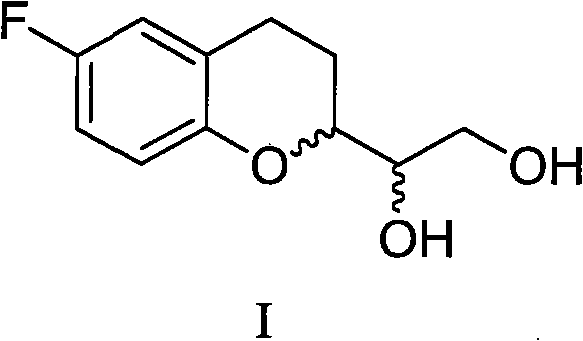
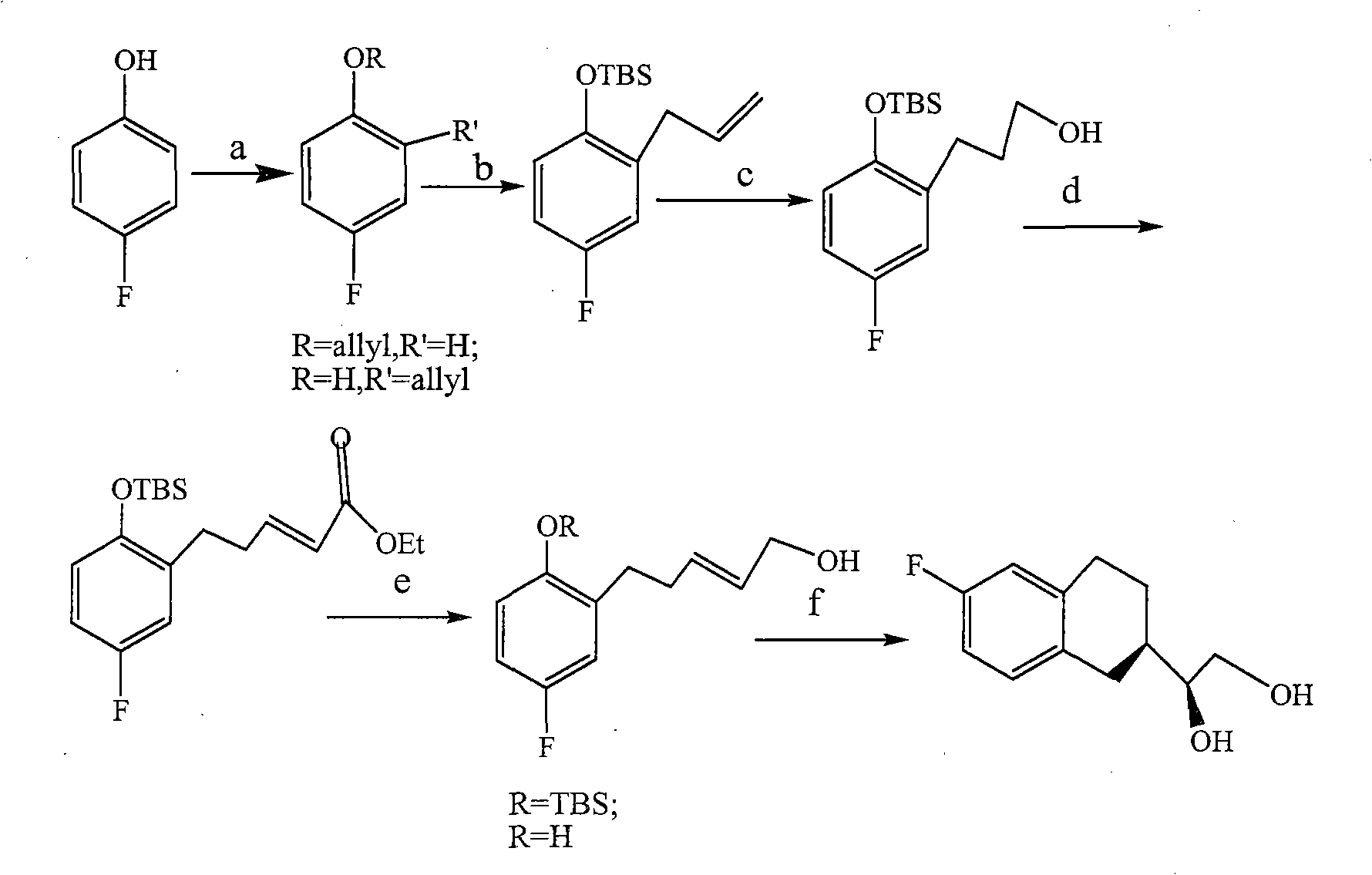
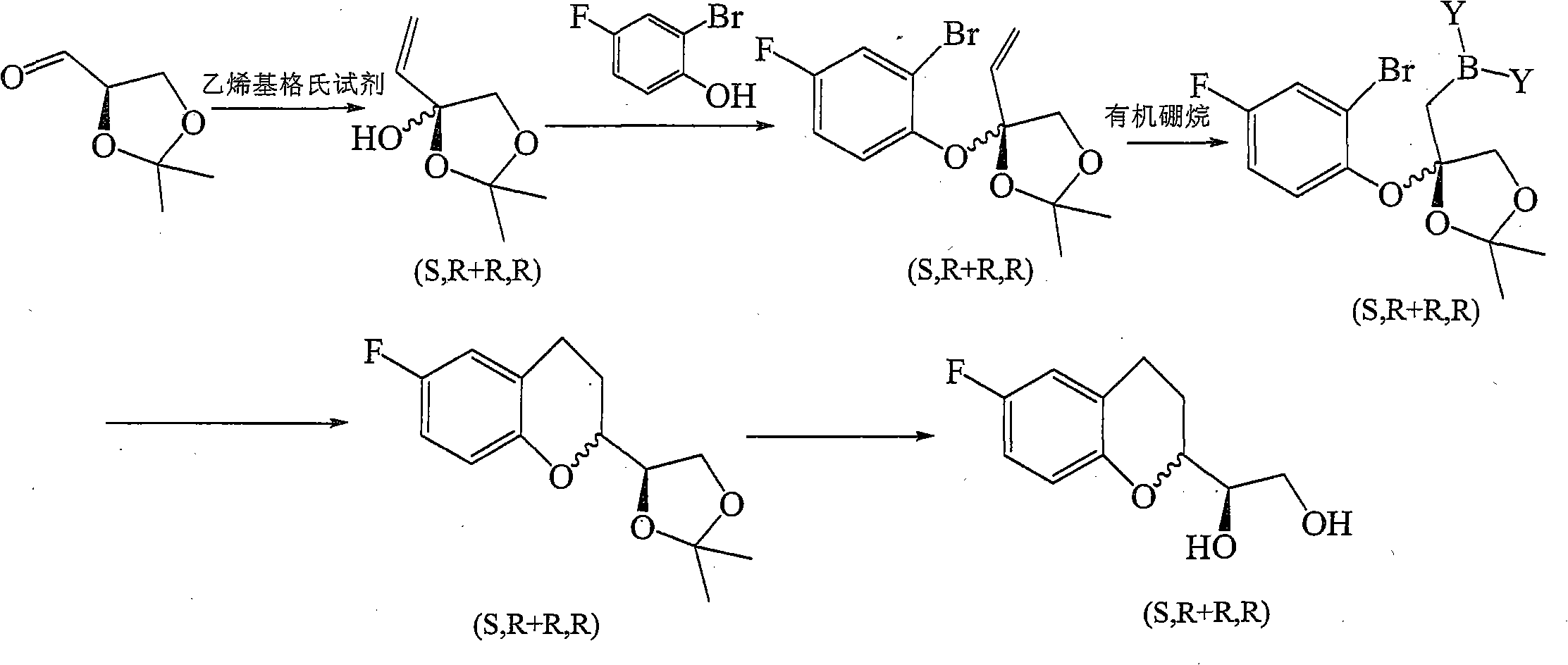
Method for preparing benzodihydropyran compound
A compound, sodium borohydride technology, applied in organic chemistry, bulk chemical production, etc., can solve the problems of harsh reaction conditions, large environmental pollution, and long synthesis steps, and achieve short reaction steps, simple post-treatment, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 1, the synthesis of formula IIa compound
[0044]
[0045] 1.1 Dissolve 5-fluoro-2-hydroxyacetophenone (80g, 0.519mol) in 1000ml of dry tetrahydrofuran, cool in an ice-water bath at 0°C, add potassium tert-butoxide (66.55g, 0.545mol) under vigorous stirring, and stir vigorously After 30 minutes, add a dichloromethane solution of D-glyceraldehyde acetone (w / w=0.432, 234.5g, 0.779mol), stir at 0°C for 3.5 hours, add concentrated hydrochloric acid dropwise, adjust the pH value to 6, and spin evaporate Solvent was removed. Ethyl acetate (1200 mL) and water (500 mL) were added to the residue, the organic phase was separated, washed with water and saturated brine, dried over anhydrous sodium sulfate, and spin-dried to obtain a yellow solid, which was recrystallized from absolute ethanol to obtain a light yellow powder Crystals (52 g, 35%).
[0046] 1.2 Dissolve 5-fluoro-2-hydroxyacetophenone (10g, 0.065mol) in 100ml of dry tetrahydrofuran, cool in an ice-water bath at 0°...
Embodiment 2
[0075] 1, the synthesis of formula IIb compound
[0076]
[0077] Dissolve 5-fluoro-2-hydroxyacetophenone (30g, 0.195mol) in 300ml of dry tetrahydrofuran, cool in an ice-water bath at 0°C, add potassium tert-butoxide (23.77g, 0.195mol) under vigorous stirring, and stir vigorously for 30 Minutes later, add a dichloromethane solution of L-glyceraldehyde acetone (w / w=0.432, 70.49g, 0.234mol), stir at 0°C for 3 hours, add concentrated hydrochloric acid dropwise, adjust the pH value to 6, and remove by rotary evaporation solvent. Ethyl acetate (400 mL) and water (300 mL) were added to the residue, the organic phase was separated, washed with water and saturated brine, dried over anhydrous sodium sulfate, and spin-dried to obtain a yellow solid, which was recrystallized from absolute ethanol to obtain a light yellow powder Crystals (25 g, 45%).
[0078] 2, the synthesis of formula IIIb compound
[0079]
[0080] Suspend the compound of formula IIb (20 g, 0.07 mol) in 100 mL...
Embodiment 3
[0097] The synthesis of embodiment three nebivolol
[0098] The nebivolol of SRRR and RSSS configuration is prepared by the compound of formula I, can refer to prior art,
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com