Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
203 results about "1H-tetrazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The delocalization energy in tetrazole is 209 kJ/mol. 1H-Tetrazole and 5-(benzylthio)-1H-tetrazole (BTT) are widely used as acidic activators of the coupling reaction in oligonucleotide synthesis. Related heterocycles. Triazoles, analogs with three nitrogen atoms
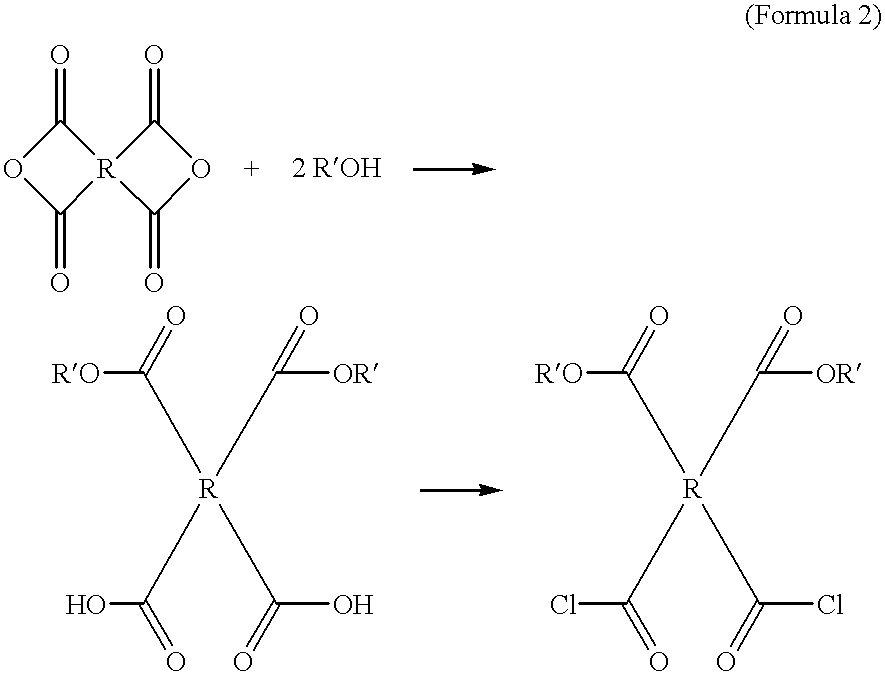
Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations
A compound of an angiotensin receptor antagonist (ARB), a neutral endopeptidase inhibitor (NEPi) and one or more monovalent cations are useful for the treatment of hypertension and / or heart failure. ARB includes S—N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine in the anion form, NEPi includes (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester in the anion form and cation includes monovalent cations such as Na+. The compound includes trisodium [3-((1S,3R)-1-biphenyl-4-ylmethyl-3-ethoxycarbonyl-1-butylcarbamoyl)propionate-(S)-3′-methyl-2′-(pentanoyl{2″-(tetrazol-5-ylate)biphenyl-4′-ylmethyl}amino)butyrate] hemipentahydrate.
Owner:NOVARTIS PHARM CORP
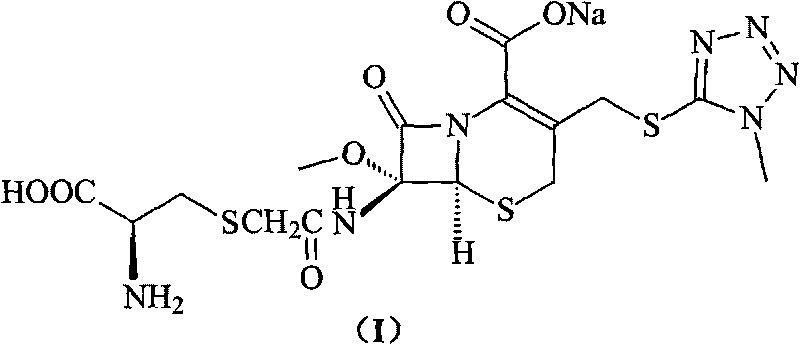
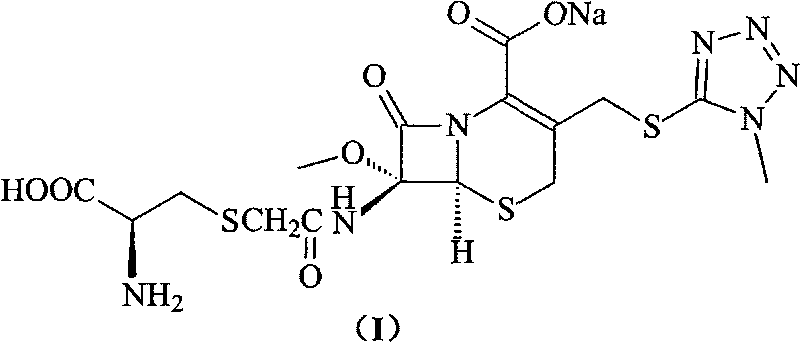
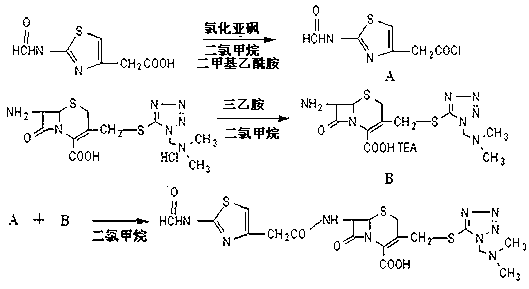
Novel method for synthesizing cefoperazone sodium compound
The invention relates to a novel preparation method for (6R,7R)-3-[[(1-methyl-1H-tetrazole-5 radial)sulphur]methyl]-7-[(R)-2-(4-ethyl-2,3-dioxo-1-piperazine carbon amido)-2-p-hydroxyl phenyl-acetamido]-8-keto-5-thia-1-polyaza[4.2.0]octane-2-alkene-2-sodiumformate(cefoperazone sodium) shown as a formula (I). The formula (I) is shown in the specifications. The invention aims to provide a novel method for synthesizing a cefoperazone sodium compound. The method has the advantages of synthesis of TZA from 7-ACA (Acetic Acid) and 1-methyl-5-sulfydryl tetrazole, mild reaction condition, easiness for operating, one-step use of recyclable dimethyl carbonate serving as an environmentally-friendly solvent, great saving in the cost, high yields of products prepared in each step, good quality, low cost, high product purity and suitability for industrial production.
Owner:哈药集团股份有限公司 +1
Method for preparing ceftezole sodium compound
ActiveCN102617606AReduce pollutionHigh yieldAntibacterial agentsOrganic chemistryCeftezole SodiumMethyl carbonate
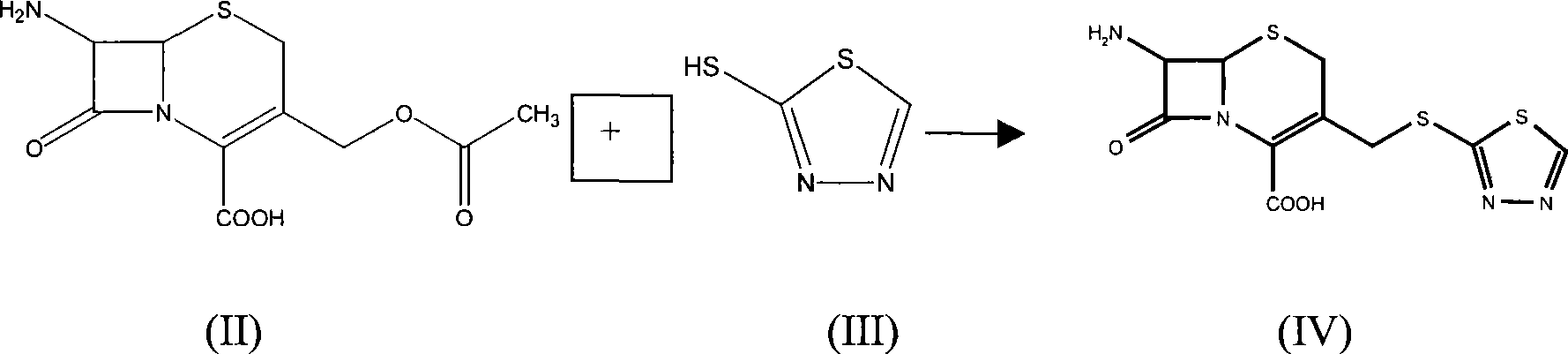
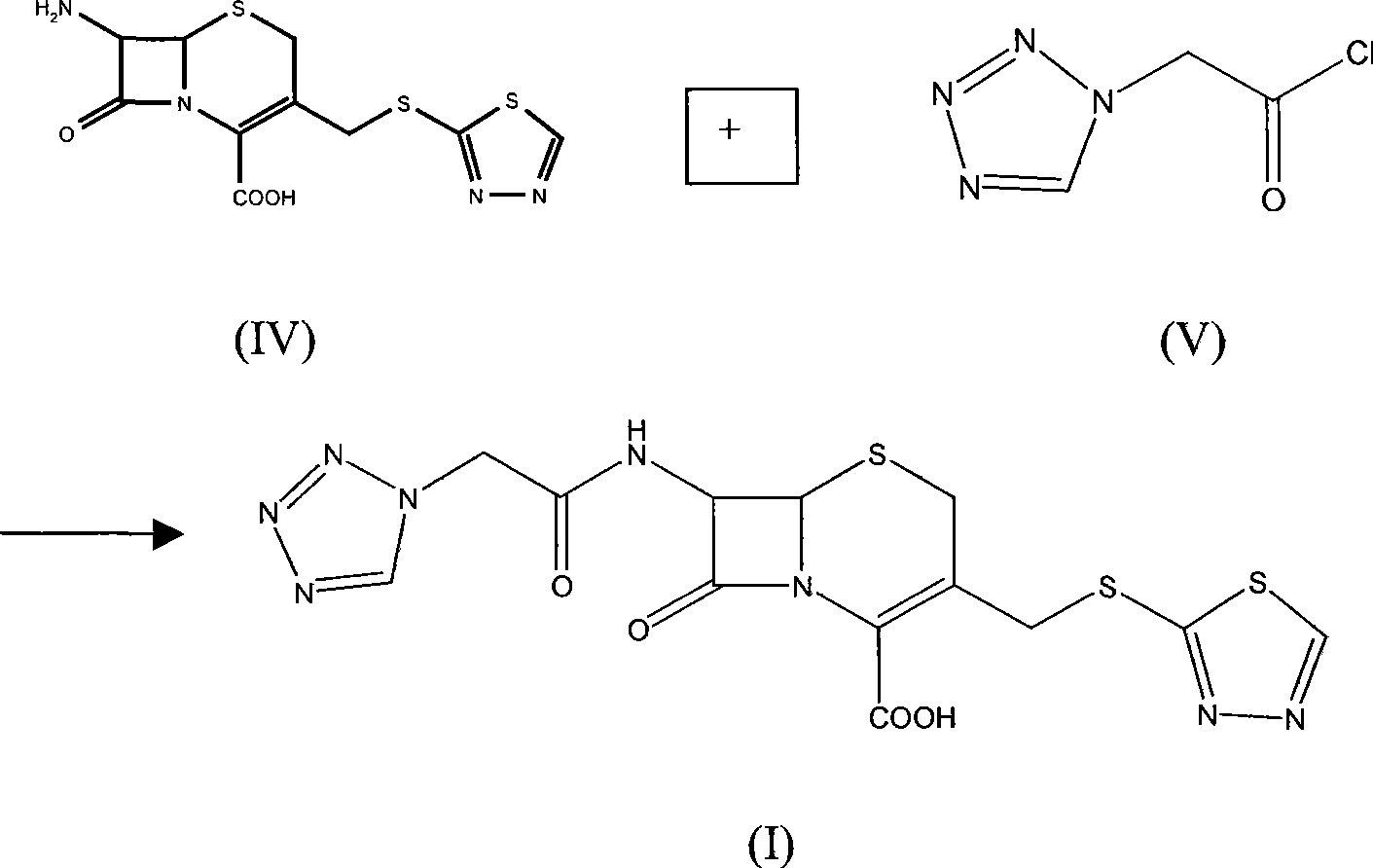
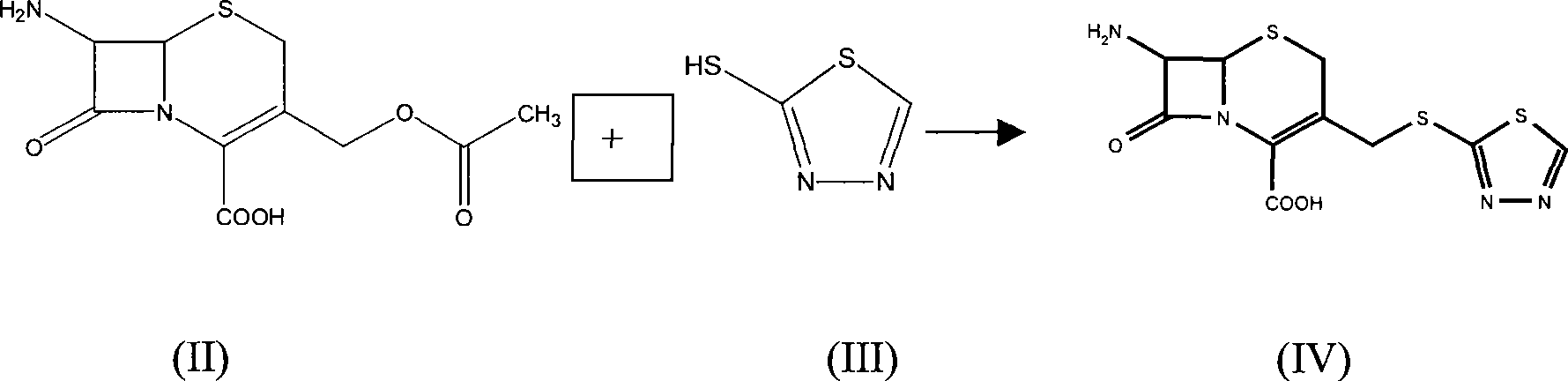
The invention relates to a method for preparing an antibacterial compound, in particular to a method for preparing a ceftezole sodium compound. The method comprises the following steps of: 1, synthesizing TZT II, and reacting 2-mercapto-1,3,4 thiadiazole and 7-aminocephalosporanic acid (ACA) to obtain TZT, wherein dimethyl carbonate is used as a reaction solvent; a boron trifluoride-dimethyl carbonate complex is used as a catalyst; after reaction, the agent used by adjusting pH of reaction liquid is sodium carbonate; the weight ratio of boron trifluoride to 7-ACA is 0.7 to 1.3; and 2, synthesizing anhydride I, namely reacting 1H-tetrazole-1-acetic acid and pivaloyl chloride to obtain anhydride; 3, synthesizing ceftezole III, namely reacting TZT II and anhydride I to obtain ceftezole; and 4, synthesizing ceftezole sodium IV, namely reacting ceftezole and sodium salt to obtain ceftezole sodium IV, wherein salt is sodium hydroxide.
Owner:哈药集团股份有限公司 +1
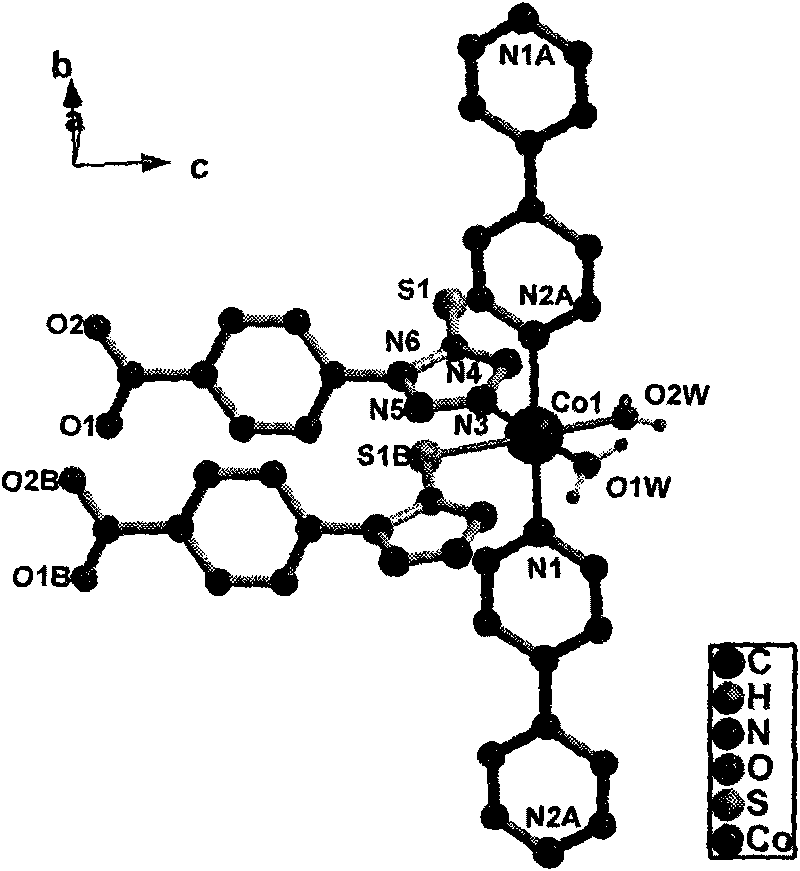
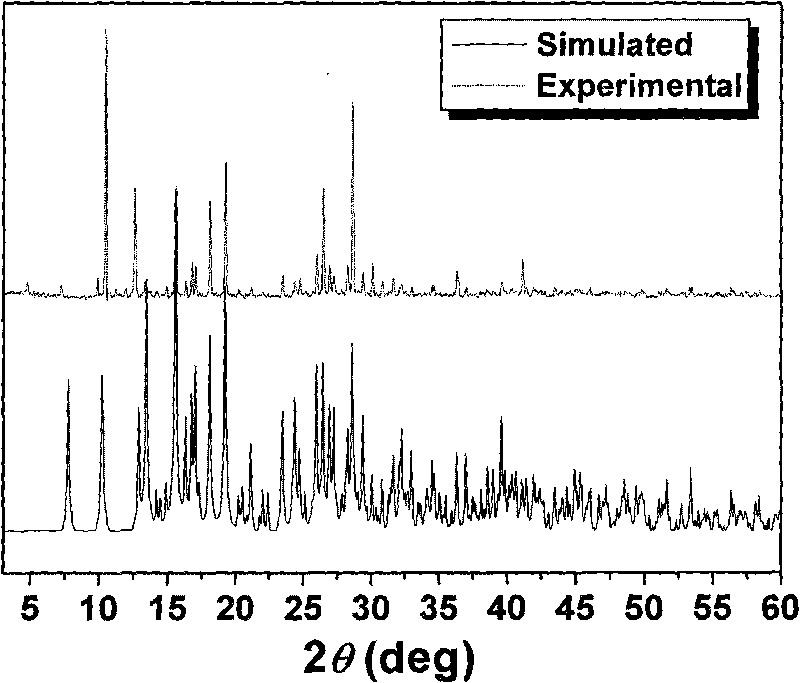
1-(4-carboxy phenyl)-5-sulfydryl-1H-tetrazole and 4, 4'-bipyridyl blending cobalt composition and preparation method thereof
InactiveCN101759723AOvercome high temperature and pressureOvercoming dangerCobalt organic compoundsOrganic/organic-metallic materials magnetismDiffusion methodsTetrazole
The invention relates to a 1-(4-carboxy phenyl)-5-sulfydryl-1H-tetrazole and 4, 4'-bipyridyl blending cobalt composition and a preparation method thereof. The composition has a chemical formula as follows: {[Co(L)(4, 4'-bipy)(H2O)2](H2O)2}, wherein L=1-(4-carboxy phenyl)-5-sulfydryl-1H-tetrazole anion ligand; and 4, 4'-bipy=4, 4'-bipyridyl. The composition is prepared by adopting a diffusion method which is different from synthesizing methods, i.e. a hydrothemal method and the like, of a conventional cobalt metal carboxylic ligand blending composition reported in the traditional literature and overcomes the technical defects of high temperature, pressure and danger, low productivity, difficult purification and separation and poor repeatability; the synthesizing method showed by the invention has moderate condition, high productivity and good repeatability at normal temperature and pressure; and in addition, a magnetism test result of a crystal sample of the composition lays the theoretical foundation for further developing a novel magnetic-function molecular material.
Owner:ZHENGZHOU UNIVERSITY OF LIGHT INDUSTRY
Cefminox sodium compound of new route
The invention provides a cefminox sodium compound of a new route, in particular a method for producing the cefminox sodium compound. The method comprises a step of generating cefminox sodium through the reaction between 7beta-bromoacetamido-7a-methoxyl-3-(1-methyl-1H-tetrazole-5-S-methyl)-3-cephem-4-carboxylic acid and D-cysteine hydrochloride, and is characterized in that: the reaction condition of the step is that the reaction is performed in aqueous solution, sodium iodide is used as a catalyst, the pH value of the reaction system is between 7.5 and 8.0, and the reaction temperature is kept at 30+ / -5 DEG C. Compared with the method for producing the cefminox sodium compound in the prior art, the method of the invention for producing the cefminox sodium compound greatly improves the product yield and the purity, and solves the problem that a cefminox sodium bulk drug always has a low purity in the prior art.
Owner:HAINAN LINGKANG PHARMA CO LTD
Liquid composition and etching method therewith
The invention relates to a liquid composition and an etching method therewith, wherein the liquid composition is used for etching copper contianing indium, gallium or zinc oxides or metal compounds with copper as main components. The etching method is cahracterized by contacting the liquid composition with the metal compound having copper or with copper as main components. The liquid composition includes A) H2O2; B) acid without fluorine atom; C) more than one from phosphonic acid, phsophates, 1H-tetrazole-1-acetic acid, 1H-tetrazole-5-acetic acid and 4-amino-1,2,4-triazole; and D) water, pH of hte composition is lwoer than 5. THe liquid composition can inhibit damage on teh indium, gallium or zinc oxides and etch copper on the oxides or the metal compounds with copper as main components.
Owner:MITSUBISHI GAS CHEM CO INC
Preparation method of cefamandole nafate
ActiveCN101880290AReduce generationImprove conversion rateOrganic chemistrySodium bicarbonateOrganic acid
The invention discloses a preparation method of cefamandole nafate, which comprises the following steps: (1) suspending 7-amino-3-[(1-methyl-1H-tetrazol-5-yl) S-methyl] -3-cephem-4-carboxylic acid and sodium bicarbonate in an acetone water solution, adding the acetone solution of alpha-formylmandeloyl chloride to carry out a condensation reaction, and preparing 7-D-(2-formyloxy phenylacetamide)-3- [(1-methyl-1H-tetrazol-5-yl) S-methyl]-3-cephem-4-carboxylic acid; and (2) dissolving the 7-D-(2-formyloxy phenylacetamide)-3- [(1-methyl-1H-tetrazol-5-yl) S-methyl]-3-cephem-4-carboxylic acid in acetone to carry out a salification reaction with the acetone solution of organic acid sodium to prepare the cefamandole nafate. The method has the advantages of simple technology, high product yield, high purity, high reaction selectivity and use of no special equipment in production, and is suitable for industrialized production.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
Cefmetazole sodium compound and synthetic method thereof
InactiveCN101550151AFew reaction stepsHigh yieldAntibacterial agentsOrganic chemistryAcetic acidCarboxylic salt
The invention relates to a cefmetazole sodium compound and a synthetic method thereof, 7 Beta-amino-7 Alpha-methoxyl-3-(1-methyl-1H-tetrazole-5-sulfidomethyl)-3-cephem-4-benzyl carboxylate and cyanomethylthio acetic acid sodium are mixed and react in the presence of p-toluenesulfonyl chloride to generate cefmetazole; sodium hydroxide is added to obtain cefmetazole sodium. Compared with the prior art, the invention has the advantages of few reaction steps, high productive rate, high product purity and low cost of raw materials, and has a broad prospect.
Owner:HAINAN LINGKANG PHARMA CO LTD
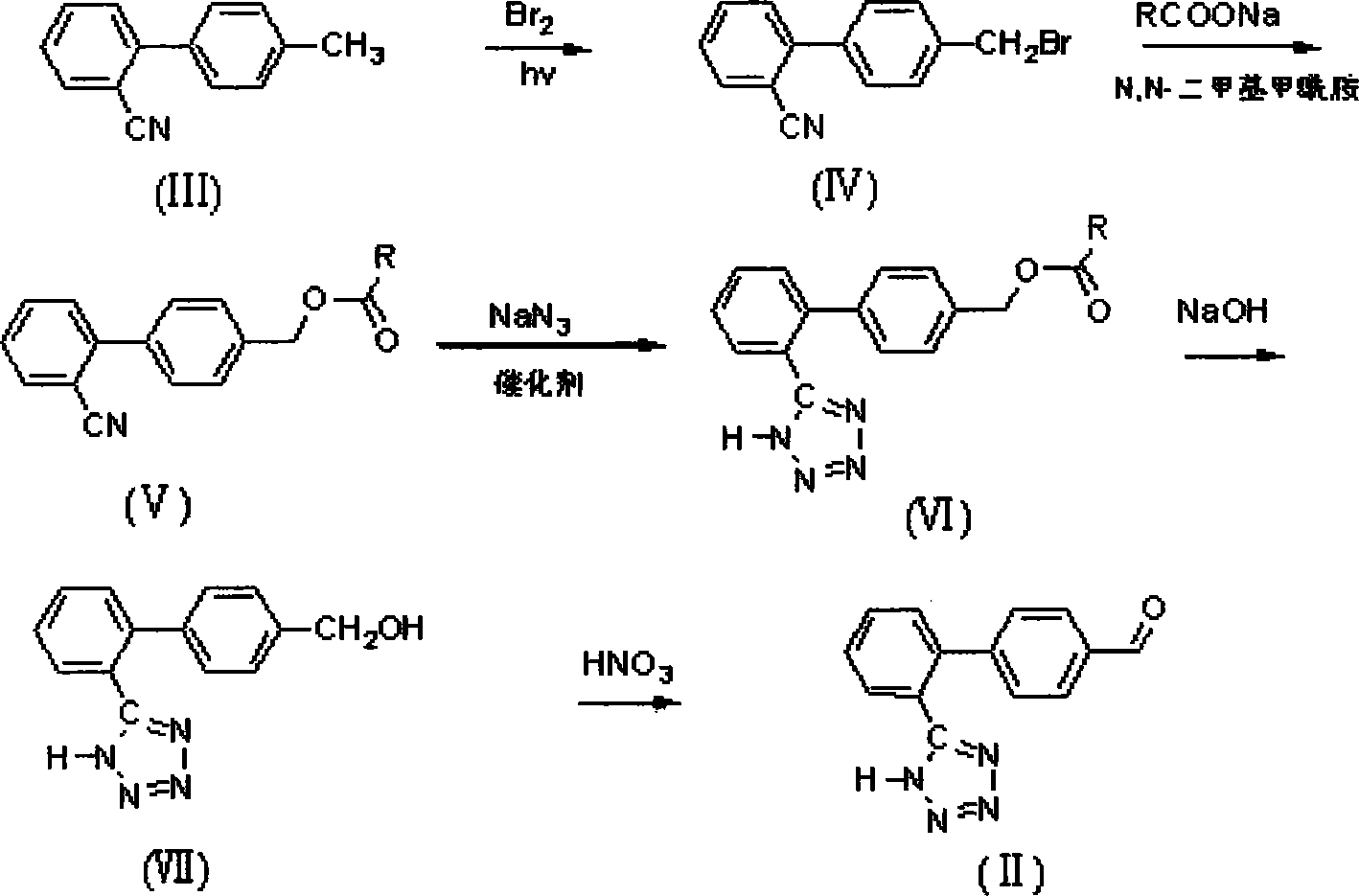
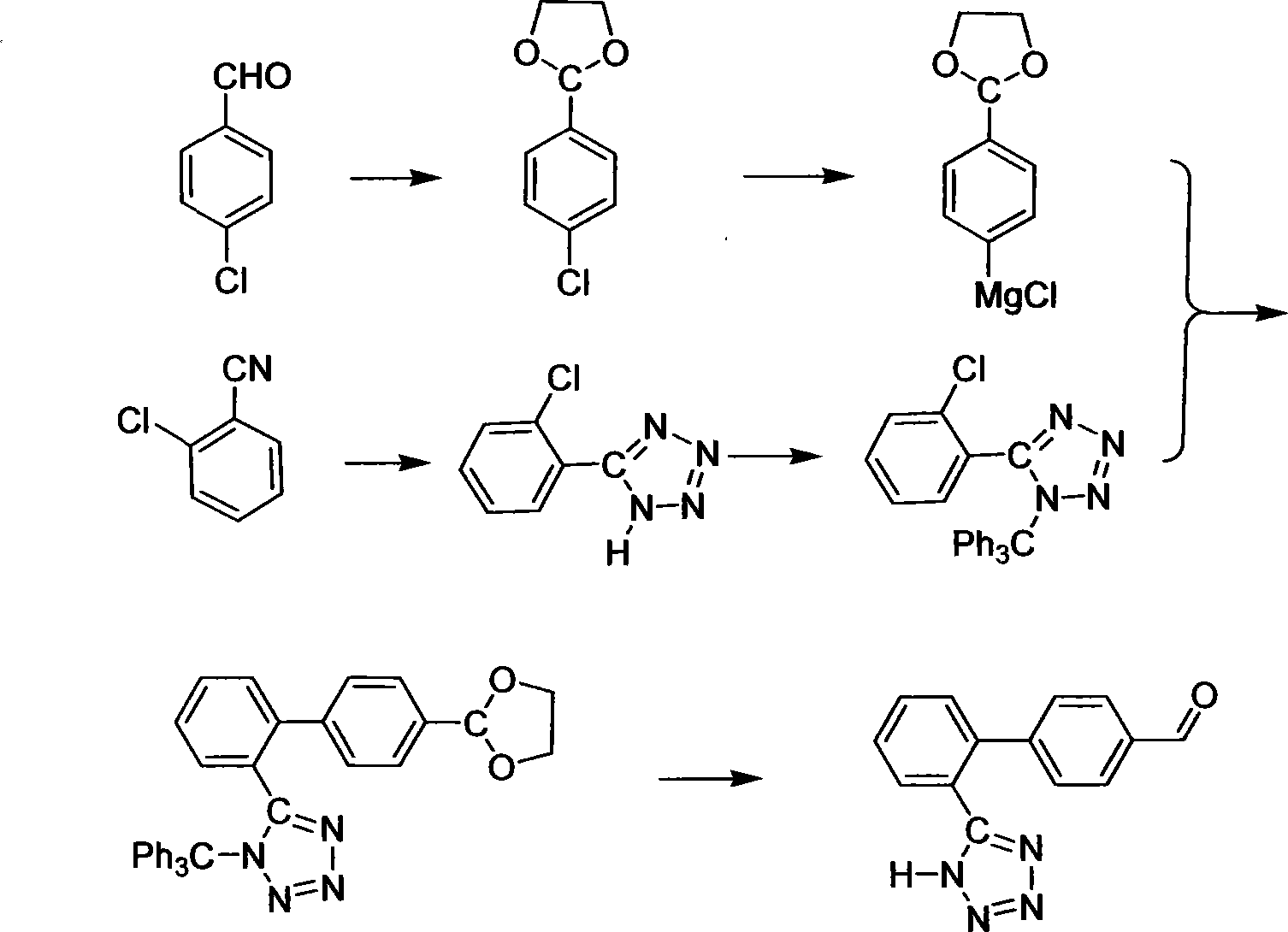
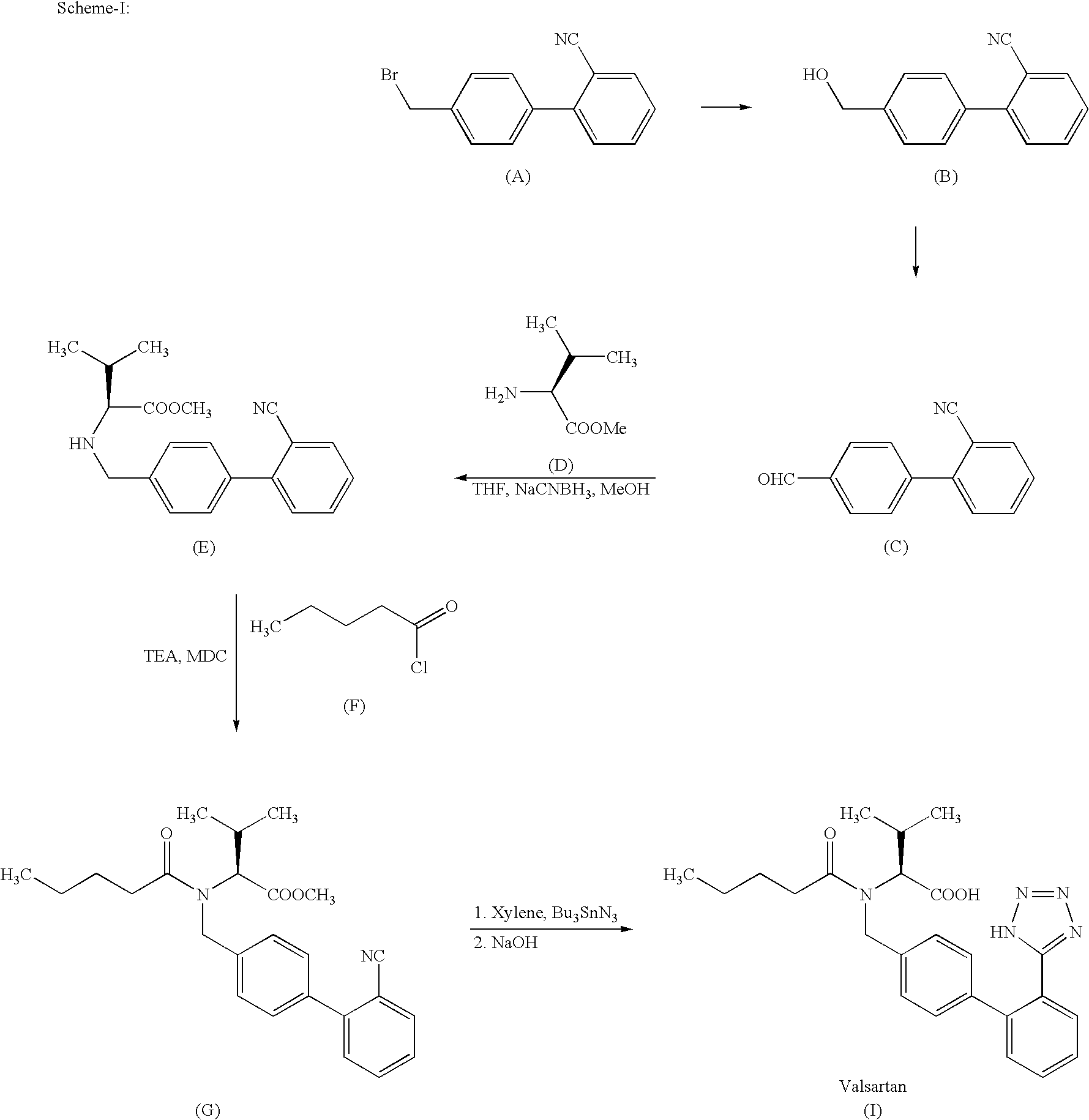
Method for preparing sartan drug main ring 5-(4'-formyl biphenyl-2-group)-1H-tetrazole treating hypertension
The invention belongs to drug synthesis, particularly relates to the main ring of Sartan drug for the treatment of the hypertension i.e. 5-(4'-formyl biphenyl-2-base)-1H-tetrazole. The preparation method comprises the following procedures, bromination, esterification, cyclization, hydrolysis and oxidation. Utilizing the triethylamine hydrochloride to serve as the catalyst, the method of the invention inhibits the side reaction, promotes the purity and the yield of the product, avoids the residue of the poisonous matter in the drug intermediate, reduces the waste discharge, realizes the clean production and protects the environment.
Owner:NANTONG SHIMEIKANG PHARMA CHEM
Process for the preparation of 5,5'-bi-1H-tetrazole salt
A process for the preparation of a 5,5'-bi-1H-tetrazole diammonium salt by dropwisely adding the aqueous hydrogen peroxide to which a small amount of weakly acidic substance has been added, to a starting aqueous solution containing hydrogen cyanide or sodium cyanide or potassium cyanide, sodium azide and a catalytic amount of copper sulfate preferably at a low temperature to maintain the pH of the reaction solution over a range of from 5 to 6, heating the reaction solution to effect the oxidation and cyclization reaction, reacting the reaction product with ammonium chloride or an aqueous solution thereof, and recovering the formed ammonium salt in the form of sparingly soluble crystals. The desired product is obtained in a high yield and in a high purity from the starting materials which are cheaply available and are easy to handle through a decreased number of steps, i.e., through a one-pot reaction without requiring cumbersome after-treatment.
Owner:JAPAN FINECHEM COMPANY +1
New preparation method of Pranlukast
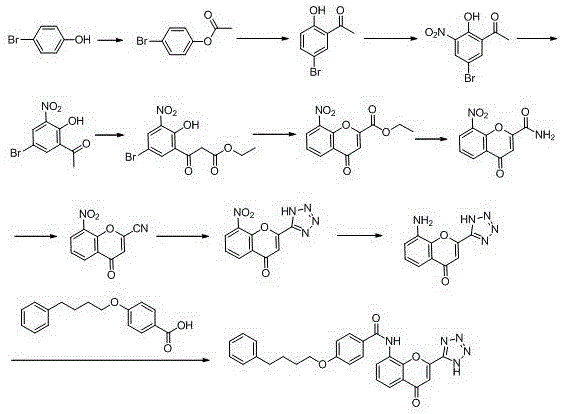
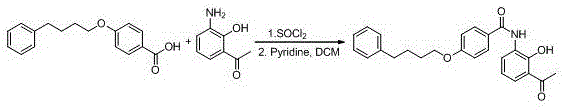
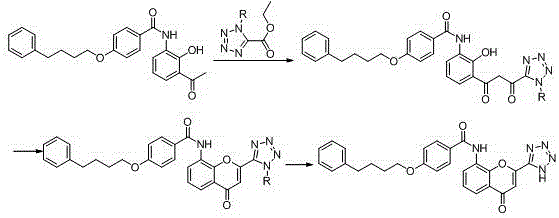
The invention provides a new preparation method of drug Pranlukast for treating asthma. The new preparation method includes the specific steps that with 2-aminophenol-4-sulfonic acid as a starting material, a key intermediate 3-amino-2-hydroxyacetophenone is prepared by means of acylation, Fries rearrangement and deprotection, then reacts with 4-(phenylbutoxy)benzoic acid, and then is subjected to condensation with ethyl 1H-tetrazole-5-acetate, and finally preparation is achieved through ring closing under the acidic condition. Compared with the prior art, the raw material used for the new preparation method is low in price and easy to obtain, industrialization of a process can be achieved easily, and the obtained final product is high in purity; and no dangerous process exists, equipment is simple, and the route is novel.
Owner:上海微巨实业有限公司
Method for preparing 5,5'-bi-1H-tetrazole salt
A process for the preparation of a 5,5'-bi-1H-tetrazole diammonium salt by using dicyan, sodium azide, ammonium chloride and water as a reaction medium. This process is industrially advantageous since the 5,5'-bi-1H-tetrazole diammonium salt is synthesized and, then, the precipitated crystals thereof are simply isolated by filtration to obtain the 5,5'-bi-1H-tetrazole diammonium salt maintaining an yield which is not lower than 90%. The 5,5'-bi-1H-tetrazole diammonium salt is a gas-generating agent for air bags, which is lowly toxic and is easy to handle.
Owner:JAPAN FINECHEM COMPANY +1
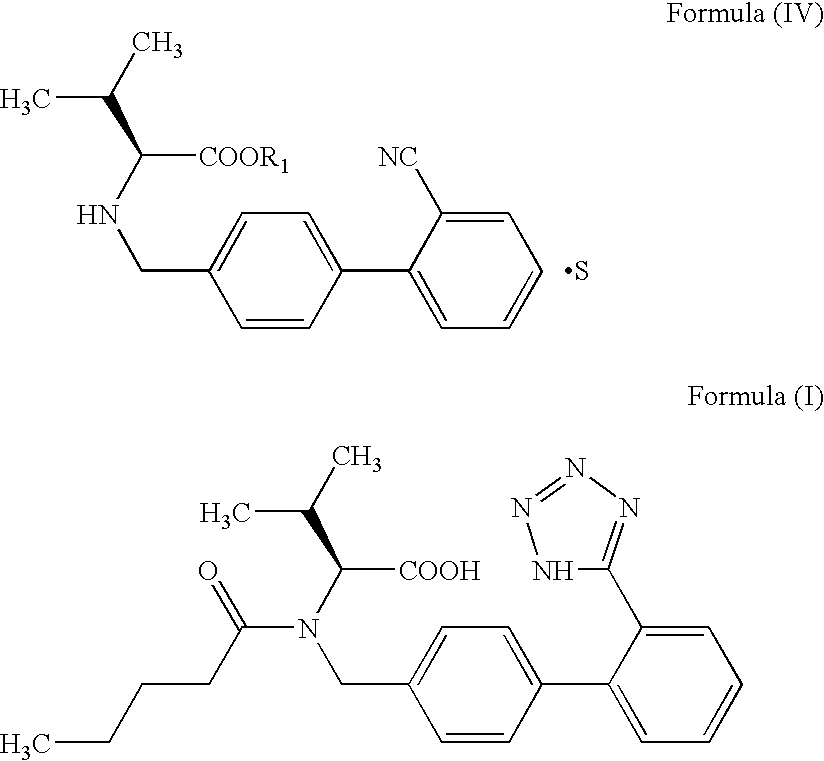
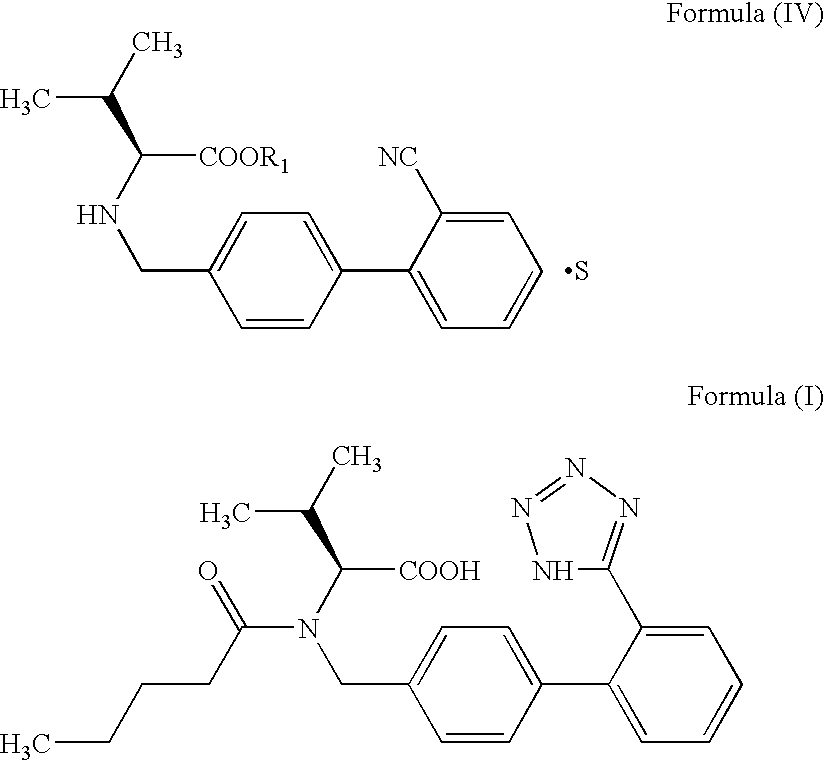
Process for preparing Valsartan
The invention relates to novel compound of formula (IV), which is an organic acid salt of N-[(2′-cyanobiphenyl-4-yl)methyl]-(L)-valine ester. This compound is an useful intermediate for process of preparation of Valsartan of formula (I), chemically known as (S)—N-(1-Carboxy-2-methylprop-1-yl)-N-pentanoyl-N-[2′-(1H-tetrazol-5-yl)biphenyl-4-ylmethyl]amine. This invention also relates to a process for preparing Valsartan using novel intermediate of formula (IV).
Owner:ALEMBIC LTD
Drug for treating or preventing duplication of hypertension with serum hyperuricemia and/or hypercholesterolemia
The purpose in this invention is to providing drugs for treating or preventing duplication of hypertension with serum hyperuricemia and / or hypercholesterolemia that is much frequency among the duplication onset in geriatric diseases. This invention is related to drugs for treating or preventing duplication of hypertension with serum hyperuricemia and / or hypercholesterolemia of which active principles are 2-propyl-3- {[2'-(1H-tetrazole -5-yl) biphenyl -4-yl]methyl}-5, 6, 7, 8-tetrahydrocyclohepta imidazole -4-(3H) - one and that prodrug or that salt. Pratosartan is able to use in combination with one or more than two diuretics chosen from sulfonamide-, phenoxyacetic acid- and thiazide-type diuretics, triamterene, amiloride, spironolactone, potassium canrenoate and traxanox sodium. Also, pratosartan is able to use in combination with one or more than two hypolipidemic drugs chosen from statins, fibrates, cholesterol absorption inhibitors, cholesterol sequestrants and cholesterol excretion enhancers.
Owner:KOTOBUKI PHARMA CO LTD
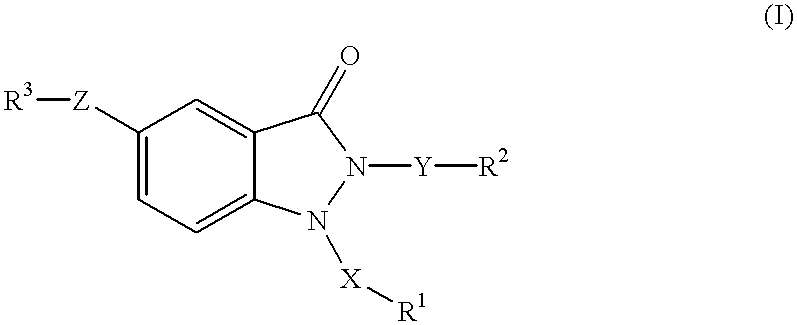
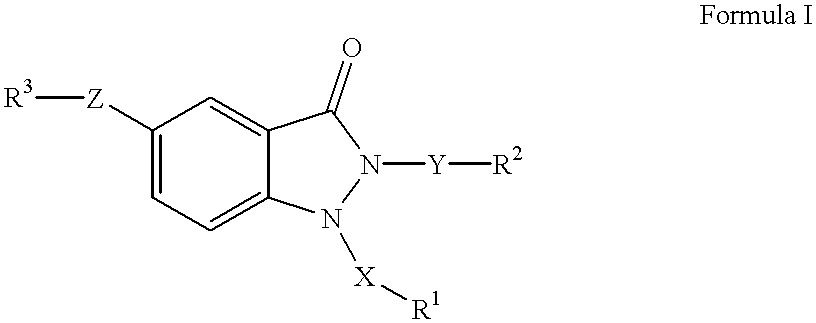
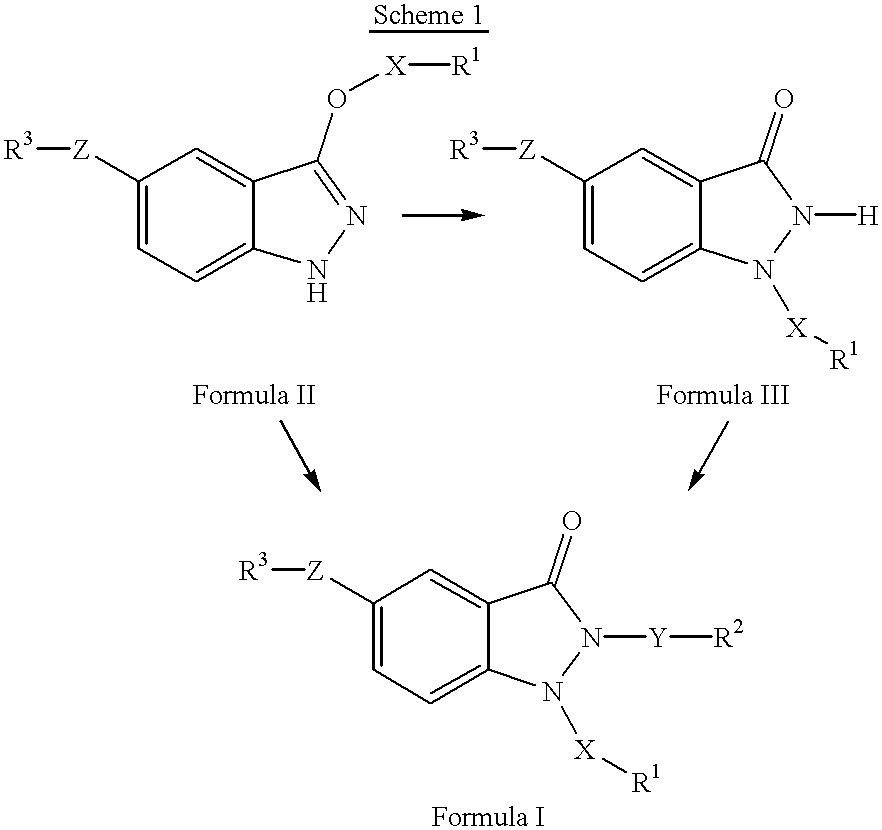
1,2,5-trisubstituted 1,2-dihydroindazol-3-ones having anti-asthmatic, anti-allergic, anti-inflammatory, immunomodulating and neuroprotective action, process for their preparation and their use as medicaments
The invention relates to 1,2,5-trisubstituted 1,2-dihydroindazol-3-ones of formula (I)whereinX is -SO2-, -SO-, -(CH2)p-, -(CH2)p-O-, -(CH2)p-(C=O)-, -(CH2)p-(C=O)-NH-, -(CH2)p-CHOH-, -CHOH-(CH2)p-, -(CH2)p-CH=CH-, -CH=CH-(CH2)p-,Y is -(C=O)-, -(C=O)-NH-, -(C=O)-NH-(CH2)p-, -C=O)-(CH2)p-, -(CH2)p-, -(CH2)p-O-, -(CH2)p-(C=O)-, -(CH2)p-(C=O)-NH-, -(CH2)p-(C=O)-NH-(CH2)p-, -(CH2)p-CHOH-, -CHOH-(CH2)p-, -(CH2)p-CH=CH-, -CH=CH-(CH2)p-,Z is -O-, -O-(CH2)p-, -NH-, -NH-(C=O)-, -NH-(C=O)-NH-, -NH-(C=O)-O-, -NH-CH2-(C=O)- and -NH-(C=O)-CH2-,P is a cardinal number from 1 to 4,R1, R2 and R3 can be the same or different and are:mono-, bi- or tricyclic saturated or mono- or polyunsaturated carbocycles having from 5 to 14 ring members; or mono-, bi- or tricyclic saturated or mono- or polyunsaturated heterocycles having from 5 to 15 ring members and from 1 to 6 heteroatoms,in which the carbocycles and the heterocycles can be mono- or polysubstituted by:C1-6-alkyl, -O-C1-6-alkyl, -O-C3-7-cycloalkyl, mono-, bi- or tricyclic saturated or mono- or polyunsaturated carbocycles having from 3 to 14 ring members, mono-, bi- or tricyclic saturated or mono- or polyunsaturated heterocycles having from 5 to 15 ring members and from 1 to 6 heteroatoms,R1 is also H, provided that when X is CH2, then R1 is not H,R3-Z is also NO2,and their pharmaceutically acceptable salts,but excluding compounds of formula (I) in whichif Z is -NH-(C=O)-, -NH-(C=O)-NH-, -NH-(C=O)-O-, -NH-(C=O)-CH2 and at the same time R1 is phenyl, monosubstituted or polysubstituted by -COOH, -COOC1-6-alkyl, -(CH2)p-COOH, -(CH2)p-COOC1-6-alkyl -CONHC1-6-alkyl, -CONHC6-14-aryl, -CONHSO2C1-6-alkyl, -CONHSO2C6-4-aryl, 1H-tetrazol-5-yl, then R2 is not phenyl, monosubstituted or polysubstituted by CN, halogen, C1-4-alkyl, C1-4-alkyloxy, CF3; andif R3-Z is NO2, then R1-X is not benzyl or 4-methoxybenzyl, and R2-Y is not benzyl or 2-picolyl at the same time; and to pharmaceutical treatment processes, and processes for making.
Owner:ARZNEIMITTELWERK DRESDEN GMBH
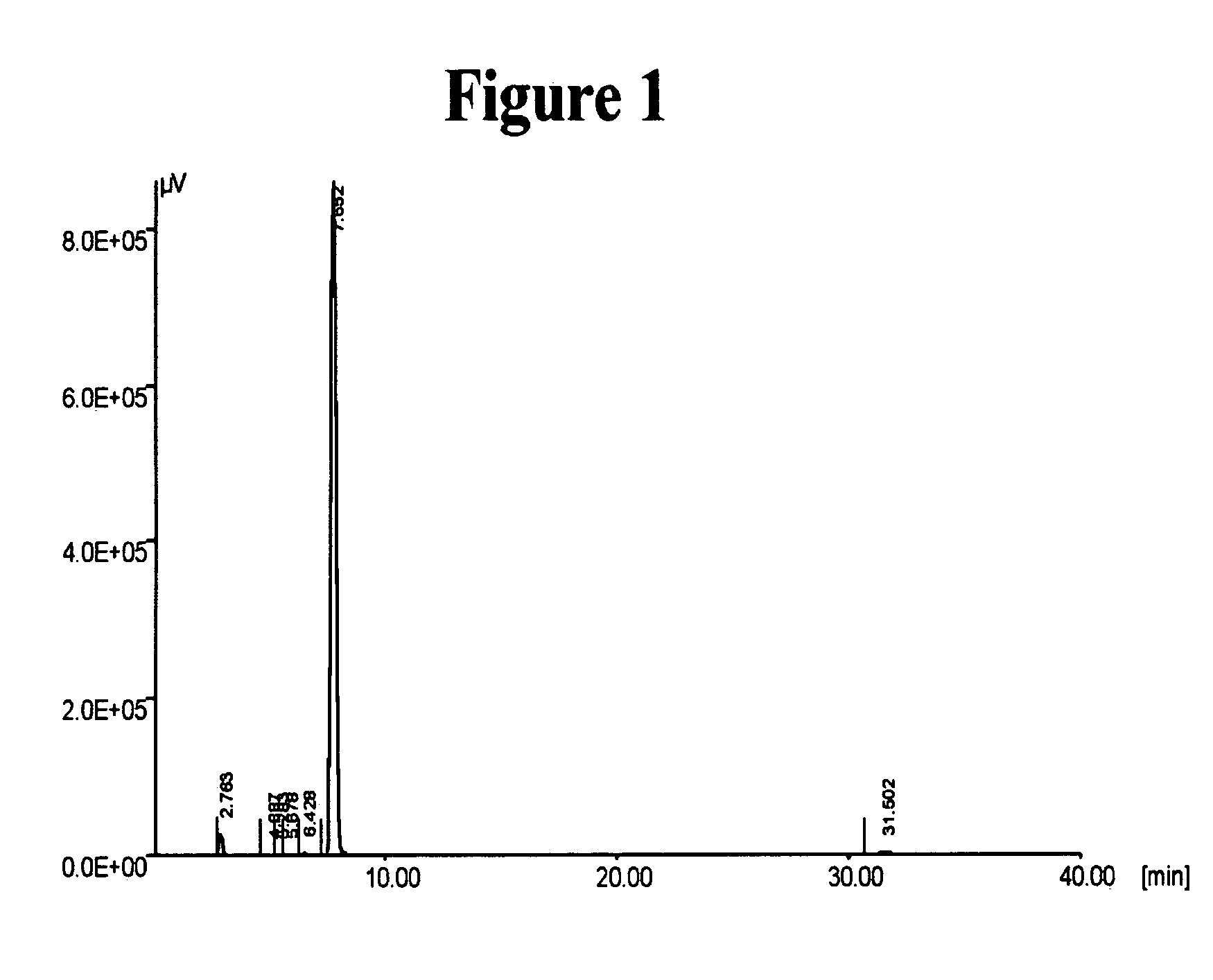
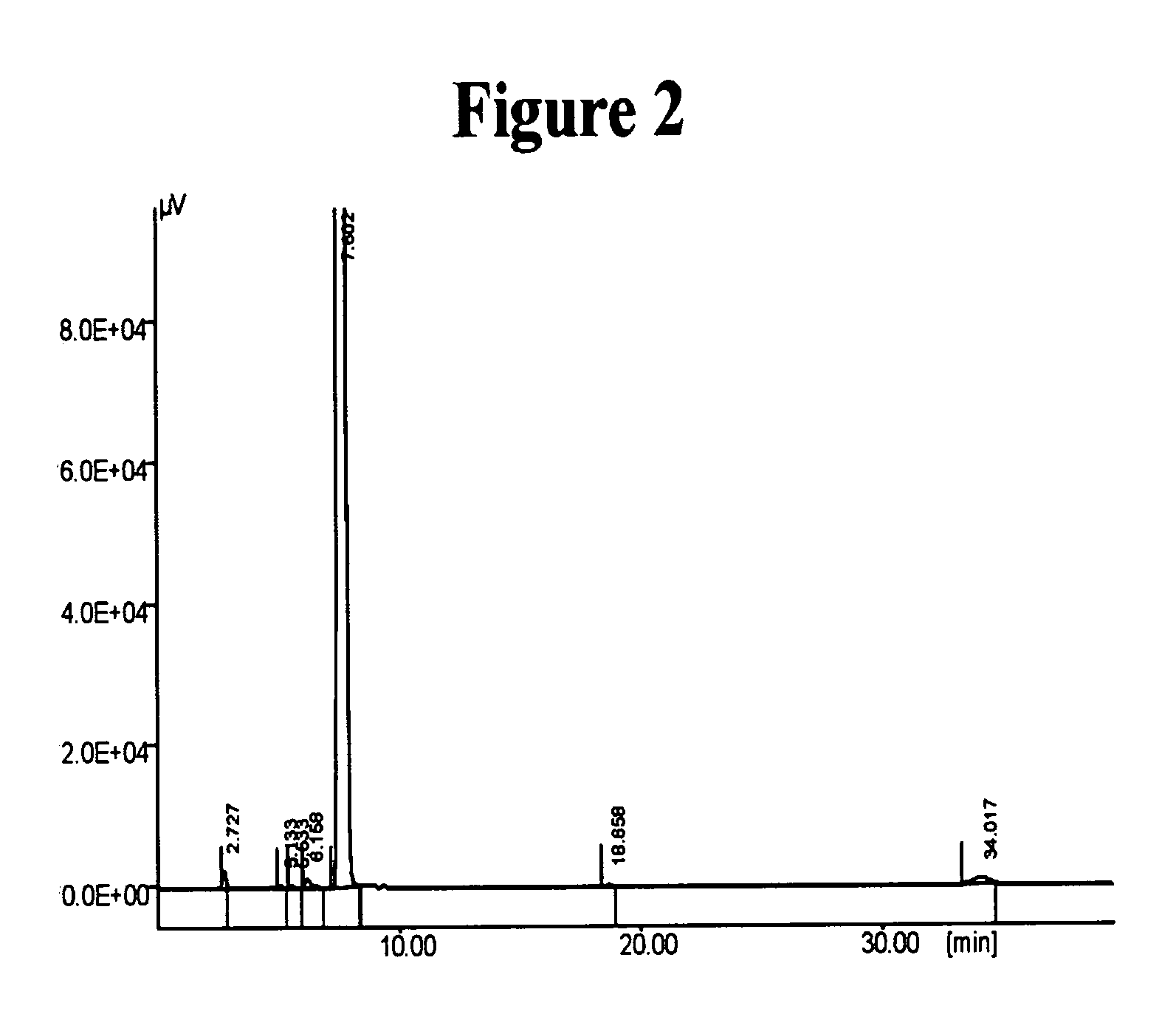
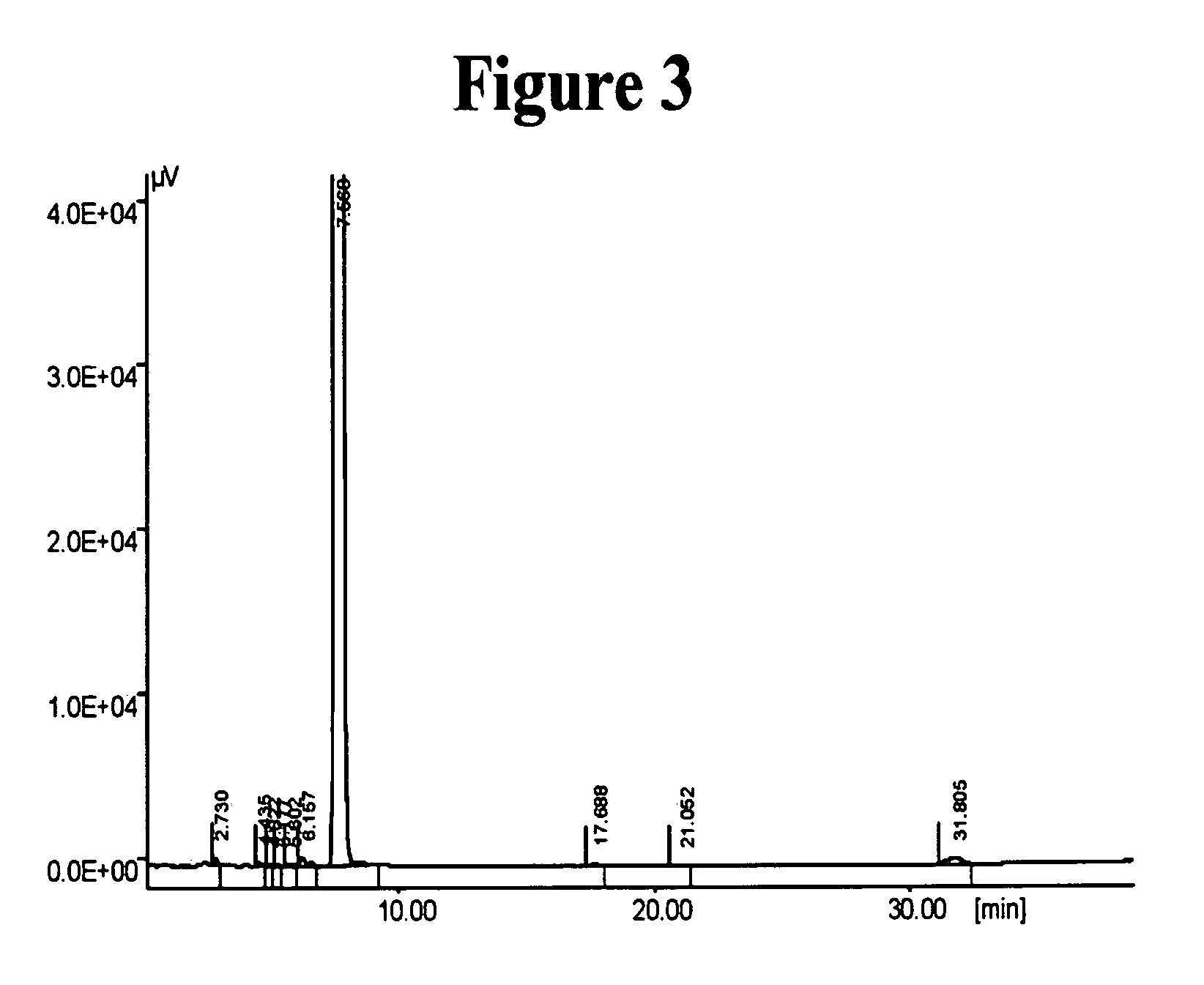
Novel crystalline forms of (S)-N-(1-Carboxy-2-methyl-prop-1-y)-N-pentanoyl-N[2'-(1H-tetrazol-5-yl)bi-phenyl-4-ylmethyl]-amine
This invention relates to novel crystalline forms of valsartan, namely Form A, Form B, Form C, Form D and their solvates thereof. Processes for the preparation of the novel forms are also provided. The present invention further relates to novel processes for preparing a stable amorphous form of valsartan, and in this connection, to the amorphous form of valsartan produced by such processes. The present invention also discloses a novel process for obtaining stable Form I crystals of valsartan.
Owner:IPCA LAB LTD
Synthetic method of cefazolin acid
The invention relates to a synthetic method of cefazolin acid. According to the synthetic method, 7-aminocephalosporanic acid is taken as a raw material, and is reacted with 1H-tetrazole-1-acetic acid in a solvent so as to obtain an intermediate; the intermediate is reacted with 5-mercapto-1-methyltetrazole under base catalysis at solution states without crystallization, washing, and drying so as to obtain cefazolin acid. One-pot method is adopted; the synthetic method is simple; solvent application amount is low; environmental pollution is low; product yield is high; cost is low; and the synthetic method is suitable for industrialized production.
Owner:QILU ANTIBIOTICS PHARMA
Preparation method of flomoxef sodium
The invention discloses a preparation method of flomoxef sodium. The preparation method comprises the following steps of dropwise adding a sodium bicarbonate solution into flomoxef acid while stirring at the temperature of 0-10 DEG C until the pH value of a reaction solution is up to 4.2-5.2, and after flomoxef acid is completely dissolved, extracting, decoloring and removing a decolorizing agent to obtain flomoxef sodium. By using the preparation method of flomoxef sodium, provided by the invention, flomoxef sodium can be effectively and uniformly produced in real time, the residues of sodium salt and flomoxef acid in a product are avoided, the purity of obtained flomoxef sodium is larger than or equal to 99%, and the residual quantity of 1-ethoxyl-5-thiol-1H-tetrazole is smaller than or equal to 0.2%, therefore, the standard requirement of Japanese pharmacopoeia JP16 is completely met.
Owner:SHANGHAI LONGXIANG BIO MEDICINE DEV CO LTD
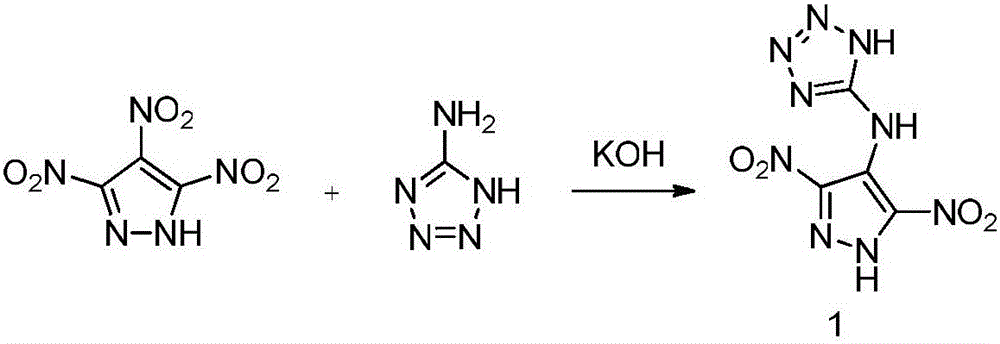
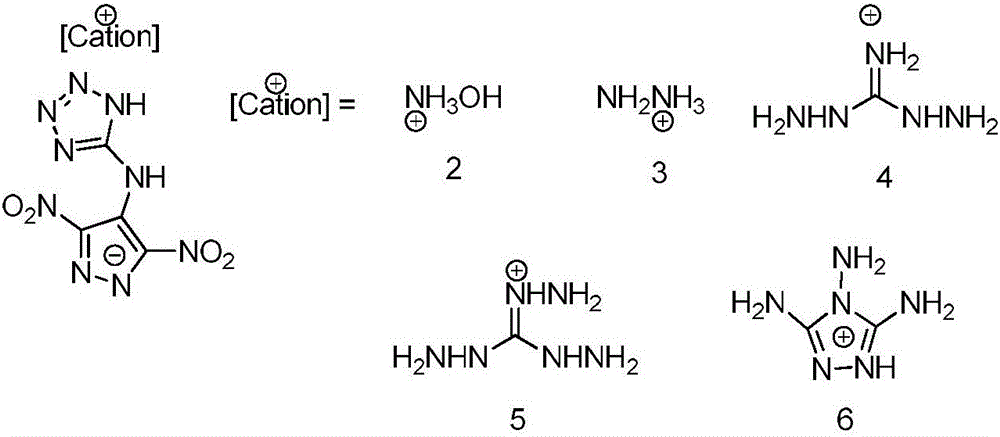
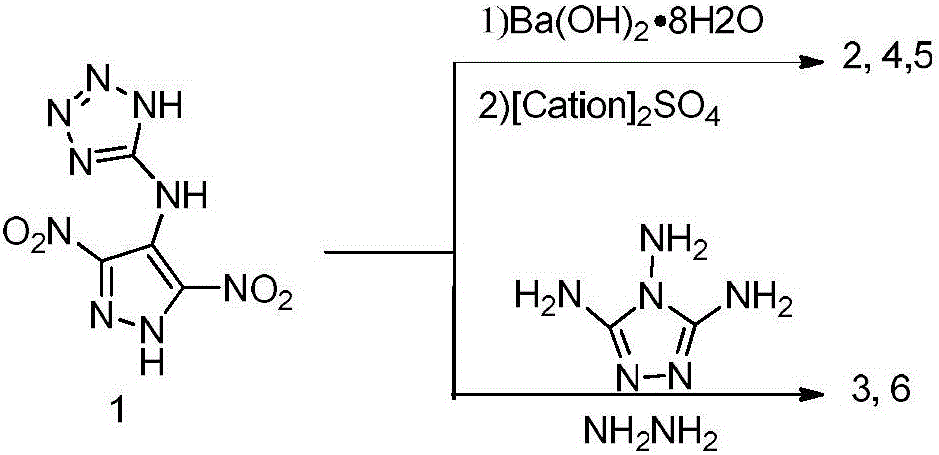
Preparation method and performance of high-energy insensitive N-(3,5-binitro-1H-pyrazol-4-yl)-1H-tetrazole-5-amine ionic salt structure
InactiveCN106432192AHigh densityHigh nitrogen contentOrganic chemistryNitrated acyclic/alicyclic/heterocyclic amine explosive compositionsFriction sensitivityHigh energy
The invention provides a preparation method and performance measurement of N-(3,5-binitro-1H-pyrazol-4-yl)-1H-tetrazole-5-amine (1) and energetic ionic salt thereof, and belongs to the technical field of energetic materials. The synthesis method comprises the steps of adding 3,4,5-trinitropyrazol, 5-amino tetrazole and water into a thick-wall sealed pipe, adjusting the pH to 7 by KOH, performing heating reaction for 48 hours, treating to obtain solid 1,1, and enabling reaction between the solid 1,1 and corresponding cationic sulfate to obtain a target product. The preparation method is simple in synthesis, stable in performance and easy in amplification. In the product, the density is high and is 1.79 to 1.86 g*cm<-3>, the decomposition temperature is high and is 279 to 296 DEG C, the impact sensitivity is 35 to 40 J, the friction sensitivity is 84 to 360 N, the detonation velocity exceeds 9,000 m*s<-1>, the nitrogen content is high and is 51.09 to 60.86 percent, the comprehensive performance is excellent, and a potential application value is achieved.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
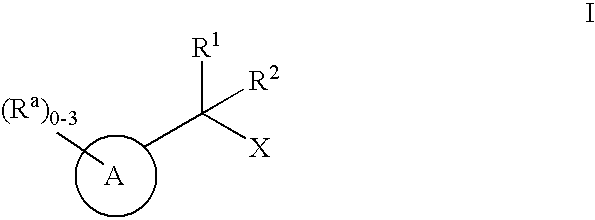
Geminally di-substituted NSAID derivatives as Abeta42 lowering agents
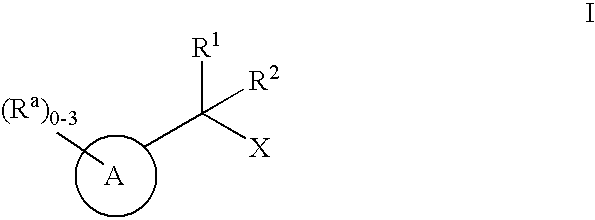
The present invention encompasses compounds of Formula I (I)or pharmaceutically acceptable salts thereof, wherein A is the base molecule of a propionic acid or acetic acid NSAID, or a derivative thereof, X is —CO2H, 1H-tetrazol-5-yl or 2H-tetrazol-5-yl and R1 and R2 are each independently selected from the group consisting of: C1-6alkyl and C3-6cycloalkyl, as well as pharmaceutical composition comprising said compounds and methods of using said compounds. The compounds of the present invention lower the level of Aβ42 and are therefore useful for preventing, delaying or reversing the progression of Alzheimer's Disease.
Owner:MERCK SHARP & DOHME CORP
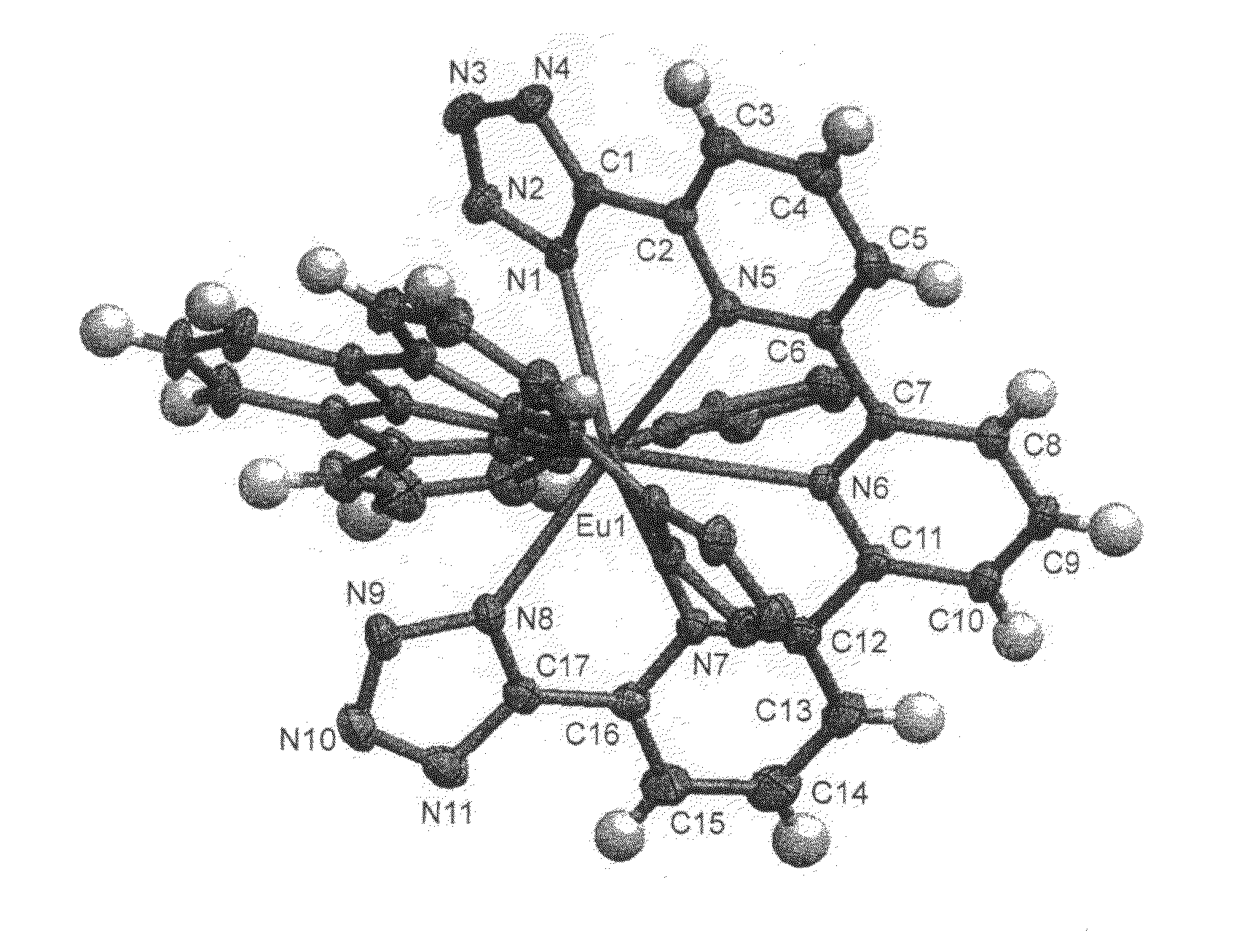
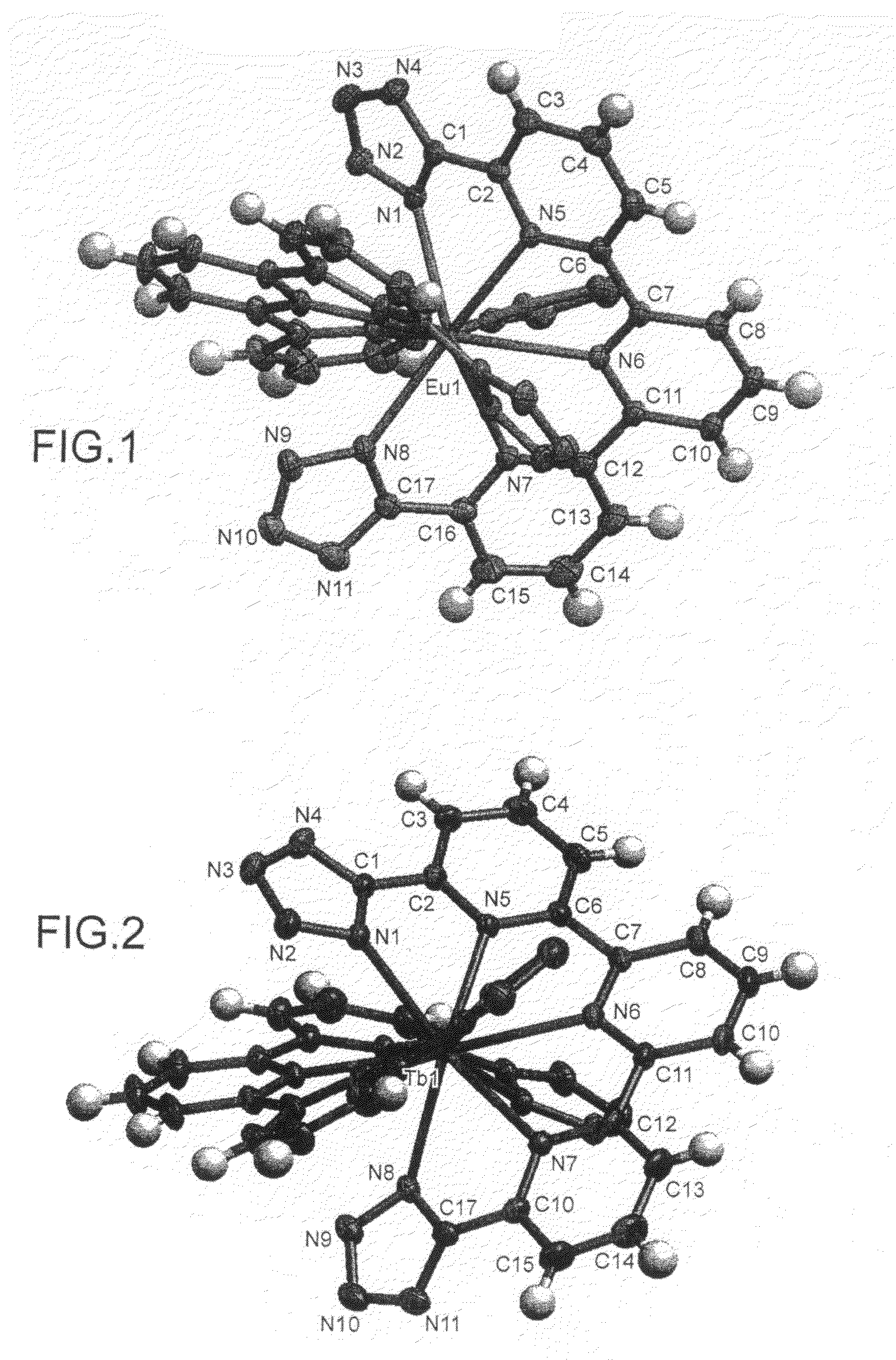
Compounds useful as ligands and particularly as organic chromophores for complexing lanthanides and applications thereof
ActiveUS20110112289A1Group 4/14 element organic compoundsGroup 8/9/10/18 element organic compoundsLanthanidePyridine
The invention relates to the use of compounds comprising at least one 2-(1H-tetrazol-5-yl)pyridine unit, of formula (I) below:as ligands for lanthanides and, more especially, as organic chromophores for complexing these elements.It also relates to lanthanide complexes using these compounds as complexing organic chromophores, and to new compounds containing one or more 2-(1H-tetrazol-5-yl)pyridine units, which are useful as ligands for lanthanides and, in particular, as organic chromophores for complexing these elements.Applications: photonics and optoelectronics, especially for forming light-emitting devices such as electroluminescent diodes; biology, as for example for the preparation of luminescent probes.
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES
Method for synthesizing cefotiam
InactiveCN102898441AHigh yieldSuitable for industrial production applicationsOrganic chemistryCefotiamWhite powder
The invention discloses a method for synthesizing cefotiam. The method comprises the following steps of: reacting acetonitrile, 7-aminocephalosporanic acid and 1- (2-bimethylaminoethyl)-1H-5-mercapto-tetrazole raw materials to obtain a 7-amino-3-[1-(2-bimethylaminoethyl)-1H-tetrazol-5-yl)thiomethyl]cefozopran dihydrochloride; reacting the product with pure water and isopropanol, decoloring the product of the reaction to obtain 7-amino-3-[1-(2-bimethylaminoethyl)-1H-tetrazole-5-yl)thiomethyl]cefozopran hydrochloride; reacting the compound and processing the product of the reaction to obtain formylamino cefotiam; reacting formylamino cefotiam with isopropanol, pure water and hydrochloric acid to obtain coarse cefotiam; and refining twice to obtain finished cefotiam. The method has the advantages that the prepared cefotiam is white powder and the cefotiam yield is as high as 33%; and the method is suitable for industrial production application.
Owner:南通康鑫药业有限公司
Preparation method of ceftezole and midbody thereof
The invention relates to a preparation method of a ceftezole and a midbody thereof. The midbody 7-amido-3-(1, 3, 4-thiadiazole-5-base) sulfidomethyl-2-cephem-2-carboxylic acid is prepared from 5-hydrosulphonyl-1, 3, 4-thiadiazole and 7-ACA under certain reaction environment; the prepared midbody 7-amido-3-(1, 3, 4-thiadiazole-5-base) sulfidomethyl-2-cephem-2-carboxylic acid reacts with 1H-tetrazole-1-acetyl chloride to prepare the ceftezole. The ceftezole can be prepared with a one-boiler method in the invention, and the preparation method has the advantages of short reaction time, gentle reaction condition, low cost and suitability of industrial production. In addition, the ceftezolet and the midbody produced by the method have high yield and good purity.
Owner:BEIJING SHENKELIANHUA TECH
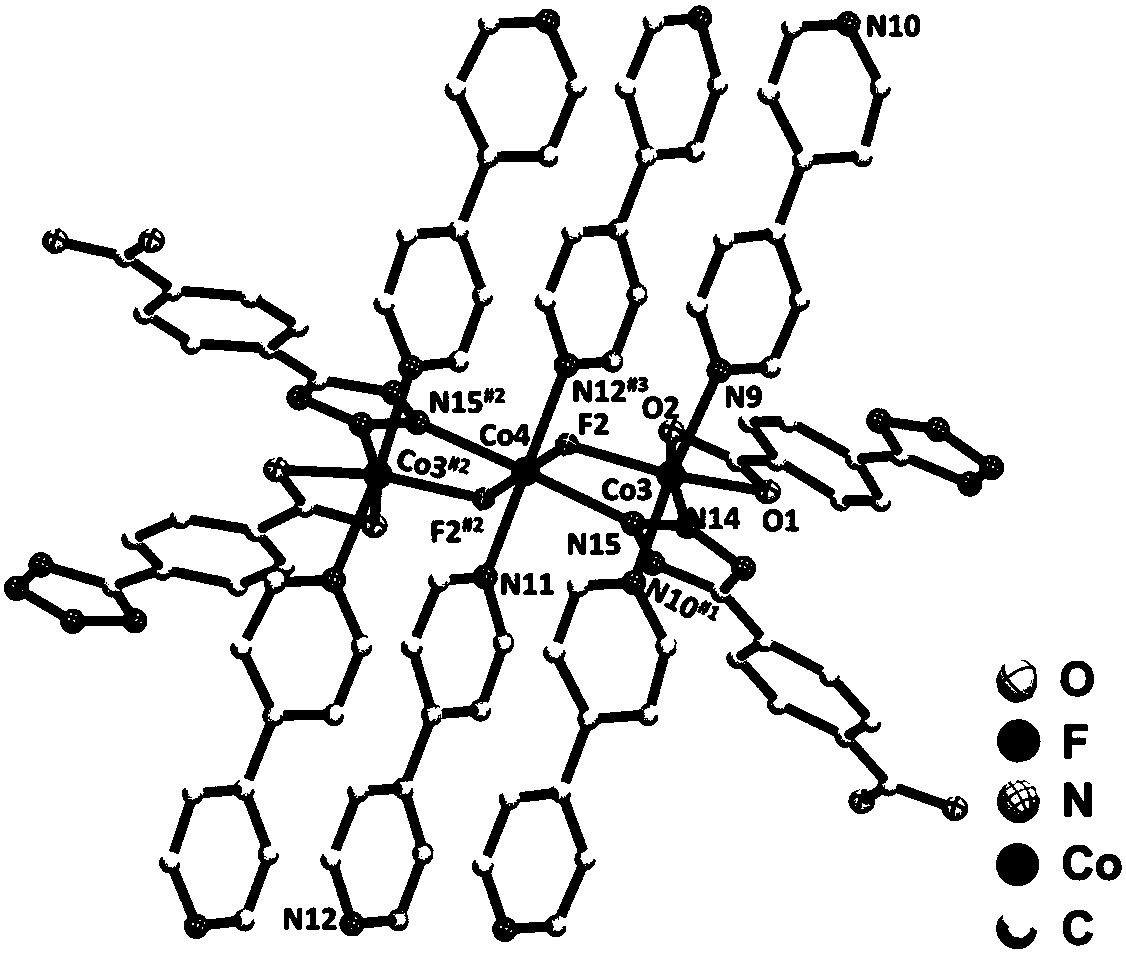
Cd(II)MOF material based on 5-(4-[1,2,4]triazole-1-phenyl)-1H-tetrazole
InactiveCN110628040ANovel structureStable structureFluorescence/phosphorescenceLuminescent compositionsDichromate ionFluorescence
The invention discloses a Cd(II) metal organic framework material based on 5-(4-[1,2,4]triazole-1-phenyl)-1H-tetrazole, a preparation method and application thereof. The metal organic framework material is prepared by taking 5-(4-[1,2,4]triazole-1-phenyl)-1H-tetrazole and CdCl2.2.5H2O as raw materials througha solvothermal method; the molecular formula of the metal organic framework is [Cd(L)2(H2O)2]n, wherein L is a ligand 5-(4-[1,2,4]triazole-1-phenyl)-1H-tetrazole; and when the metal organic framework material is used for the detection of nitrobenzene (NB), acetone (ACE), iron ions (Fe<3+>)and dichromate ions (Cr2O7<2->) as a fluorescent sensing material, high sensitivity is shown.
Owner:ANYANG NORMAL UNIV
Highly pure cilostazol and an improved process for obtaining same
InactiveUS20050222202A1Easy to operateImprove efficiencyBiocideOrganic chemistry1H-tetrazoleCilostazol
A novel process for preparing highly pure cilostazol, effected by reacting 6-hydroxy-3,4-dihydroquinolinone and 5-(4-chlorobutyl)-1-cyclohexyl-1H-tetrazole in the presence of a hydrated inorganic base, is disclosed. Further disclosed is highly pure cilostazol, and particularly highly pure cilostazol that is substantially free of 6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)-butoxy]-1-[4-(1-cyclohexyl-1H-tetrazol-5-yl)-butyl]-3,4-dihydro-1H-quinolin-2-one.
Owner:CHEMAGIS
Flexible metal organic frame material and preparation method and application thereof
InactiveCN108219159ASimple preparation processMild reaction conditionsProductsGas treatmentBenzoic acidN dimethylformamide
The invention discloses a preparation method for a flexible metal organic frame material. The method comprises the following steps: (1) mixing N,N-dimethylformamide, ethyl alcohol, and water to form mixed liquid, and adding fluoroboric acid to form a mixed solvent; (2) adding cobalt-nitrate hexahydrate, 4,4'-dipyridyl, and 4-(1H-tetrazole-5-yl) benzoic acid to the mixed solvent, and stirring to form a mixture; (3) placing the mixture in a high-pressure reaction kettle, heating in a closed space, cooling to the room temperature, to obtain reaction liquid; (4) filtering the reaction liquid to obtain a filtrated body, and washing the filtrated body by using the ethyl alcohol, to obtain a pink rodlike crystal; and (5) activating the pink rodlike crystal, to obtain the flexible metal organic frame material. The flexible metal organic frame material is used for adsorbing and separating CO2 and CH4. The disclosed flexible metal organic frame material and the preparation method and the application thereof have the advantages of the simple and convenient preparation technology, the moderate reaction conditions, the easily obtained raw materials, and the good adsorptive selectivity and so on.
Owner:NORTHWEST UNIV
Nitro containing and halogen benzene substituted 1H-tetrazole-1-acetic acid structure, and preparation method and use thereof
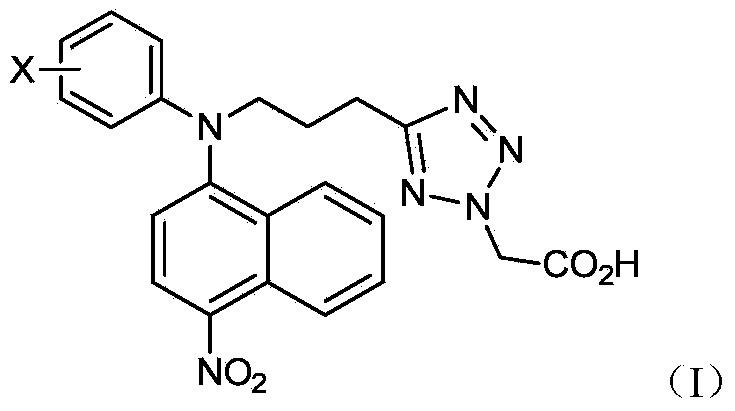
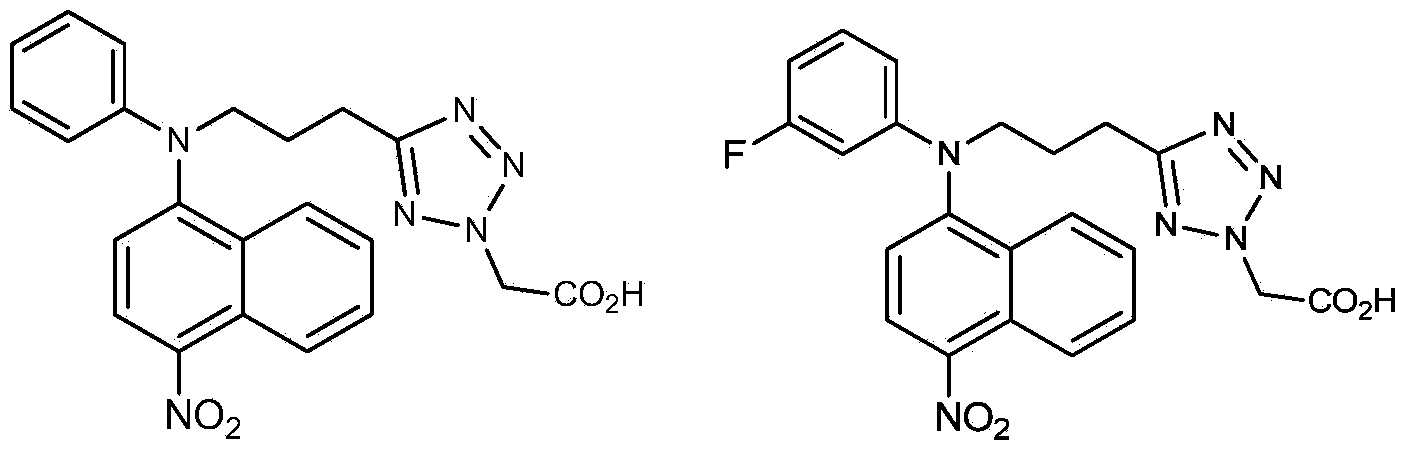
The invention relates to the field of medicines relative to hyperuricemia and gout, particularly to an inhibitor for a urate transporter 1 in a nitro containing and halogen benzene substituted 1H-tetrazole-1-acetic acid structure, a preparation method of the urate transporter 1, a medicine composition containing the inhibitor, and the application of the inhibitor in preparing diabetes medicines. The general formula (refer to the Specification) of the compound is disclosed by the invention, wherein X is selected from halogen substituent.
Owner:浙江西塘实业有限公司
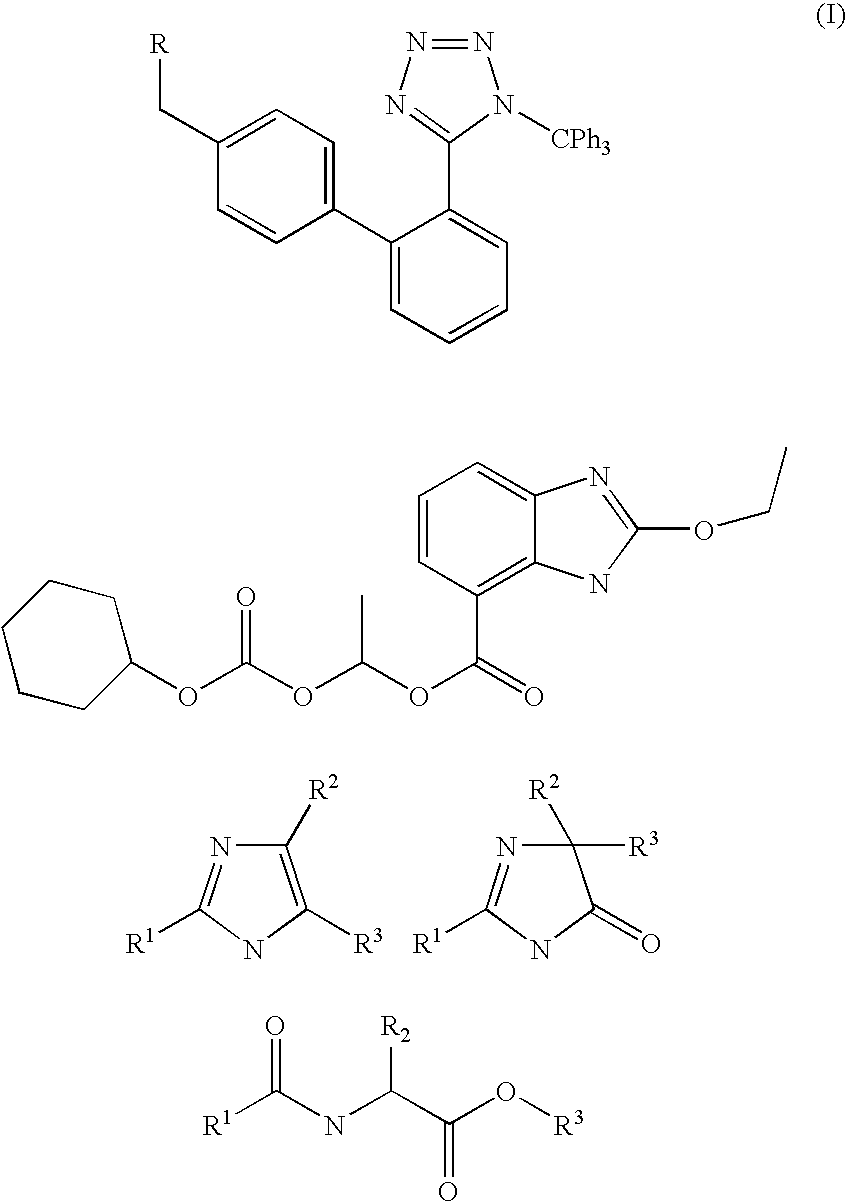
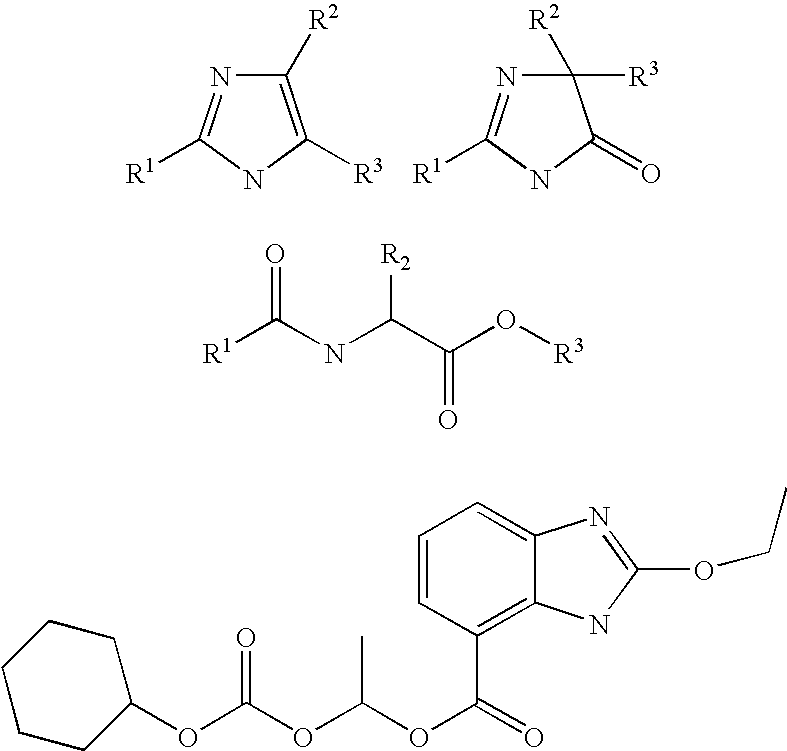
Method of removing the triphenylmethane protecting group
A method of removing the triphenylmethane protecting group from 1-triphenylmethy1-5-(4′-subst. methyl-1,1′-biphenyl-2-yl)-1H-tetrazoles of general formula I wherein R represents the groups of formulae and where R1, R2 and R3 can be H, a halogen, an unbranched or branched C1-C5 alkyl, C1-C5 hydroxyalkyl, C1-C5 alkoxy, C1-C5 alkoxymethyl or benzyl, or wherein R2 and R3 can form together a saturated or unsaturated C5-C7 ring, optionally an unsubstituted or substituted aromatic ring, is carried out by solvolysis in a simple anhydrous C1 to C5 alcohol in a neutral or slightly basic medium. The method is suitable for the preparation of drugs, such as the potassium salts of losartan, irbesartan or valsartan or candesartan cilexetil.
Owner:ZENTIVA AS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/eb239ae2-f451-4c9e-8101-694067a7ab8d/US08877938-20141104-D00000.png)
![Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/eb239ae2-f451-4c9e-8101-694067a7ab8d/US08877938-20141104-D00001.png)
![Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/eb239ae2-f451-4c9e-8101-694067a7ab8d/US08877938-20141104-C00001.png)














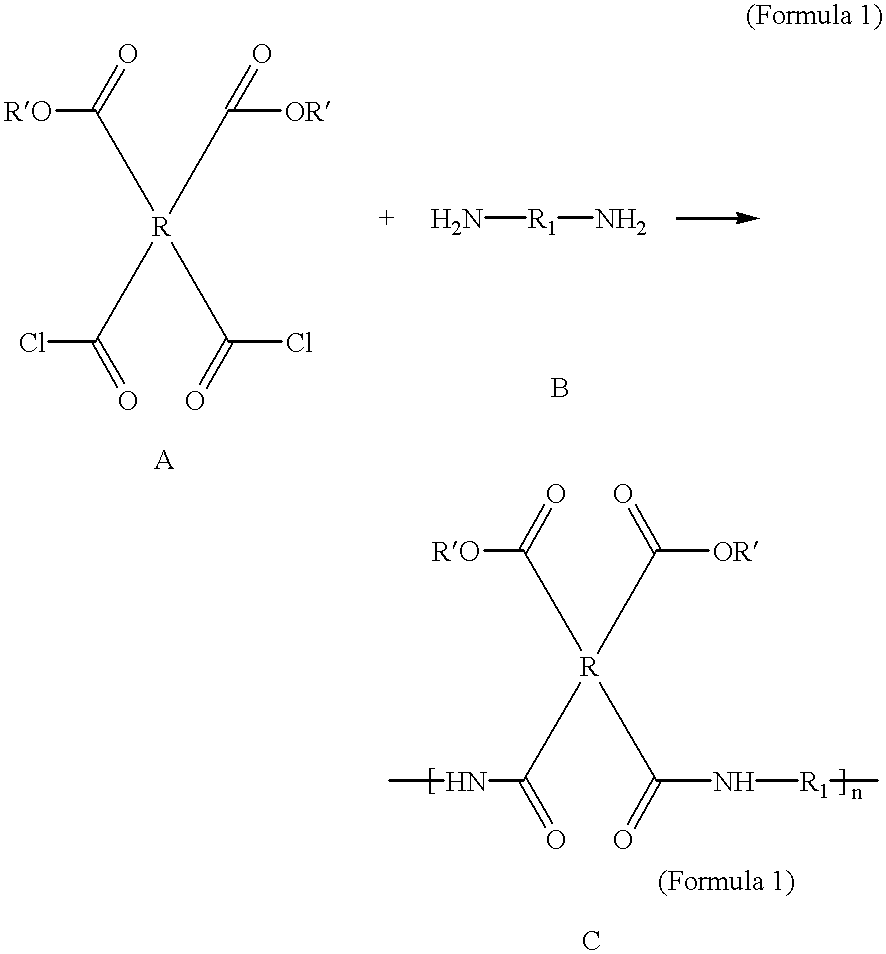


![Novel crystalline forms of (S)-N-(1-Carboxy-2-methyl-prop-1-y)-N-pentanoyl-N[2'-(1H-tetrazol-5-yl)bi-phenyl-4-ylmethyl]-amine Novel crystalline forms of (S)-N-(1-Carboxy-2-methyl-prop-1-y)-N-pentanoyl-N[2'-(1H-tetrazol-5-yl)bi-phenyl-4-ylmethyl]-amine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b02b7cac-da91-4c80-ba4e-e47c597f6199/US20080261959A1-20081023-D00001.png)
![Novel crystalline forms of (S)-N-(1-Carboxy-2-methyl-prop-1-y)-N-pentanoyl-N[2'-(1H-tetrazol-5-yl)bi-phenyl-4-ylmethyl]-amine Novel crystalline forms of (S)-N-(1-Carboxy-2-methyl-prop-1-y)-N-pentanoyl-N[2'-(1H-tetrazol-5-yl)bi-phenyl-4-ylmethyl]-amine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b02b7cac-da91-4c80-ba4e-e47c597f6199/US20080261959A1-20081023-D00002.png)
![Novel crystalline forms of (S)-N-(1-Carboxy-2-methyl-prop-1-y)-N-pentanoyl-N[2'-(1H-tetrazol-5-yl)bi-phenyl-4-ylmethyl]-amine Novel crystalline forms of (S)-N-(1-Carboxy-2-methyl-prop-1-y)-N-pentanoyl-N[2'-(1H-tetrazol-5-yl)bi-phenyl-4-ylmethyl]-amine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b02b7cac-da91-4c80-ba4e-e47c597f6199/US20080261959A1-20081023-D00003.png)





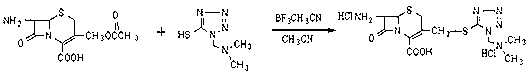

![Cd(II)MOF material based on 5-(4-[1,2,4]triazole-1-phenyl)-1H-tetrazole Cd(II)MOF material based on 5-(4-[1,2,4]triazole-1-phenyl)-1H-tetrazole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ec8592a4-9a73-43f8-b6e8-9500586d6134/191015093338.png)
![Cd(II)MOF material based on 5-(4-[1,2,4]triazole-1-phenyl)-1H-tetrazole Cd(II)MOF material based on 5-(4-[1,2,4]triazole-1-phenyl)-1H-tetrazole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ec8592a4-9a73-43f8-b6e8-9500586d6134/191015093342.png)
![Cd(II)MOF material based on 5-(4-[1,2,4]triazole-1-phenyl)-1H-tetrazole Cd(II)MOF material based on 5-(4-[1,2,4]triazole-1-phenyl)-1H-tetrazole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ec8592a4-9a73-43f8-b6e8-9500586d6134/191015093346.png)