Preparation method and performance of high-energy insensitive N-(3,5-binitro-1H-pyrazol-4-yl)-1H-tetrazole-5-amine ionic salt structure
A technology of trinitropyrazole and dinitro, applied in the directions of nitrated acyclic/alicyclic/heterocyclic amine explosive compositions, organic chemistry, etc., can solve problems such as poor thermal stability, achieve high yield, Simple synthetic method and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
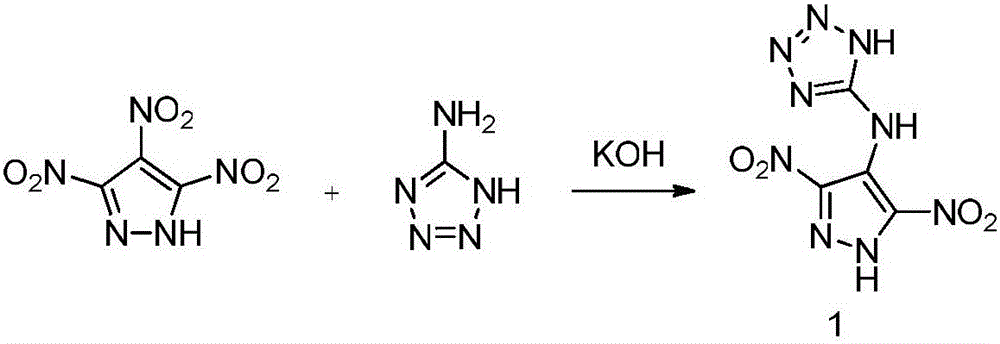
[0027] Example 1 Preparation of N-(3,5-dinitro-1H-pyrazol-4-yl)-1H-tetrazol-5-amine (1)
[0028] Its structural formula is as follows:
[0029]
[0030] Into a 50mL thick-walled sealed glass tube, add 0.203g (1mmol) 3,4,5-trinitropyrazole, 0.255g (3mmol) 5-aminotetrazole, 0.224g (5mmol) KOH and 5mL water in sequence, seal Stir, heat up to 160°C, react for 30h, cool with 20% H 2 SO 4 The solution was acidified to pH=1, filtered to obtain a yellow solid, and recrystallized from water / methanol to obtain 0.152 g of a yellow crystal, with a yield of 63%. Density ρ = 1.86g cm -3 , decomposition temperature T d =279℃; IR(neat):3301,3211,3084,2760,1624,1472,1327,1079,837,556cm -1 ; 1 H NMR (500MHz,D 6 -DMSO): δ=9.24 (s, 2NH), 7.11 (t, NH) ppm; 13 C NMR (100MHz,D 6 -DMSO): δ=149.3, 115.52, 99.9ppm; MS (ESI): m / z (%): 240 [M-H] - .
Embodiment 2
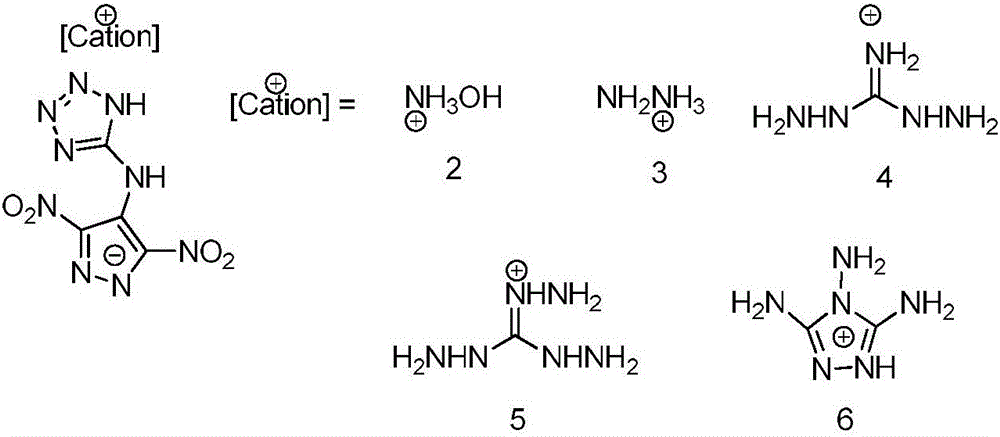
[0031] Example 2 Preparation of N-(3,5-dinitro-1H-pyrazol-4-yl)-1H-tetrazolium-5-amine hydroxylamine salt (2)
[0032] Its structural formula is as follows:
[0033]
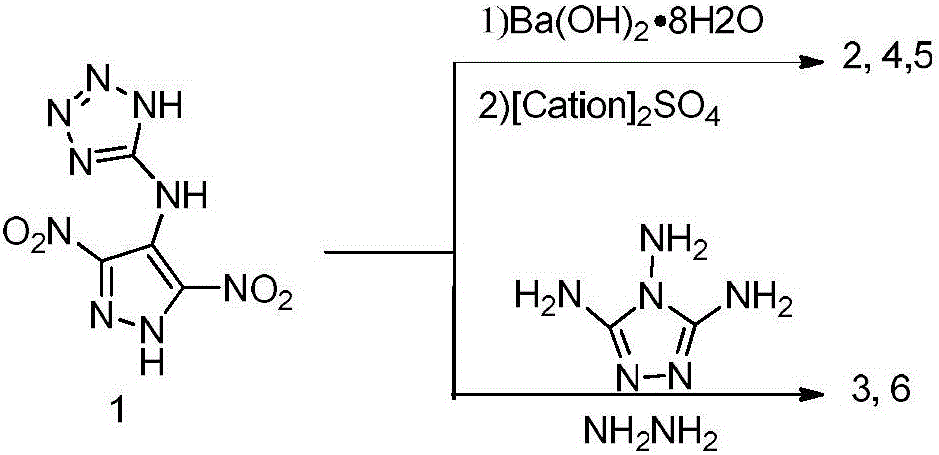
[0034] Dissolve 0.241g (1.0mmol) of 1 in 50mL of hot water and methanol mixture, add 0.158g (0.5mmol) of Ba(OH) 2 ·8H 2 O, after the reaction mixture was reacted at 60° C. for 2 h, 0.5 mmol of hydroxylamine sulfate was added thereto, the mixture continued to react for 2 h, and the insoluble matter was filtered, the filtrate was concentrated, and the resulting solid was recrystallized from water to obtain the product (0.213 g, 78%). Density: ρ=1.84g cm -3 ;Decomposition temperature: T d =296°C (DSC); IR (neat): 3393, 3246, 3077, 1631, 1474, 1327, 1080, 1008, 841, 730, 556cm -1 . 1 H NMR (500MHz, d 6 -DMSO): δ=14.86(s, NH), 9.24(s, NH), 7.11(t, NH 2 ) ppm; 13 C NMR (100MHz,D 6 -DMSO): δ=157.1,155.8,149.4,115.5ppm; Anal.calcd for C 4 h 6 N 10 o 5 (274.15)%: C 17.50, H 2.23, N51.12; found C 17.52, H ...
Embodiment 3
[0035] Example 3 Preparation of N-(3,5-dinitro-1H-pyrazol-4-yl)-1H-tetrazolium-5-amine hydrazine salt (3)
[0036] Its structural formula is as follows:
[0037]
[0038] Dissolve 0.241g (1.0mmol) 1 in hot water and methanol mixture, add 0.050g (1.0mmol) hydrazine hydrate (99%) to it, stir and react at room temperature for 2h, concentrate the reaction solution and recrystallize the crude product with water Yellow crystals (0.194 g, yield: 71%) were obtained. Density: ρ=1.84g·cm -3 . Decomposition temperature: T d = 290°C. IR(neat):3326,3301,3166,2918,2649,2077,1579,1456,1318,1104,1016,945,615cm -1 . 1 H NMR (500MHz,D 6 -DMSO): δ=6.63(s), ppm; 13 C NMR (100MHz,D 6 -DMSO): δ=155.8,149.4,115.5ppm; Anal.calcd for C 4 h 7 N 11 o 4 (273.17)%: C 17.57, H 2.54, N 56.45; found C 17.59, H 2.58, N 56.40.
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
| impact sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com