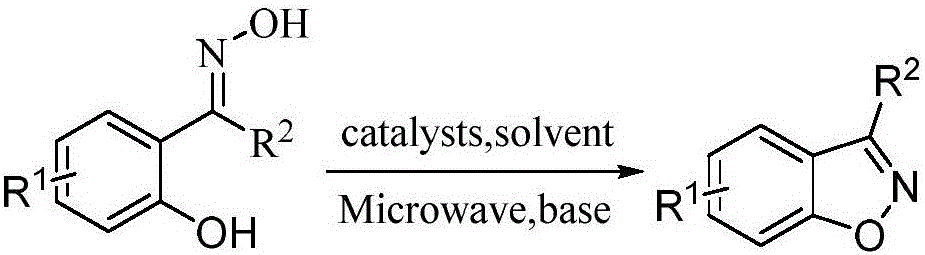
Method for preparing benzisoxazole in one pot based on 2-hydroxyacetophenone oxime and derivatives thereof
A technology of hydroxyacetophenone oxime and benzisoxazole, which is applied in the field of medicinal chemistry, can solve problems such as limiting practical application value, and achieve the effects of high product yield, short synthesis steps, and easy-to-obtain raw material sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
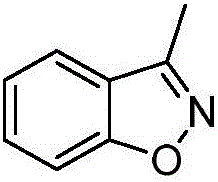
[0018] Example 1: 3-Methylbenzisoxazole
[0019]
[0020] Weigh 0.304g (2mmol) of 2-hydroxyacetophenone oxime, 0.457g (3mmol) of sodium difluorochloroacetate, and 0.417g (3mmol) of potassium carbonate into a 50mL three-necked flask, and add 2mL of N,N-dimethylethyl The amide was used as the solvent, stirred evenly by magnetic force, put it into a microwave catalytic synthesizer, manually set the parameters (microwave power 300W, temperature 85°C, microwave time 35min), and carried out microwave reaction. After the reaction was completed, it was cooled to room temperature, 10 mL of deionized water and 10 mL of ethyl acetate were added to the reaction system for extraction, and the organic phase was separated and washed three times with 10 mL of deionized water. The organic phase was dried over anhydrous magnesium sulfate, filtered, and the solvent was evaporated in vacuo to obtain a crude product. The crude product was purified by silica gel column chromatography (ethyl ace...
example 2
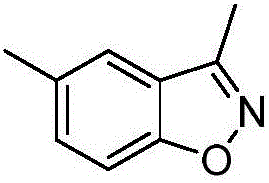
[0021] Example 2: 3,5-Dimethylbenzisoxazole
[0022]
[0023] Weigh respectively 0.332g (2mmol) of 5-methoxy-2-hydroxyacetophenone oxime, 0.609g (2mmol) of potassium difluorobromoacetate, and 0.424g (4mmol) of sodium carbonate in a 50mL three-necked flask, and add 4mL of dimethyl Sulfoxide is used as a solvent, and it is heated to 100°C for 240 minutes. After the reaction was completed, it was cooled to room temperature, 10 mL of deionized water and 10 mL of ethyl acetate were added to the reaction system for extraction, and the organic phase was separated and washed three times with 10 mL of deionized water. The organic phase was dried over anhydrous magnesium sulfate, filtered, and the organic phase was removed by vacuum rotary evaporation to obtain a crude product. The crude product was purified by silica gel column chromatography (ethyl acetate / petroleum ether=1:20) to obtain 0.133g of 3,5-dimethylbenzisoxazole with a yield of 45%. 1 HNMR (400MHz, CDCl 3 )δ7.30~7.40 ...
example 3
[0024] Example 3: 5-Chloro-3-methylbenzisoxazole
[0025]
[0026] Synthesized in a method similar to Example 1, raw material is 0.373g (2mmol) 5-chloro-2-hydroxyacetophenone oxime, promotor is 0.812g (4mmol) ethyl bromodifluoroacetate, alkali is 0.326g (1mmol) carbonic acid Cesium, the solvent is 1mL N-methylpyrrolidone, and the power is 200W, the temperature is 70°C, microwave reaction is 60min, and 0.125g of 5-chloro-3-methylbenzisoxazole is obtained, and the yield is 37%. 1 HNMR (400MHz, CDCl 3 )δ7.60(s,1H),7.48(s,2H),2.57(s,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com