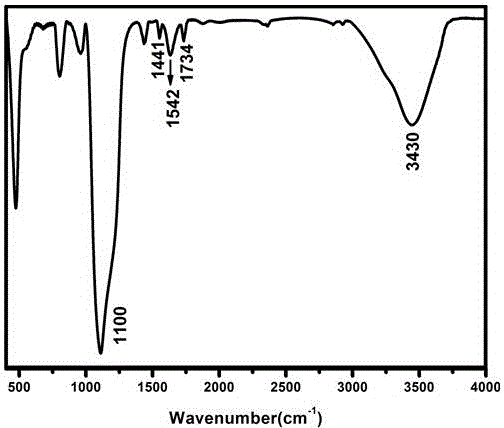
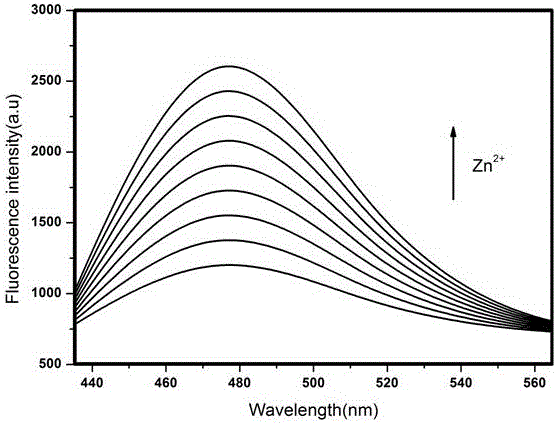
A fluorescent sensor for detecting zinc ions and its preparation method
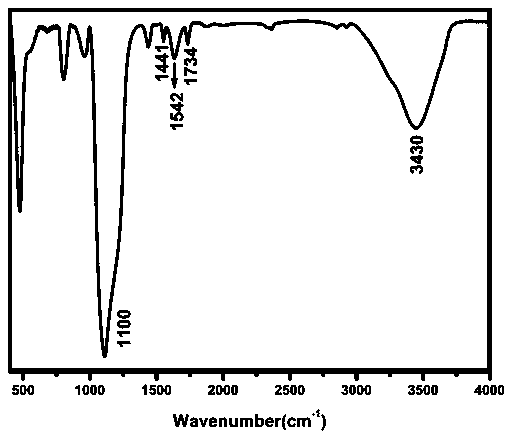
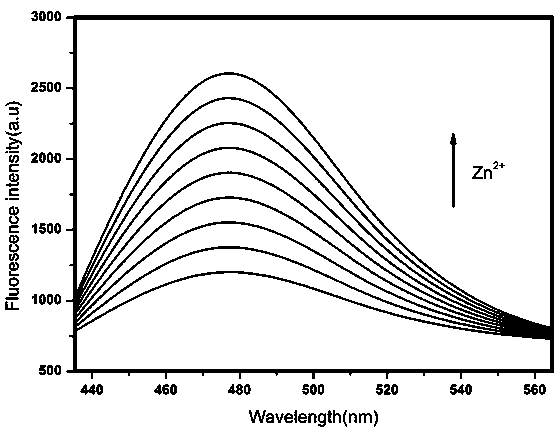
ActiveCN106770124BQuick responseLow detection limitFluorescence/phosphorescenceNitrogen gasNanostructure
The invention relates to a fluorescence sensor for detecting zinc ions and a preparation method thereof, and belongs to the technical field of fluorescence chemical sensors with nano structures. The preparation method comprises the following steps: putting nano silicon dioxide in ethanol, then adding 3-chloropropyltrimethoxysilane and performing refluxing reaction to obtain a product A; putting the product A in acetonitrile, adding indole-3-acetaldehyde and performing refluxing reaction under nitrogen protection to obtain a product B; putting the product B in methanol, then adding sodium hydroxide and 2-hydroxyacetophenone, and performing refluxing reaction to obtain a product C; putting the product C in methanol, then adding sodium hydroxide and a hydrogen peroxide solution, reacting under an ice bath and performing centrifugal separation to obtain a target product. The fluorescence sensor prepared by the preparation method provided by the invention has good selectivity for the zinc ions and has the advantages of being low in detection limit and high in response speed.
Owner:KUNMING UNIV OF SCI & TECH
Method for preparing benzodihydropyran compound
ActiveCN102311417AShort reaction stepsMild reaction conditionsOrganic chemistryBulk chemical productionBenzeneAfter treatment
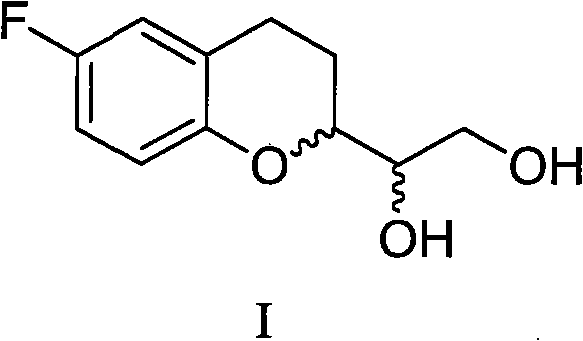
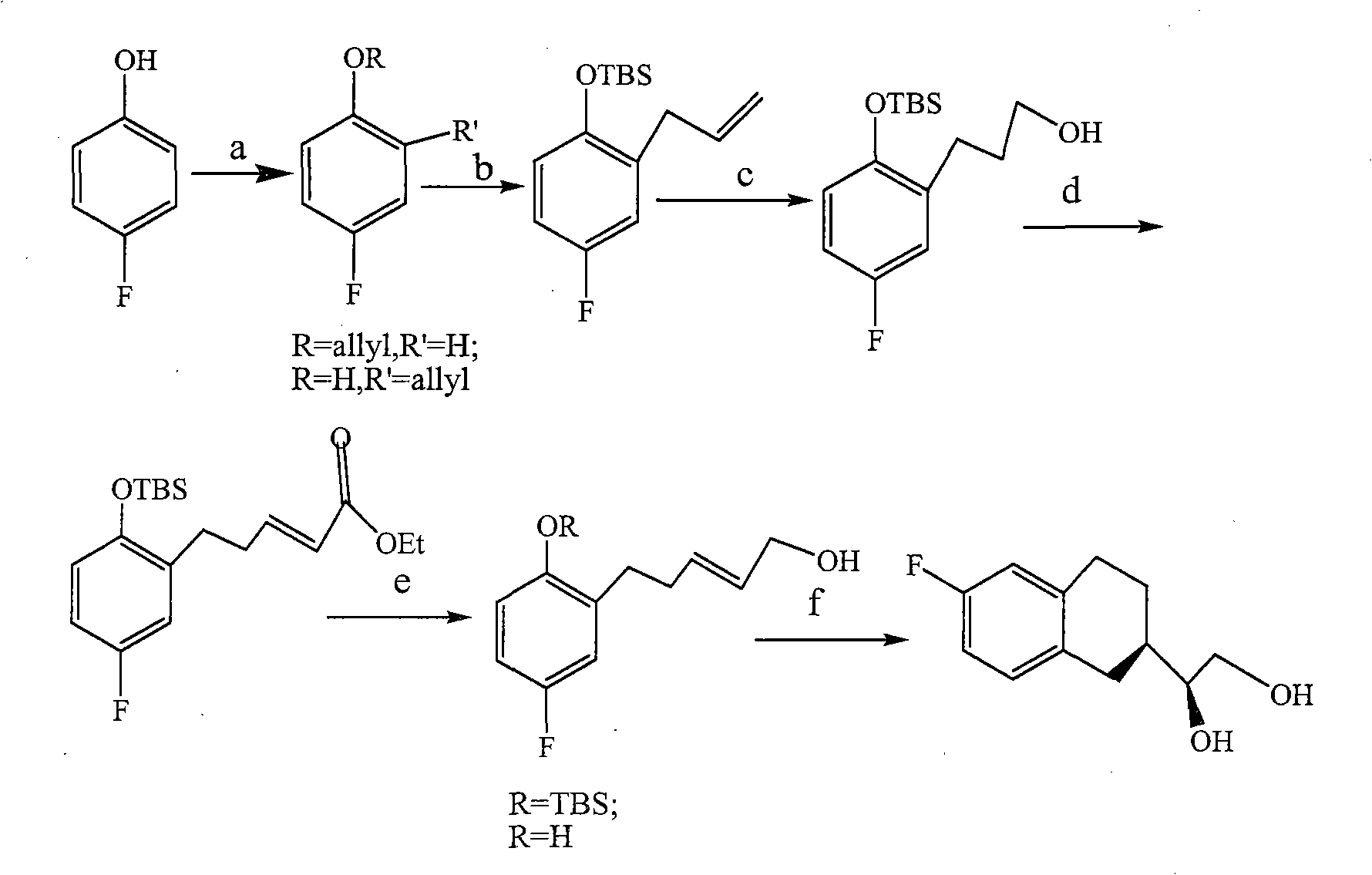
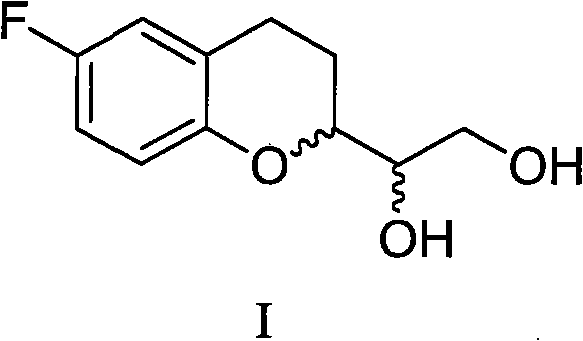
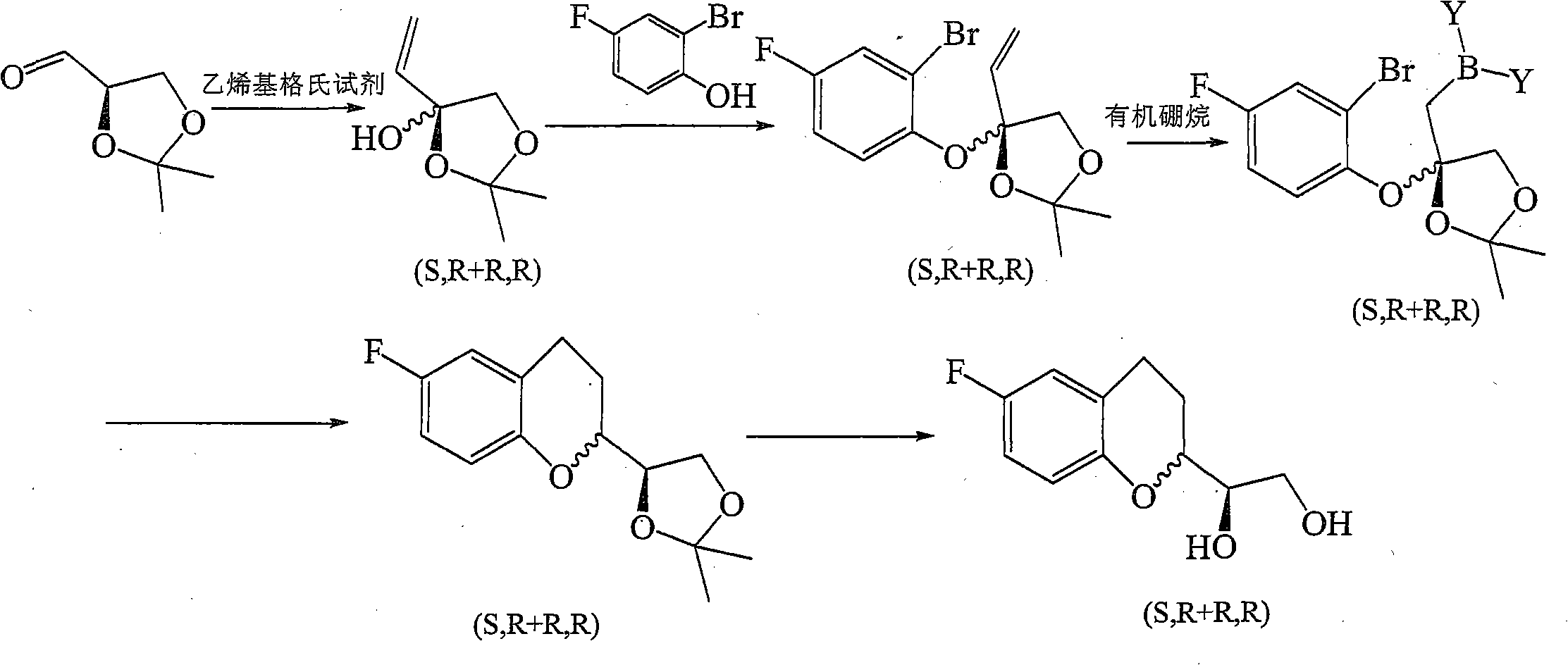
The invention provides a method for preparing 1-(6-fluorine benzopyranyl) ethane-1,2-diol. In the preparation method, 5-fluorine-2-hydroxyacetophenone and glyceraldehyde acetonide are mainly used as raw materials, and reduction, cyclization, hydrogenation and propylidene protective group removal are carried out on a compound as shown in a formula II in the reaction. The method provided by the invention has the advantages of short whole reaction processes, mild reaction conditions, high yield, simple after-treatment such as purification and the like, and cheap and available raw materials.
Owner:四川弘远药业有限公司
A method for one-step synthesis of flavonoids catalyzed by 1,3-dialkylimidazolium oxometalates
ActiveCN105294627BHigh yieldIncrease production capacityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsBenzaldehydeOxygen
The invention discloses a method for catalyzing one-step synthesis of flavonoids by using 1,3-dialkylimidazole oxometalate. ‑Hydroxyacetophenone or 2‑hydroxyacetophenone derivatives, ionic liquid catalyst, and organic solvent, stirred and heated to 50-90°C, then using oxygen or air as oxidant, reacting at constant temperature for 2-7 hours, cooling, and depressurizing After separation by distillation, column chromatography and recrystallization, the target flavonoids are obtained. The biggest feature of the present invention is that ionic liquid is used as a catalyst, and the yield of one-step synthesis of flavonoids is as high as 85%. Therefore, compared with the existing synthesis method, the present invention shortens the reaction steps and significantly improves the synthesis of flavonoids Efficiency, with the advantages of high product yield, low production cost, simple operation steps, mild reaction conditions, etc., so it is considered to be a new method for efficiently synthesizing flavonoids.
Owner:JIANGXI NORMAL UNIV
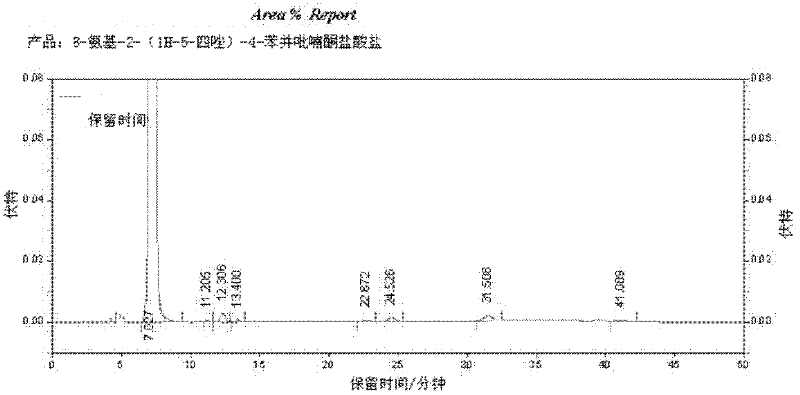
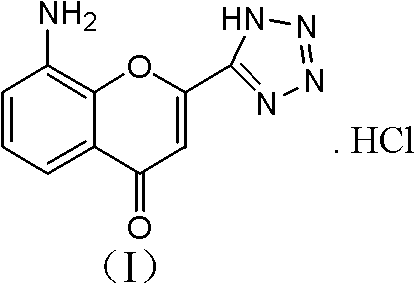
Preparation method of 8-amino-2-(1H-tetrazol-5-yl)-chromen hydrochloride
InactiveCN102358737ARaw materials are easy to getIncrease added valueOrganic chemistrySodium methoxideSpite
The invention discloses a preparation method of 8-amino-2-(1H-tetrazol-5-yl)-chromen hydrochloride. The method comprises the steps of: (1) preparing a compound (III) N-(3-acetyl-2-hydroxyl-phenyl)-acetamide: adding a compound (II) 3-amino-2-hydroxyacetophenone, a solvent, a sodium hydroxide or potassium hydroxide aqueous solution and an acid binding agent into a reaction container for uniform mixing, and adding acetyl chloride dropwisely at a temperature of 0-5DEG C, then keeping the temperature at 1-5DEG C for reaction till the end; (2) preparing an end product: adding sodium methoxide or potassium tert-butoxide, DMSO (dimethylsulfoxide) into a reaction container and stirring them well, and adding the compound (III) and a compound (IV) 1H-tetrazol-5-carboxylic acid ethyl ester in order, then leaving the mixture to react at a temperature of 80-85DEG C till the end. The method of the invention has easily available raw materials and high added value, and can obtain high value-added products. In spite of multi-step reactions, the reactions can be completed in one or two steps. Therefore, the method has an obviously simplified process, and boasts industrial production value.
Owner:中国中化股份有限公司 +1
Preparation method for benzodihydropyran compound
The invention provides a preparation method for 1-(6-fluorobenzopyranyl) ethane-1,2-diol, an intermediate of nebivolol. In the preparation method, 5-fluoro-2-hydroxyacetophenone and glyceraldehyde acetonide are mainly used as raw materials; the raw materials are reacted to obtain a compound of a formula II; the compound of the formula II is then reduced, dehydrated, hydrogenated and cyclized; and a propylidene protection group is removed. The preparation method has the advantages of short entire reaction process, mild reaction conditions, high yield, simple post-treatment after purification and the like and cheap and readily available raw materials.
Owner:CHENGDU KANGHONG PHARMA GRP
2'-Hydroxyl-3phenyl propiophenone compound and preparation method and application thereof
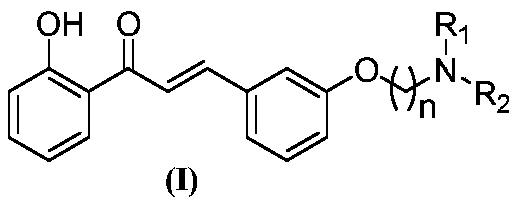
ActiveCN110272349ASignificant improvementEnhanced inhibitory effectNervous disorderOrganic chemistry3-HydroxybenzaldehydeHydrogen atmosphere
The invention relates to a 2'-hydroxyl-3phenyl propiophenone compound and a preparation method and application thereof. The preparation method comprises the following steps that 1, 3-hydroxybenzaldehyde is used as an initial raw material, and under a first solvent and a first alkaline condition, the 3-hydroxybenzaldehyde reacts with dibromide to obtain bromine alkoxy benzaldehyde; 2, the bromine alkoxy benzaldehyde reacts with secondary amine under a second solvent and a second alkaline condition to obtain amine alkoxy benzaldehyde; 3, the amine alkoxy benzaldehyde and 2-hydroxyacetophenone react under a third solvent and a third alkaline condition to obtain amine alkoxy chalcone; 4, Pd / C is added to the amine alkoxy chalcone, and under the hydrogen atmosphere, under the condition of a fourth solvent, catalytic hydrogenation is conducted to obtain the 2'-hydroxyl-3phenyl propiophenone compound. The compound has obvious selective butyrycholine enzyme inhibition activity and can be applied to treatment and / or prevention of related neurodegeneration diseases.
Owner:NANYANG NORMAL UNIV
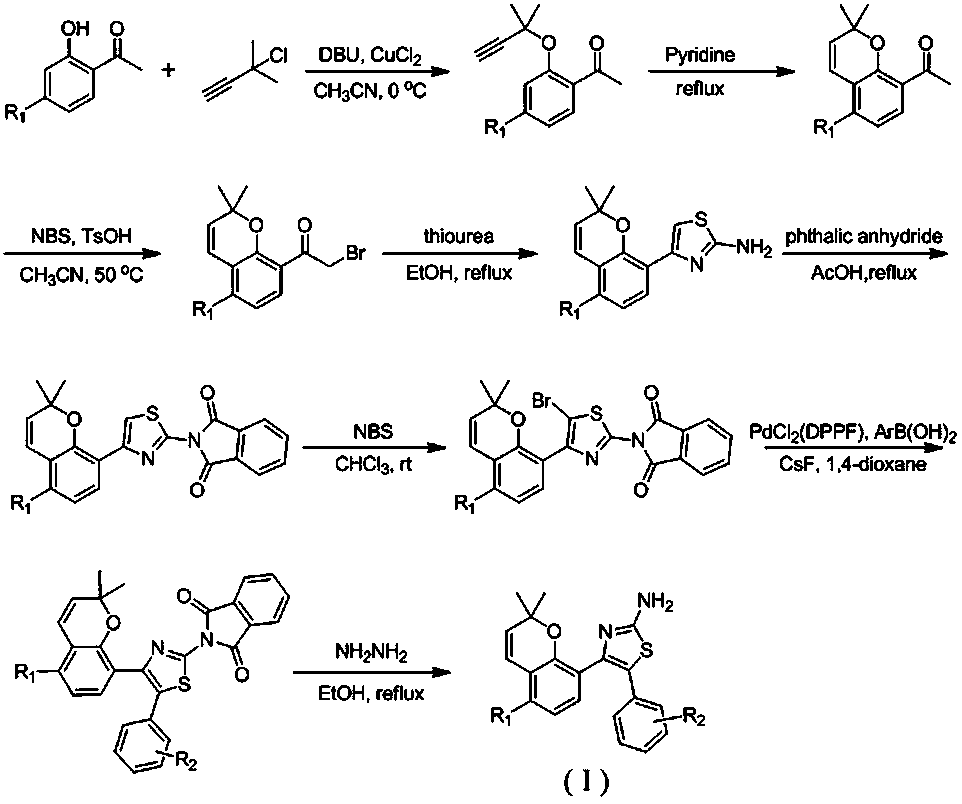
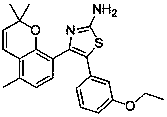
A kind of 2-amino-4,5-diarylthiazole type compound and its preparation method and application
InactiveCN105712988BStrong inhibitory activityOrganic active ingredientsOrganic chemistryArylThiazole
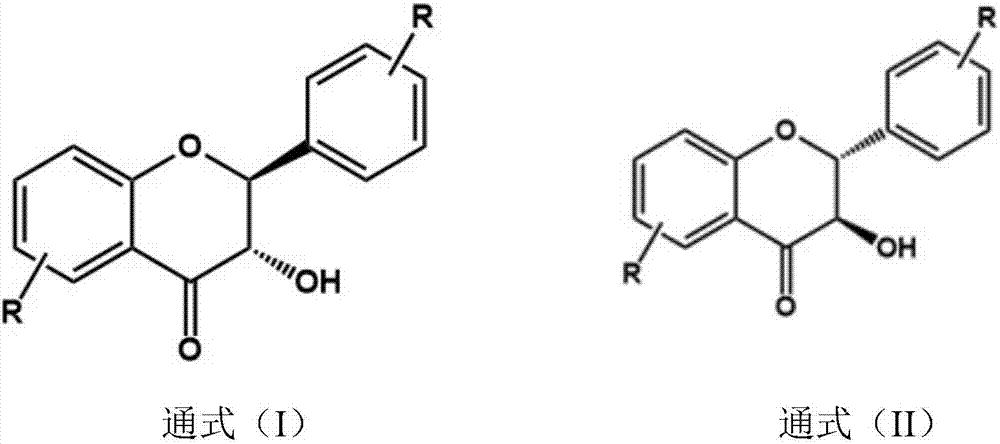
The invention discloses a 2-amino-4,5-diaryl-thiazole-type compound and a preparing method and application thereof. The 2-amino-4,5-diaryl-thiazole-type compound has the structure shown in the formula (I) (please see the specification). The preparing method includes the steps that substituted 2-hydroxyacetophenone serves as a raw material, and is etherified, rearranged, brominated, subjected to ring closing through thiourea, and reacted with phthalic anhydride, the reacted product is brominated to obtain substituted 2-(5-bromine-4-(2,2-dimethyl-2H-chromene-8-base) thiazole-2-base) isoindoline-1,3-diketone, the substituted 2-(5-bromine-4-(2,2-dimethyl-2H-chromene-8-base) thiazole-2-base) isoindoline-1,3-diketone and arylboronic acid are coupled to obtain substituted 2-(4-(2,2-dimethyl-2H-chromene-8-base)-5-aryl thiazole-2-base) isoindoline-1,3-diketone, and finally protection is removed to obtain the target compound. The 2-amino-4,5-diaryl-thiazole-type compound can serve as a raw material of antitumor medicine, and raw materials of the preparing method are simple and easy to obtain, and operation is convenient.
Owner:南京信卓化工科技有限公司
Method for preparing p-hydroxy phenyl ethyl ketone
InactiveCN109369359AReduce backmixingSmall footprintOrganic compound preparationCarbonyl compound preparationPhenylacetatesOrganic solvent
The invention discloses a method for preparing p-hydroxy phenyl ethyl ketone. The method comprises steps as follows: phenyl acetate represented as formula (I) and Lewis acid are dispersed in an organic solvent, a mixed raw material solution is subjected to a Fries rearrangement reaction in a tubular reactor, the solution obtained after the reaction is subjected to aftertreatment, and p-hydroxy phenyl ethyl ketone represented as formula (II) is prepared; the reaction formula of the Fries rearrangement reaction is shown in the description. With the adoption of a mode of tubular reaction similarto plug flow, backmixing of the materials in the tubular reaction is almost avoided, mass and heat transfer efficiency is high, side reactions are obviously reduced, reaction selectivity is good, 2-hydroxyacetophenone as a rearrangement byproduct is obviously reduced, and product yield and purity are both higher.
Owner:ZHEJIANG UNIV OF TECH
Fluorescence sensor for detecting zinc ions and preparation method thereof
ActiveCN106770124AQuick responseLow detection limitFluorescence/phosphorescenceNano structuringSodium hydroxide
The invention relates to a fluorescence sensor for detecting zinc ions and a preparation method thereof, and belongs to the technical field of fluorescence chemical sensors with nano structures. The preparation method comprises the following steps: putting nano silicon dioxide in ethanol, then adding 3-chloropropyltrimethoxysilane and performing refluxing reaction to obtain a product A; putting the product A in acetonitrile, adding indole-3-acetaldehyde and performing refluxing reaction under nitrogen protection to obtain a product B; putting the product B in methanol, then adding sodium hydroxide and 2-hydroxyacetophenone, and performing refluxing reaction to obtain a product C; putting the product C in methanol, then adding sodium hydroxide and a hydrogen peroxide solution, reacting under an ice bath and performing centrifugal separation to obtain a target product. The fluorescence sensor prepared by the preparation method provided by the invention has good selectivity for the zinc ions and has the advantages of being low in detection limit and high in response speed.
Owner:KUNMING UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com