Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31results about How to "The steps are closely connected" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
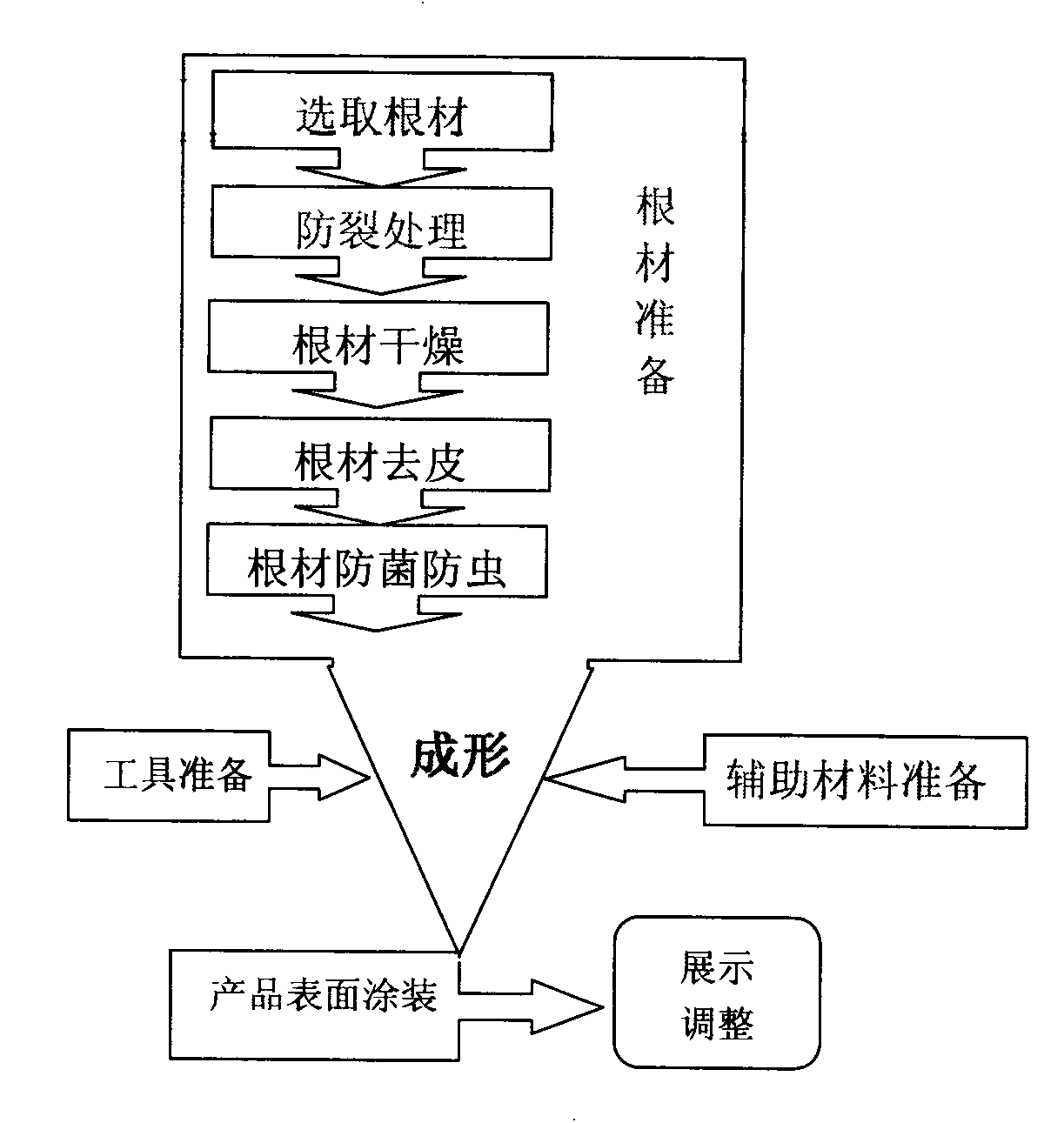
Standard preparation technology for root carving product
InactiveCN103350604AIncrease useReduce wasteDecorative surface effectsMaterials preparationEngineering
The invention provides a standard preparation technology for a root carving product, which comprises the six steps, namely root timber preparation, tool preparation, auxiliary material preparation, forming, product surface coating and show adjusting. Strong applicability system production measure is adopted, root timber can be widely utilized, universality is strong, waste root timbers in drought and cold areas like Sinkiang such as birch, populus and diversifolia are adopted, and the technology is rich in local style, standard in technology, tight in step join, short in carving treatment period, high in manufacturing efficiency, less in material wastage, and rich in style, can better express root carving technology, and is suitable for environment protection, and convenient to operate, manual consumption is greatly decreased, and the product quality is easy to control.
Owner:YUEPUHU FULE ENVIRONMENTAL PROTECTION TECH DEV
Composite lubricant for drilling fluid, as well as preparation method and application of composite lubricant
ActiveCN107011876AImprove temperature resistanceImprove thermal stabilityDrilling compositionBiodieselTemperature resistance
The invention discloses a composite lubricant for drilling fluid. The composite lubricant is mainly prepared from the following raw materials in parts by mass: 60 to 90 parts of fatty alcohol-polyoxyethylene ether, 30 to 60 parts of fatty acid ester, 20 to 40 parts of waste animal fat, 5 to 10 parts of vinyl benzene, 5 to 9 parts of water-soluble carbon black, 2 to 5 parts of graphene substances, 1 to 3 parts of an extreme pressure anti-wear reagent, 1 to 3 parts of a dispersing agent, and 1 to 3 parts of a defoamer. The preparation method comprises the following steps: uniformly mixing and stirring the fatty alcohol-polyoxyethylene ether, the fatty alcohol-polyoxyethylene ether, the waste animal fat and vinyl benzene; adding the water-soluble carbon black, the graphene substances, the extreme pressure anti-wear reagent, the dispersing agent and the defoamer sequentially, performing mixing and stirring, and cooling the raw materials to 50 to 70 DEG C while stirring, so as to form uniform emulsion. The composite lubricant is high in temperature resistance and anti-pressure capacity, and can still keep good lubricating property under high-temperature and high-pressure conditions, biodiesel fuel is taken as a raw material for the main components of the composite lubricant, and no polluted component is contained.
Owner:任丘市力科节能材料有限公司
High-performance magnesia carbon brick and preparation method thereof
The invention provides a high-performance magnesia carbon brick and a preparation method thereof. The high-performance magnesia carbon brick is mainly prepared from the following raw materials in parts by mass: 60-75 parts of fused magnesite particles, 10-25 parts of 120-400-mesh fused magnesite fine powder, 1-5 parts of aluminum powder, 0.1-5 parts of a spinel-calcium aluminate multiphase material, 10-16 parts of crystalline flake graphite and 2-4 parts of a binding agent, wherein the fused magnesite particles are prepared by mixing three kinds of particles with the particle size of 3-5 mm, 1-3 mm and 0.075-1 mm according to the mass ratio of (3-5):(3-5):(2-4). According to the magnesia carbon brick, the spinel-calcium aluminate multiphase material is introduced, so that a low-melting-point substance is generated on the surface of the magnesia carbon brick at a high temperature, thermal stress is relieved, thermal shock damage and mechanical damage are reduced, and therefore the purpose of preventing the magnesia carbon brick from cracking is achieved.
Owner:上海新泰山高温工程材料有限公司
Amino acid ionic liquid molecules and preparation method and application thereof
InactiveCN105669474AImprove thermal stabilityReduce volatilityOrganic compound preparationDispersed particle separationAlkaneQuaternary ammonium cation
The invention provides amino acid ionic liquid molecules and a preparation method and an application thereof; the amino acid ionic liquid molecules have the chemical structural general formula defined in the specification, wherein R1 is alkane, R2 is hydrocarbonyl of halide, and X is amino acid. The preparation method comprises the steps: carrying out an anion exchange reaction of a quaternary ammonium compound with an alkali for 1-4 h under a condition of the temperature of 25-30 DEG C to obtain a hydroxyl compound of quaternary ammonium salt; carrying out a dehydration reaction of the hydroxyl compound of the quaternary ammonium salt with the amino acid for 4-8 h under a condition of the temperature of 10-50 DEG C, and thus obtaining the amino acid ionic liquid molecules. The amino acid ionic liquid molecules automatically perform phase separation after absorption of acid gas and are directly repeatedly recycled, and the energy consumption is low.
Owner:HUBEI UNIV
Acetaminophen pharmaceutical composition preparation and preparation method thereof
ActiveCN108451916ARaise the pHAvoid irritation damageOrganic active ingredientsAntipyreticDrugChemistry
The invention provides a acetaminophen pharmaceutical composition preparation and a preparation method thereof. The preparation method comprises the following steps: uniformly mixing acetaminophen, crospovidone, povidone K30, methylparaben, ethylparaben and propyl hydroxybenzoate, and adding a binding agent aqueous solution to pelletize; drying through a gradient variable-temperature method afterpelletizing, controlling the temperature of each gradient to 20-40 DEG C, adding alginic acid, calcium carbonate and colloidal silicon dioxide to pre-mix for 3-5 minutes after drying, adding magnesiumstearate to mix for 3-5 minutes, and tabletting and covering. The preparation method for the acetaminophen pharmaceutical composition preparation is simple in operation step, and is gentle in operation condition; and the preparation method can realize quick dissolving-out of effective ingredients in the preparation, increases a dissolution rate, and is worthy of being widely popularized and applied.
Owner:重庆国泰康宁制药有限责任公司
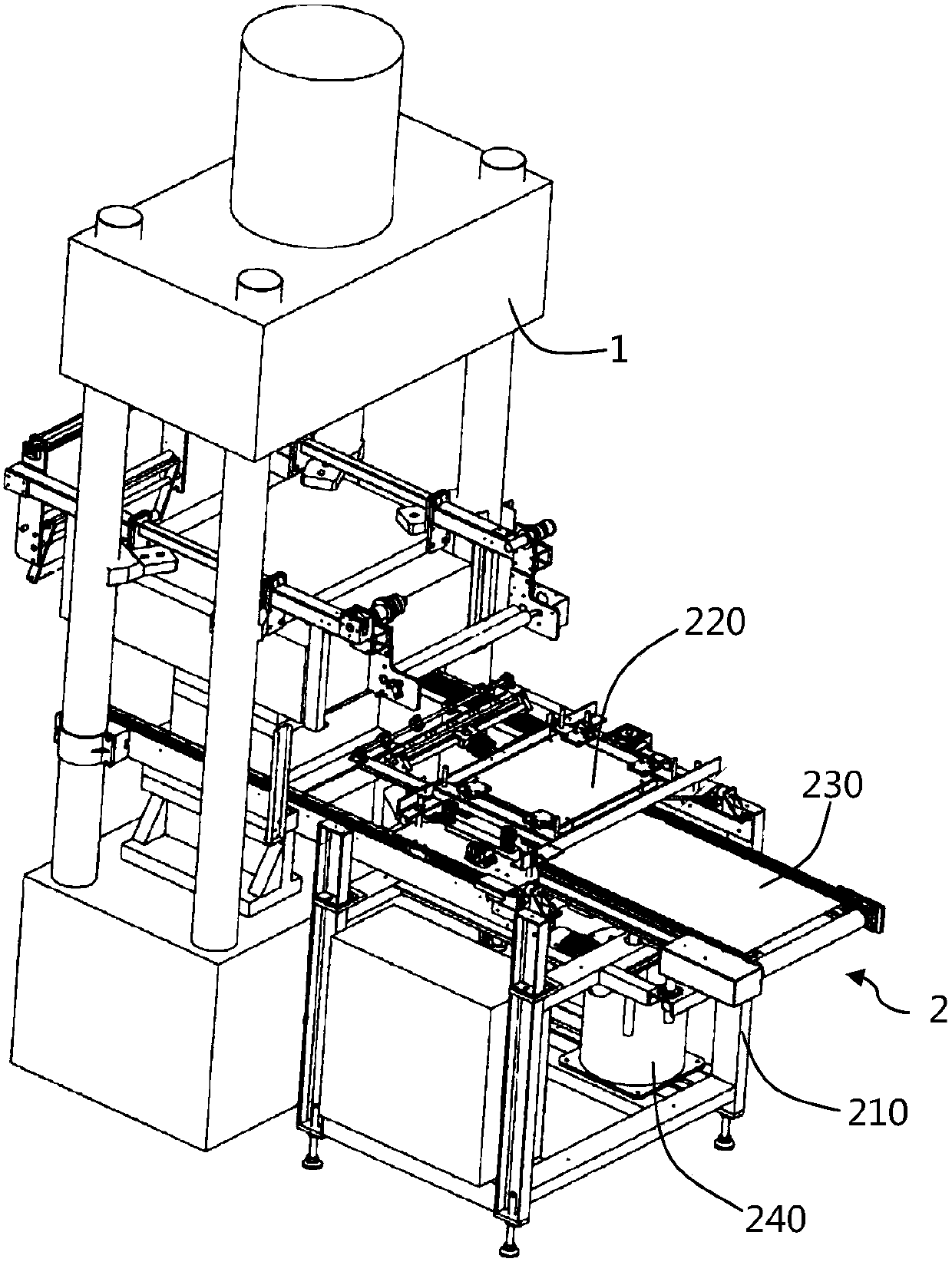
Automatic blank fetching equipment and method for magnetic material oil hydraulic machine
PendingCN107718692AReduce volumeSuitable for large-scale productionPressesEngineeringSmall footprint
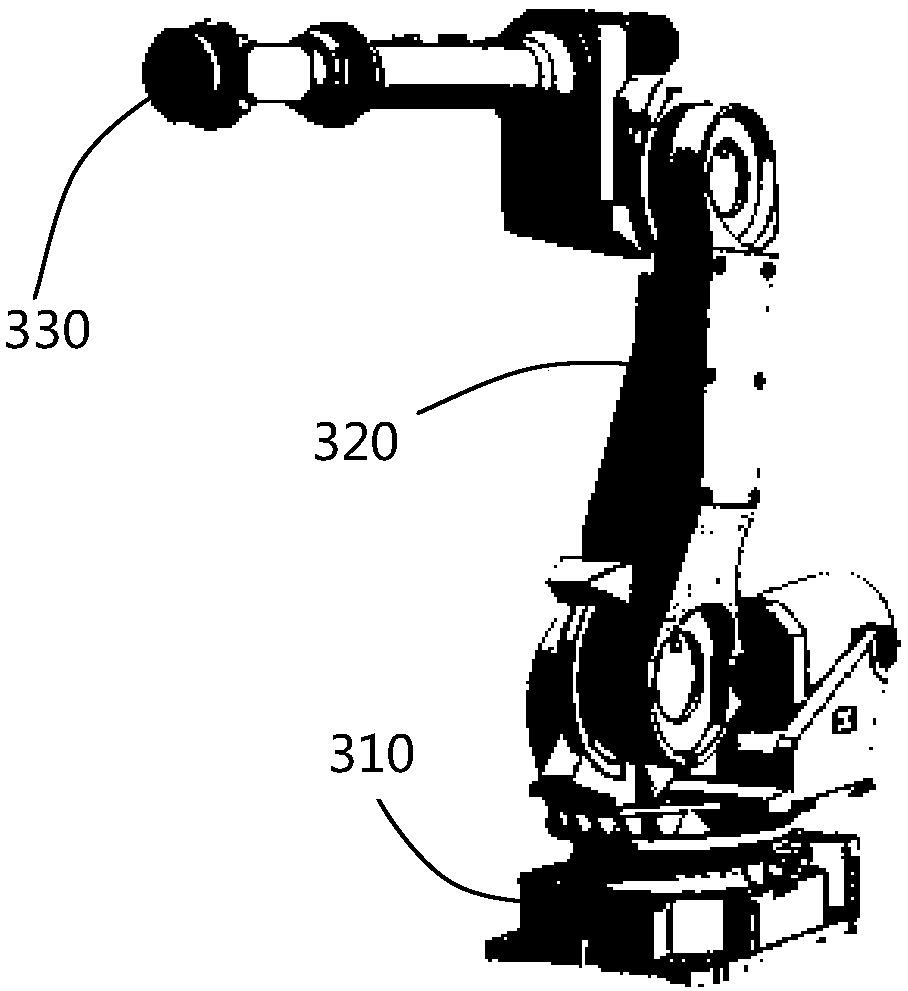
The invention discloses automatic blank fetching equipment and method for a magnetic material oil hydraulic machine and belongs to the technical field of magnetic material production equipment, and the automatic blank fetching equipment comprises a controller, a blank fetching machine and a blank fetching robot, wherein the controller is used for controlling the whole equipment, the blank fetchingmachine is arranged on one side of the material output of the oil hydraulic machine, and the blank fetching robot is arranged on any side of the blank fetching machine. The blank fetching robot and the blank fetching machine are in electrical connection with the controller correspondingly, and at least one layer of magnetic molded blank parts which are put in good order on the blank fetching machine are taken away by the blank fetching robot in sequence. According to the automatic blank fetching equipment and method for the magnetic material oil hydraulic machine, the blank fetching robot adopted by the invention is small in volume and land occupied area, for some enterprises with relatively narrow space, the requirements of intelligent production can be better met, and the blank fetchingrobot is suitable for the large-scale production of enterprises; at least one layer of magnetic molded blank parts which are put in good order on the blank fetching machine are taken away by the blank fetching robot in sequence and blank materials are neatly put in good order on a tray, the magnetic molded blank parts are not prone to breakage, the production qualification rate of the blank partsis high, the artificial blank fetching is reduced, and the work efficiency is effectively improved.
Owner:SINOSTEEL TIANYUAN MAANSHAN TONGLI MAGNETIC MATERIAL
Corrosion-resistant freeze-thaw resistant mortar and preparation method thereof
The invention provides corrosion-resistant freeze-thaw resistant mortar which is mainly composed of powder and emulsion according to a mass ratio of (4-6):1. The powder comprises the following components in parts by weight: 300-400 parts of cement, 400-500 parts of coarse sand, 100-300 parts of fine sand, 1-2 parts of a water reducing agent, 3-8 parts of an expanding agent, 1-2 parts of an antifoaming agent, 1-3 parts of HPMC (Hydroxy Propyl Methyl Cellulose), 1-2 parts of starch ether, 1-2 parts of a water repellent, 1-3 parts of polypropylene fibers, 1-2 parts of hollow fibers and 30-50 parts of rubber powder; and the emulsion comprises the following components in percentage by weight: 20-30% of cationic chloroprene emulsion, 20-30% of vinyl acetate-ethylene copolymer emulsion and the balance of drinking water. The preparation method comprises the following steps: respectively preparing the powder and emulsion, and uniformly stirring and mixing according to a certain ratio, thereby obtaining the product. The corrosion-resistant freeze-thaw resistant mortar disclosed by the invention has dual performances of corrosion resistance and freeze-thaw resistance and is worthy of wide popularization and application.
Owner:内蒙古绰勒水利水电有限责任公司 +1
Fine spinning fabric and processing method thereof
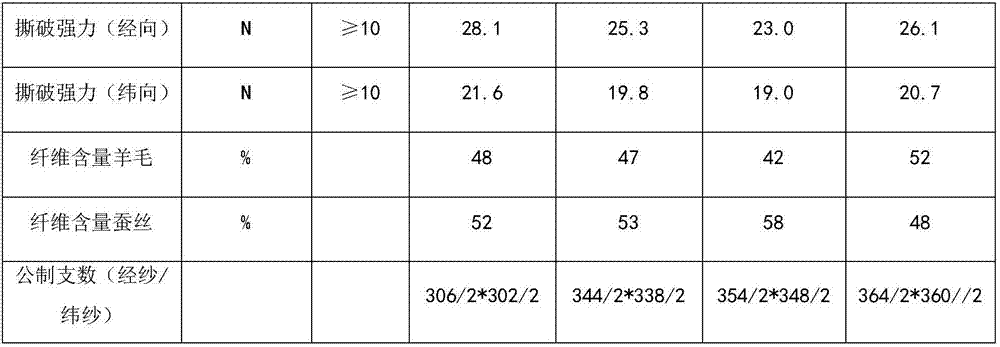
InactiveCN107268154AStrong competitive positionSimple processing methodWoven fabricsYarnFiberWorsted
The invention provides a fine spinning fabric. In the fine spinning fabric, the wool content is controlled in 40-50 wt%, the mulberry silk content is controlled in 50-60 wt%, and the metric count is above 300 s / 2. The processing method includes: (A), blending mulberry silk, water-soluble fibers and wool, plying the mixture and the mulberry silk to form yarns, and weaving the yarns into an ultrathin fabric; (B), soaking the ultrathin fabric in water at the temperature of 70-80 DEG C, taking the ultrathin fabric out of water and allowing the ultrathin fabric to stand for 4-5 hours, allowing the water-soluble fibers to fully expand, soaking the ultrathin fabric in water at the temperature of 80-90 DEG C for 40-60 min, and performing vinylon dissolution to acquire the fine spinning fabric. The metric count of fine spinning fabric can reach above 300s / 2; and the fine spinning fabric has advantages of fine and smooth performance, warming performance and the elasticity of the wool, has the advantages of light and soft performance and natural gloss of the mulberry silk, can meet the ultrathin demand, and has high serviceability.
Owner:SHANDONG NANSHAN TEXTILE GARMENT +1
Metformin hydrochloride medicinal composition preparation and preparation method thereof
ActiveCN108420807ANot easy to loseMoisture stableOrganic active ingredientsMetabolism disorderAdhesiveMetformin Hydrochloride
The invention provides a metformin hydrochloride medicinal composition preparation and a preparation method thereof. The preparation method comprises the following steps: (A) pelletizing melbine withan adhesive solution; (B) pelletizing at a stirring speed within 200-400rpm for 90-180 seconds; (C) drying by using a gradient temperature variation method, after drying, adding corn starch and mannitol, premixing for 3-5 minutes, further mixing with magnesium stearate for 3-5 minutes, tabletting, and coating, thereby obtaining the metformin hydrochloride medicinal composition preparation, whereineach gradient of temperatures is controlled within 20-40 DEG C, drying, the temperatures are changed in a tendency that temperatures of a former gradient and a later gradient are alternatively changed, and the temperatures of two adjacent gradients are 4-5 DEG C different. The preparation method of the metformin hydrochloride medicinal composition preparation provided by the embodiment of the invention is simple in operation step and gentle in operation condition, and effective components in the preparation prepared by using the preparation method can be rapidly dissolved, and the dissolutionrate can be increased.
Owner:重庆希尔安药业有限公司
Coumarins compound and extracting method thereof
The invention provides a coumarins compound. The chemical name is 4-hydroxyphenyl-5-para hydroxyphenylethyl-7-hydroxyl-3,4-dihydrocoumarin. An extracting method comprises the steps that oil tea leaves are extracted in a heating mode and concentrated to obtain a concentrated solution, and the concentrated solution is adsorbed by D101 macro-porous resin, and then eluted sequentially with distilled water and an ethanol solution; silica gel chromatography is carried out on eluant eluted through the ethanol solution, and the eluant is collected with 400-500 mL as one unit; silica gel chromatography is carried out on the second unit of the collected eluant, dichloromethane-methyl alcohol is adopted as a moving phase, the eluant is collected with 90-100 mL as one unit, and after the eluant of the units from 12 to 14 is merged, separation and extraction continue to be carried out to obtain the compound. The coumarins compound is a novel single compound, purity is high, components are definite, no impurity exists, and the compound is obtained for the first time through extraction and purification, which has pioneering significance.
Owner:CENTRAL SOUTH UNIVERSITY OF FORESTRY AND TECHNOLOGY
Feed additive for improving growth performance and meat quality of pigs as well as preparation method and application of feed additive
PendingCN113974006AGreenImprove disease resistanceFood processingAnimal feeding stuffBiotechnologyAnimal science
The invention relates to a feed additive for improving growth performance and meat quality of pigs as well as a preparation method and application of the feed additive. The feed additive comprises the following raw materials in parts by weight: 5-12 parts of roxburgh rose residues, 6-13 parts of honeysuckle flowers, 5-15 parts of tea leaf residues, 8-14 parts of a spanishneedles herb extract, 5-10 parts of a grape seed extract, 7-15 parts of a traditional Chinese medicine negative ion preparation, 5-13 parts of perilla oil and 8-51 parts of a carrier. All the components of the feed additive are safe and free of residues, and after the components are used together, the growth performance, immunity and antioxidant function of pigs can be improved, the pork quality can be improved, and intestinal flora balance can be maintained. The preparation method comprises the step of mixing the raw materials according to the proportion, and is simple, rapid and good in condition controllability. When being used for preparing a pig feed, the feed additive can obviously improve the disease resistance, growth performance, immunity and antioxidant function of pigs, and in addition, the feed additive can also improve the flavor of pork, prolong the shelf life and further improve the breeding benefits.
Owner:辽宁傲农饲料有限公司 +1
Slag pretreatment method and preparation method of supercapacitor electrode material
PendingCN113380558AReduce manufacturing costTechnology environmental protectionMaterial nanotechnologyHybrid capacitor electrodesPretreatment methodSlag
The invention provides a slag pretreatment method and a preparation method of a supercapacitor electrode material. The slag pretreatment method comprises the steps of: carrying out high-temperature sintering on nickel-molybdenum slag at 800-1000 DEG C for 4-6 hours, then reducing the temperature to 600 DEG C, and placing the sintered nickel-molybdenum slag in pure water for washing the slag, and drying the slag; grinding the dried nickel-molybdenum slag, and screening with the ground slag with a screen of 800 meshes or above to obtain slag powder; and soaking the slag powder in hydrochloric acid, adding NH3 water to carry out hydrothermal reaction with thiourea, and cooling and drying a reaction product after finishing the reaction. According to the slag pretreatment method and the preparation method of the supercapacitor electrode material of the invention, the slag is used for preparing the supercapacitor electrode material, so that energy recycling, energy conservation and environmental protection are realized; the slag is pretreated to a certain degree, so that the slag is more suitable for being used as a raw material of the supercapacitor electrode material, and the obtained material has very strong electrochemical performance through detection.
Owner:GUIZHOU CHEM IND BUILDING CORP +1
Refractory brick double-station fast-switching forming equipment
InactiveCN109291213AAvoid manual penetration into the upper mold seatAvoid security issuesMould auxillary partsFeeding arrangmentsLeveling effectBrick
The invention relates to refractory brick double-station fast-switching forming equipment comprising a press. The press comprises an upper mold seat, a lower mold seat and a male mold; a loading tablea and a loading table b are arranged on the two sides of the press correspondingly; the press further comprises a female mold assembly, the female mold assembly comprises a female mold a and a femalemold b movably connected with the female mold a, the female mold a and the female mold b are both provided with guiding-in grooves, and matching blocks are arranged in the guiding-in grooves; the loading table a is provided with an adding mechanism a and a vibrating mechanism a; the adding mechanism a adds sandy soil into the female mold a, and after the sandy soil is added, the vibrating mechanism a vibrates the loading table a; the loading table b is provided with an adding mechanism b and a vibrating mechanism b; and the adding mechanism b adds sandy soil into the female mold b, and afterthe sandy soil is added, the vibrating mechanism b vibrates the loading table b. According to the refractory brick double-station fast-switching forming equipment, the technical problems that potential safety hazards exist when manual penetrating is conducted between mold seats for adding, the adding efficiency is low, the added sandy soil needs to be leveled manually, the leveling effect is poor,and the function that ceramic strips are placed into the sandy soil to be integrally formed is not achieved are solved.
Owner:ZHEJIANG ZHONGXIN NEW MATERIAL CO LTD
A kind of amino acid ionic liquid molecule and its preparation method and application
InactiveCN105669474BImprove thermal stabilityReduce volatilityOrganic compound preparationDispersed particle separationAlkaneHalohydrocarbon
The invention provides amino acid ionic liquid molecules and a preparation method and an application thereof; the amino acid ionic liquid molecules have the chemical structural general formula defined in the specification, wherein R1 is alkane, R2 is hydrocarbonyl of halide, and X is amino acid. The preparation method comprises the steps: carrying out an anion exchange reaction of a quaternary ammonium compound with an alkali for 1-4 h under a condition of the temperature of 25-30 DEG C to obtain a hydroxyl compound of quaternary ammonium salt; carrying out a dehydration reaction of the hydroxyl compound of the quaternary ammonium salt with the amino acid for 4-8 h under a condition of the temperature of 10-50 DEG C, and thus obtaining the amino acid ionic liquid molecules. The amino acid ionic liquid molecules automatically perform phase separation after absorption of acid gas and are directly repeatedly recycled, and the energy consumption is low.
Owner:HUBEI UNIV
A modified carbon fiber/sio 2 Preparation method of airgel composite material
ActiveCN113860848BIncrease the content of functional groupsIncrease the lengthCeramicwareFiberCarbon fibers
The invention provides a modified carbon fiber / SiO 2 The preparation method of the airgel composite material comprises the steps of: desizing the carbon fiber, and after nitric acid oxidation treatment, using silane coupling agents KH-550 and KH-560 to carry out surface modification and grafting treatment to obtain the modified carbon fiber; Stir and disperse the modified carbon fiber and silicon dioxide wet gel evenly, and freeze-dry after drying, aging, hydrophobic modification and soaking. The preparation method of airgel composite material of the present invention strengthens SiO by adopting the modified carbon fiber 2 The performance of airgel, the chemical cross-linking combination of the two has better mechanical properties than the previous physical combination, the whole operation method is simple, the operating conditions are mild, the steps are closely connected, and the prepared material has good performance.
Owner:GUIZHOU MATERIAL IND TECH INSTITUE
Method for enhancing strength of high-aluminum preform
The invention provides a method for enhancing the strength of a high-aluminum preform, comprising the following steps: (A) adding magnesium sulfate heptahydrate into water, thoroughly stirring to formsoaking liquid, and cooling for standby application; (B) demoulding the aluminum preform, then naturally curing, soaking in the soaking liquid until bubbles on the surface of the aluminum preform disappear, and baking at a temperature of 200 DEG C or lower for 30 hours or shorter. The method provided by the embodiment of the invention can greatly increase the normal temperature strength of the preform subjected to natural curing by adding salt and rare earth oxide in the high aluminum soaking process, and also can reduce the addition amounts of binding agents such as calcium aluminate cementand rho-Al2O3 micropowder in the high aluminum material, thus promoting the slag corrosion resistance of the material, finally greatly improving the intermediate temperature strength of the soaked preform, and being capable of effectively alleviating structural flaking caused by thermal stress concentration of the preform during use.
Owner:SHANGHAI LIER REFRACTORY MATERIAL
Preparation method of modified carbon fiber/SiO2 aerogel composite material
ActiveCN113860848AIncrease the content of functional groupsIncrease the lengthCeramicwareFiberCarbon fibers
The invention provides a preparation method of a modified carbon fiber / SiO2 aerogel composite material. The preparation method comprises the following steps: carrying out desizing and nitric acid oxidation treatment on carbon fibers, and carrying out surface modification grafting treatment on the carbon fibers by using silane coupling agents KH-550 and KH-560 to obtain modified carbon fibers; and uniformly stirring and dispersing the modified carbon fibers and silicon dioxide wet gel, drying, aging, carrying out hydrophobic modification, soaking, and carrying out freeze drying. According to the preparation method of the aerogel composite material, the modified carbon fibers are adopted to enhance the performance of the SiO2 aerogel, the SiO2 aerogel and the modified carbon fibers are subjected to chemical crosslinking combination, compared with a previous physical combination mode, the mechanical performance of the aerogel composite material is more excellent, the whole operation method is simple, the operation condition is mild, step linkage is tight, and the prepared material is good in performance.
Owner:GUIZHOU MATERIAL IND TECH INSTITUE
A kind of high performance magnesia carbon brick and preparation method thereof
The invention provides a high-performance magnesia-carbon brick and a preparation method thereof, mainly comprising the following raw materials in parts by mass: 60-75 parts of fused magnesia particles, 10-25 parts of fused magnesia fine powder of 120 mesh-400 mesh , 1-5 parts of aluminum powder, 0.1-5 parts of spinel-calcium aluminate composite material, 10-16 parts of flake graphite, 2-4 parts of binding agent; the fused magnesia particles are 3-5mm, 1 ‑3mm and 0.075‑1mm particles are mixed in mass ratio (3‑5): (3‑5): (2‑4). The magnesia-carbon brick of the embodiment of the present invention introduces a spinel-calcium aluminate composite material, thereby producing low-melting-point substances on the surface of the magnesia-carbon brick at high temperature, relieving thermal stress, reducing thermal shock damage and mechanical damage, thereby achieving The purpose of preventing cracking of the magnesia carbon brick body.
Owner:上海新泰山高温工程材料有限公司
Feed additive for improving production performance of lactating sows, preparation method and application thereof
ActiveCN107183385BImprove immunityGood for healthAnimal feeding stuffAccessory food factorsBiotechnologyMotherwort
The invention relates to a feed additive for improving the production performance of lactating sows, a preparation method and application thereof, and belongs to the technical field of sow feeding. The above-mentioned feed additives include enteromorpha, pyrroloquinoline quinone, kelp, motherwort, buliuxing, malt, brown sugar, licorice, purslane, Bupleurum and compound amino acids. Its preparation method includes: mixing kelp, motherwort, blancia, malt, brown sugar, licorice, purslane and Bupleurum, decocting, concentrating, and mixing with enteromorpha, pyrroloquinoline quinone and compound amino acids. The preparation method is simple, low in cost and good in controllability. The prepared feed additive can effectively improve the immune level of lactating sows and promote lactation; and its raw materials are all green additives, which are safe and environment-friendly. Applying it to the feed of lactating sows can pass benefits through the mother and offspring, improve the health status of suckling piglets, improve the growth performance of piglets and the economic benefits of breeding.
Owner:FUJIAN AONONG BIOLOGICAL TECH GRP CO LTD +2
A functional synthetic material and its preparation method and product
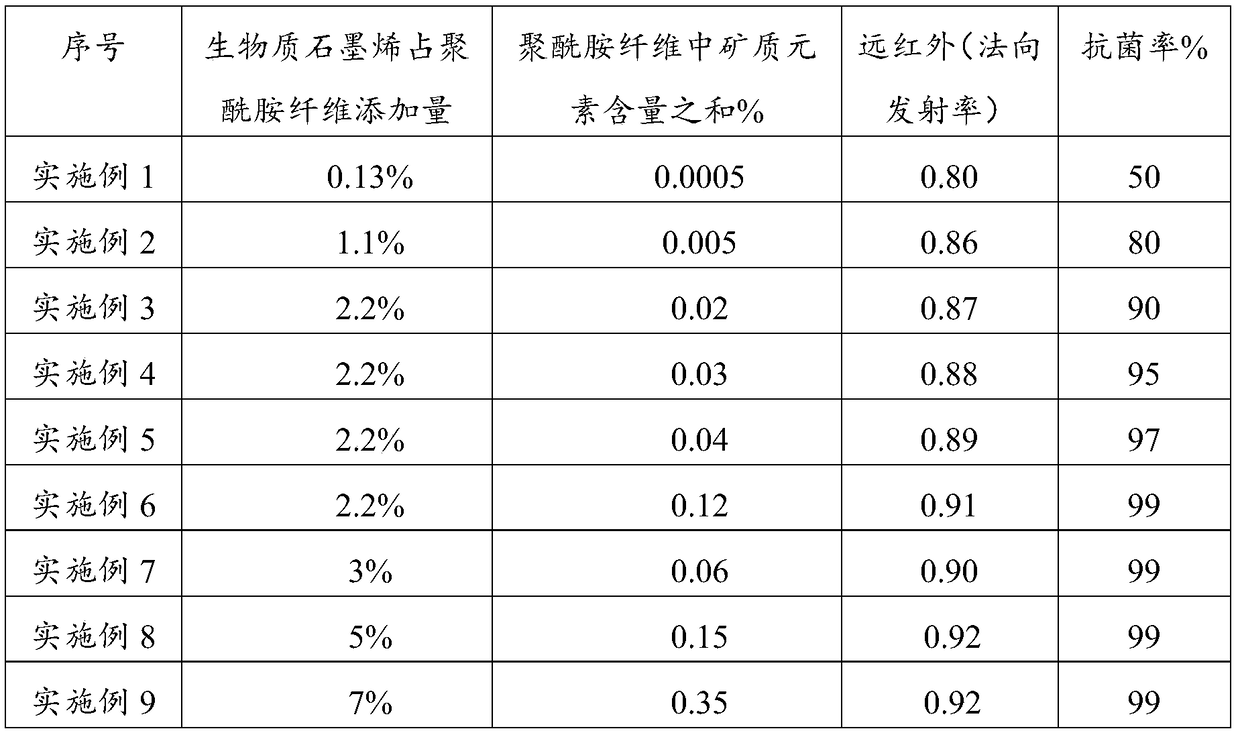
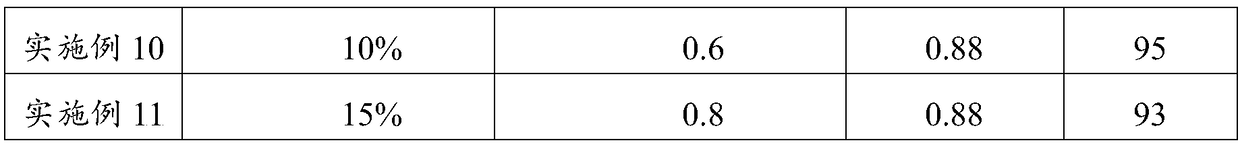
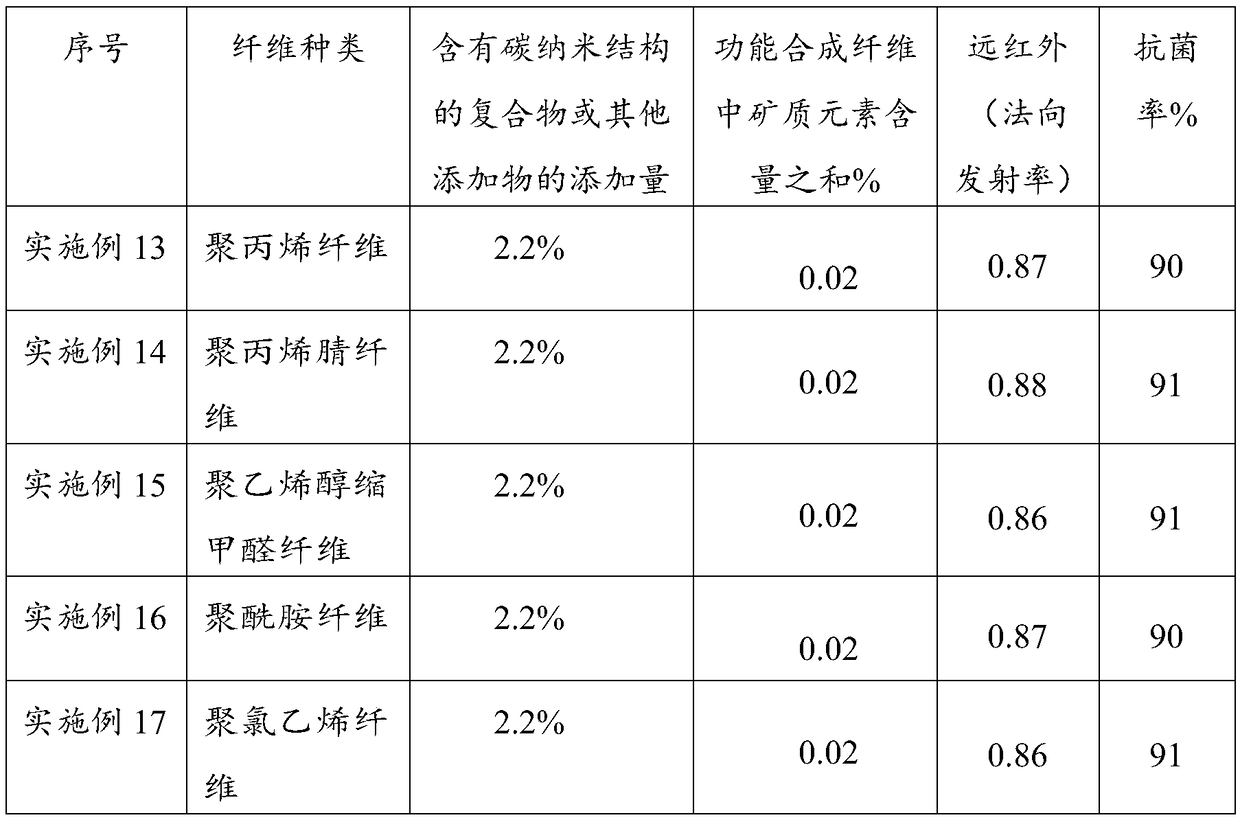
ActiveCN106245140BIncrease added valueEnhanced Far Infrared PerformanceGrapheneMonocomponent polyolefin artificial filamentFiberFunctional synthesis
The invention provides a functional synthesis material. The functional synthesis material has a grapheme structure and mineral elements, wherein the mineral elements include Fe, Si and Al elements. The functional synthesis material is wide in application, and the fiber product of the functional synthesis material can be used for making garment for civil use, home textiles, ultraviolet armored fabric and industrial special protective garment, and has excellent far infrared, anti-radiation, anti-static, antimicrobial and antibacterial performances.
Owner:JINAN SHENGQUAN GRP SHARE HLDG CO LTD
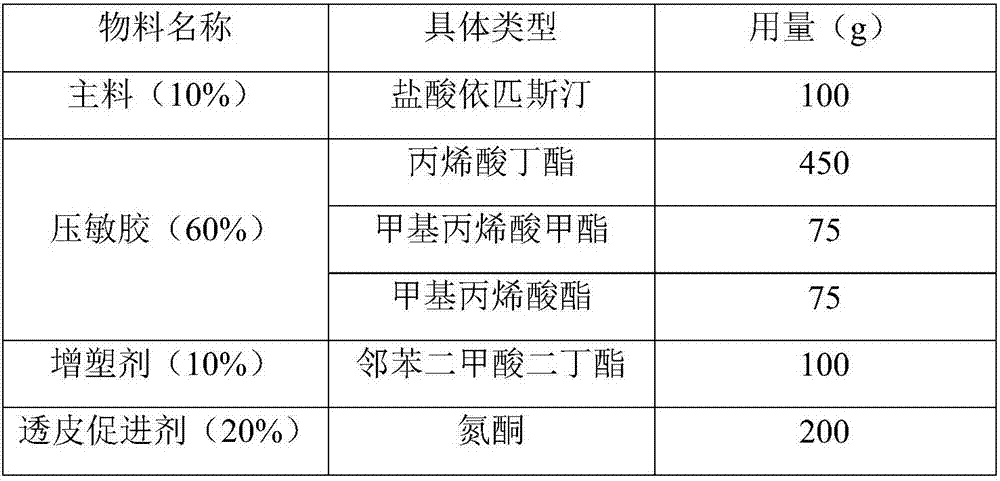
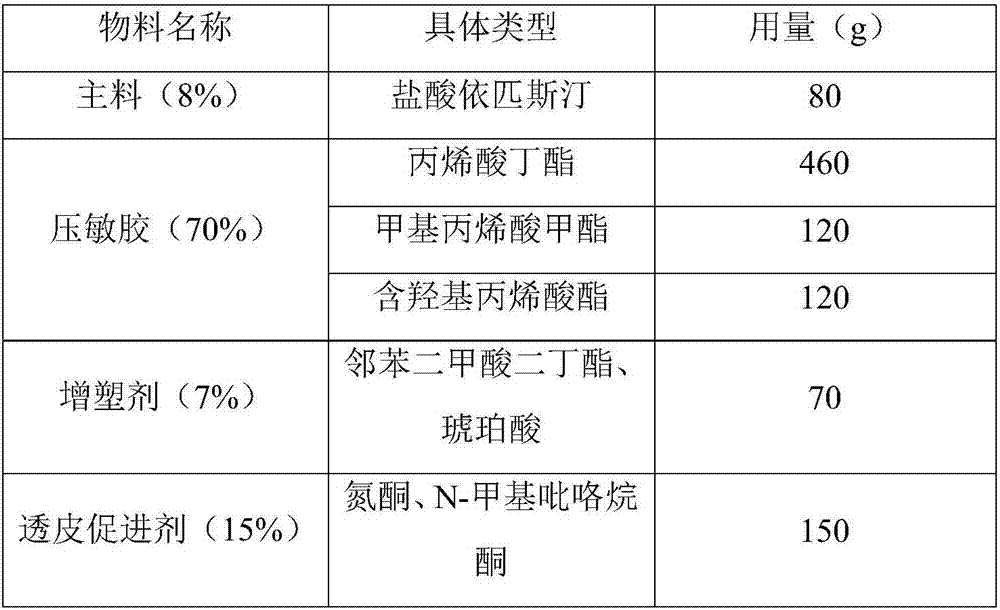
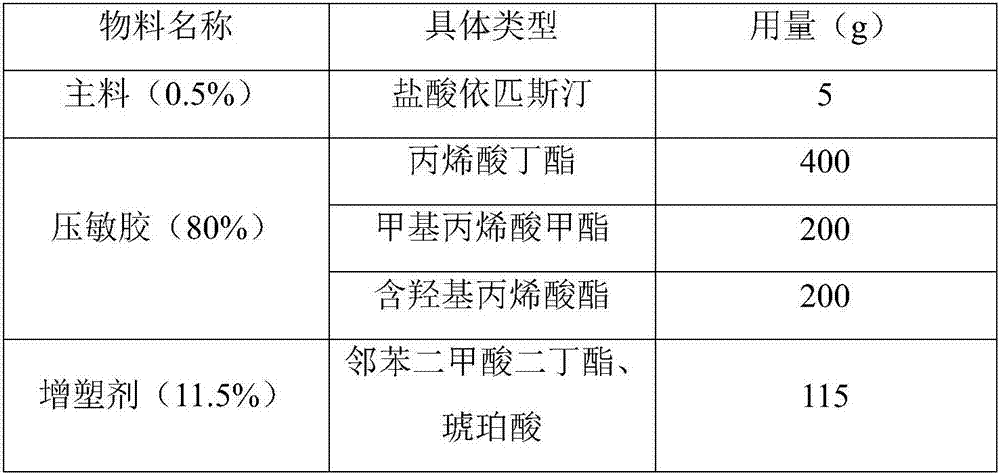
Epinastine hydrochloride transdermal patch and preparation method thereof
InactiveCN107233333AReduce dosageStable in naturePharmaceutical non-active ingredientsRespiratory disorderTransdermal patchOral medicine
The invention provides an epinastine hydrochloride transdermal patch. The epinastine hydrochloride transdermal patch is composed of a back lining layer, a drug containing layer and a anti-adhesion layer, wherein the drug containing layer is composed of, by weight percentage, 0.5-10% of epinastine hydrochloride, 60-90% of pressure-sensitive adhesives, 5-20% of plasticizer and 4-30% of transdermal enhancer. The preparation method of the epinastine hydrochloride transdermal patch comprises stirring and dissolving the plasticizer and the transdermal enhancer, sequentially add in and uniformly mixing the epinastine hydrochloride and the pressure-sensitive adhesives, performing degassing and drying processes, applying anti-adhesion materials to form the anti-adhesion layer, drying the anti-adhesion layer and covering the back lining layer to obtain the epinastine hydrochloride transdermal patch. The epinastine hydrochloride transdermal patch is prepared by mixing and matching water-soluble polymer materials and main drugs and is low in dosage; the epinastine hydrochloride transdermal patch can be prepared into thin patches, which are stable in properties, safe, free from irritation and good in skin tolerance; compared with traditional oral medicines, the epinastine hydrochloride transdermal patch is good in effects, beneficial to absorption by patients and capable of avoiding first-pass effects of liver of the patients.
Owner:重庆瑞泊莱医药科技有限公司
Composite lubricant for drilling fluid, preparation method and application thereof
ActiveCN107011876BImprove temperature resistanceImprove thermal stabilityDrilling compositionBiodieselHigh pressure
The invention discloses a composite lubricant for drilling fluid. The composite lubricant is mainly prepared from the following raw materials in parts by mass: 60 to 90 parts of fatty alcohol-polyoxyethylene ether, 30 to 60 parts of fatty acid ester, 20 to 40 parts of waste animal fat, 5 to 10 parts of vinyl benzene, 5 to 9 parts of water-soluble carbon black, 2 to 5 parts of graphene substances, 1 to 3 parts of an extreme pressure anti-wear reagent, 1 to 3 parts of a dispersing agent, and 1 to 3 parts of a defoamer. The preparation method comprises the following steps: uniformly mixing and stirring the fatty alcohol-polyoxyethylene ether, the fatty alcohol-polyoxyethylene ether, the waste animal fat and vinyl benzene; adding the water-soluble carbon black, the graphene substances, the extreme pressure anti-wear reagent, the dispersing agent and the defoamer sequentially, performing mixing and stirring, and cooling the raw materials to 50 to 70 DEG C while stirring, so as to form uniform emulsion. The composite lubricant is high in temperature resistance and anti-pressure capacity, and can still keep good lubricating property under high-temperature and high-pressure conditions, biodiesel fuel is taken as a raw material for the main components of the composite lubricant, and no polluted component is contained.
Owner:任丘市力科节能材料有限公司
A manufacturing method and production line of needle-punched felt for thermoplastic resin
ActiveCN104191789BMelt Dipping FastImprove vertical and horizontal strengthLamination ancillary operationsLaminationProduction linePorosity
The invention relates to a manufacturing method of a needled felt for thermoplastic resin. The manufacturing method comprises the following steps: uncoiling a thermoplastic glass-fiber fabric through an uncoiling shaft, placing thermoplastic short-cut glass fibers on a yarn frame behind an uncoiling device, and conveying the thermoplastic short-cut glass fibers to a short cutting device above the uncoiling device through a yarn guide pipe of the yarn frame; uniformly scattering the thermoplastic short-cut glass fibers through a rotating mace to enable the scattered thermoplastic short-cut glass fibers to fall on the upper surface of the thermoplastic glass-fiber fabric on the uncoiling device below; and conveying the thermoplastic glass-fiber fabric with the thermoplastic short-cut glass fibers into a needling device, an edge cutting device and a coiling device through conveying belts to obtain the finished product. The prepared needled felt for thermoplastic resin adopts the thermoplastic glass-fiber fabric as basic cloth, and the thermoplastic short-cut glass fibers are combined with the thermoplastic glass-fiber fabric in a needling manner, so that the prepared needled felt for thermoplastic resin has advantages of high longitudinal and transverse strength, low probability of deformation, high porosity, rapid melting and soaking of thermoplastic resin and the like. The production line has advantages of compact and reasonable flow design and convenience in operation.
Owner:ZHEJIANG LIANYANG NEW MATERIAL
A kind of paracetamol pharmaceutical composition preparation and preparation method thereof
ActiveCN108451916BDisintegrates quicklyPlay the role of sealing water retentionOrganic active ingredientsAntipyreticMagnesium stearateStearic acid
The invention provides a acetaminophen pharmaceutical composition preparation and a preparation method thereof. The preparation method comprises the following steps: uniformly mixing acetaminophen, crospovidone, povidone K30, methylparaben, ethylparaben and propyl hydroxybenzoate, and adding a binding agent aqueous solution to pelletize; drying through a gradient variable-temperature method afterpelletizing, controlling the temperature of each gradient to 20-40 DEG C, adding alginic acid, calcium carbonate and colloidal silicon dioxide to pre-mix for 3-5 minutes after drying, adding magnesiumstearate to mix for 3-5 minutes, and tabletting and covering. The preparation method for the acetaminophen pharmaceutical composition preparation is simple in operation step, and is gentle in operation condition; and the preparation method can realize quick dissolving-out of effective ingredients in the preparation, increases a dissolution rate, and is worthy of being widely popularized and applied.
Owner:重庆国泰康宁制药有限责任公司
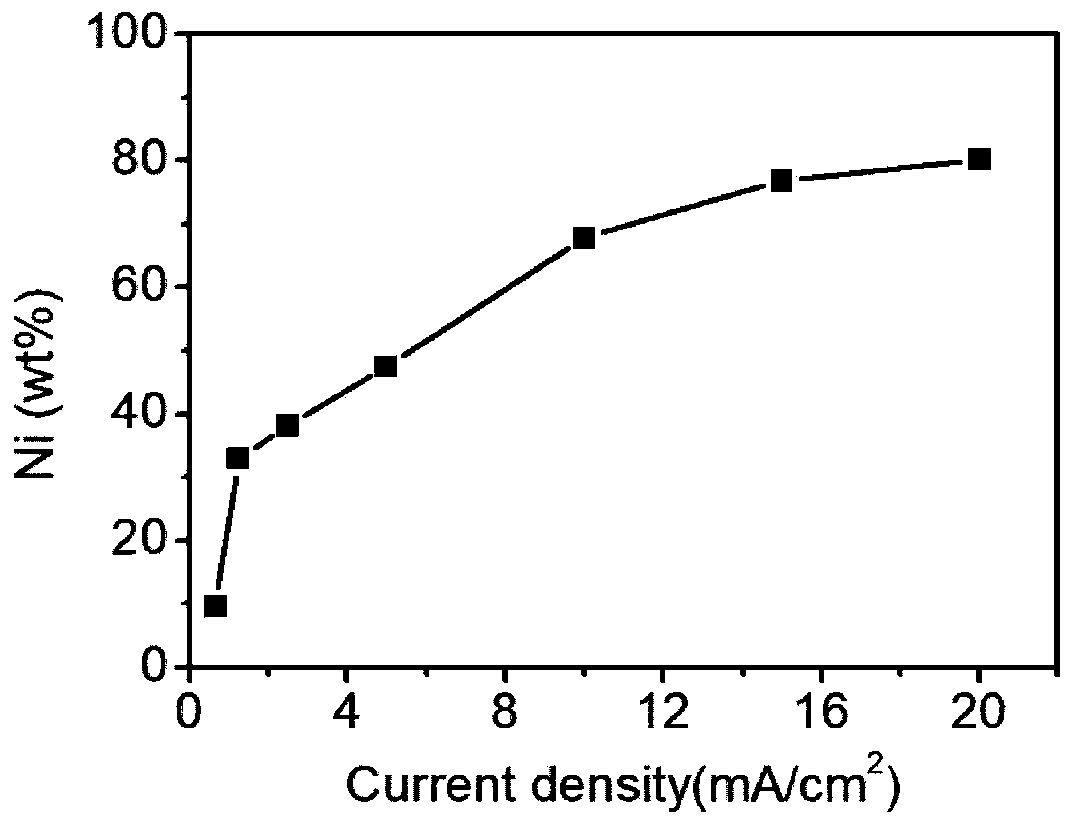
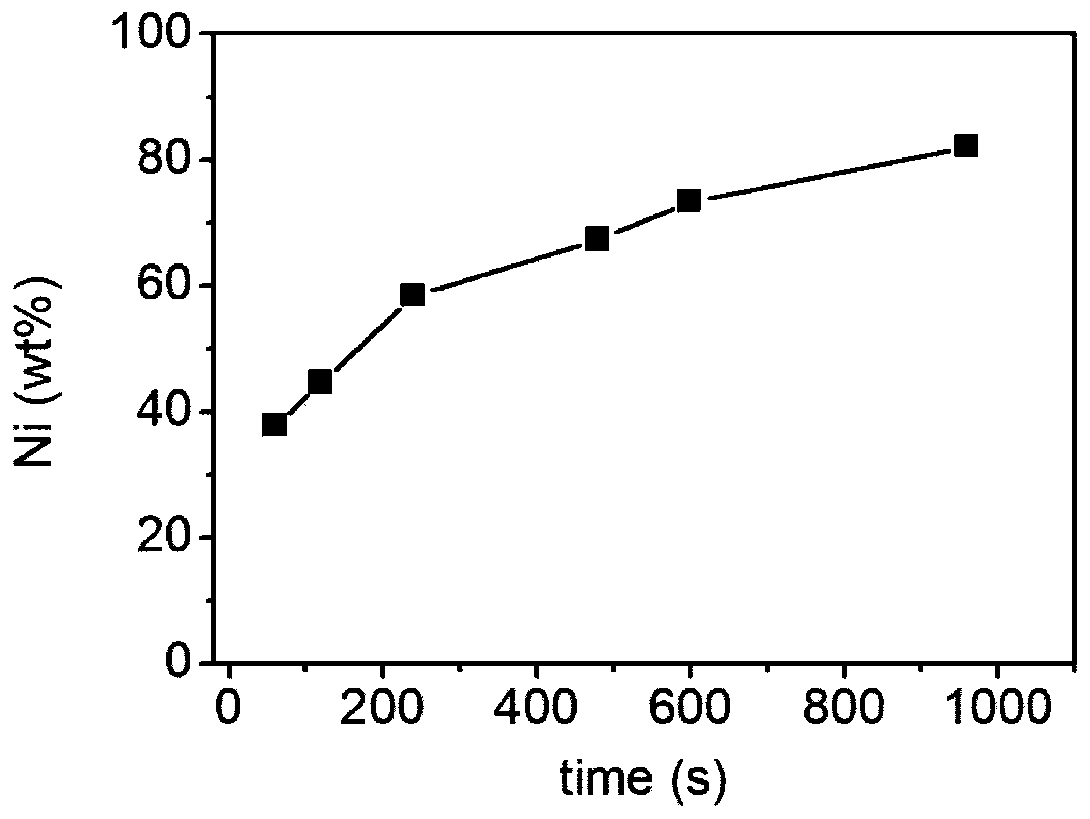
A kind of preparation method of nano-nickel/array carbon nanotube composite material
ActiveCN109136986BAvoid preprocessingGood dispersionMaterial nanotechnologyPhotography auxillary processesMetallic foilElectrochemistry
The invention discloses a preparation method of a nano-nickel / array carbon nanotube composite material. The method comprises the following steps: using an array carbon nanotube as a cathode, and making nickel ions subjected to electrochemical reduction reaction on the array carbon nanotube at a certain current density to form the nano-nickel / array carbon nanotube composite material. The method provided by the invention uses the array carbon nanotube without any pretreatment on a metal foil as a substrate, obtains nano-nickel with controllable morphology and size on the cathode carbon nanotubeaccording to a constant current continuous electrochemical method, adopts closely linked steps, avoids a pretreatment process of the carbon nanotubes in the conventional method, and shortens the process flow; in addition, the constant current method is easier to control and easy to continuously and massively produce the nano-nickel / array carbon nanotube composite material. The method provided by the invention can effectively control the morphology and the size of the nano-nickel and the content of nickel by adjusting components and concentrations of the solution system, the current magnitude and the energization time, and is a simple, continuous and scalable preparation method of the nano-nickel / carbon nanotube composite material.
Owner:高彪峰
A linear motor type portable forming equipment and portable forming method
ActiveCN108656635BRealize gluingComplete quickly and efficientlyPaper/cardboard articlesEngineeringLinear motor
The invention relates to the field of portable processing and manufacturing, in particular to a linear motor type portable forming device and a portable forming method. The linear motor type portableforming device comprises a working plate, a transmission mechanism, a gluing mechanism, a linear motor, a cutter, a folding mechanism, a pressing block and a separating mechanism; the transmission mechanism is arranged on a transverse side plate of the working plate; the linear motor is arranged at the upper part of the transverse side plate of the working plate and is arranged at the rear end ofthe gluing mechanism; the cutter is arranged on one side of the tail end of a paper groove below the gluing mechanism, and is arranged below a motor sliding block; and the folding mechanism is arranged at the rear end of the cutter. According to the portable forming device and the portable forming method, the portable manufacturing can be rapidly and effectively finished by utilizing the orderly process steps which are tightly connected.
Owner:JIANGSU NANJIANG MACHINERY CO LTD
Medicinal liquor production line
InactiveCN103451080ASimple structureEasy to operateAlcoholic beverage preparationProduction lineMedicine
The invention relates to medicinal liquor infusion field and specifically relates to a medicinal liquor production line. The medicinal liquor production line is characterized by comprising a medicine machine, a winemaking machine, a capper and a below conveyor belt which are arranged in sequence. The medicinal liquor production line is simple in structure, convenient to operate and capable of realizing mass production. Moreover, the steps are tightly connected, and the infusion effect is good.
Owner:陈启章
A kind of anticorrosion antifreeze-thaw mortar and preparation method thereof
Owner:内蒙古绰勒水利水电有限责任公司 +1
A kind of metformin hydrochloride pharmaceutical composition preparation and preparation method thereof
ActiveCN108420807BNot easy to loseMoisture stableOrganic active ingredientsMetabolism disorderMetformin hclPharmaceutical drug
The invention provides a metformin hydrochloride medicinal composition preparation and a preparation method thereof. The preparation method comprises the following steps: (A) pelletizing melbine withan adhesive solution; (B) pelletizing at a stirring speed within 200-400rpm for 90-180 seconds; (C) drying by using a gradient temperature variation method, after drying, adding corn starch and mannitol, premixing for 3-5 minutes, further mixing with magnesium stearate for 3-5 minutes, tabletting, and coating, thereby obtaining the metformin hydrochloride medicinal composition preparation, whereineach gradient of temperatures is controlled within 20-40 DEG C, drying, the temperatures are changed in a tendency that temperatures of a former gradient and a later gradient are alternatively changed, and the temperatures of two adjacent gradients are 4-5 DEG C different. The preparation method of the metformin hydrochloride medicinal composition preparation provided by the embodiment of the invention is simple in operation step and gentle in operation condition, and effective components in the preparation prepared by using the preparation method can be rapidly dissolved, and the dissolutionrate can be increased.
Owner:重庆希尔安药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com