Metformin hydrochloride medicinal composition preparation and preparation method thereof
A technology of metformin hydrochloride and metformin, which is applied in the direction of drug combination, pharmaceutical formula, pill delivery, etc., can solve the problems of inability to achieve rapid dissolution, low dissolution rate of preparations, increased labor intensity, etc., and achieve remarkable therapeutic effect, remarkable drug effect, The effect of enhancing the potency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] According to one aspect of the present invention, a kind of preparation method of the pharmaceutical composition of metformin hydrochloride comprises the steps:
[0031] (A) adding metformin to an aqueous binder solution for granulation;
[0032] (B) The stirring speed of granulation is between 200-400rpm, and the granulation time is 90-180s;
[0033] (C) The method of gradient temperature change is used for drying, and the temperature of each gradient is controlled between 20-40°C, and the temperature of the previous gradient and the subsequent gradient tend to alternate back and forth, and the difference between the two adjacent gradients 4-5°C, after drying, add cornstarch and mannitol to pre-mix for 3-5 minutes, then add magnesium stearate and mix for 3-5 minutes, press into tablets, and coat.
[0034] In this preparation method, magnesium stearate is added after drying, which is added separately from other previously added substances. This method of operation can ...
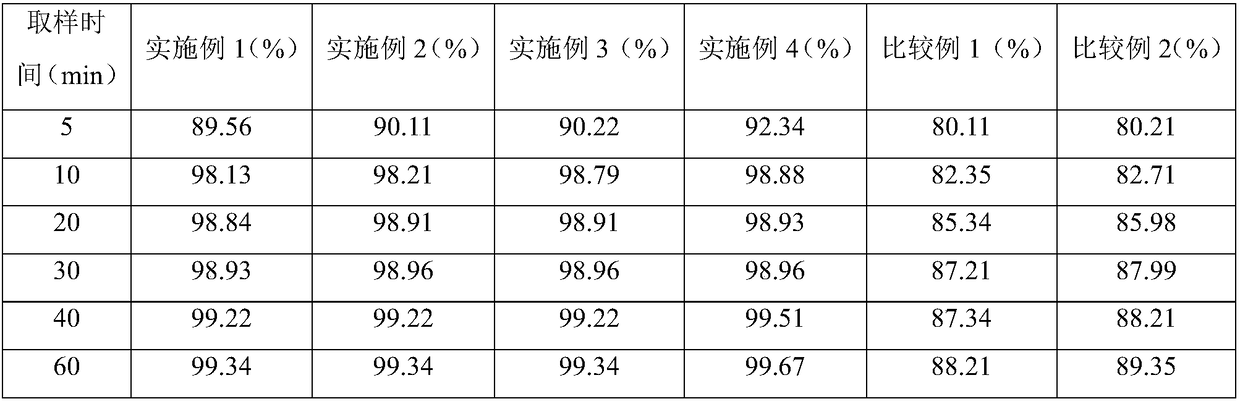
Embodiment 1
[0048] 1) According to the production requirements, the materials such as metformin, mannitol, corn starch and magnesium stearate are weighed;
[0049] 2) Adhesive preparation: Weigh the hypromellose of the adhesive prepared in the prescription amount, add an appropriate amount of boiling water to disperse and dissolve, and cool to room temperature to obtain an aqueous hypromellose solution;
[0050] 3) Mixing and granulation: add metformin to the hypromellose aqueous solution, start stirring and shearing, the stirring rate is 200rpm, and the granulation time is controlled within 90s;
[0051] 4) Pour all the wet granules into the boiling drying granulator, and perform variable temperature drying operation. The variable temperature drying interval is three intervals. The temperature of the first gradient is set at 35°C, the temperature of the second gradient is set at 40°C, and the third gradient The temperature of the gradient is set at 35°C, and after drying to a moisture co...
Embodiment 2
[0056] 1) According to the production requirements, the materials such as metformin, mannitol, corn starch and magnesium stearate were weighed, the D90 index of metformin was controlled at 90 μm, the mesh particle size of corn starch was 60 mesh, and the mesh particle size of mannitol was 80 mesh;
[0057] 2) Adhesive preparation: Weigh the hypromellose of the adhesive prepared in the prescription amount, add an appropriate amount of boiling water to disperse and dissolve, and cool to room temperature to obtain a 5% (w / w) hypromellose aqueous solution;
[0058] 3) Mixing and granulation: add metformin to the hypromellose aqueous solution, start stirring and shearing, the stirring rate is 400rpm, and the granulation time is controlled within 180s;
[0059] 4) Pour all the wet granules into the boiling drying granulator, and carry out variable temperature drying operation. The variable temperature drying interval is four intervals. The temperature of the first gradient is set at ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com