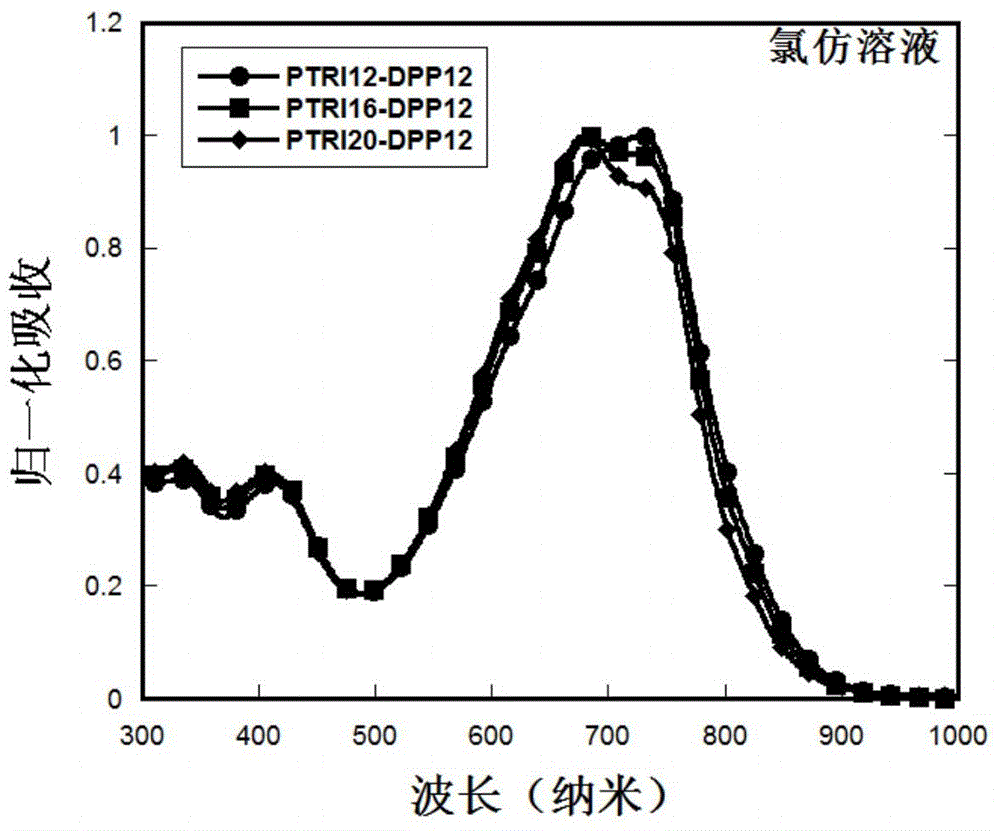
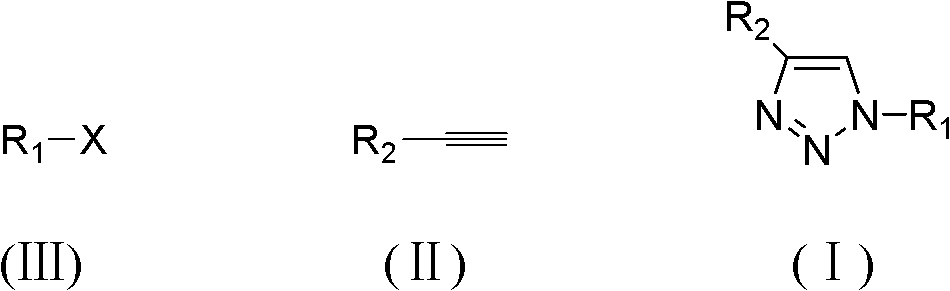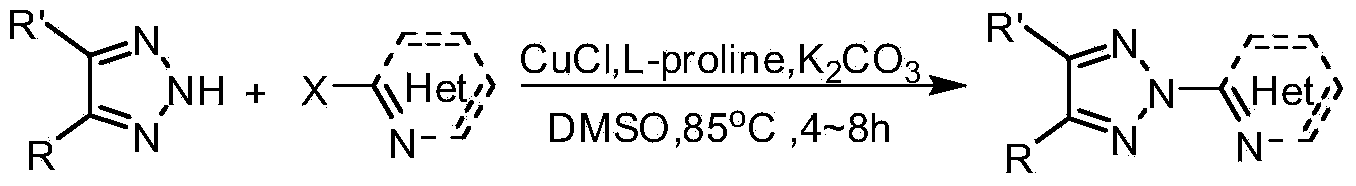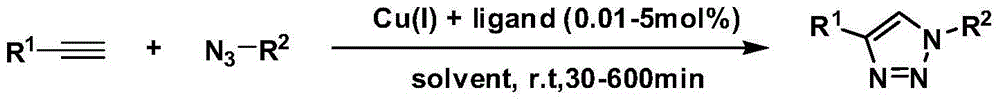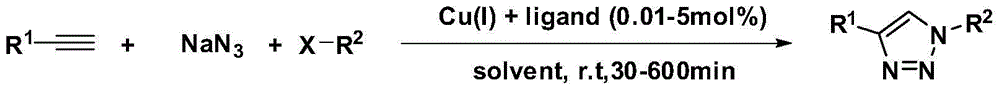Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
212 results about "1,2,3-Triazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
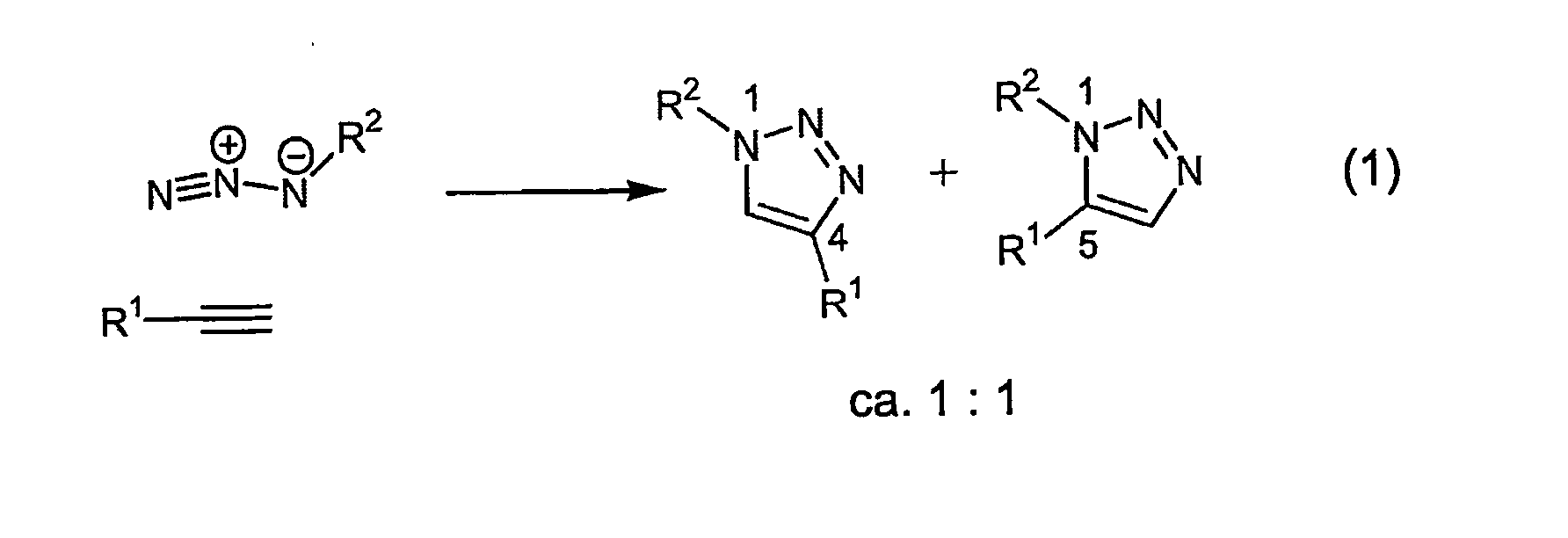
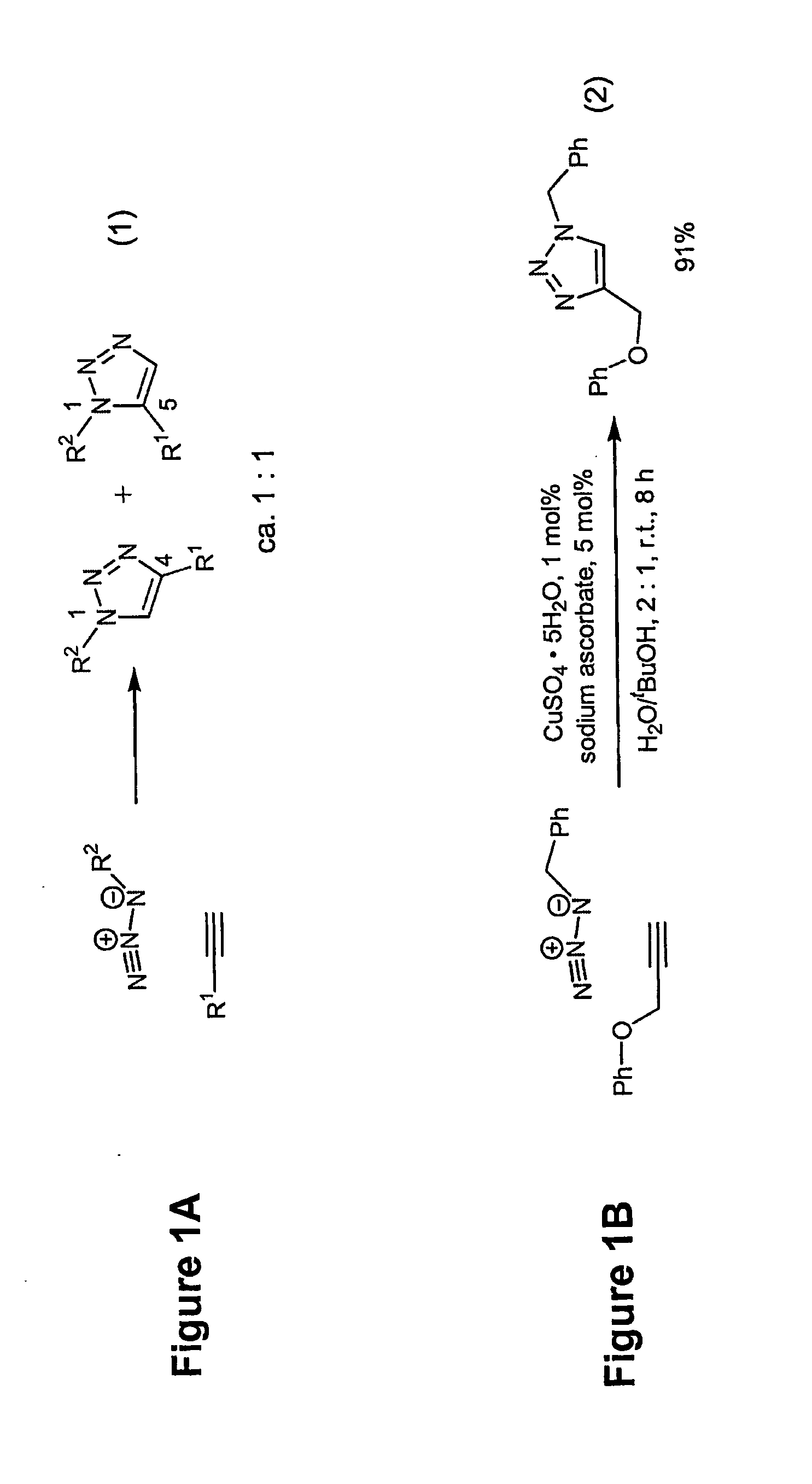
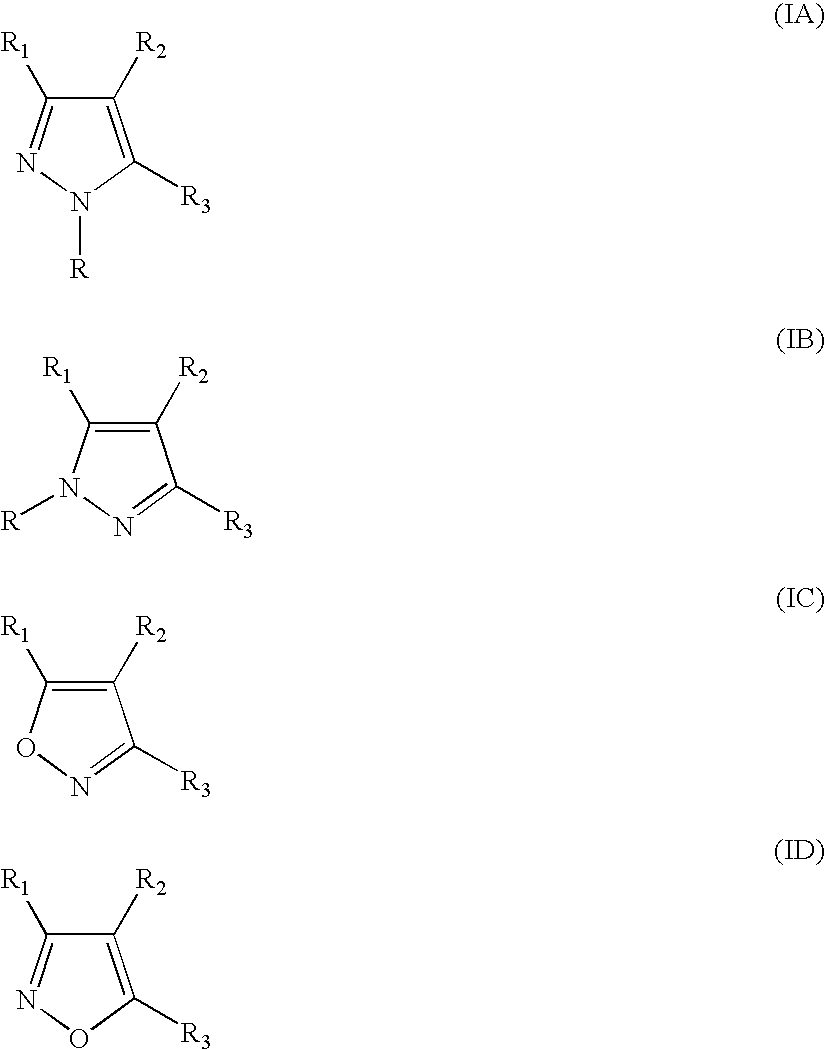
1,2,3-Triazole is one of a pair of isomeric chemical compounds with molecular formula C₂H₃N₃, called triazoles, which have a five-membered ring of two carbon atoms and three nitrogen atoms. 1,2,3-Triazole is a basic aromatic heterocycle.
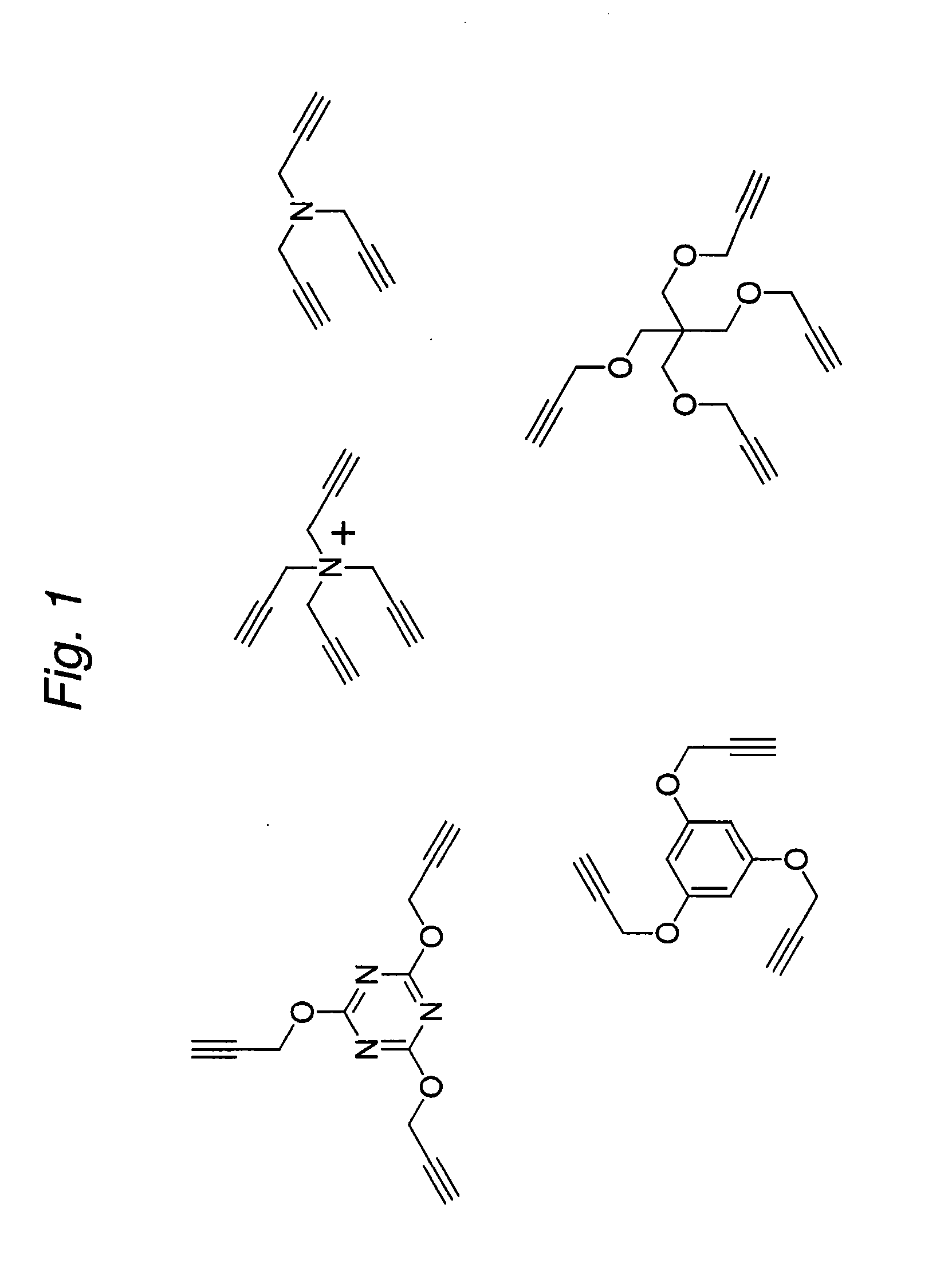
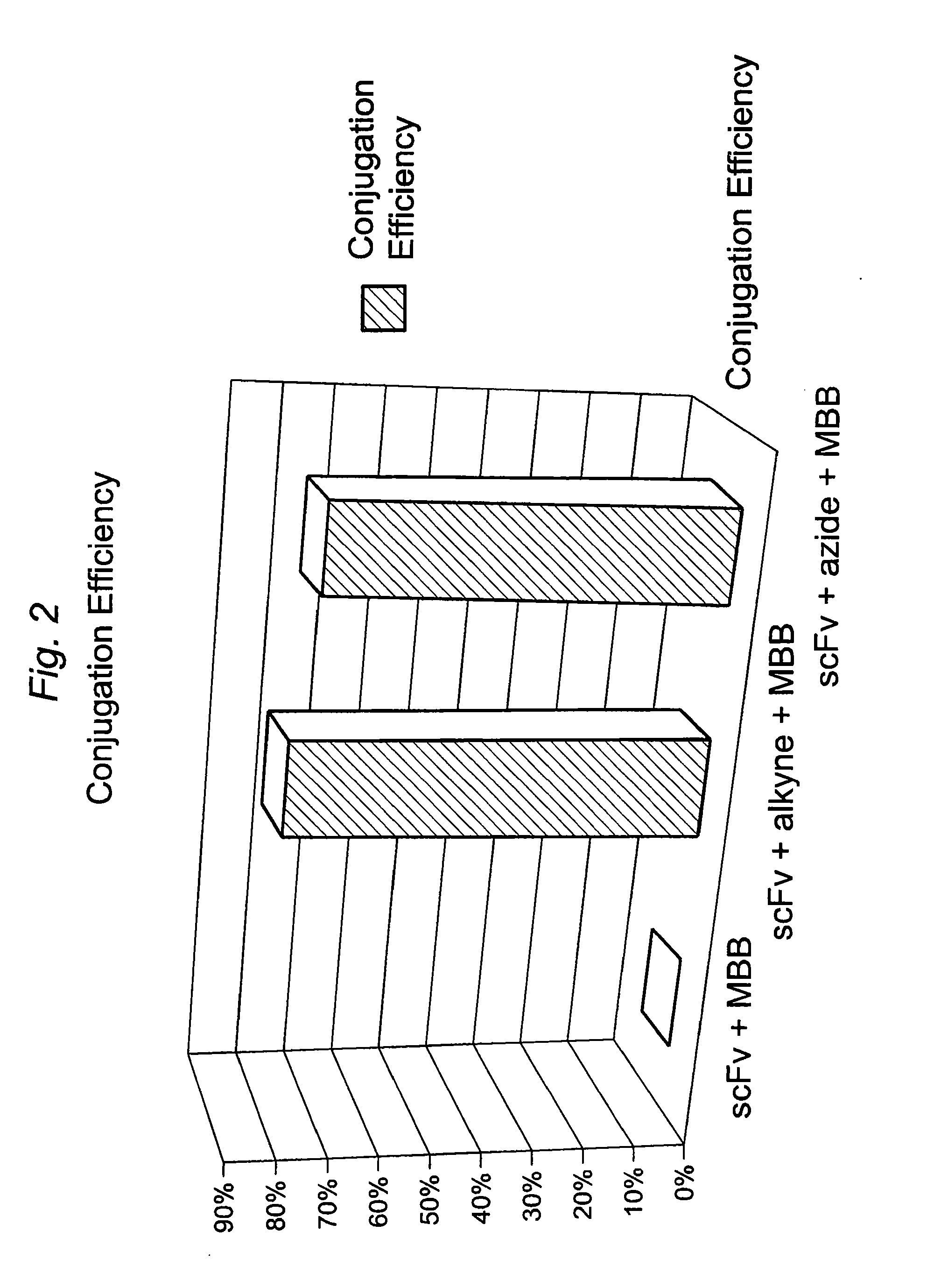
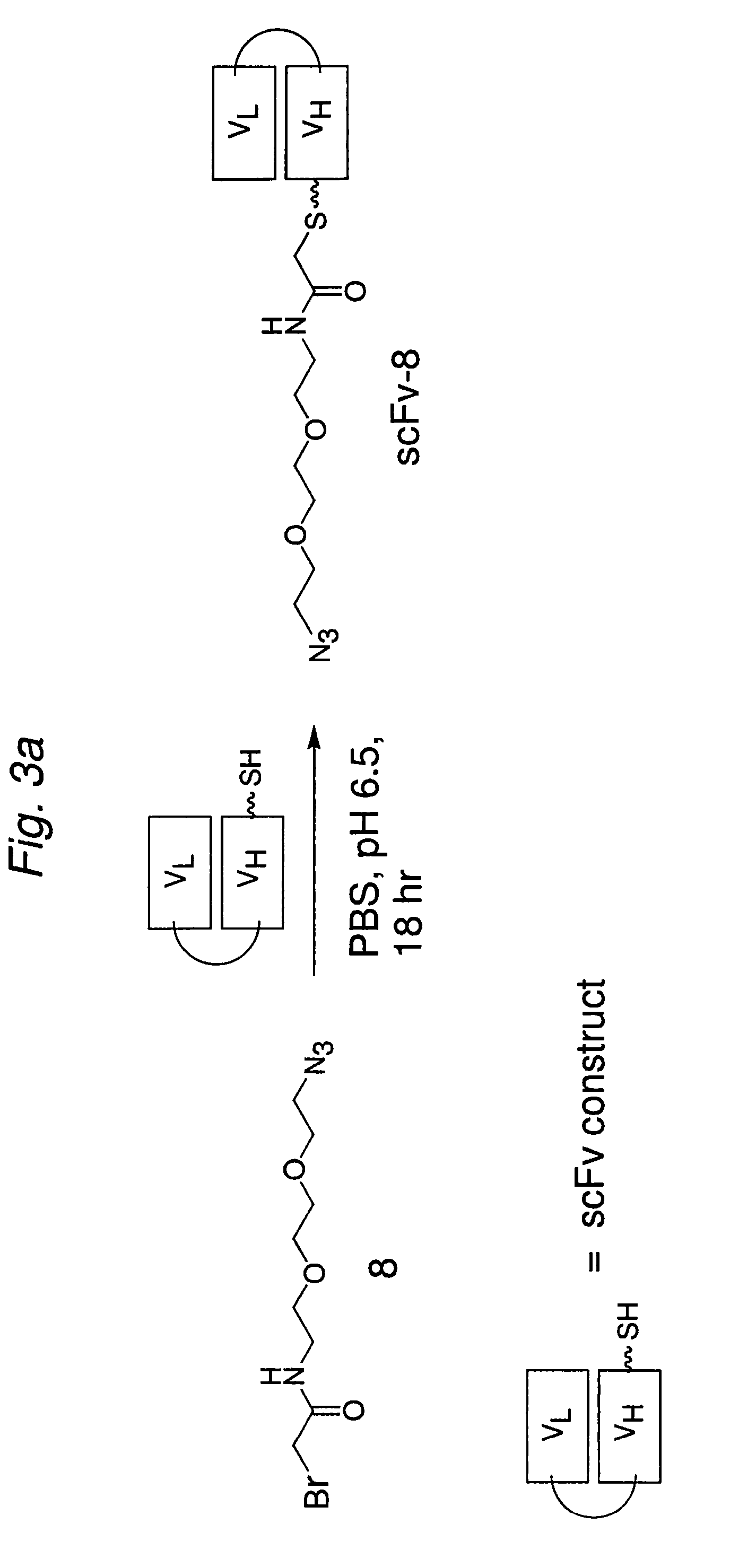
Construction of a Multivalent SCFV Through Alkyne-Azide 1,3-Dipolar Cycloaddition
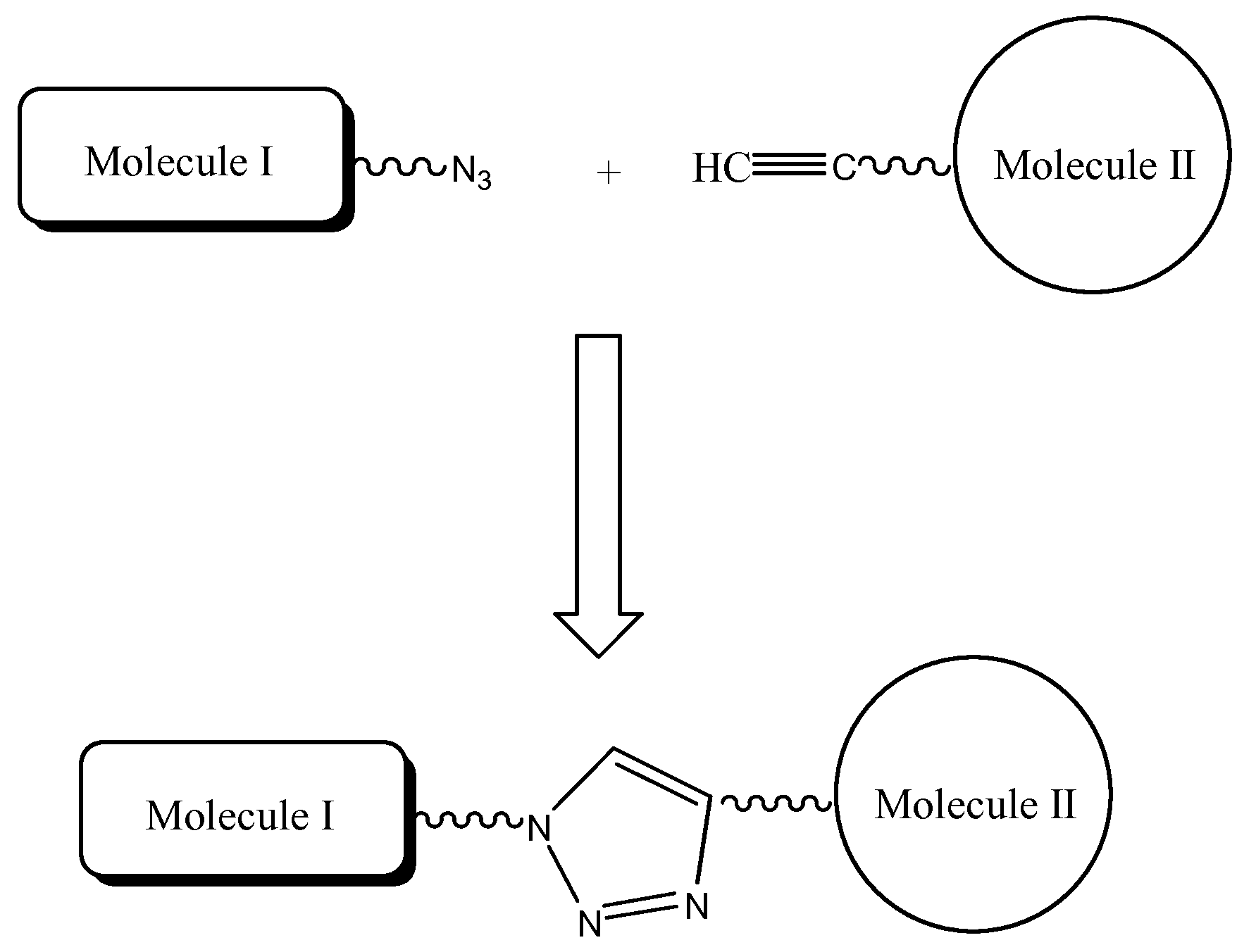
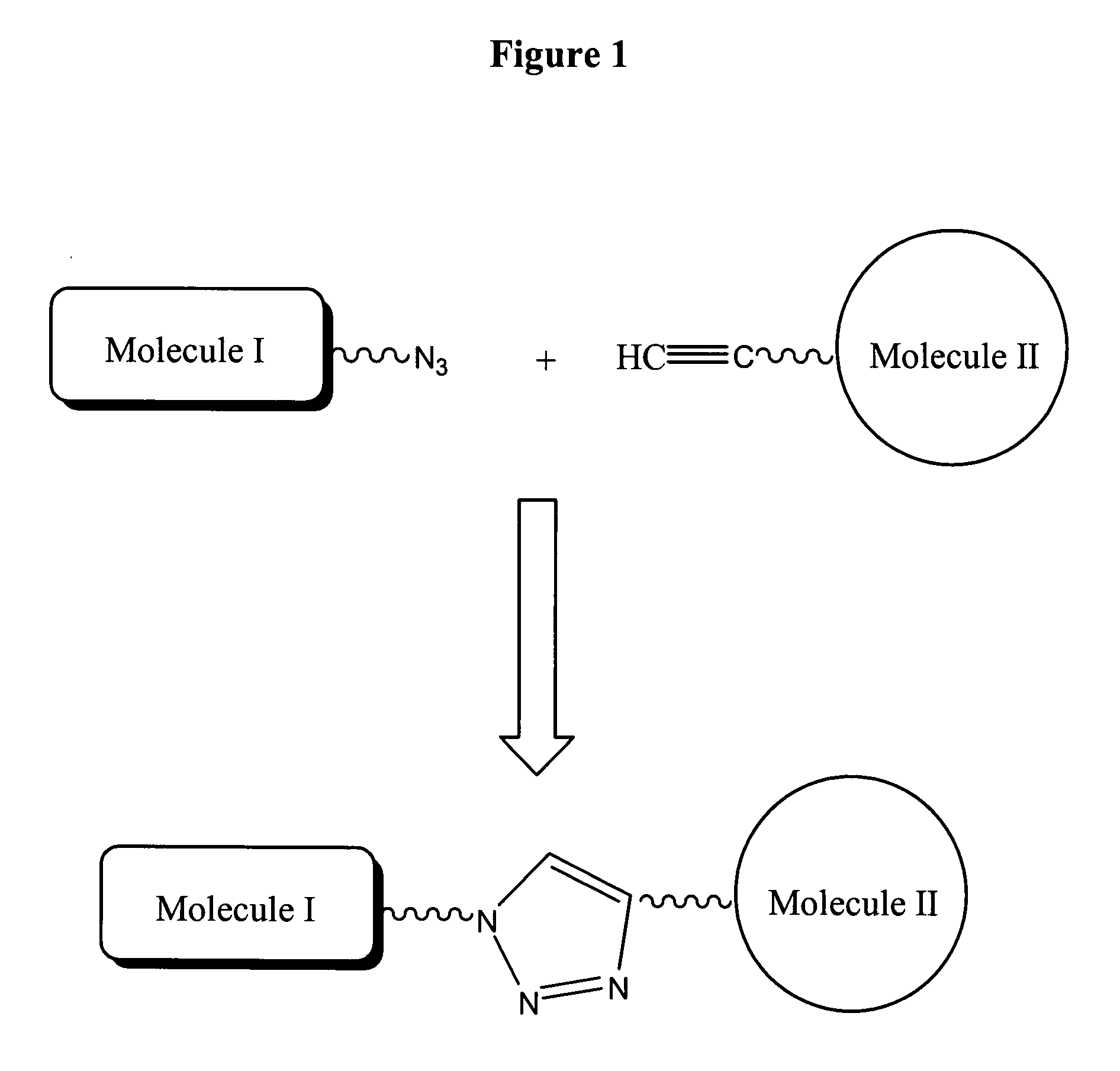
The present invention provides for a practical, universal and efficient method to ligate two large macromolecules (e.g., proteins) using the alkyne-azide 1,3-dipolar cycloaddition reaction to produce a conjugated macromolecule, such as a multivalent scFv. The present invention also provides for conjugate macromolecules comprising a plurality of macromolecule components cross-linked through at least one linking group comprising at least one 1,2,3-triazole moiety, wherein at least 50 percent of the macromolecule components in the conjugate macromolecule has only one site available for cross-linking.
Owner:RGT UNIV OF CALIFORNIA
Nicotinamide acids, amides, and their mimetics active as inhibitors of PDE4 isozymes
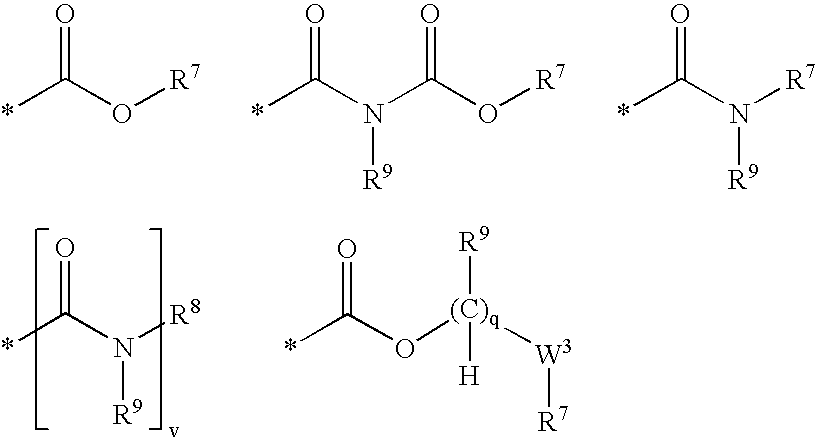
Compounds useful as inhibitors of PDE4 in the treatment of diseases regulated by the activation and degranulation of eosinophils, especially asthma, chronic bronchitis, and chronic obstructuive pulmonary disease, of the formula: wherein j is 0 or 1, k is 0 or 1, m is 0, 1, or 2; n is 1 or 2; A is selected from the partial Formulas: where q is 1, 2, or 3, W3 is -O-; -N(R9)-; or -OC(=O)-; R7 is selected from -H; -(C1-C6) alkyl, -(C2-C6) alkenyl, or -(C2-C6) alkynyl substituted by 0 to 3 substituents R10; -(CH2)u-(C3-C7) cycloalkyl where u is 0, 1 or 2, substituted by 0 to 3 R10; and phenyl or benzyl substituted by 0 to 3 R14; R8 is tetrazol-5-yl; 1,2,4-triazol-3-yl; 1,2,4-triazol-3-on-5-yl; 1,2,3-triazol-5-yl; imidazol-2-yl; imidazol-4-yl; imidazolidin-2-on-4-yl; 1,3,4-oxadiazolyl; 1,3,4-oxadiazol-2-on-5-yl; 1,2,4-oxadiazol-3-yl; 1,2,4-oxadiazol-5-on-3-yl; 1,2,4-oxadiazol-5-yl; 1,2,4-oxadiazol-3-on-5-yl; 1,2,5-thiadiazolyl; 1,3,4-thiadiazolyl; morpholinyl; parathiazinyl; oxazolyl; isoxazolyl; thiazolyl; isothiazolyl; pyrrolyl; pyrazolyl; succinimidyl; glutarimidyl; pyrrolidonyl; 2-piperidonyl; 2-pyridonyl; 4-pyridonyl; pyridazin-3-onyl; pyridyl; pyrimidinyl; pyrazinyl; pyridazinyl; indolyl; indolinyl; isoindolinyl; benzo[b]furanyl; 2,3-dihydrobenzofuranyl; 1,3-dihydroisobenzofuranyl; 2H-1-benzopyranyl; 2-H-chromenyl; chromanyl; benzothienyl; 1H-indazolyl; benzimidazolyl; benzoxazolyl; benzisoxazolyl; benzothiazolyl; benzotriazolyl; benzotriazinyl; phthalazinyl; 1,8-naphthyridinyl; quinolinyl; isoquinolinyl; quinazolinyl; quinoxalinyl; pyrazolo[3,4-d]pyrimidinyl; pyrimido[4,5-d]pyrimidinyl; imidazo[1,2-a]pyridinyl; pyridopyridinyl; pteridinyl; or 1H-purinyl; or A is selected from phosphorous and sulfur acid groups; W is -O-; -S(=O)t-, where t is 0, 1, or 2; or -N(R3)-; Y is =C(R1a)-, or -[N<custom-character file="US20020111495A1-20020815-P00900.TIF" wi="20" he="20" id="custom-character-00001" / >(O)k] where k is 0 or 1; R4, R5 and R6 are (1) -H; provided that R5 and R6 are not both -H at the same time, -F; -Cl; -(C2-C4) alkynyl; -R16; -OR16; -S(=O)pR16; -C(=O)R16, -C(=O)OR16, -C(=O)OR<highlight><sup
Owner:PFIZER INC
Copper-catalysed ligation of azides and acetylenes
InactiveUS20080214831A1Organic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsMetalloleSufficient time
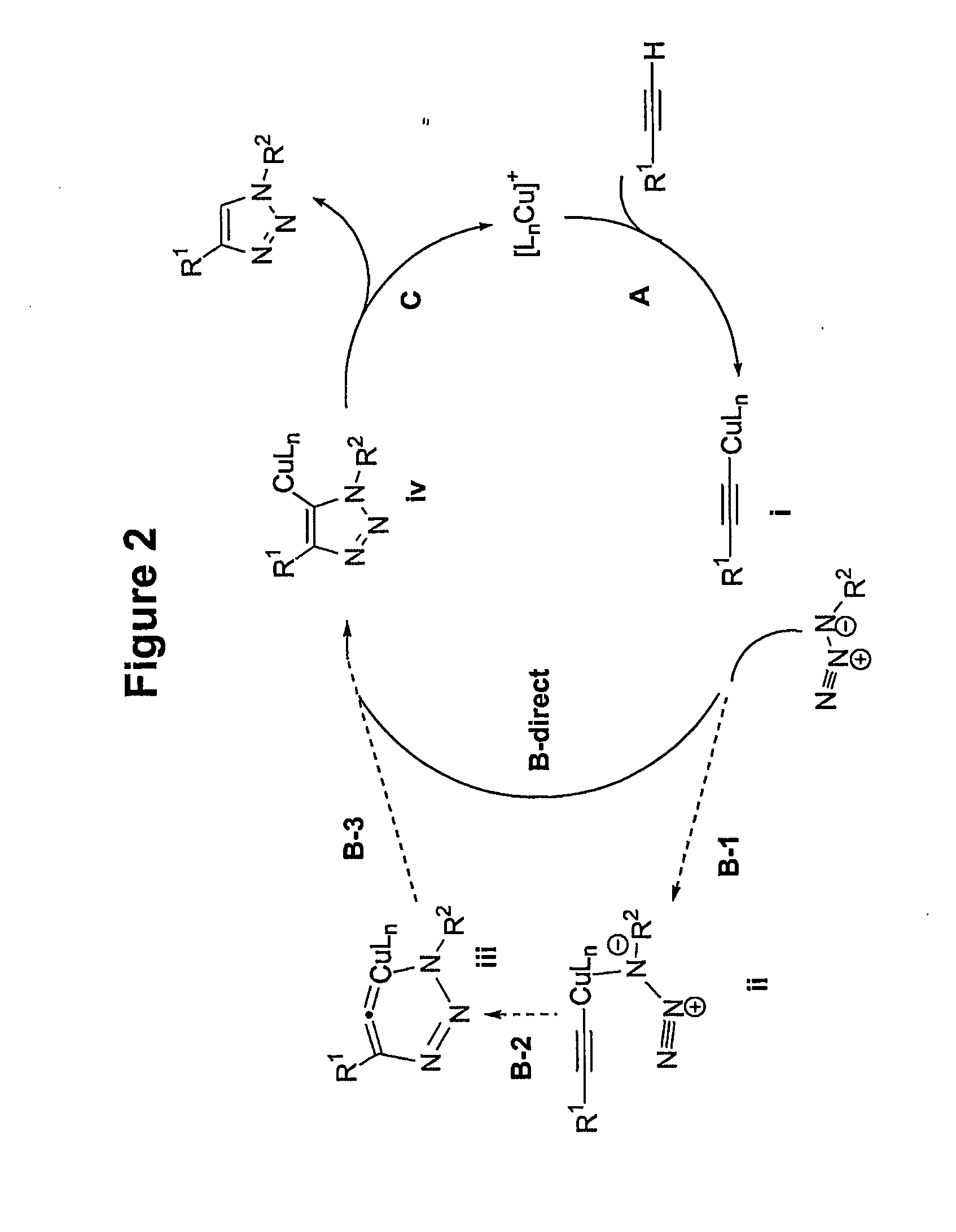
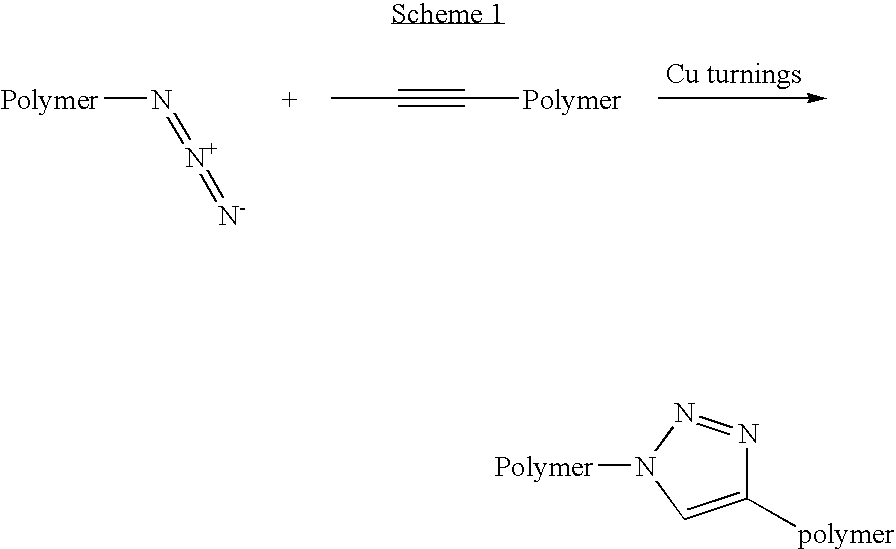
A copper catalyzed click chemistry ligation process is employed to bind azides and terminal acetylenes to provide 1,4-disubstituted 1,2,3-triazole triazoles. The process comprises contacting an organic azide and a terminal alkyne with a source of reactive Cu(I) ion for a time sufficient to form by cycloaddition a 1,4-disubstituted 1,2,3-triazole. The source of reactive Cu(I) ion can be, for example, a Cu(I) salt or copper metal. The process is preferably carried out in a solvent, such as an aqueous alcohol. Optionally, the process can be performed in a solvent that comprises a ligand for Cu(I) and an amine.
Owner:TETARD INC
Preparation of organosilicon-containing triazoles
InactiveUS20110077365A1Improve responseSlow performanceSilicon organic compoundsGroup 3/13 element organic compounds1,2,3-TriazoleAzide
The disclosure includes methods for preparing organosilicon-containing 1,2,3-triazoles by reacting an organosilicon containing azide with an alkyne compound or an organosilicon containing alkyne with an azide compound under thermal reaction conditions.
Owner:MCMASTER UNIV
Polytriazoles constructed by 1, 3-dipolar cycloaddition
A process of synthesizing hyperbranched polytriazoles, linear and hyperbranched poly(aroyltriazoles) by Huisgen 1,3-dipolar cycloaddition. The polytriazoles were prepared by A2+B3 method to avoid self-polymerization during monomer preparation and storage. The polymers are light emissive and can be crosslinked to generate well-resolution photopatterns upon UV irradiation. White light emission patterns were observed with fluorescence microscopy. The high molecular weight poly(aroyltriazoles) (up to 26000 Da) are prepared in high yields (up to 92.0%) and with high regioselectivity (the ratio of 1,4- and 1,5-disubstituted 1,2,3-triazole is equal or larger than 9:1). The polycyclomerization is not moisture or oxygen sensitive and therefore, no special precautions are necessary before and during the reaction. All the polymers are processible, easily film-forming, and curable into thermosets by heat or irradiation. The hyperbranched polymers can act as fluorescent adhesive materials with large tensile strength.
Owner:THE HONG KONG UNIV OF SCI & TECH
Substituted 5-membered ring compounds and their use
Compound of a compound of formula (1) or a salt, N-oxide, hydrate or solvate thereof, in the preparation of a composition for inhibition of HSP90 activity: wherein ring A is an aromatic or non-aromatic carbocyclic or heterocyclic ring having 5 ring atoms, for example 1,2,3-triazolyl or a 1,2,4-triazolyl or a tetrazolyl ring; and R1 R2 R3 are as defined in the specification are inhibitors of HSP90 and therefore of use in the treatment of, for example, cancers, viral disease, inflammatory diseases such as rheumatoid arthritis, asthma, multiple sclerosis, Type I diabetes, lupus, psoriasis and inflammatory bowel disease; cystic fibrosis angiogenesis-related disease such as diabetic retinopathy, haemangiomas, and endometriosis; or for protection of normal cells against chemotherapy-induced toxicity; or diseases where failure to undergo apoptosis is an underlying factor, or protection from hypoxia-ischemic injury due to elevation of Hsp70 in the heart and brain; scrapie / CJD, Huntingdon's and Alzheimer's disease.
Owner:VERNALIS (R&D) LTD +2
Preparation method of 1H-1,2,3-triazole compound
ActiveCN102603660AMild reaction conditionsEasy to operateOrganic chemistryN dimethylformamideSodium azide
The invention discloses a preparation method of a 1H-1,2,3-triazole compound as shown in a formula (I), which comprises the following steps: mixing halide as shown in a formula (III) with alkyne-terminated compound as shown in a formula (II) and sodium azide, reacting for 5-85 hours at 25-80 DEG C in a reaction solvent under the catalytic action of porous copper; after finishing the reaction, performing after-treatment of the reaction solution to obtain the 1H-1,2,3-triazole compound as shown in the formula (I), wherein the solvent is de-ionized water, acetonitrile, anhydrous ethanol, toluene, acetone, N,N-dimethylformamide, tetrahydrofuran or dioxane. The preparation method provided by the invention adopts one-pot operation, the catalyst can be reused for multiple times, the reaction condition is mild, and the operation is easy and simple.
Owner:平邑仁安中医药产业发展有限公司
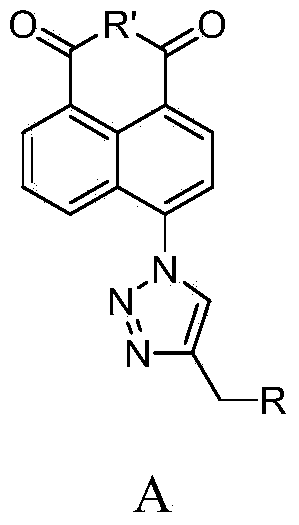
Synthesis of naphthalene nucleus 4-position 1,2,3-triazole containing naphthalimide derivative and application thereof
The invention relates to a synthesis of naphthalene nucleus 4-position 1,2,3-triazole containing naphthalimide derivative and application thereof, and belongs to the field of organic synthesis. The derivative has a compound in a general formula A structure; the preparation method of the derivative comprises the following steps: by using 4-bromo-1,8-anhydride naphthalene as raw material, amidating with the corresponding chain amine through azidation, finally reacting with an amido-propyne connected with cyclic amine; the obtained derivative is applied to a tumor cell inhibition drug.
Owner:DALIAN UNIV OF TECH
Method for increasing and regulating light emission from a chemiluminescent reaction
ActiveUS9040252B2Microbiological testing/measurementChemiluminescene/bioluminescencePeroxidase1-Methylimidazole
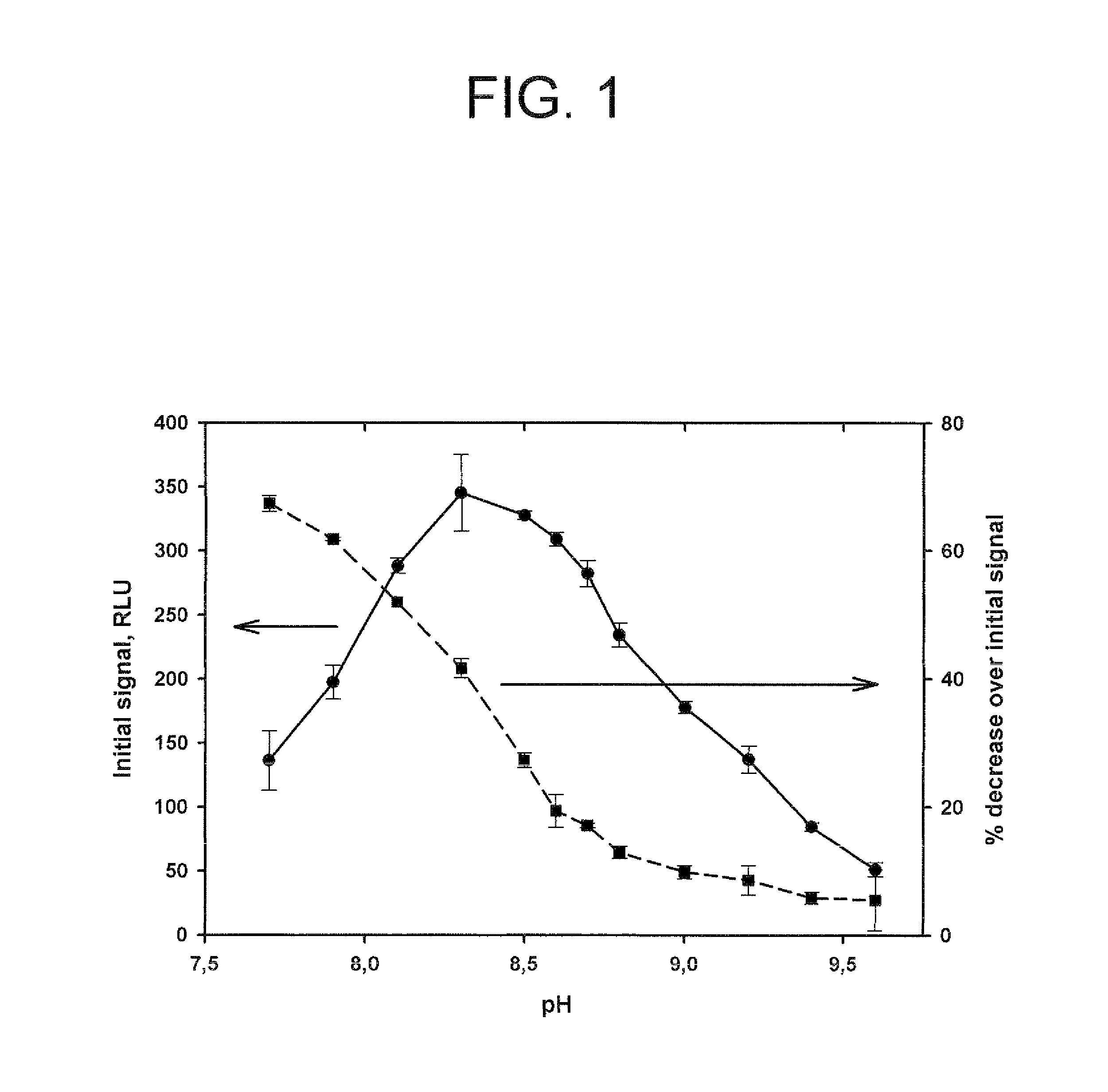
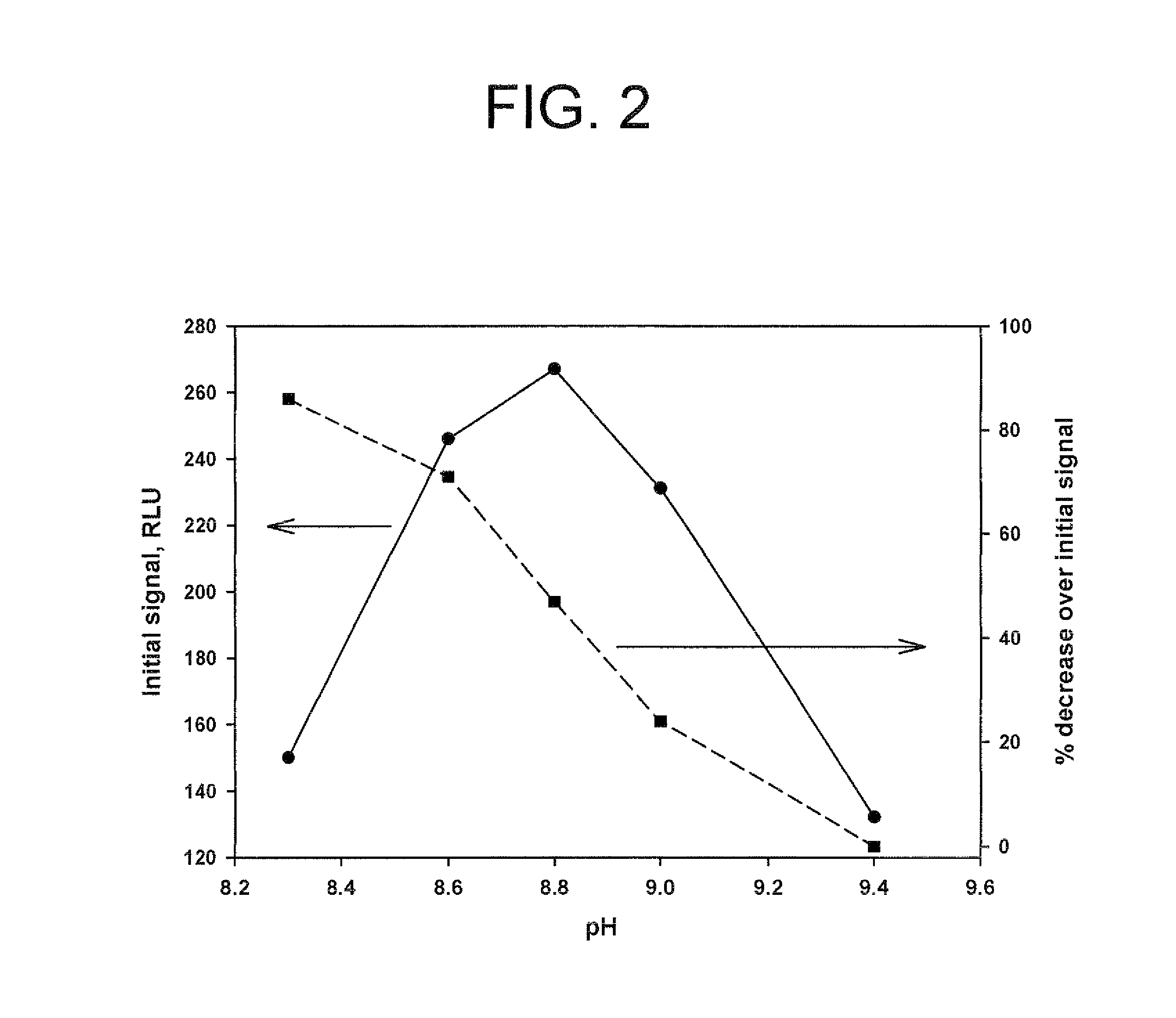
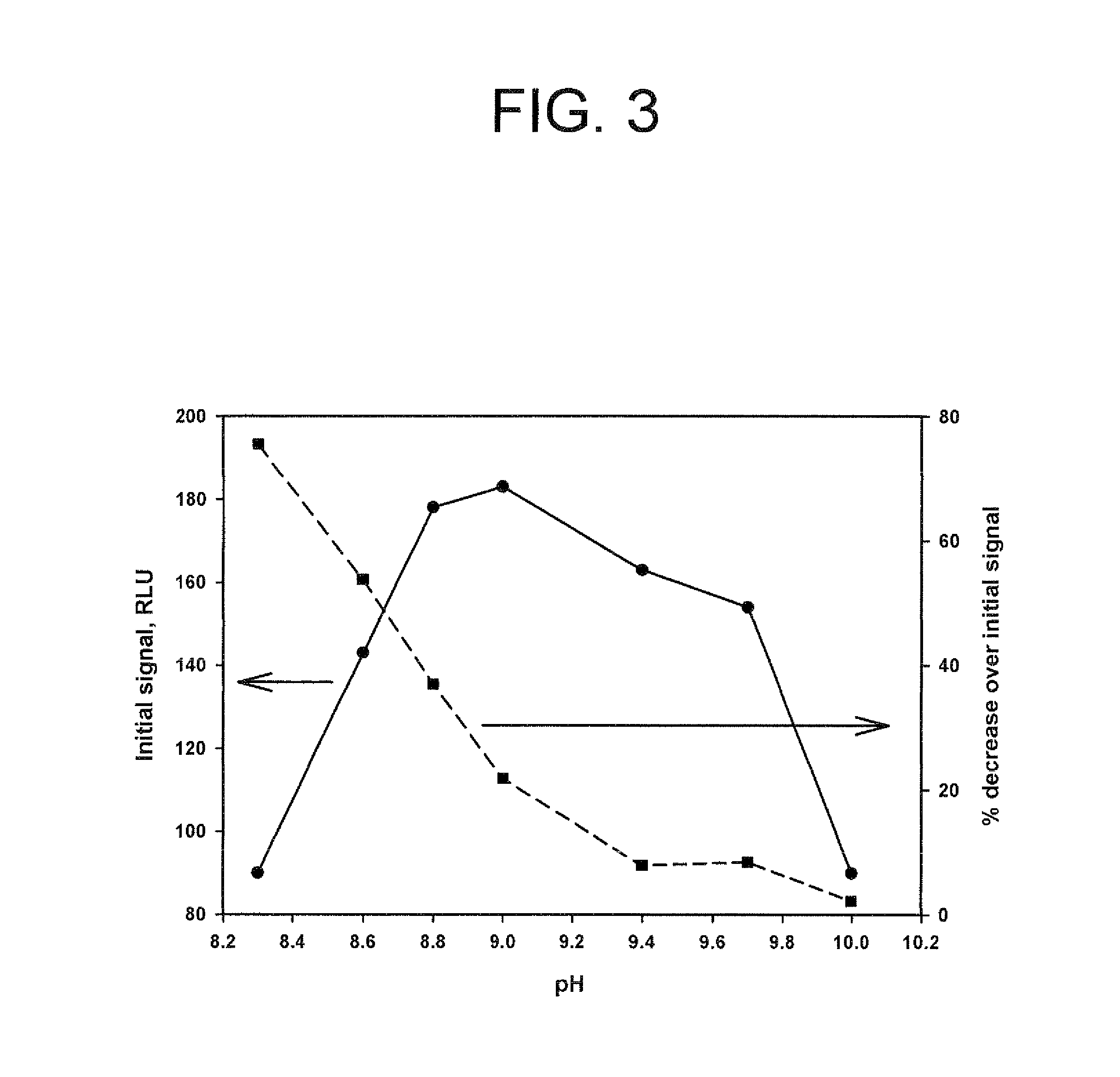
Method for increasing and regulating the emission of light from a chemiluminescent reaction including luminol, a peroxidase enzyme, an oxidant and an electron mediator (primary enhancer) through the use of an acylation catalyst (secondary enhancer) belonging to the class of N-azoles, i.e., a class of five-membered nitrogen heteroaromatic ring compounds containing at least one other atom of nitrogen. N-azoles, which are especially useful as secondary enhancers are imidazole, 1-methylimidazole, 1,2,3-triazole and 1,2,4-triazole. The invention also describes the use in diagnostic assays of chemiluminescent substrates containing said N-azoles, as secondary enhancers.
Owner:CYANAGEN
Proton exchange membranes using cycloaddition reaction between azide and alkyne containing components
ActiveUS7208243B2Improve stabilityReduce volumeIon-exchanger regenerationFinal product manufactureCross-linkFuel cells
A process for making an ion-conducting polymer comprises cross-linking polymers having functional groups such as alkyne groups and azide groups. An example ion-conducting polymer has cross-links including nitrogen-containing heterocycles formed by the reaction between the functional groups, such as 1,2,3-triazole groups formed by a cycloaddition reaction between alkyne and azide groups. The ion-conducting polymer may be used in an ion-electrolyte membrane. Examples include a proton-electrolyte membrane useful for fuel cells.
Owner:TOYOTA MOTOR CO LTD
Compounds for the Treatment of Inflammation of the Central Nervous System
KATP channel openers (KCOs) are useful for the prophylactic and / or therapeutic treatment of CNS chronic inflammation associated with a disease or state in a mammal, including a human. The administration of KCOs, including the groups of benzopirans, cyanoguanidines, thioformamides, benzothiadiazines, pyridyl nitrates, pyrimidine sulfates, cyclobutenediones, DHP-related compounds, tertiary carbinols, 6-sulfonil-chromenes, 1,2,3-triazoles, pyridothiadiazines, benzothiazines, halogenquinazolins and phenylbenzimidazoles, and in particular, the compound diazoxide, result in a reduction of reactive microglial response in various CNS pathologies such as axonal injury, brain tumors, traumatic damage, neurodegeneration, spinal cord injury, infectious and autoimmune diseases. KCOs, isotopically modified, are also useful for the preparation of diagnostic agents for detection and follow-up of CNS chronic inflammation.
Owner:NEUROTEC PHARMA
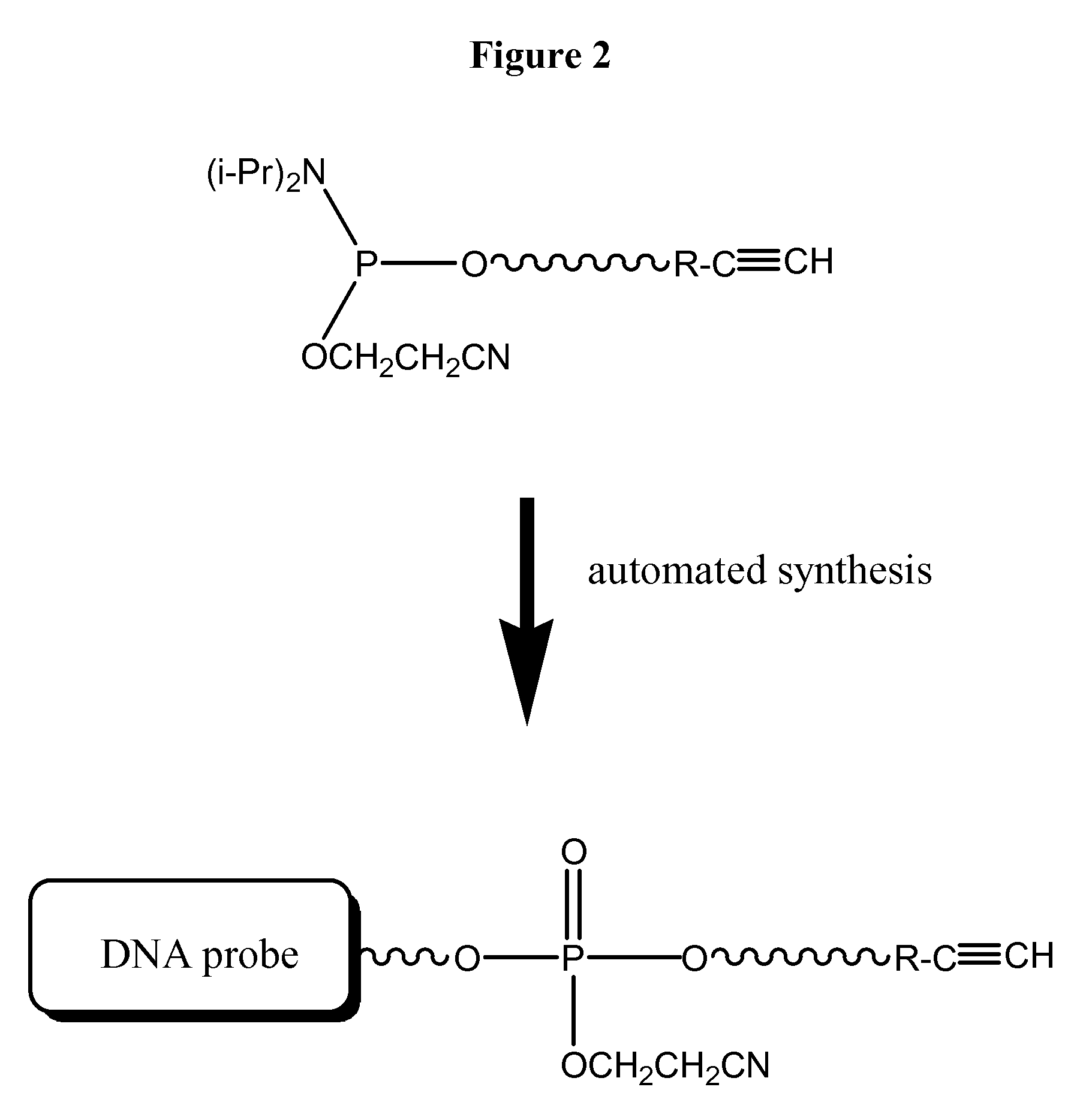
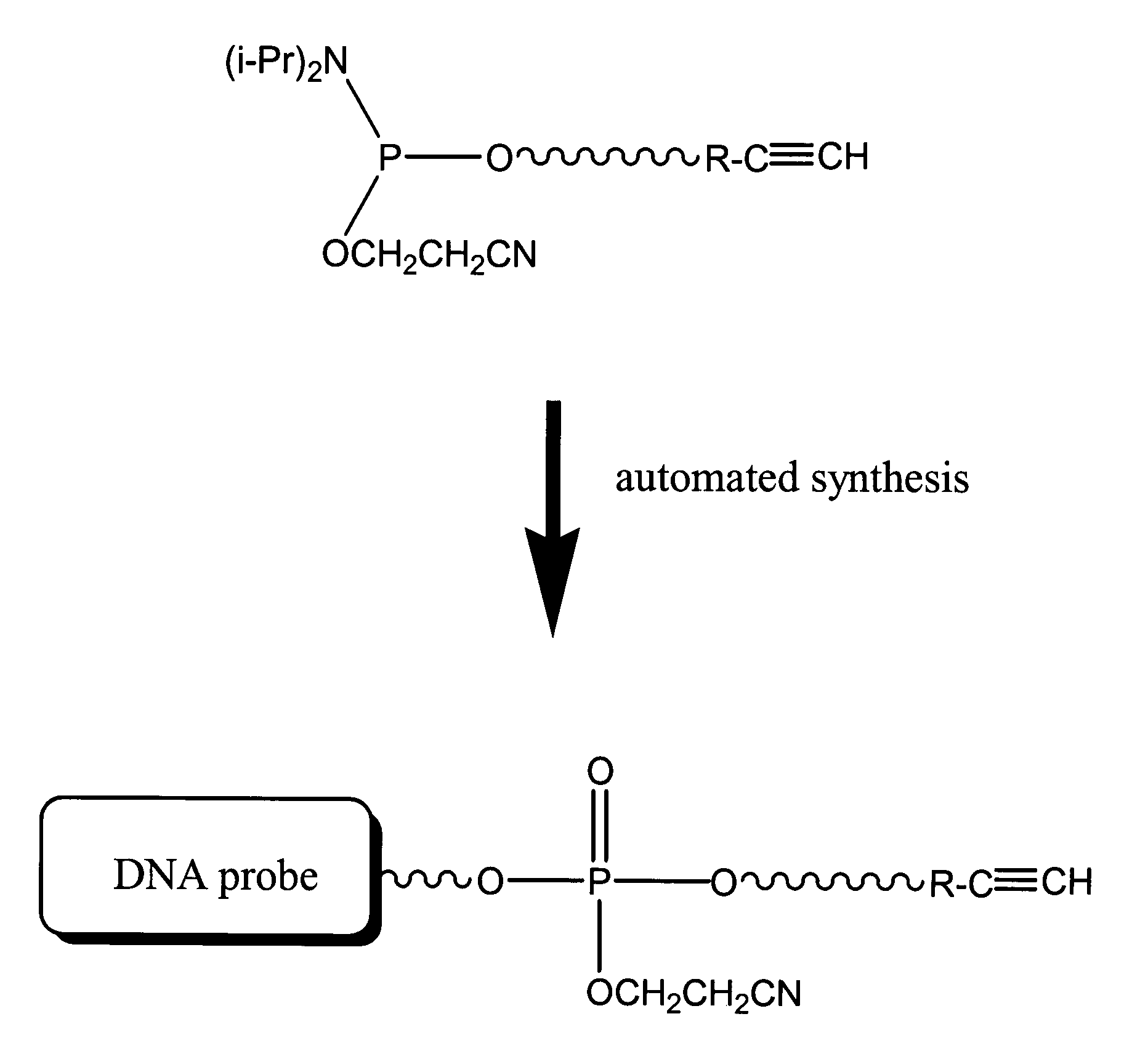
Compositions, probes and conjugates and uses thereof
The present invention relates to compositions useful as probes and in other applications and methods of their use. In some embodiments, nucleotides are prepared and functionalized with dyes. In some embodiments a first molecule is functionalized with an alkynyl group, a second molecule is functionalized with an azide group, and said first and second molecules are mixed under conditions to form a conjugate with a 1,2,3-triazol group. In further embodiments, a nucleotide is functionalized with an alkynyl group, a dye is functionalized with an azide group, and mixing the nucleotide and the dye forms a conjugate capable of emitting light.
Owner:GEN PROBE INC
Compositions, probes, and conjugates and uses thereof
The present invention relates to compositions useful as probes and in other applications and methods of their use. In some embodiments, nucleotides are prepared and functionalized with dyes. In some embodiments a first molecule is functionalized with an alkynyl group, a second molecule is functionalized with an azide group, and said first and second molecules are mixed under conditions to form a conjugate with a 1,2,3-triazol group. In further embodiments, a nucleotide is functionalized with an alkynyl group, a dye is functionalized with an azide group, and mixing the nucleotide and the dye forms a conjugate capable of emitting light.
Owner:GEN PROBE INC
Organic semiconductor material and preparation method and application thereof
ActiveCN104672434AEasy transferGood planaritySolid-state devicesSemiconductor/solid-state device manufacturingAfter treatmentSemiconductor materials
The invention discloses an organic semiconductor material. The organic semiconductor material contains a 4, 4'-bi-1H-1, 2, 3-triazolyl dibenzothiophene unit and an aromatic group which are connected by a conjugating manner. The invention further discloses a preparation method of the inorganic semiconductor material. The inorganic semiconductor material is prepared from a bialkynyl-containing dibenzothiophene monomer and an alkyl chain containing azide by click chemistry. Compared with the prior art, the organic semiconductor material is beneficial for pi-pi accumulation of molecules and enables good planarity of a polymer, and the performances of a device can be improved. The preparation method is simple and convenient, high in yield, simple in after-treatment, and convenient to purify.
Owner:SOUTH CHINA UNIV OF TECH
N2-substituted-1,2,3-triazole ligand auxiliary Cu (I) catalysts and applications thereof
InactiveCN103521267AReduce dosageImprove adaptabilityOrganic-compounds/hydrides/coordination-complexes catalystsCopper organic compoundsCycloadditionAlkyne
The invention relates to N2-substituted-1,2,3-triazole ligand auxiliary Cu (I) catalysts and applications thereof in reaction of azides and terminal alkynes. The catalysts comprise N2-substituted-1,2,3-triazole ligands and Cu(I) salts, wherein the molar ratio of N2-substituted-1,2,3-triazole ligands to Cu(I) salts is 1:0.5-1:3. The catalysts are advantaged in that the middle nitrogen on the ring of the N2-substituted-1,2,3-triazole ligand has lower electron cloud density than the other two nitrogens, the middle nitrogen is substituted by a nitrogen heterocyclic ring, and the ligand utilizes triazole nitrogen atom with high electron cloud density and other heterocyclic ring nitrogen atom as a ligand effectively, the coordination capability with monovalent copper ions is enhanced, and monovalent copper ions is stabilized. The synthesis is simple and the properties are stable. In the catalysis of monovalent copper ions to a cycloaddition reaction of terminal alkynes and azides, the catalyst dosage is small, the reaction conditions are mild, the product yield is high and the substrate adaptability is strong.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
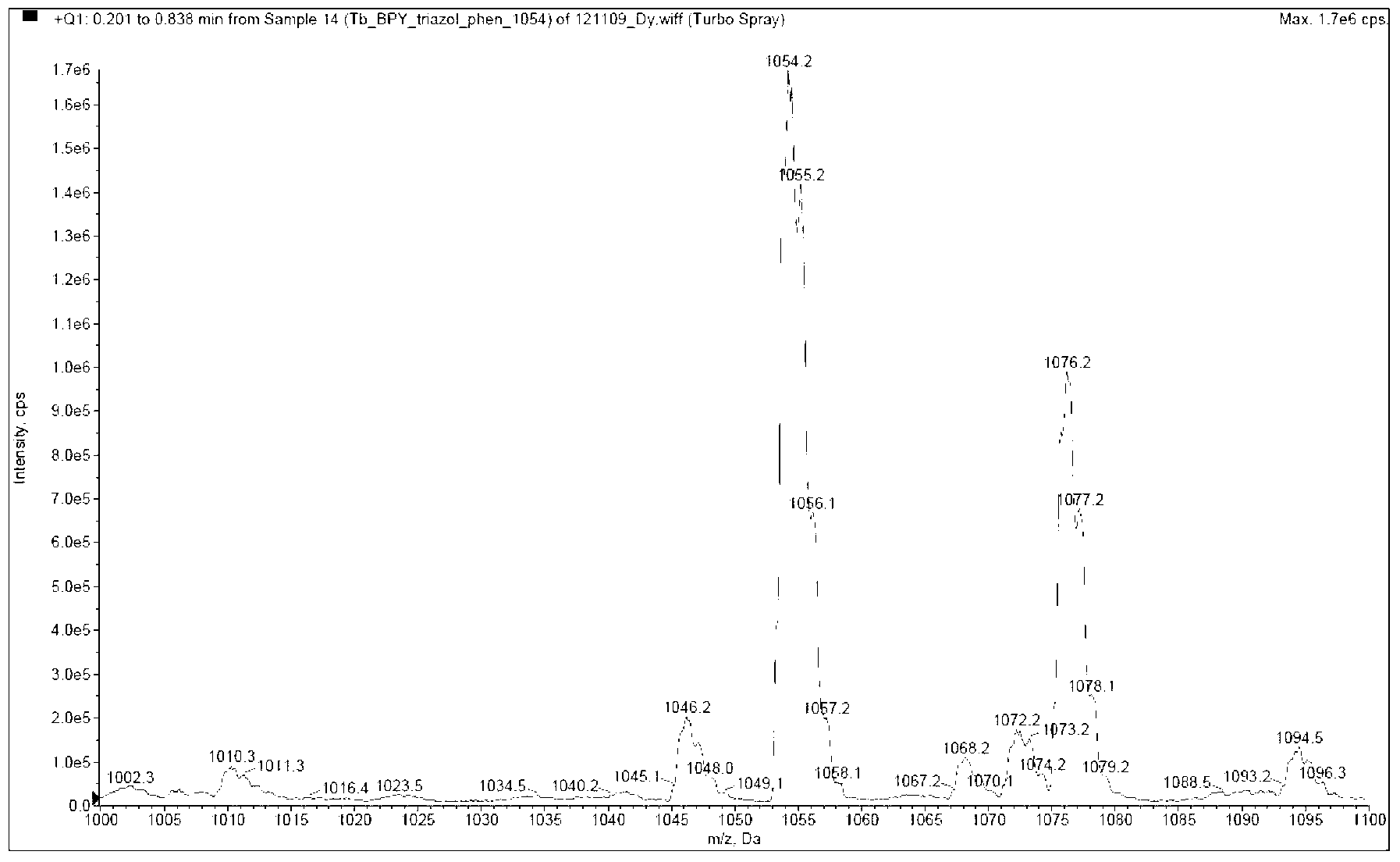
Nitrogen-containing bidentate heterocycle-substituted 1,2,3-triazole rare earth complex and synthetic method thereof
ActiveCN103265567AHigh purityHigh yieldGroup 3/13 element organic compoundsLuminescent compositionsLutetiumCerium
The invention discloses a nitrogen-containing bidentate heterocycle-substituted 1,2,3-triazole rare earth complex LnL3 having a structure represented by formula 1, and a preparation method thereof. In the formula 1, Ar is a nitrogen-containing bidentate heterocyclic ring; R1 is selected from H, alkyl groups, halogenated alkyl groups and aryl groups; and a central rare earth Ln is anyone selected from yttrium, lanthanum, cerium, praseodymium, neodymium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium and lutetium. The preparation method is characterized in that the adoption of one of nitrogen-containing bidentate heterocycle-substituted triazole compounds comprising dipyridine, phenanthroline triazole and the like as a single ligand can also satisfy the coordination saturation, and the triazolyl group in the complex adopted as a negative ion and the central rare earth metal cation realize the charge balance to obtain the electric neutrality. The preparation method has the advantages of high yield, short reaction time, simple operation and great cost reduction, and the obtained rare earth complex has the advantages of good purity and high thermal stability, and can be processed through an evaporation film forming technology or a solution film forming technology to make devices.
Owner:GUANGDONG SYNYOO NEW MATERIAL
Triazolyl-containing amino-dithio formic ether compound as well as preparation method and application of compound
ActiveCN102850283ASignificant in vitro antitumor activityEnhanced inhibitory effectOrganic active ingredientsOrganic chemistryClick chemistryEther
The invention discloses a triazolyl-containing amino-dithio formic ether compound as well as a preparation method and application of the compound which is taken as a new antineoplastic medicament lead compound, belonging to the field of pharmaceutical chemistry. According to the invention, 1, 2, 3-triazole active fragment is introduced into the structure of amino-dithio formic ether by click chemistry, thus the preparation method is simple, efficient and environment-friendly. The triazolyl-containing amino-dithio formic ether compound has the structure general formula shown in the specification, and has excellent antineoplastic activity for multiple tumour cells, can be used as a candidate or lead compound for the further development to be applied to the preparation of the antineoplastic medicaments.
Owner:ZHENGZHOU UNIV
Penam crystals and process for producing the same
InactiveUS7547777B2Improve efficiencyPoor compatibilityOrganic chemistryOrganic solventCarboxylic acid
The present invention provides novel 2α-methyl-2β-[(1,2,3-triazol-1-yl)methyl]penam-3α-carboxylic acid benzhydryl ester (TMPB)-acetone crystals for use in the production of 2α-methyl-2β-[(1,2,3-triazol-1-yl)methyl]penam-3α-carboxylic acid 1,1-dioxide benzhydryl ester (TAZB); a process for producing the TMPB-acetone crystals comprising the steps of (A) concentrating a TMPB-containing organic solvent solution, (B) dissolving the resulting concentrate in acetone, and (C) precipitating TMPB-acetone crystals from the acetone solution thus obtained; and a process for producing TAZB comprising the step of reacting the TMPB-acetone crystals with an oxidizing agent.
Owner:OTSUKA CHEM CO LTD +1
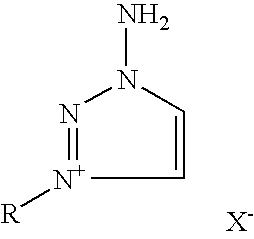
Preparation of substituted-1,2,3-triazoles
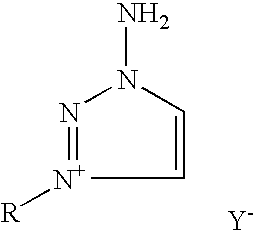
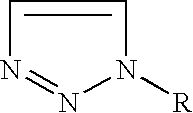
The invention provides a high yield / isomerically pure synthesis of new 1-amino-3-substituted-1,2,3-triazolium salts and their subsequent transformation into isomerically pure 1-substituted-1,2,3-triazoles. These new 1-amino-3-substituted-1,2,3-triazolium salts are easily isolated and can be stored at ambient conditions with no degradation or isomerization. These quarternary salts are easily converted with appropriate silver salts to a wide array of new quarternary salts with various anions, with many of these new salts having melting points below 100 C, classifying them as ionic liquids. Subsequent diazotization of these 1-amino-3-substituted-1,2,3-triazolium salts results in high yields of isomerically pure 1-substituted-1,2,3-triazoles, that until this invention were problematic to make pure without tedious reagents or workup procedures. Both classes of materials (1-amino-3-substituted-1,2,3-triazolium salts as well as 1-substituted-1,2,3-triazoles) should be of high interest as these classes of materials are known to be very important pharmaceutical materials for a wide array of medical and agricultural applications as well as possible propellant applications.
Owner:GOVERNMENT OF THE UNITED STATE OF AMERICA AS REPRESENTED BY THE SEC OF THE AIR FORCE
Polytriazoles constructed by 1,3-dipolar cycloaddition
A process of synthesizing hyperbranched polytriazoles, linear and hyperbranched poly(aroyltriazoles) by Huisgen 1,3-dipolar cycloaddition. The polytriazoles were prepared by A2+B3 method to avoid self-polymerization during monomer preparation and storage. The polymers are light emissive and can be crosslinked to generate well-resolution photopatterns upon UV irradiation. White light emission patterns were observed with fluorescence microscopy. The high molecular weight poly(aroyltriazoles) (up to 26000 Da) are prepared in high yields (up to 92.0%) and with high regioselectivity (the ratio of 1,4- and 1,5-disubstituted 1,2,3-triazole is equal or larger than 9:1). The polycyclomerization is not moisture or oxygen sensitive and therefore, no special precautions are necessary before and during the reaction. All the polymers are processible, easily film-forming, and curable into thermosets by heat or irradiation. The hyperbranched polymers can act as fluorescent adhesive materials with large tensile strength.
Owner:THE HONG KONG UNIV OF SCI & TECH
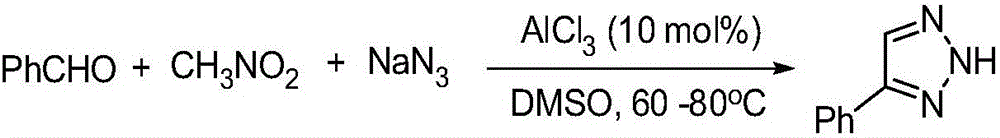
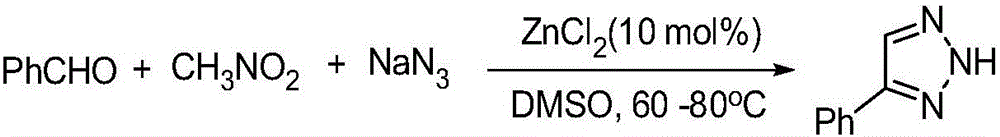
Synthesis method of NH-1,2,3-triazole compound
ActiveCN105669569ARaw materials are easy to getMild reaction conditionsOrganic chemistrySynthesis methodsSodium azide
The invention relates to a synthesis method of an NH-1,2,3-triazole compound and belongs to the technical field of organic and drug synthesis. In the presence of a Lewis acid catalyst, the NH-1,2,3-triazole compound is prepared from aromatic aldehyde, nitro-hydrocarbon and sodium azide by adopting a one-pot process. The synthesis method of the NH-1,2,3-triazole compound has the beneficial effects that available Lewis acids such as AlCl3 are taken as the catalyst, one-pot reaction is carried out on aromatic aldehyde, a nitro-hydrocarbon compound containing alpha hydrogen (wherein hydrocarbon is C1-C6 alkyls, C1-C6 alkoxys or ethyl formate) and sodium azide, reaction conditions are mild, yield is high, raw materials are available, the NH-1,2,3-triazole compound is conveniently and effectively synthesized, and compared with an existing method, the synthesis method provided by the invention is mild in reaction conditions, short in reaction time, good in safety, simple in operation, wide in substrate range and high in reaction efficiency and adopts a cheap catalyst, so that the synthesis method provided by the invention has potential application value.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
1,2,3-Triazole-flavonoid compound-sophocarpidine ternary conjugate and use
The invention discloses a 1,2,3-triazole-flavonoid compound-sophocarpidine ternary conjugate and the use. The 1,2,3-triazole-flavonoid compound-sophocarpidine ternary conjugate has a structure as shown in the specification. The 1,2,3-triazole-flavonoid compound-sophocarpidine ternary conjugate has high-efficiency antitumor activity and a potential application value in the aspect of antitumor drug development. The 1,2,3-triazole-flavonoid compound-sophocarpidine ternary conjugate has a remarkable effect in the antitumor aspect, and can be applied to preventing and treating a plurality of clinically common tumors such as breast cancer, lung cancer, cervical cancer and liver cancer.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
Preparation and application of combined antibacterial peptide having high stability and anti-drug-resistance activity
ActiveCN105440105AImprove stabilityHigh activityAntibacterial agentsPeptide/protein ingredientsSodium ascorbateAntioxidant
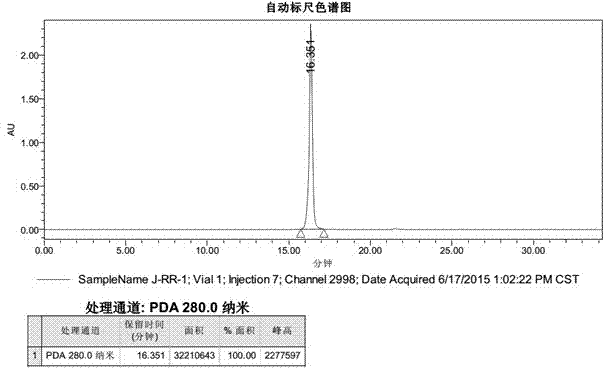
The invention discloses a combined antibacterial peptide having high stability and anti-drug-resistance activity, and belongs to the biochemical technical field. With precursor peptides containing propargyl glycine and azide lysine as a substrate, CuSO4.5H2O as a catalyst, sodium ascorbate as an antioxidant, and a water-N,N-dimethyl formamide mixed liquid as a solvent, a 1,3-dipolar cycloaddition reaction of click chemistry is carried out for 24-28 hours, and a 1,4-disubstituted-1H-1,2,3-triazole structure is generated and links the two precursor peptide chains to obtain the product. Antibacterial experiments of common standard bacteria and clinically isolated drug-resistant bacteria indicate that the synthesized J-RR-1 peptide has relatively strong antibacterial activity and clinically isolated drug-resistant bacteria resisting activity, and also has relatively high stability on pancreatin. Therefore, the peptide has a good application prospect in preparation of clinical treatment drugs.
Owner:倪京满 +1
Method for forming through-base wafer vias
InactiveUS20130344696A1High yieldExcellently reproducibleSemiconductor/solid-state device detailsSemiconductor/solid-state device manufacturingEngineering1,2,3-Triazole
Method for manufacturing semiconductor wafers having at least one through-base wafer via, the said method comprising the steps of (1) providing a semiconductor wafer having at least one electrically conductive via comprising an electrically conductive metal and extending from the front side of the semiconductor wafer at least partially through the semiconductor wafer; (2) affixing the frontside of the semiconductor wafer to a carrier; (3) contacting the backside of the semiconductor wafer with a polishing pad and an aqueous chemical mechanical polishing composition having a pH of equal to or greater than 9 and comprising (A) abrasive particles; (B) an oxidizing agent containing at least one peroxide group; and (C) an additive acting both as metal chelating agent and metal corrosion inhibitor; (4) chemically mechanically polishing the backside of the semiconductor wafer until at least one electrically conductive via is exposed. Preferably, the additive (C) is 1,2,3-triazole.
Owner:BASF AG
Combination therapy with 4-(3-(2h-1,2,3-triazol-2-yl)phenylamino)-2-((1r,2s)-2-aminocyclohexylamino)pyrimidine-5-carboxamide
InactiveUS20130244963A1Good treatment effectReduce the amount requiredBiocideCarbohydrate active ingredientsAnaphylaxisSystemic lupus erythematosus
The present invention is directed to pharmaceutical compositions and methods of using combination therapies containing a SYK inhibitor, or a pharmaceutically acceptable salt thereof, and a antineoplastic or antiinflammatory agent for the treatment of inflammatory, autoimmune and cell proliferative diseases, such as allergic reaction, transplant rejection, rheumatoid arthritis (RA), lupus, multiple sclerosis (MS) or psoriasis undesired acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), non-Hodgkin lymphoma (NHL) (including diffuse large B cell lymphoma (DLBCL)), mantle cell lymphoma, acute lymphocytic leukemia (ALL), follicular lymphoma, Burkitt's lymphoma, small Lymphocytic (SLL), Lymphoma, multiple myeloma, asthma, vasculitis, Idiopathic thrombocytopenic purpura (ITP), Heparin Induced Thrombocytopenia (HIT) and hemolytic anemia.
Owner:ALEXION PHARMA INC
Synthesis method for substituted triazole compound
InactiveCN103613551ARaw materials are easy to getEasy to operateOrganic chemistrySynthesis methodsCerium
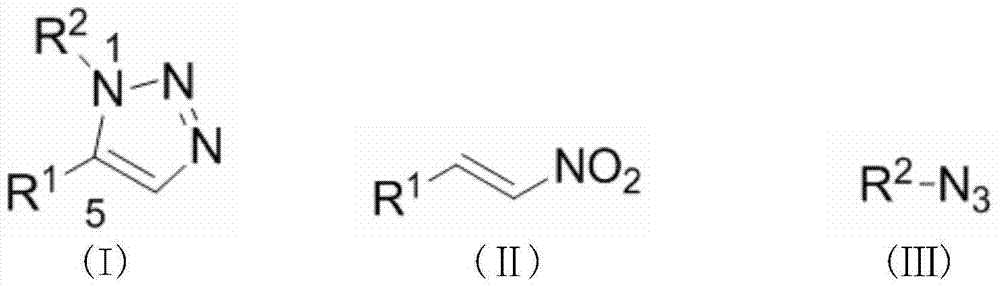
The invention discloses a synthesis method for a substituted triazole compound. The method mainly comprises the following steps: under the existence of a rare earth metal catalyst, performing backflow reaction to nitroolefin and organic azido in an organic solvent at 80-110DEG C, to obtain a target crude product, wherein the rare earth metal catalyst is cerium trifluoromethanesulfonate, samarium trifluoromethanesulfonate, europium trifluoromethanesulfonate, ytterbium trifluoromethanesulfonate, praseodymium trifluoromethanesulfonate, lanthanum trifluoromethanesulfonate, neodymium trifluoromethanesulfonate, erbium trifluoromethanesulfonate or dysprosium trifluoromethanesulfonate. According to the synthesis method, 1,5-disubstituted-1,2,3-triazole compound can be efficiently selectively synthesized by taking nitroolefin and organic azido as active ingredients under the action of a rare earth metal catalyst. The method is easily available for active ingredients, and simple to operate, and the productivity can achieve more than 70%.
Owner:GUANGXI NORMAL UNIV
1,2,3-Triazole unit-based micro-molecular luminescent material and application thereof
ActiveCN105176521ASingle structureMolecular weight determinationOrganic chemistrySolid-state devicesEvaporationLight-emitting diode
The invention belongs to the technical field of organic photoelectric materials, and discloses a 1,2,3-triazole unit-based micro-molecular luminescent material and an application thereof. The molecular weight, the pi electron conjugate degree, the intramolecular charge transfer and other properties of the material can be adjusted through adopting 1,2,3-triazole as a skeleton unit and changing the kind, the position and the quantity of units connected with the skeleton unit. The organic donor-receptor connection luminescent material can realize intramolecular charge transfer, and reduces the carrier imbalance problem of unipolar luminescent materials in order to simplify the structure of a device and improve the performances of the device. Two adjacent units connected with the triazole unit have spatial distortion due to steric hindrance, so the structural non-planarity is reduced, and the accumulation degree of the material is reduced, thereby the aggregation quenching of the material and luminescence of an exciplex are inhibited. The material can be used in evaporation organic micro-molecular electroluminescent diodes.
Owner:SOUTH CHINA UNIV OF TECH
N1 substituted 1,2,3-triazole derivative for ligand of Cu(I) as well as preparation method and application of N1 substituted 1,2,3-triazole derivative
InactiveCN104016968AReduce dosageHigh yieldOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsSodium ascorbateCycloaddition
The invention relates to an N1 substituted 1,2,3-triazole derivative for a ligand of Cu(I) as well as a preparation method and application of the N1 substituted 1,2,3-triazole derivative. The structural general formula I of the N1 substituted 1,2,3-triazole derivative is as shown in the specification: R is alkyl or aryl; R' is an H atom, alkyl or aryl; X is a CH or N atom. The preparation method comprises the steps of mixing nitrogen-containing heterocyclic ring substituted terminal alkyne with azide, CuSO4 and sodium ascorbate, stirring based on absolute methanol as a solvent in the air under room temperature, reacting for 0.5-1 hour, and then directly generating a solid namely the N1 substituted 1,2,3-triazole derivative. Compared with the prior art, the N1 substituted 1,2,3-triazole derivative disclosed by the invention has the advantages that compared with other ligands, the ligand is simple in synthesis and stable in property. In a cycloaddition reaction between mono-valent copper ion catalytic terminal alkyne and azide, the ligand is small in catalyst dose, mild in reaction condition, high in product yield and strong in substrate adaptability.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
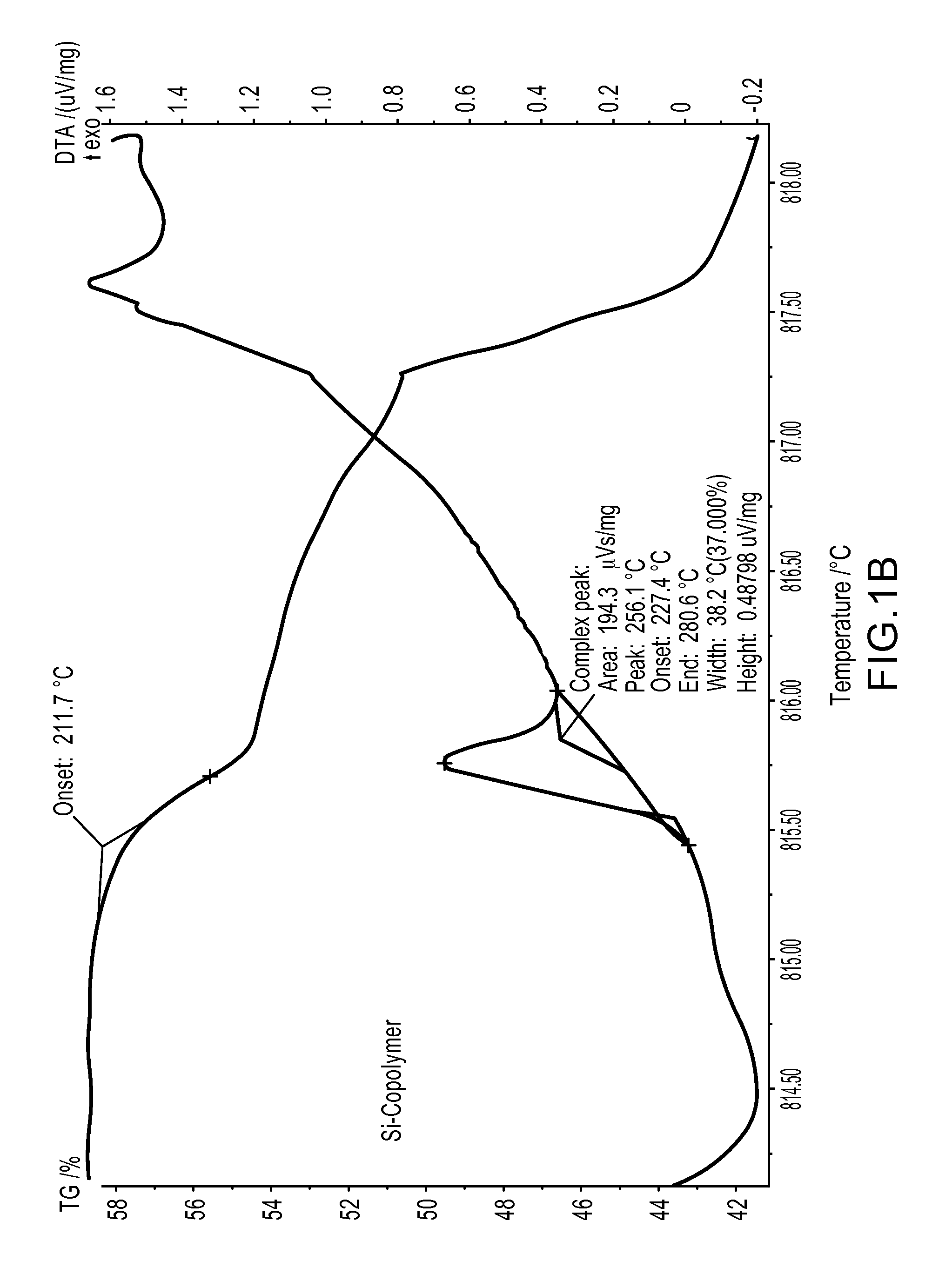
Triazole-containing fluorocarbon-grafted polysiloxanes
A polymer for imparting repellency and stain resistance to substrates is disclosed of Formula 1: (W)a(R2)b—Si(R1)2—O—[Si(R1)2—O]n—[Si(W)c(R1)d(R2)e—O]m—Si(R1)2—(W)f(R2)g wherein: a, b, c, d, e, f and g are each independently 0 or 1, provided that (a+b) is 1, (f+g) is 1, and (c+d+e) is 2, each R1 is divalently a C1 to C8 alkyl, each R2 is independently H or C1 to C8 alkyl, optionally containing oxygen, nitrogen, or sulfur, or a combination thereof, n=0 to 500, m=1 to 100, each W is independently (Rf—X-A-X)p—Y wherein: Rf is a straight or branched perfluoroalkyl group having from about 2 to about 20 carbon atoms, or a mixture thereof, which is optionally interrupted by at least one oxygen atom, each X is independently an organic divalent linking group having from about 1 to about 20 carbon atoms, optionally containing oxygen, nitrogen, or sulfur, or a combination thereof, A is a 1, 2, 3-triazole, p=0 to 2, and Y is O, OR, N, NR or N(R)2 wherein R is H or C1 to C20 alkyl, optionally containing oxygen, nitrogen, or sulfur, or a combination thereof.
Owner:EI DU PONT DE NEMOURS & CO
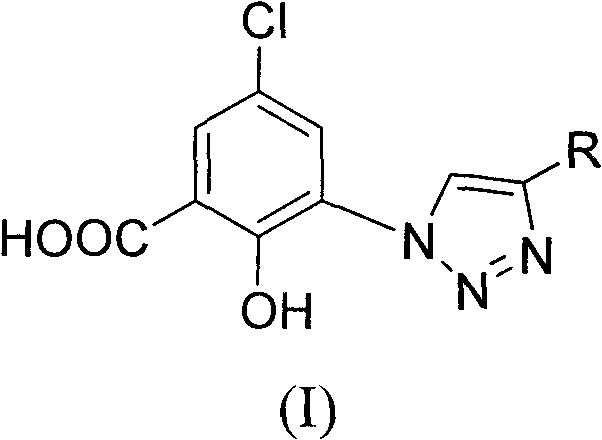
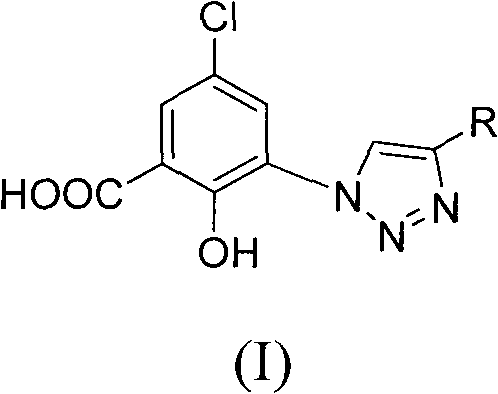
5-chlorol-2-hydroxyl-3-(4-substituted-1h-1,2,3-triazole) benzoic acid compound as well as preparation method and application thereof
InactiveCN101812028AGood inhibitory effectReduce diseaseOrganic active ingredientsOrganic chemistry5-chlorosalicylic acidBenzoic acid
The invention relates to a 5-chlorol-2-hydroxyl-3-(4-substituted-1H-1,2,3-triazole) benzoic acid compound expressed in the formula (I) as well as a preparation method and application thereof. The definition of R is shown as the specification. In the preparation method, 5-chlorolsalicylic acid used as a raw material is nitrified and reduced, is subjected to azidation and reacts with end alkyne to obtain the corresponding 5-chlorol-2-hydroxyl-3-(4-substituted-1H-1,2,3-triazole) benzoic acid compound. The compound has the inhibiting function on HIV-1 integrase, and the IC50 of part of the compound reaches 1.6 mircograms / mL, therefore, the compound is a stronger HIV-1 integrase inhibiting agent and is expected to be developed into a new HIV virus resistant medicament.
Owner:BEIJING UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com