Flavonone compound synthesized through one-pot process and preparation method thereof
A technology of dihydroflavones and compounds, which is applied in the field of synthesis of flavone derivatives, can solve problems such as many steps, difficult conditions, and insufficient production capacity, and achieve the effects of mild reaction conditions, reduced use of harmful solvents, and reduced emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
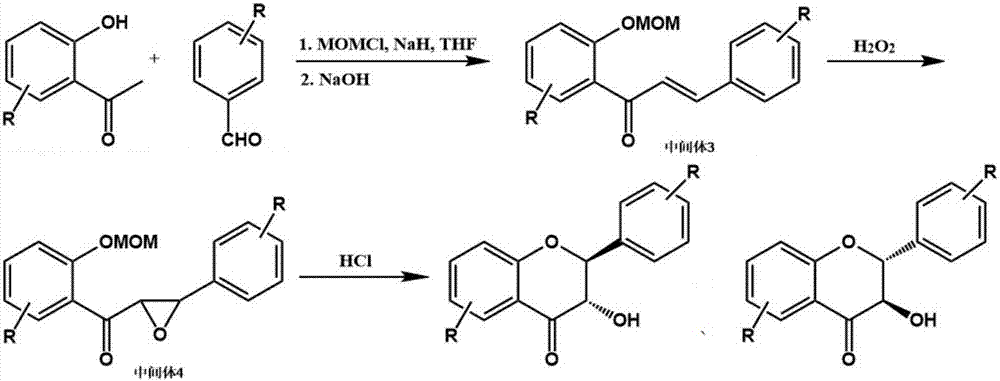
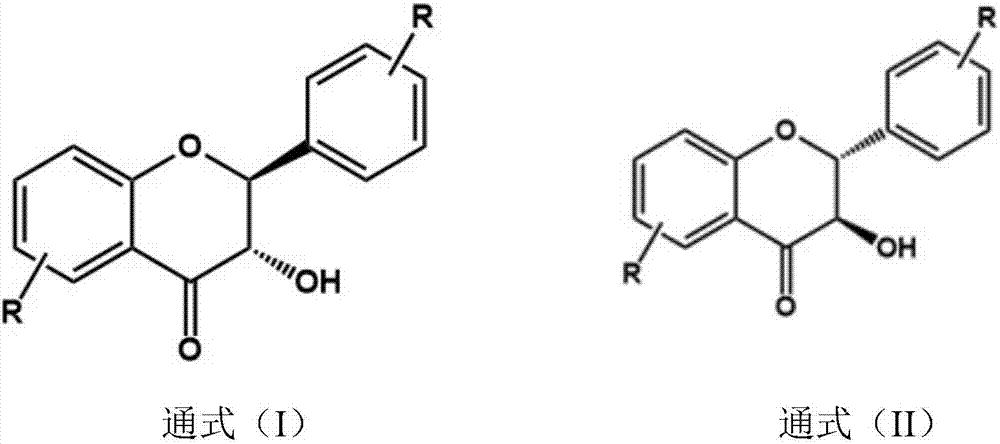
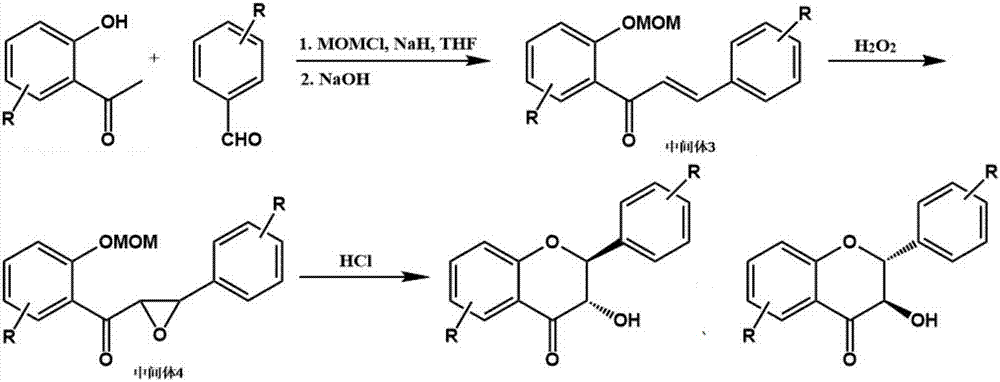
[0024] refer to figure 1 , a preparation method of said 3',4',5,7-tetrahydroxydihydroflavone (taxifolin), said preparation method comprising the following specific steps:
[0025] (1) Dissolve 10g of 2,4,6-trihydroxyacetophenone and 8.2g of 3,4-dihydroxybenzaldehyde in 100mL of anhydrous tetrahydrofuran, then add 36g of chloromethyl methyl ether and 18g of 60% sodium hydride , reacted at 5°C for 2h, then added 6.0mL of 1.0mol / L sodium hydroxide aqueous solution to the reaction solution, and condensed at 25°C for 4h to obtain intermediate 3;
[0026] (2) Add 6.8 mL of 30% hydrogen peroxide to the reaction liquid containing intermediate 3 prepared in step (1), and react at 25° C. for 4 hours to obtain intermediate 4;
[0027] (3) Charge hydrogen chloride gas into the reaction solution containing intermediate 4 obtained through step (2), control the temperature at 55° C., stop charging HCl gas when the pH in the reaction solution is less than 1, and continue the reaction for 30 ...
Embodiment 2
[0032] refer to figure 1 , a preparation method of the 2', 5'-dimethoxy-5,7-dihydroxyflavone, the preparation method comprising the following specific steps:
[0033] (1) Dissolve 10g of 2,4,6-trihydroxyacetophenone and 9.9g of 2,5-dimethoxybenzaldehyde in anhydrous tetrahydrofuran, then add 21.6g of chloromethyl methyl ether and 10.7g of 60% Sodium hydride, react at 5°C for 2h, then add 6.0mL of 1.0mol / L sodium hydroxide aqueous solution to the reaction solution, and condense at 25°C for 4h to obtain intermediate 3;
[0034] (2) Add 6.8 mL of 30% hydrogen peroxide to the reaction liquid containing intermediate 3 prepared in step (1), and react at 25° C. for 4 hours to obtain intermediate 4;
[0035] (3) Charge hydrogen chloride gas into the reaction solution containing intermediate 4 obtained through step (2), control the temperature at 55° C., stop charging HCl gas when the pH in the reaction solution is less than 1, and continue the reaction for 30 minutes , and finally a...
Embodiment 3
[0040] refer to figure 1 , a preparation method of said 2', 3', 5-trihydroxyflavones, said preparation method comprising the following specific steps:
[0041] (1) Dissolve 10g of 2,6-dihydroxyacetophenone and 9.1g of 2,3-dihydroxybenzaldehyde in 100mL of anhydrous tetrahydrofuran, then add 31.8g of chloromethyl methyl ether and 22.4g of 60% sodium hydride , reacted at 5°C for 2h, then added 6.6mL of 1.0mol / L sodium hydroxide aqueous solution to the reaction solution, and condensed at 25°C for 4h to obtain intermediate 3;
[0042] (2) Add 7.4 mL of 30% hydrogen peroxide to the reaction liquid containing intermediate 3 prepared in step (1), and react at 25° C. for 4 hours to obtain intermediate 4;
[0043] (3) Charge hydrogen chloride gas into the reaction solution containing intermediate 4 obtained through step (2), control the temperature at 55° C., stop charging HCl gas when the pH in the reaction solution is less than 1, and continue the reaction for 30 minutes , finally ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com