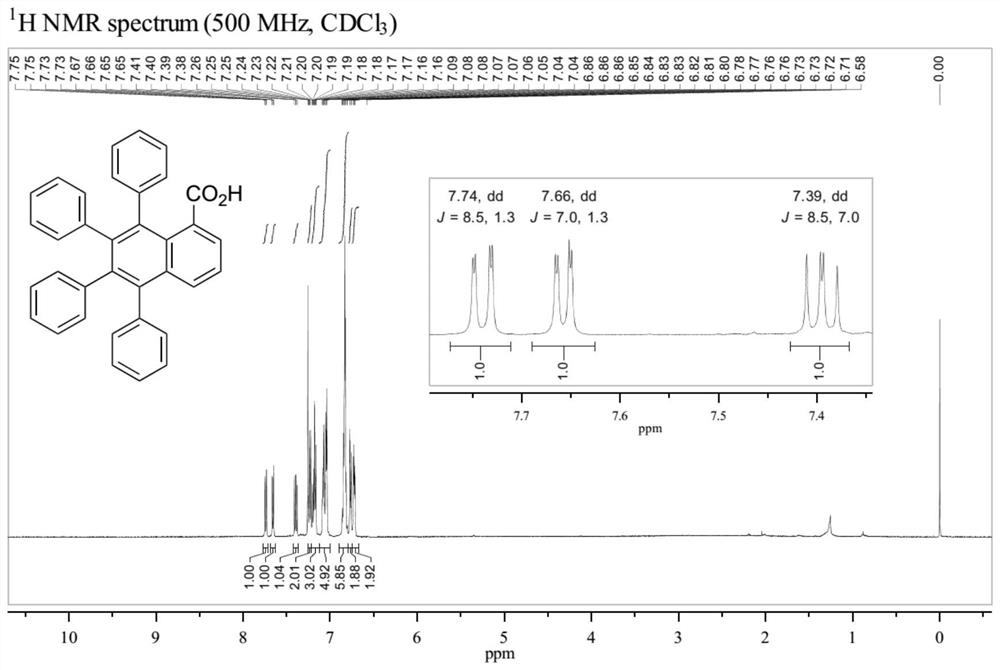
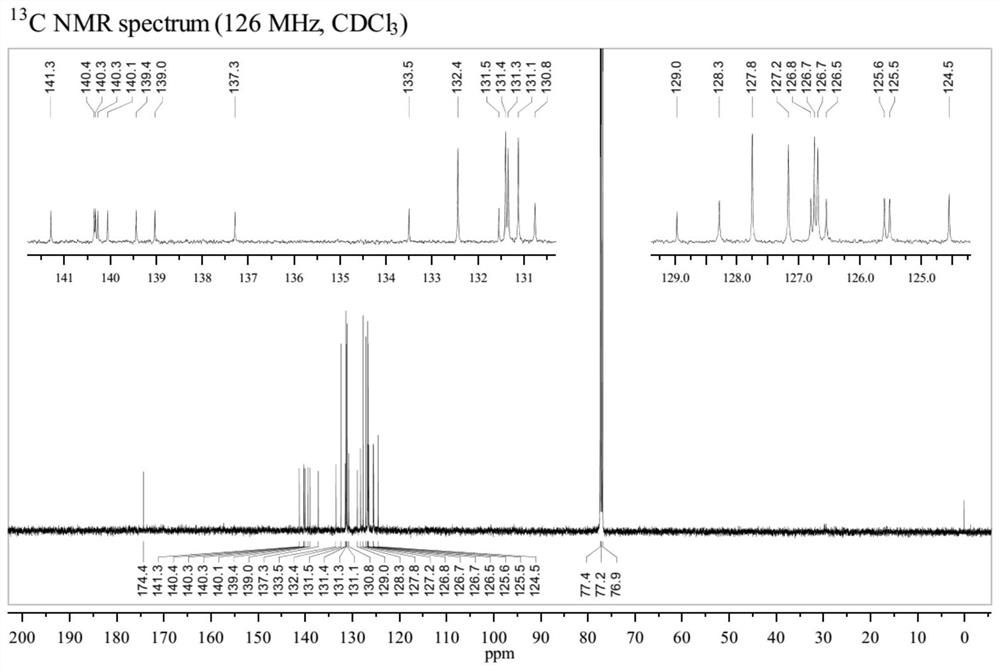
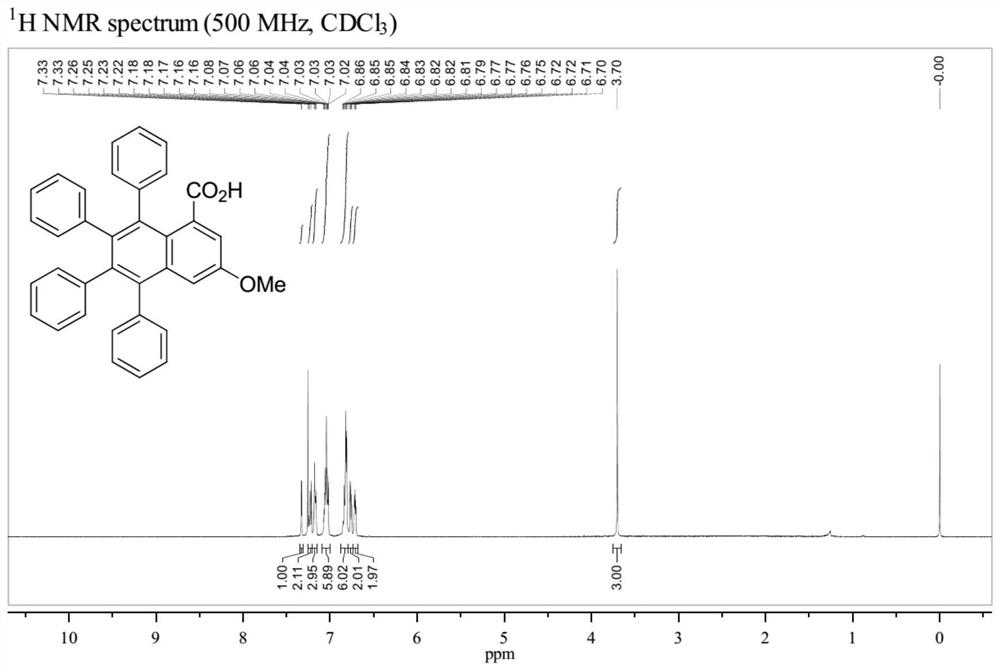
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
41results about How to "React Operational Security" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
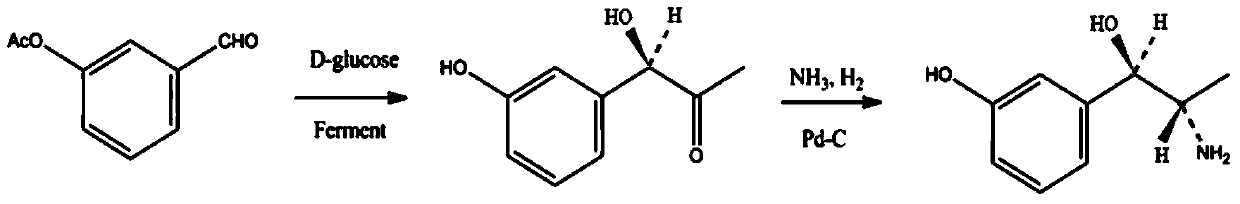
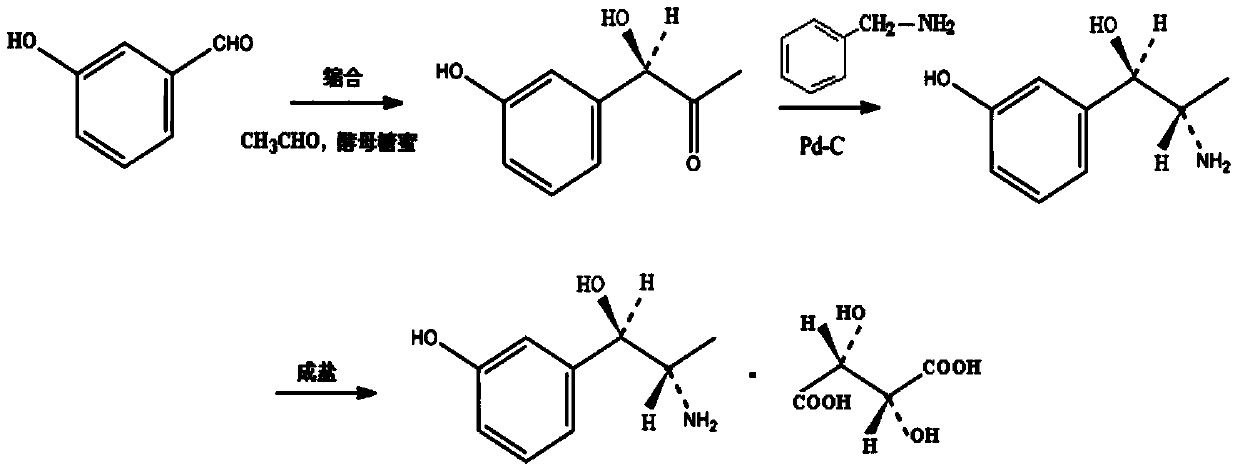
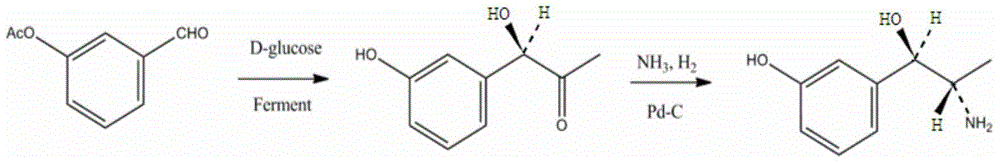
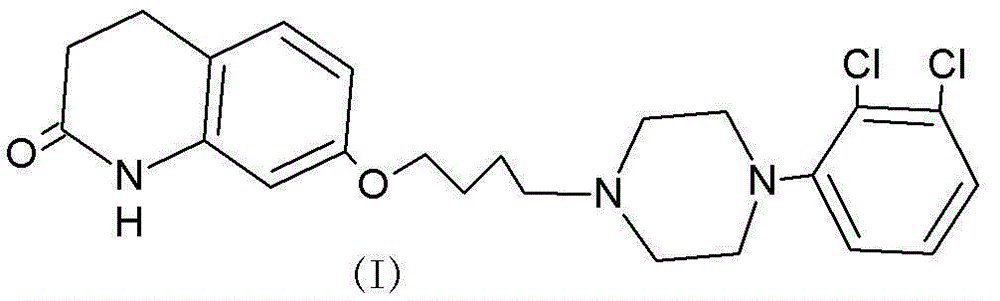
Synthesis method of metaraminol bitartrate
ActiveCN103739504AEasy to controlFew synthetic stepsOrganic compound preparationCarboxylic acid salt preparationSynthesis methodsSpatial configuration
The invention discloses a synthesis method of metaraminol bitartrate, and in particular provides a method for synthesizing metaraminol bitartrate by using a chiral catalysis method. The synthesis method comprises the steps: catalyzing a chiral addition reaction of hydroxybenzaldehyde and nitroethane by using a chiral catalyst system consisting of cinchona alkaloid, copper acetate hydrate and less imidazole to obtain an addition product with a dominant required spatial configuration, and then reducing nitro by using hydrogen in the presence of Pd-C to obtain amine to obtain aramine, and salifying the aramine with L(+)-tartaric acid to obtain a final product metaraminol bitartrate. According to the synthesis method, an enzyme catalyst is prevented from being used, a raw material of the synthesis reaction is easily available, the chiral catalyst is easily purchased or prepared self, the synthesis steps are relatively less, the chiral control efficiency is higher, the enantioselectivity is high, the yield is good, the reaction operation is easily controlled, and is safe and reliable, and the foundation is laid for the later industrialized amplification production.
Owner:广州普星药业有限公司
Synthetic method of pregabalin
InactiveCN104496832AImprove conversion rateShort reaction timeOrganic compound preparationAmino-carboxyl compound preparationPregabalinHydrolysis
The invention discloses a synthetic method of pregabalin. According to the synthetic method, isovaleraldehyde and cyanoacetic alkyl ester are used as starting materials; condensation reaction, Michael addition reaction, hydrolysis reaction, amidation reaction and the like are carried out successively to obtain 3-(carbamoyl methyl)-5-methyl-hexanoic acid; and resolution and Hofmann elimination are carried out to obtain pregabalin. The greatest improvement of the synthetic method is to carry out the hydrolysis reaction and the amidation reaction under the condition of near-critical water. Thus, addition of a catalyst is avoided, and yield of the reaction is raised. In addition, a flow reactor can be applied to the reaction so as to obtain a better reaction effect.
Owner:ZHEJIANG MENOVO PHARMA
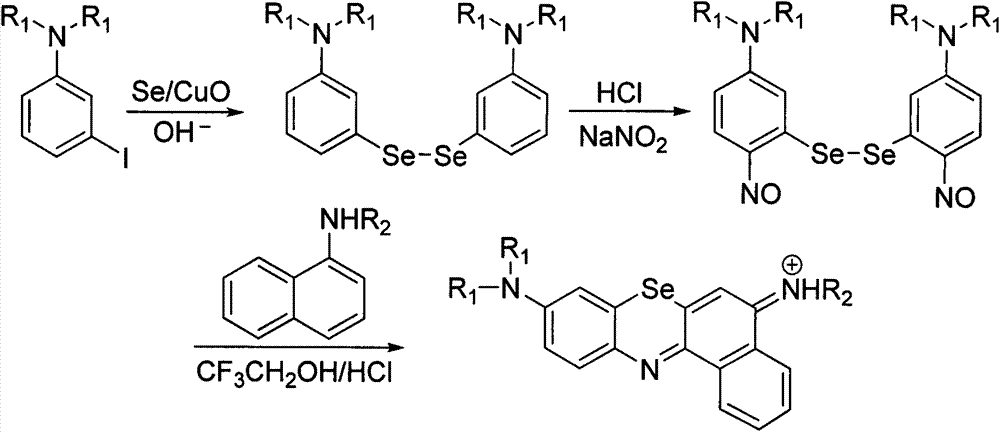
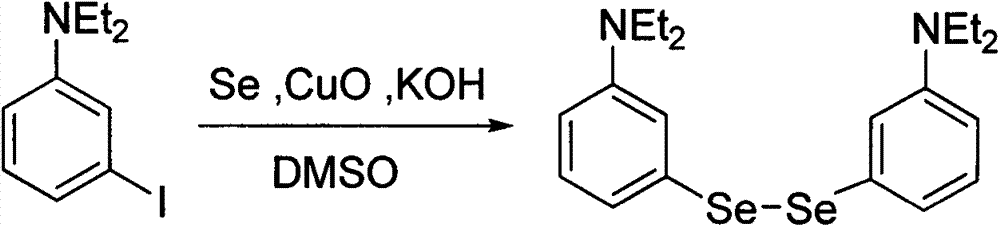
Method for preparing benzo-phenoselenazine photosensitizer
InactiveCN103242260AEnhanced couplingThe reaction is easy to operateOrganic chemistryPtru catalystNitrobenzene
The invention relates to a method for synthesizing 5-(alkylamide-)-9-(N, N-dialkylamide) benzo-phenoselenazine hydrochloride shown in a formula I by using nanometer copper oxide as a catalyst. A benzo-phenoselenazine derivant, namely the 5-(alkylamide-)-9-(N, N-dialkylamide) benzo-phenoselenazine hydrochloride is an excellent photosensitizer for a photodynamic therapy, has the absorption wavelength above 650 nanometers, good water solubility, larger absorption intensity, high tumor cell selectivity and short metabolic time and is easy for synthesis and structure modification, thereby being an ideal novel photosensitizer. The method comprises the following steps of: with 3-iodo-N,N-dialkylaniline and selenium powder as raw materials, preparing di-(3-N,N-dialkylaniline) diselenide shown in a formula II under the catalysis of copper oxide, carrying out a nitrosation reaction on the bi-(3-N,N-dialkylaniline) diselenide and sodium nitrite to generate a bi-(3-N, N-dialkylamide-6-nitrosobenzene) diselenide, and finally carrying out a cyclization reaction on the bi-(3-N,N-dialkylamide-6-nitrosobenzene) diselenide and N-alkyl-1-naphthylamine to prepare the benzo-phenoselenazine derivant shown in the formula I. The method for synthesizing the benzo-phenoselenazine derivant is mild in reaction conditions, simple to operate and high in yield; and the raw materials are available.
Owner:CENT SOUTH UNIV
Preparation process of polyhydroxy isoflavone
ActiveCN103087027AHarm reductionAchieve room temperature reactionOrganic chemistryCyanideBenzyl cyanide
The invention relates to a preparation method of polyhydroxy isoflavone. The polyhydroxy isoflavone comprises 4',6,7-trihydroxy isoflavone and 3',4',6,7-tetrahydroxy isoflavone. According to the invention, the large-scale preparation of 4',6,7-trihydroxy isoflavone and 3',4',6,7-tetrahydroxy isoflavone can be realized by treating cheap and easily-acquired chemical raw materials including 3,4-dimethoxy phenol, 3,4-dimethoxy benzyl cyanide and hydroxybenzyl cyanide as starting materials through optimal research on Hoesch reaction, carburization n-cyclohexylmaleimide reaction and demethylation protection. The preparation method of polyhydroxy isoflavone disclosed by the invention is economic, efficient, environment-friendly, safe and easy to industrialize. The 4',6,7-trihydroxy isoflavone and the 3',4',6,7-tetrahydroxy isoflavone can be applied to the research and the development of new medicines in the aspects of medicines, food hygiene and the like.
Owner:中国人民解放军第三军医大学军事预防医学院
Method for preparing cyclic carbonate from olefin and carbon dioxide by electrochemical method
InactiveCN102877086AOvercoming the problem of anode consumabilitySolve bottlenecksElectrolysis componentsOrganic chemistryElectrochemical responsePtru catalyst
The invention discloses a method for preparing cyclic carbonate from olefin and carbon dioxide by an electrochemical method. By the method, the olefin and the carbon dioxide are used as raw materials, and an inert anode is used for converting the olefin and the carbon dioxide into the cyclic carbonate at a time through an electrochemical reaction at normal temperature and normal pressure in a diaphragm-free single chamber electrolytic tank. According to the method, the olefin and the carbon dioxide which are low in price and readily available are used as the raw materials, so that an oxidant and a catalyst are not required to be added additionally; a sacrificial metal anode is replaced by the inert anode, so that the problem of consumption performance of metal anodes is solved radically; and the method is mild in reaction condition, simple in process operation and low in production cost, has the characteristics of high selectivity, high yield, high efficiency, high economy and environment friendliness, is suitable for appreciation processing of olefin products in petrochemical industries and conversion utilization of the carbon dioxide serving as waste gas generated in petrifaction oil refineries, thermal power plants and other industries, and has economic and environment-friendly benefits.
Owner:SOUTH CHINA UNIV OF TECH
Preparation method of diphosphite
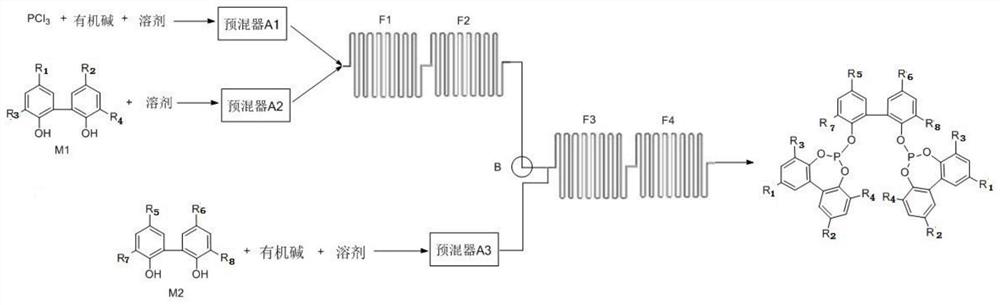
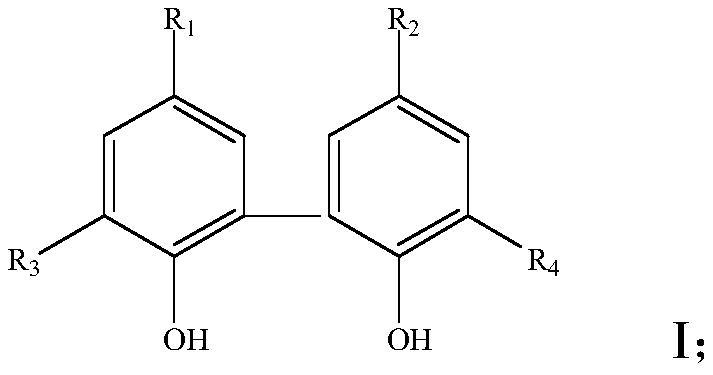
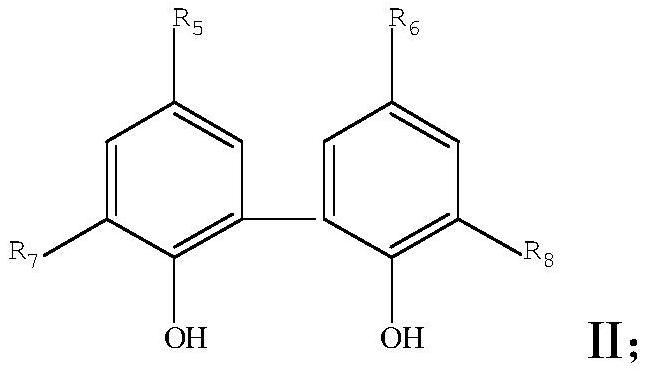
ActiveCN109369722APrecise ratioAccurate timeGroup 5/15 element organic compoundsHazardous substancePhysical chemistry
The invention discloses a preparation method of diphosphate. The method is characterized by mixing reaction raw materials based on agent dosage, and performing continuous flow micro-reaction to obtainthe diphosphate. According to the preparation method, the reaction process can be accurately controlled, and intermediate separation and exposing can be avoided, so that the risk of harmful substances to environmental protection and health safety can be avoided; in addition, the mass transferring and heat transferring are quick in the reaction process; little byproducts are produced; the reactionyield, the reaction purity and the reaction efficiency are extremely increased.
Owner:CHINA NAT OFFSHORE OIL CORP +3
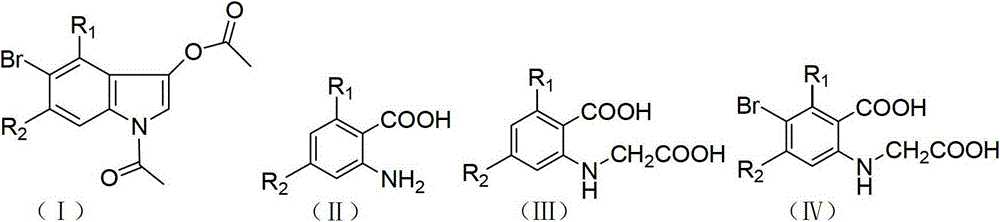
Method for synthesizing 1-acetyl-halo-indolyl-3-acetate
InactiveCN103145605AHigh yieldThe reaction is easy to operateOrganic chemistryStrong acidsP-Toluenesulfonic acid
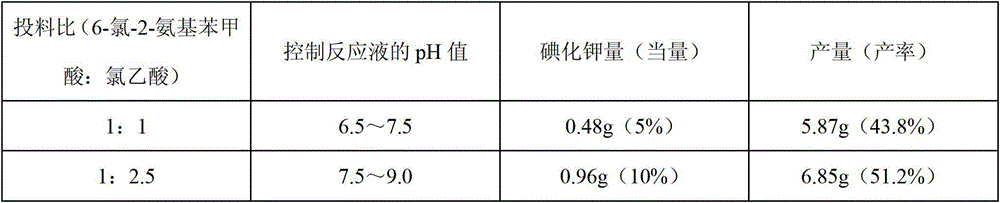
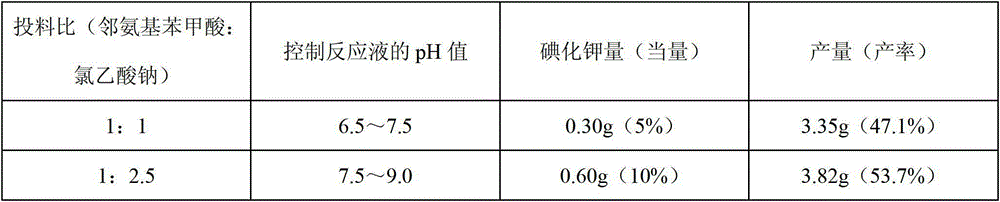
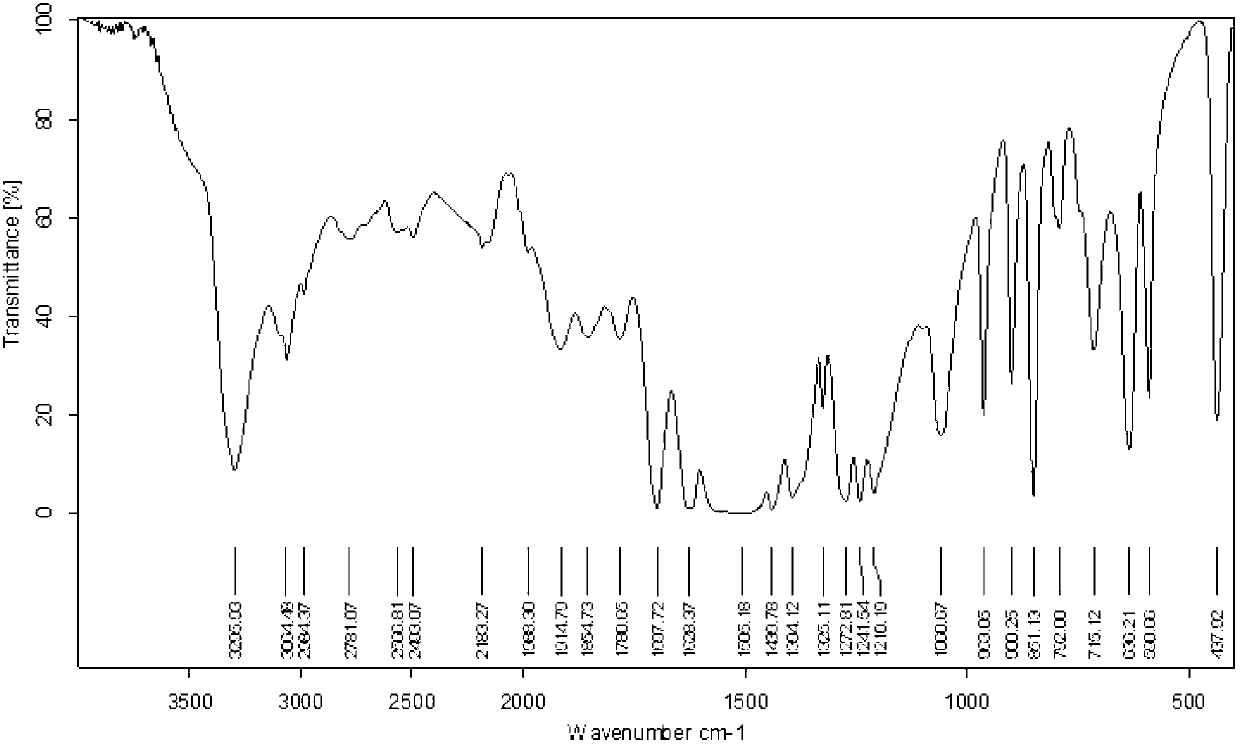
The invention discloses a method for synthesizing 1-acetyl-halo-indolyl-3-acetate. According to the method, ortho-aminobenzoic acid with a general formula (II) and a chloride of ortho-aminobenzoic acid which serve as starting raw materials react with ClCH2COOH, NaOH, Na2CO3 and a catalyst KI (or NaI or the like) to produce N-(2-carboxyl)phenylglycine with a general formula (III) and a chloride of N-(2-carboxyl)phenylglycine, N-(2-carboxyl)phenylglycine bromine of a general formula (IV) and a chloride of N-(2-carboxyl)phenylglycine bromine are produced in a manner that N-(2-carboxyl)phenylglycine and the chloride of N-(2-carboxyl)phenylglycine react with N-bromosuccinimide (NBS), CH3OH, H2O and a catalyst, namely ammonium nitrate (or strong acids and weak-base salts, such as ammonium chloride, ammonium sulfate, hydrochloric acid, p-toluenesulfonic acid (p-TSA) and sulfuric acid, or protonic acid), and N-(2-carboxyl)phenylglycine bromine and the chloride of N-(2-carboxyl)phenylglycine bromine react with Ac2O and anhydrous AcNa to produce 1-acetyl-halo-indolyl-3-acetate. The method has the advantages of being safer, more environment-friendly, more time-saving, more convenient in operation, and the like.
Owner:GUANGDONG INST OF MICROORGANISM +1
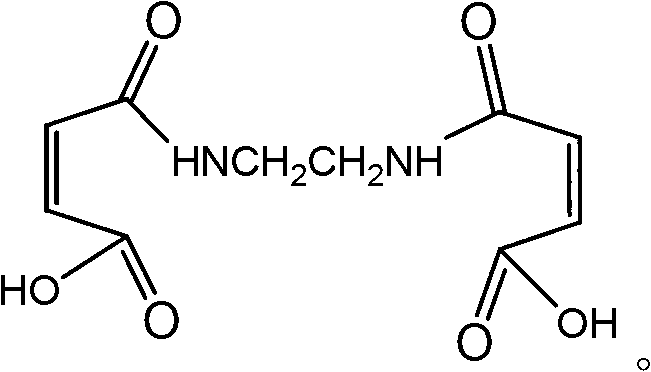
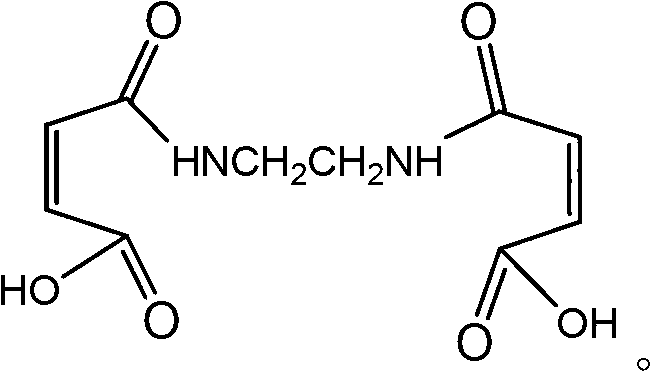
Ethylenedimaleamic acid and preparation method thereof
InactiveCN102757358AThe reaction is easy to operateReact Operational SecurityOrganic compound preparationCarboxylic acid amides preparationEthylenediamineFiltration
The invention discloses an ethylenedimaleamic acid and a preparation method thereof. The structural formula of the ethylenedimaleamic acid is disclosed in the specification. The preparation method comprises the following steps: adding A mol of maleic anhydride and B ml of tetrahydrofuran into a dry three-neck flask, dropwisely adding C mol of ethylenediamine while stirring, reacting while stirring at room temperature for two hours to generate white powder, carrying out vacuum filtration, and drying to obtain the ethylenedimaleamic acid, wherein A:B:C=(2-2.8):10:1. The method disclosed by the invention has the advantages of simple and safe reaction operation, simple after-treatment and high yield; and the prepared ethylenedimaleamic acid as a bran-new amide compound can be used as an organic chemical intermediate and a water treatment material.
Owner:SHAANXI UNIV OF SCI & TECH
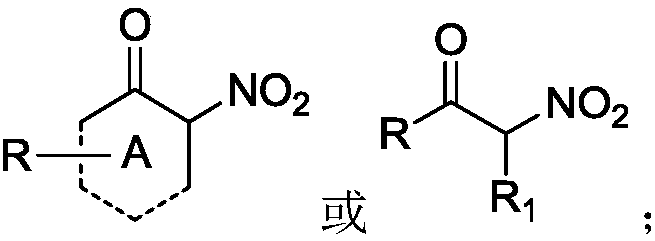
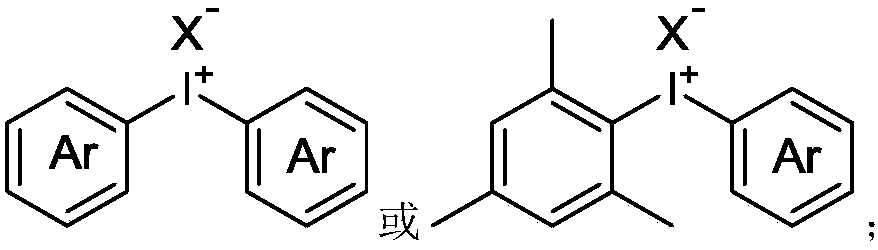
Synthesis method of alpha-nitro-alpha-aryl ketone compound
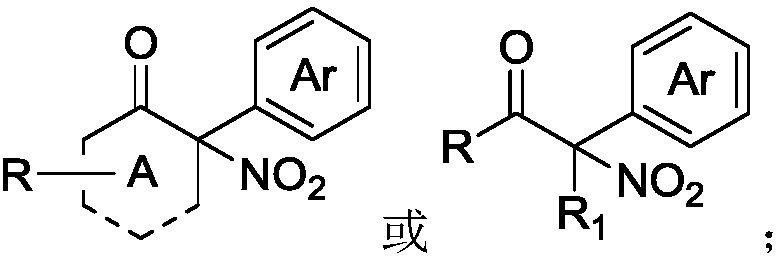
InactiveCN109053455ASynthesize efficientlyAchieve synthesisOrganic chemistryOrganic compound preparationSynthesis methodsKetone
The invention discloses a synthesis method of an alpha-nitro-alpha-aryl ketone compound. The method comprises the following steps: under the effect of alkali, enabling alpha-nitro ketone compound anddiaryliodonium salt to react, and obtaining the alpha-nitro-alpha-aryl ketone compound. The structural formula of the alpha-nitro ketone compound is as follows: (shown in the description) The structural formula of the diaryliodonium salt is as follows: (shown in the description). The structural formula of the alpha-nitro-alpha-aryl ketone compound is as follows: (shown in the description), whereinA is 5-ring to 12-ring, R is alkyl or aryl, R1 is alkyl or aryl, Ar is aryl, and X- is anions. On the premise of no transitional metal catalytic condition, the synthesis of the alpha-nitro-alpha-arylketone compound can be economically and efficiently realized, and the reaction operation is simple and safe.
Owner:LANZHOU UNIVERSITY
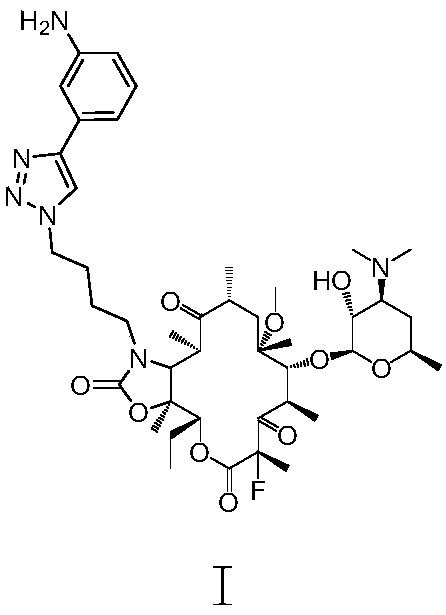
Preparation method of solithromycin
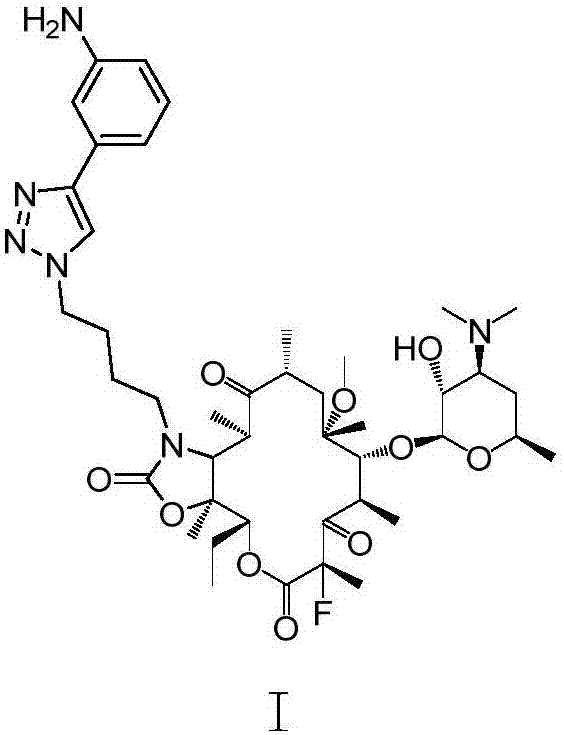
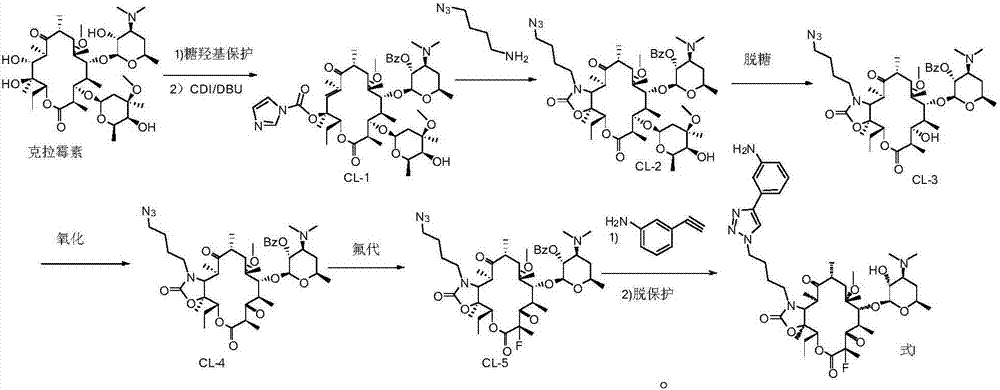
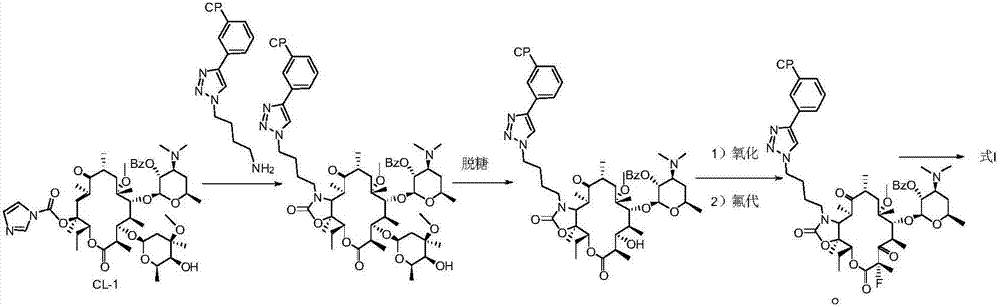
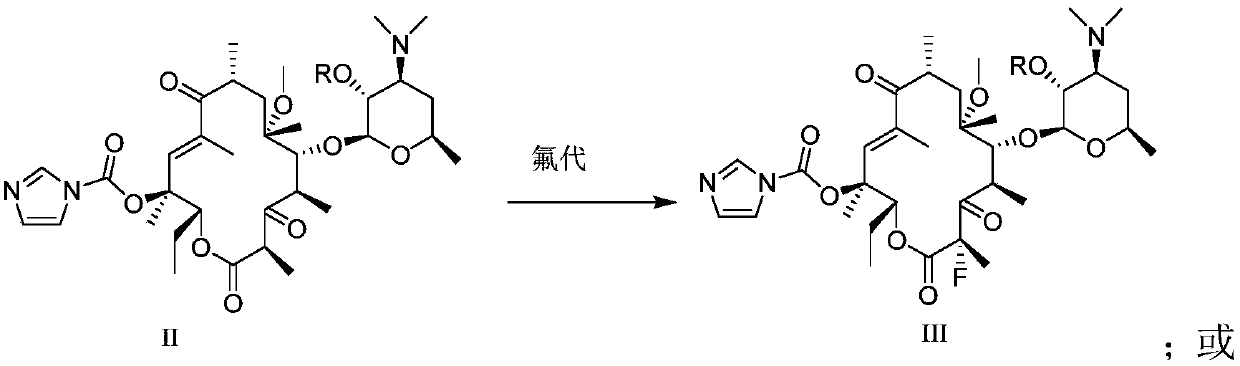
InactiveCN107216361AImprove conversion rateReact Operational SecuritySugar derivativesSugar derivatives preparationSolubilityButt joint
The invention provides a preparation method of solithromycin. The method comprises the following steps: performing fluorination reaction on a compound II to generate a compound III; forming a butt joint on the compound III and 4-azidobutylamine to form an oxazole ring compound VI; and performing ring-closing reaction on the compound VI and 3-aminophenylacetylene to obtain the solithromycin I. The method abandons the explosive and hazardous azido intermediate oxidization in the prior art, so the reaction operation is safer. Especially, it is pleasantly surprising that in the process of preparing oxazole-ring and five-membered ring triazoles, since the reactant solubility is higher, the reactant conversion rate is high, and the side reactions are fewer; and thus, the method lowers the production cost, is beneficial to environmental protection and suitable for industrial production, and has great application value.
Owner:ZHEJIANG JINGXIN PHARMA +1
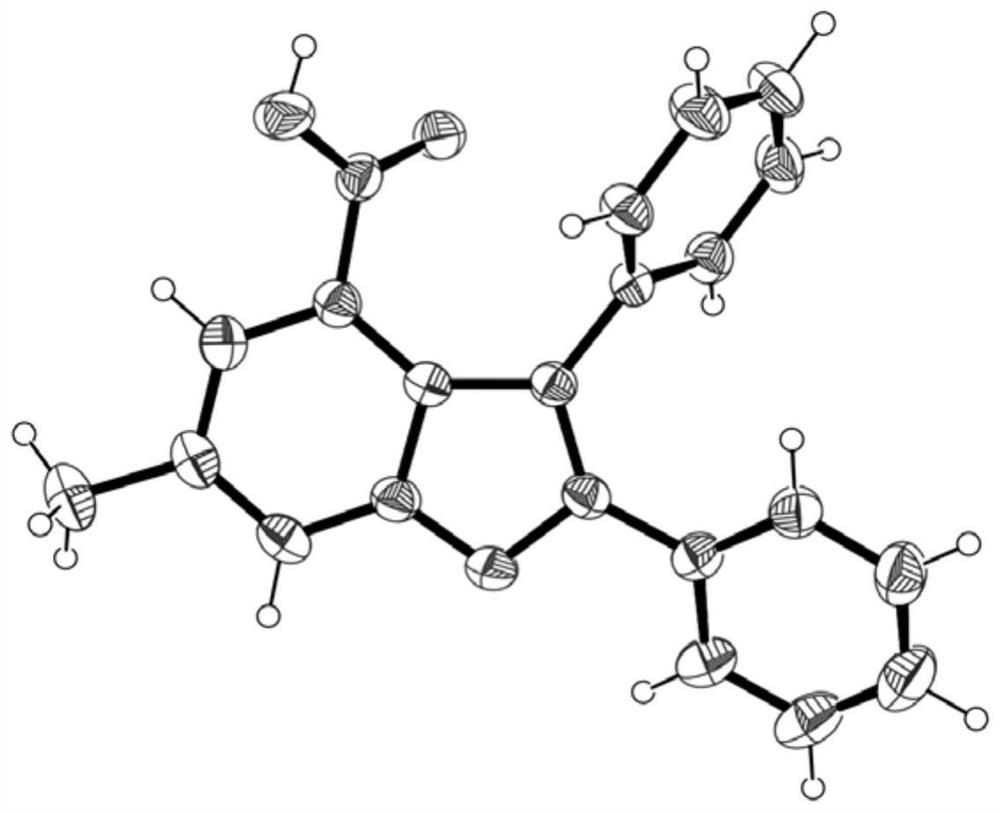
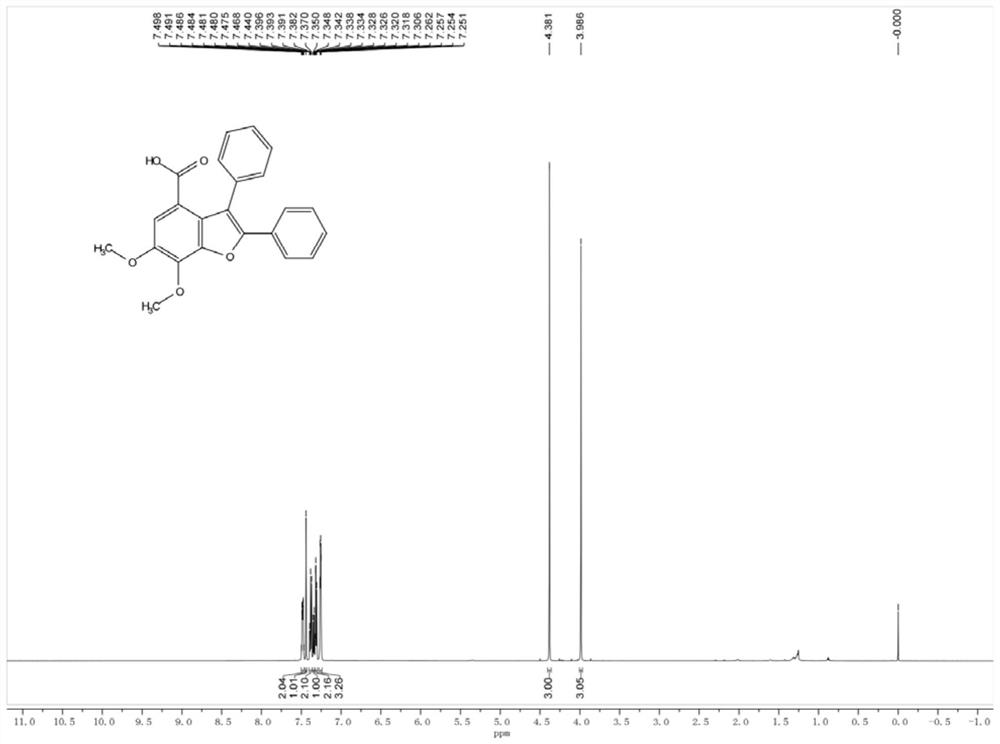
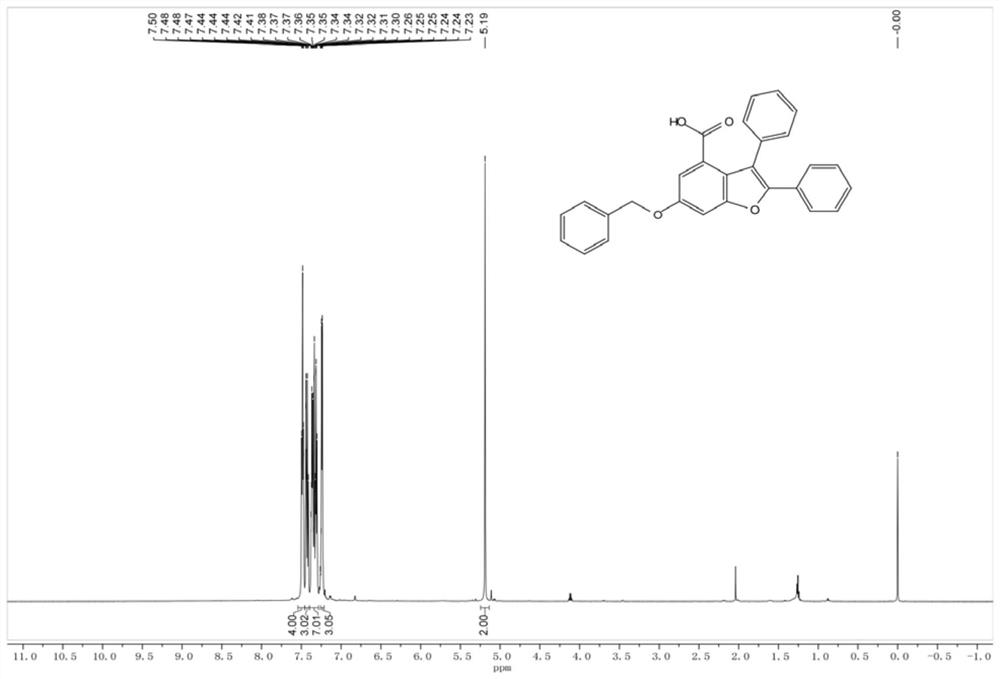
Method for preparing polysubstituted benzofuran-4-formic acid compound through ruthenium catalysis
The invention belongs to the technical field of organic synthesis, and discloses a method for preparing a polysubstituted benzofuran-4-formic acid compound through ruthenium catalysis. An m-hydroxybenzoic acid compound, a diaryl alkyne compound, a ruthenium catalyst, an additive and a solvent are subjected to a heating reaction in an air or oxygen environment, and a reaction product is separated and purified, so that the polysubstituted benzofuran-4-formic acid compound is obtained. The ruthenium catalytic system used in the method has high catalytic activity, so that air or oxygen can be used as a green oxidant in the reaction, and toxic, dangerous or expensive oxidants are prevented from being used. The reaction raw materials are easy to obtain, the solvent amount is small, the solvent is insensitive to water, operation is easy, convenient and safe, the method can be well amplified to the gram-level scale, and industrialization is convenient to achieve. The reaction is taken as a key step, the natural product diptoindonesin G with biological activity can be conveniently synthesized, and the diptoindonesinG has a good application prospect.
Owner:GUANGZHOU UNIVERSITY
Method for preparing solithromycin
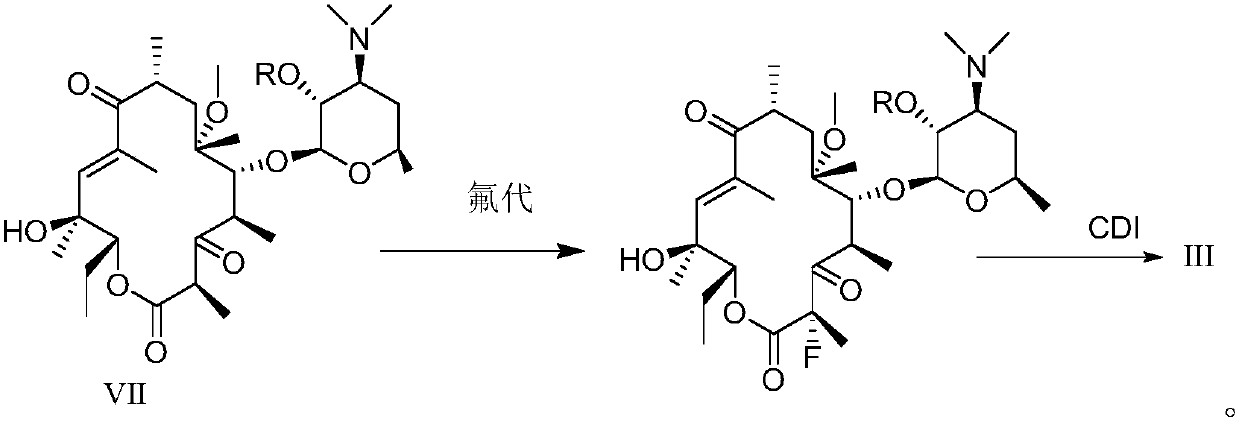
InactiveCN107216360AImprove conversion rateReact Operational SecuritySugar derivativesSugar derivatives preparationSolubilitySide chain
The invention provided a method for preparing solithromycin. The method comprises the following steps: a compound II is subjected to a fluorination reaction, so that a compound III is produced, and the compound III docks with an amino group in a side chain of a compound IV five-member ring triazole (1-(4-aminobutyl)-4-(3-protected aminophenyl)-1H-1,2,3-triazole), so that a compound V containing an oxazole ring is formed; and the compound V is subjected to corresponding de-protection or reduction to form an amino group, so that solithromycin I is obtained. The method for preparing the solithromycin gets rid of easy-explosion dangerous oxidation and fluorination reactions of azido intermediates in the prior art, and reaction operation is more safe; and it is surprised that when the oxazole ring triazole and the five-member ring triazole are prepared, solubility of reactants is high, so that the conversion rate of the reactants is high, side reactions are less, and the production cost is reduced. The method facilitates environmental protection, is suitable for industrialized production, and has a large application value.
Owner:ZHEJIANG JINGXIN PHARMA +1
Method for efficiently synthesizing alpha, beta-unsaturated aldehyde without synthesis gas
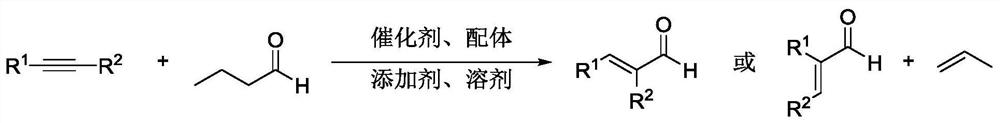
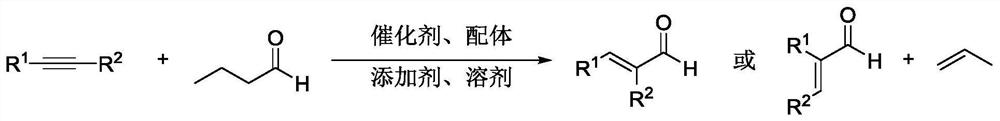
ActiveCN111825537AReduce usageEasy to operateOrganic compound preparationOrganic chemistry methodsPtru catalystFormylation reaction
The invention relates to a method for efficiently synthesizing alpha, beta-unsaturated aldehyde. A series of alkynes can be efficiently and rapidly converted into alpha, beta-unsaturated aldehydes through a hydroformyl transfer reaction between simple alkyl aldehydes and alkynes by using cheap and easily available n-butyraldehyde as a donor of hydroformyl. Compared with a traditional hydroformylation reaction system, the method does not need to use flammable and combustible synthesis gas, does not need to use a special device to contain the synthesis gas, adopts commercially available catalysts and ligands, is simple and convenient to operate, mild in condition and low in cost, and thoroughly inhibits side reaction products such as saturated aldehyde and alkyne hydrogenation products; andthe compound has specific chemoselectivity and excellent regioselectivity and stereoselectivity, and has huge application potential.
Owner:SICHUAN UNIV
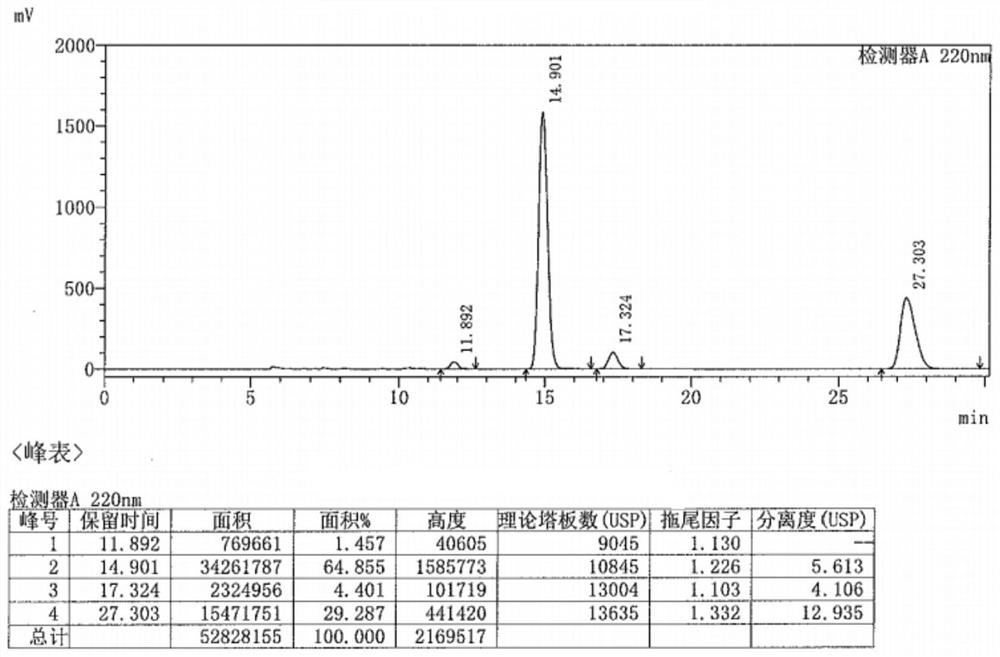
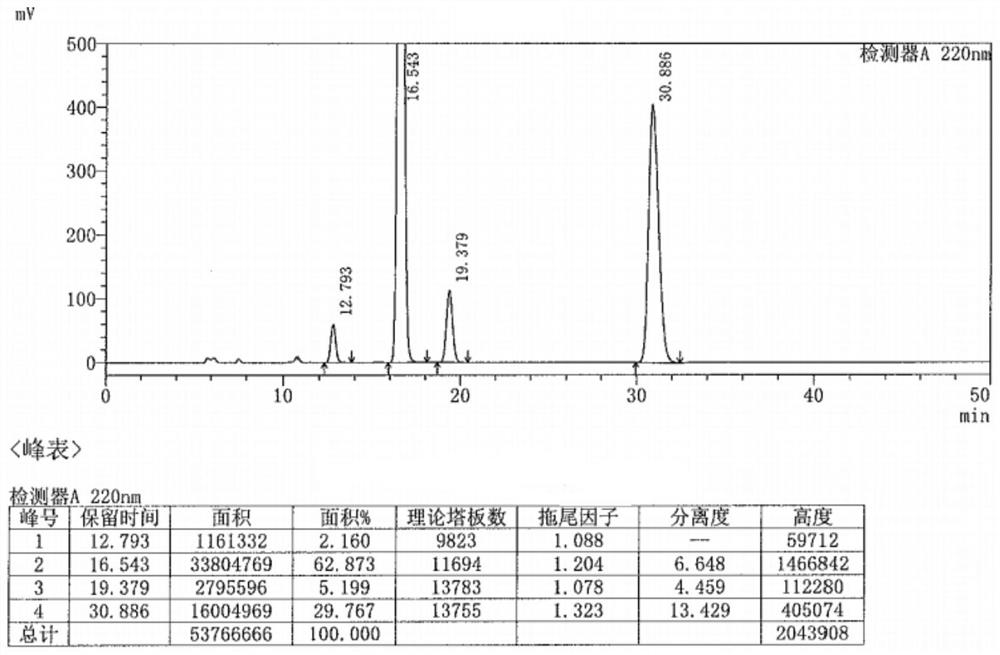
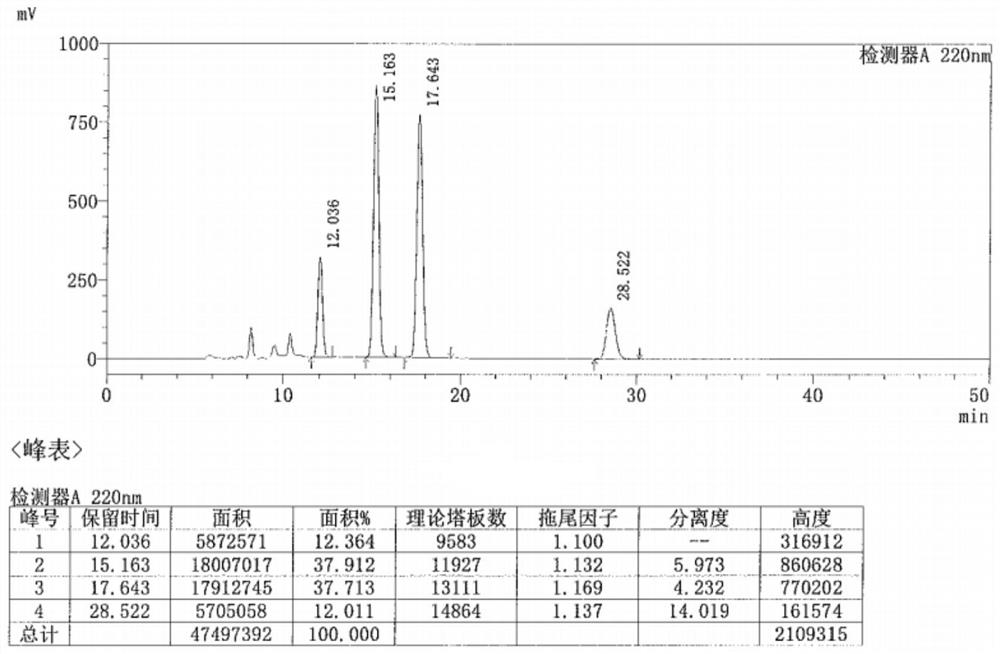
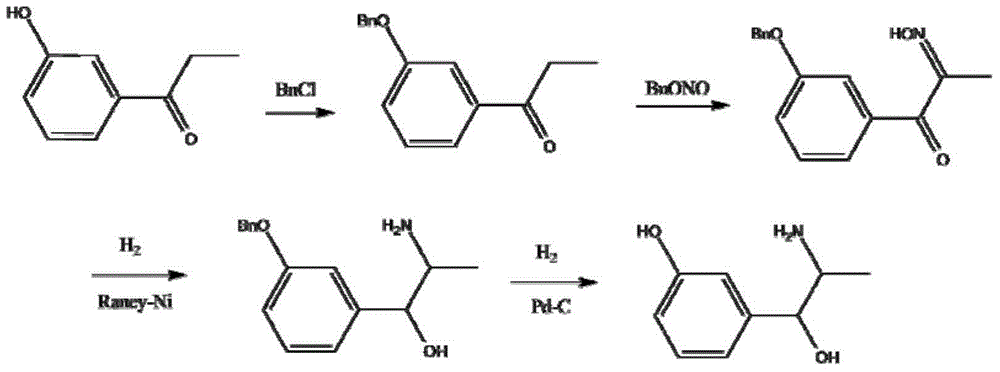
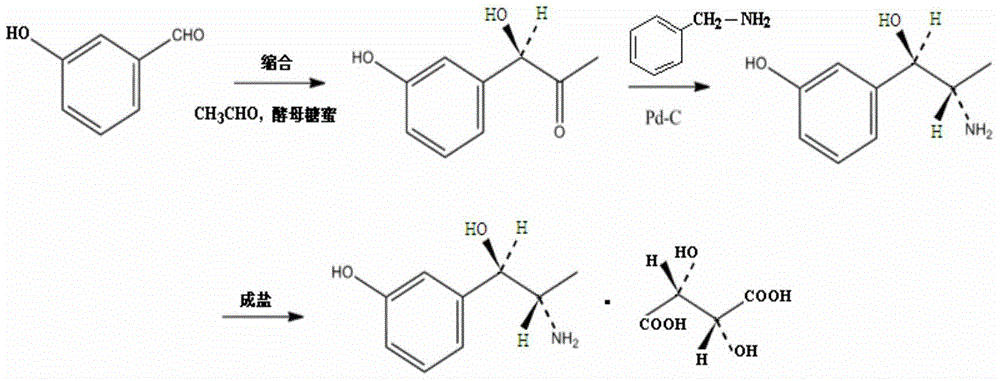
Preparation method of metaraminol bitartrate
ActiveCN114835592AReduce usageLow costOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHydroxylaminePtru catalyst
The invention belongs to the technical field of drug synthesis, and discloses a preparation method of metaraminol bitartrate, which comprises the following steps: under an alkaline condition, a compound V and a compound IV are subjected to a Henry reaction under the action of a metal catalyst and a chiral ligand to obtain a compound III, the compound III is subjected to catalytic hydrogenation to obtain a compound II, and the compound II is subjected to recrystallization to obtain metaraminol bitartrate. And salifying the compound II and L-tartaric acid to obtain metaraminol bitartrate I, wherein the chiral ligands are a compound VI and a compound VII. The method can effectively control the three-dimensional configuration in the production process, can obviously improve the product quality, and is simple in production process, high in yield, low in cost and suitable for industrial production.
Owner:BEIJING XINLINGXIAN MEDICAL TECH DEV CO LTD
A kind of synthetic method of metaraminol tartrate
ActiveCN103739504BEasy to controlFew synthetic stepsOrganic compound preparationCarboxylic acid salt preparationHydroxylamineSynthesis methods
The invention discloses a synthesis method of metaraminol bitartrate, and in particular provides a method for synthesizing metaraminol bitartrate by using a chiral catalysis method. The synthesis method comprises the steps: catalyzing a chiral addition reaction of hydroxybenzaldehyde and nitroethane by using a chiral catalyst system consisting of cinchona alkaloid, copper acetate hydrate and less imidazole to obtain an addition product with a dominant required spatial configuration, and then reducing nitro by using hydrogen in the presence of Pd-C to obtain amine to obtain aramine, and salifying the aramine with L(+)-tartaric acid to obtain a final product metaraminol bitartrate. According to the synthesis method, an enzyme catalyst is prevented from being used, a raw material of the synthesis reaction is easily available, the chiral catalyst is easily purchased or prepared self, the synthesis steps are relatively less, the chiral control efficiency is higher, the enantioselectivity is high, the yield is good, the reaction operation is easily controlled, and is safe and reliable, and the foundation is laid for the later industrialized amplification production.
Owner:广州普星药业有限公司
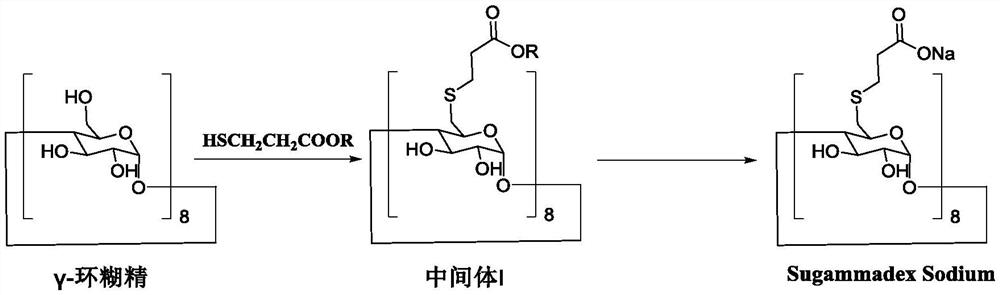
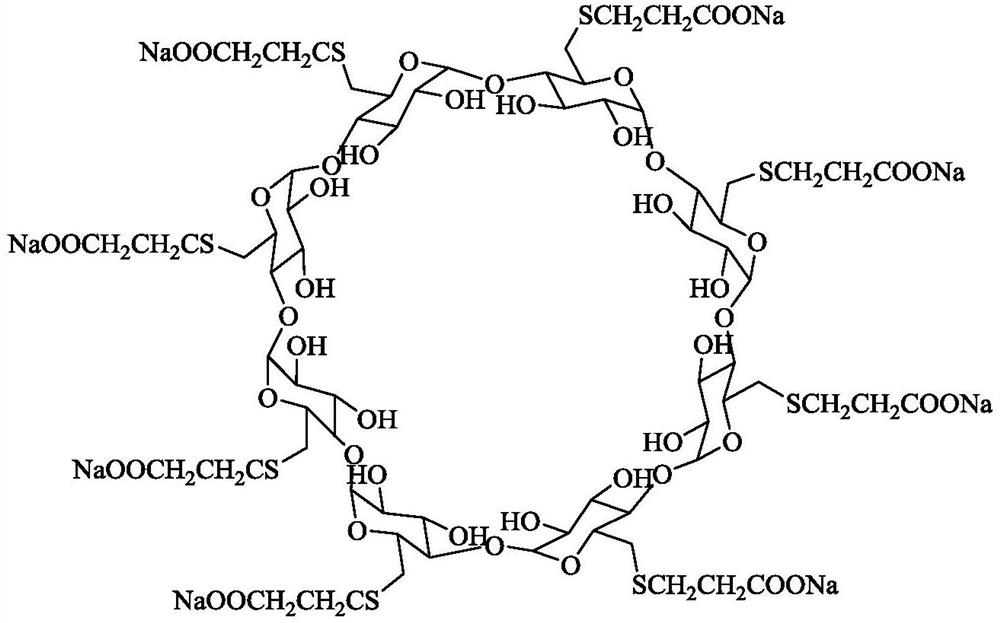
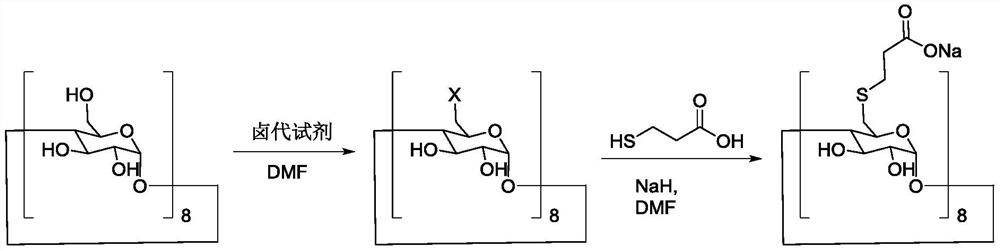
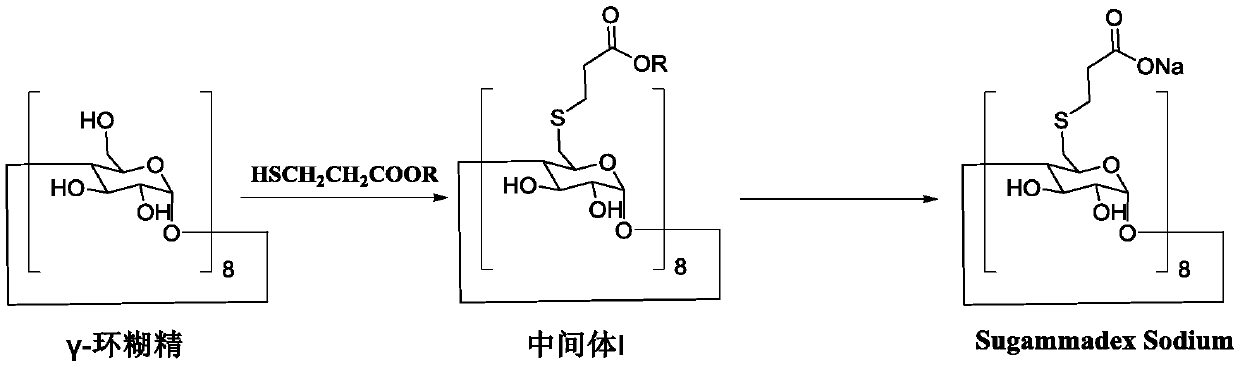
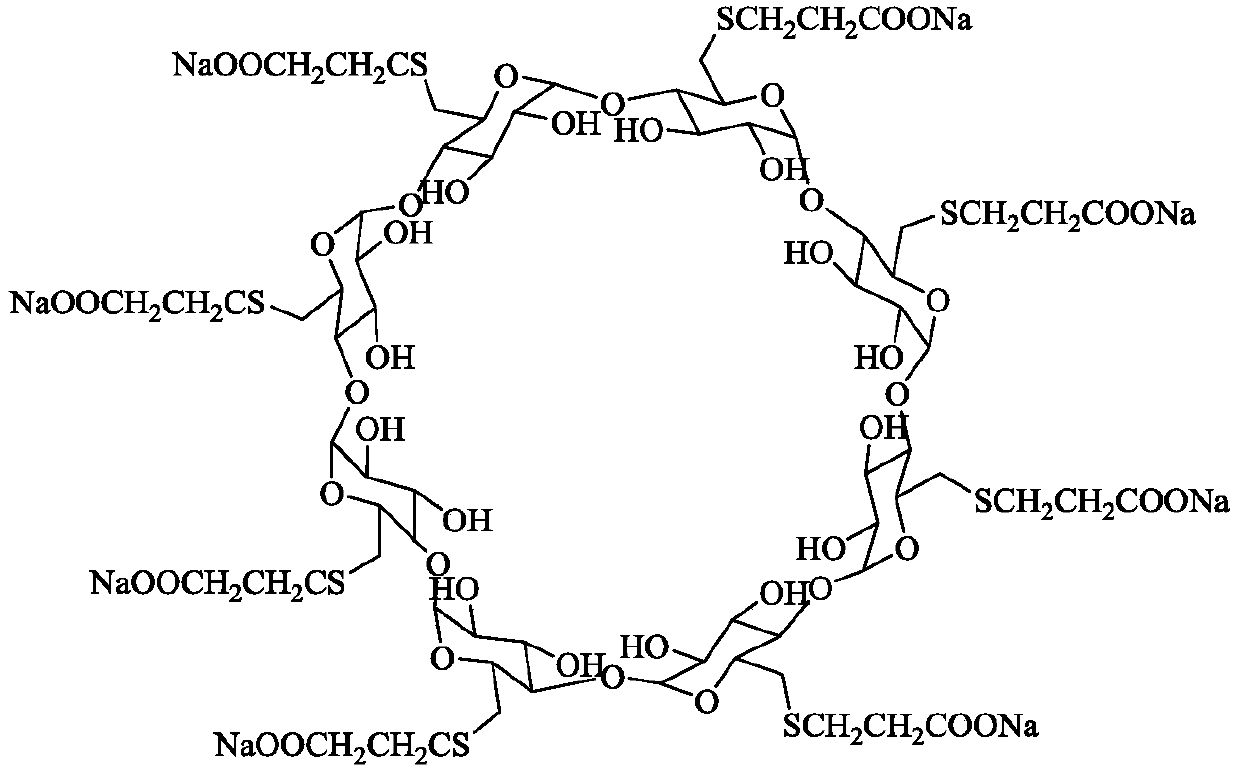
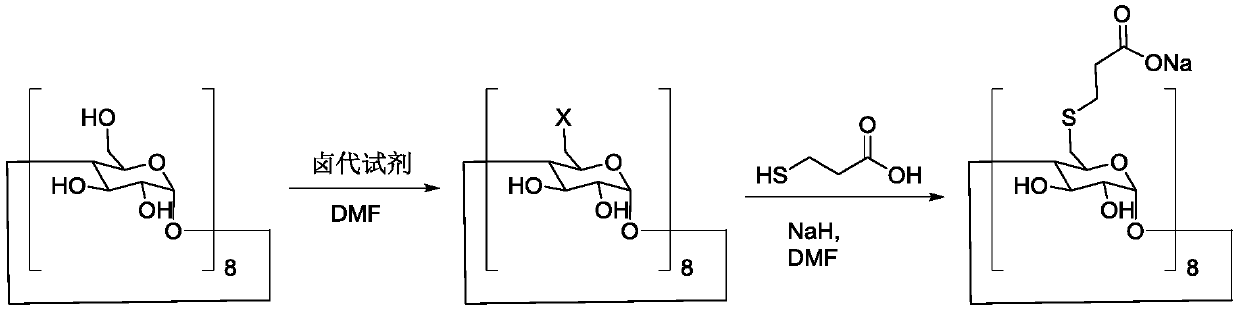
Preparation method of sugammadex sodium
The invention discloses a preparation method of sugammadex sodium. The method comprises the following steps: taking gamma-cyclodextrin as a raw material, reacting gamma-cyclodextrin with 3-mercaptopropionic acid substituted ester under the action of a phosphine reagent and an azo reagent to generate sugammadex ester; and hydrolyzing the sugammadex ester and an alkaline reagent in a solvent at a certain temperature to obtain the target product sugammadex sodium. The preparation method of sugammadex sodium is mild in reaction, a gamma-cyclodextrin perhalogenation reaction process is omitted, theoperation is simple, convenient and time-saving, the yield of the obtained target product is high, and the method is suitable for industrial scale-up production.
Owner:LUNAN PHARMA GROUP CORPORATION
Process for continuously and industrially producing terephthalaldehyde by using micro-channel reactor
InactiveCN111704534AHigh yieldPrecise control of the reaction processChemical/physical/physico-chemical microreactorsCarbonyl compound preparation by hydrolysisHexamethylenetetramineProcess engineering
The invention relates to the technical field of terephthalaldehyde preparation, and specifically relates to a process for continuously and industrially producing terephthalaldehyde by using a micro-channel reactor. The micro-channel reactor comprises a plurality of groups of reaction chips, which are communicated in sequence; each reaction chip comprises an outer panel I, a reaction channel I, a reaction channel II, a reaction channel III and an outer panel II, which are laminated at a time; and the process comprises the following steps: crushing, dosing, synthesis reaction, cooling-crystallization, filter-pressing I, sodium carbonate solution washing, filter-pressing II, secondary water washing, filter-pressing, centrifugal separation, drying, packaging, and storage. The yield of terephthalaldehyde produced by an urotropine method is greatly increased, and due to continuous flow production and the high reaction rate, the production efficiency is also greatly improved.
Owner:杭州迈科瑞科技有限公司
Preparation method of 5a-androstane-2-ene-17-ketone
ActiveCN112375120AThe synthesis steps are simpleReaction operation is green and environmentally friendlyOrganic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationPtru catalystHeteropoly acid
The invention discloses a preparation method of 5a-androstane-2-ene-17-ketone. The method comprises the following two steps: (1) a supported heteropolyacid catalyst is prepared by adopting an impregnation method; and (2) epiandrosterone is refluxed in an organic solvent under the catalysis of the supported heteropoly acid catalyst to remove 3-hydroxyl to obtain 5a-androstane-2-ene-17-ketone and anisomer thereof, and a pure product of 5a-androstane-2-ene-17-ketone is obtained by recrystallization with ethanol. The yield can reach 90% or above, the HPLC is 98% or above, and green and environment-friendly industrial production can be achieved.
Owner:湖南省湘中制药有限公司
A kind of preparation method of bisphosphite
ActiveCN109369722BPrecise ratioAccurate timeGroup 5/15 element organic compoundsPhosphoric Acid EstersHazardous substance
The invention discloses a method for preparing bisphosphite. The preparation method is prepared by mixing reaction raw materials according to reagent amounts and adopting continuous flow micro-reaction. The reaction process of the preparation method can be precisely controlled without intermediate separation and exposure, avoiding the environmental protection and health and safety caused by harmful substances; at the same time, the reaction process of the preparation method has fast mass transfer and heat transfer speed and few by-products , the reaction yield, product purity and reaction efficiency are greatly improved.
Owner:CHINA NAT OFFSHORE OIL CORP +3
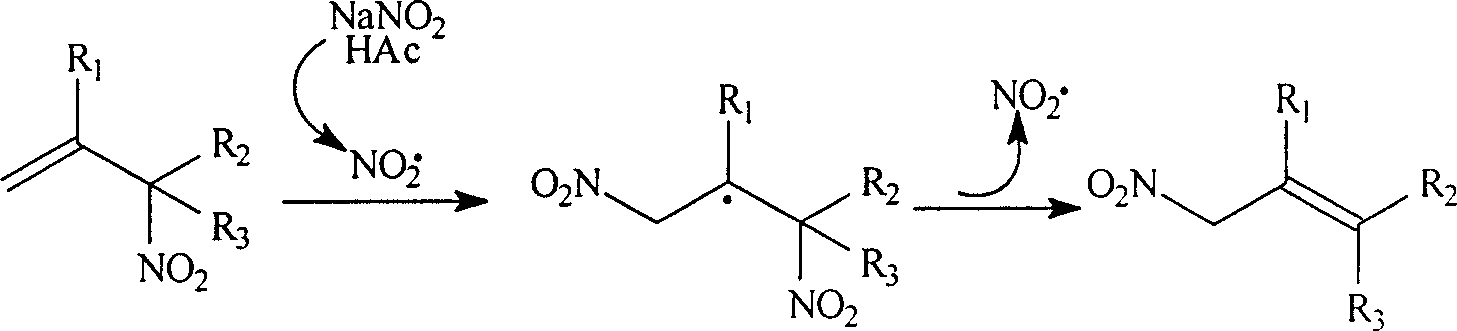
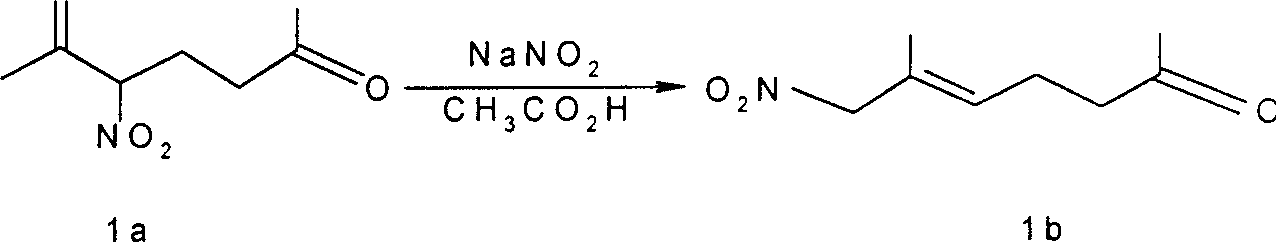
Process for preparing allylic primary nitro compound
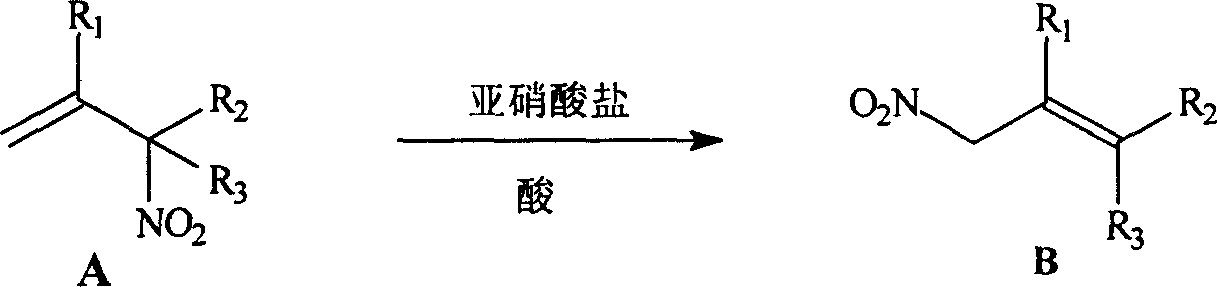
InactiveCN1166621CSimple stepsThe reaction is easy to operateOrganic chemistryOrganic compound preparationNitro compoundOrganic acid
A process for preparing allytrinitro compound includes such steps as reaction of the allybinitro or ally tetranitro compound on excess nitrite and inorganic or organic acid in hydrocarbon or halohydrocarbon solvent to obtain NOX, and further reaction at 50-60 deg.c to obtain allytrinitro compound. Its advantages are simple process and high output rate (50-90%).
Owner:HUBEI UNIV
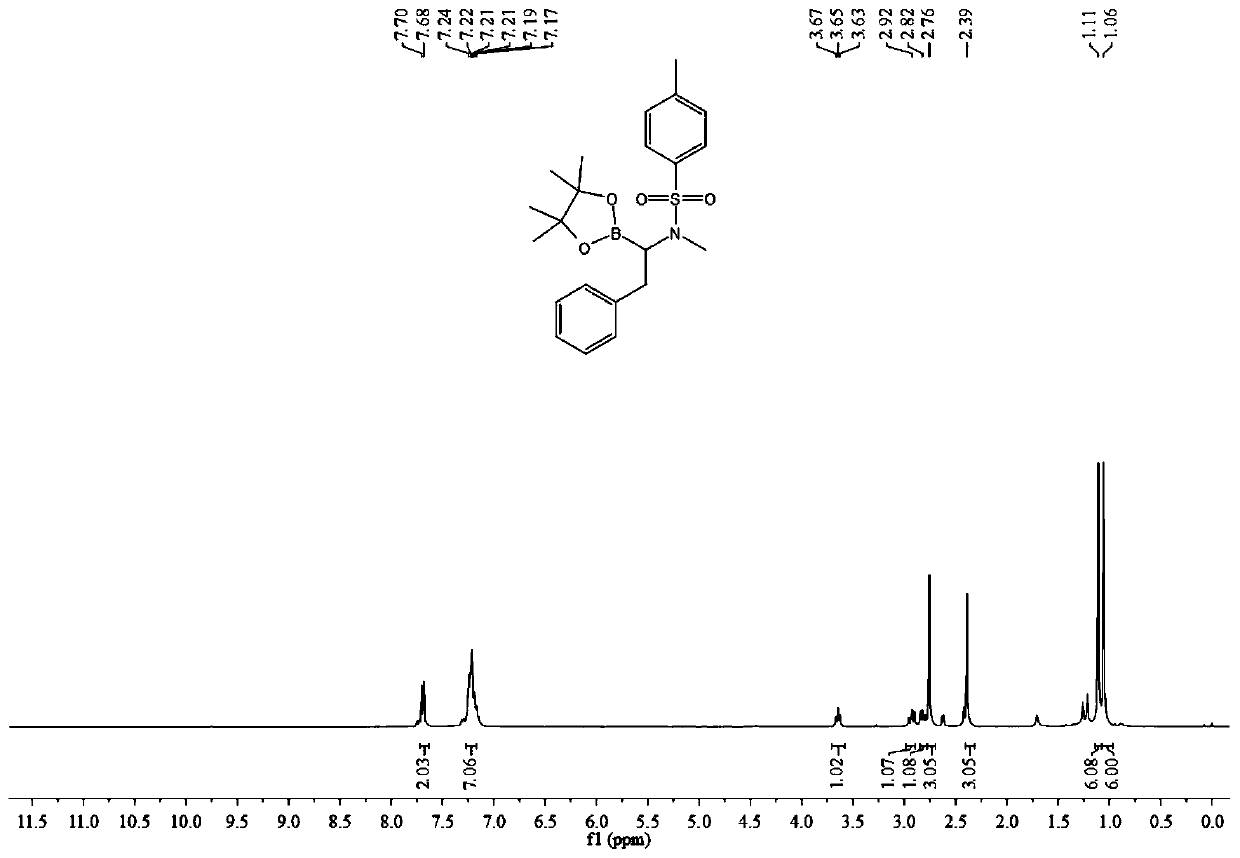
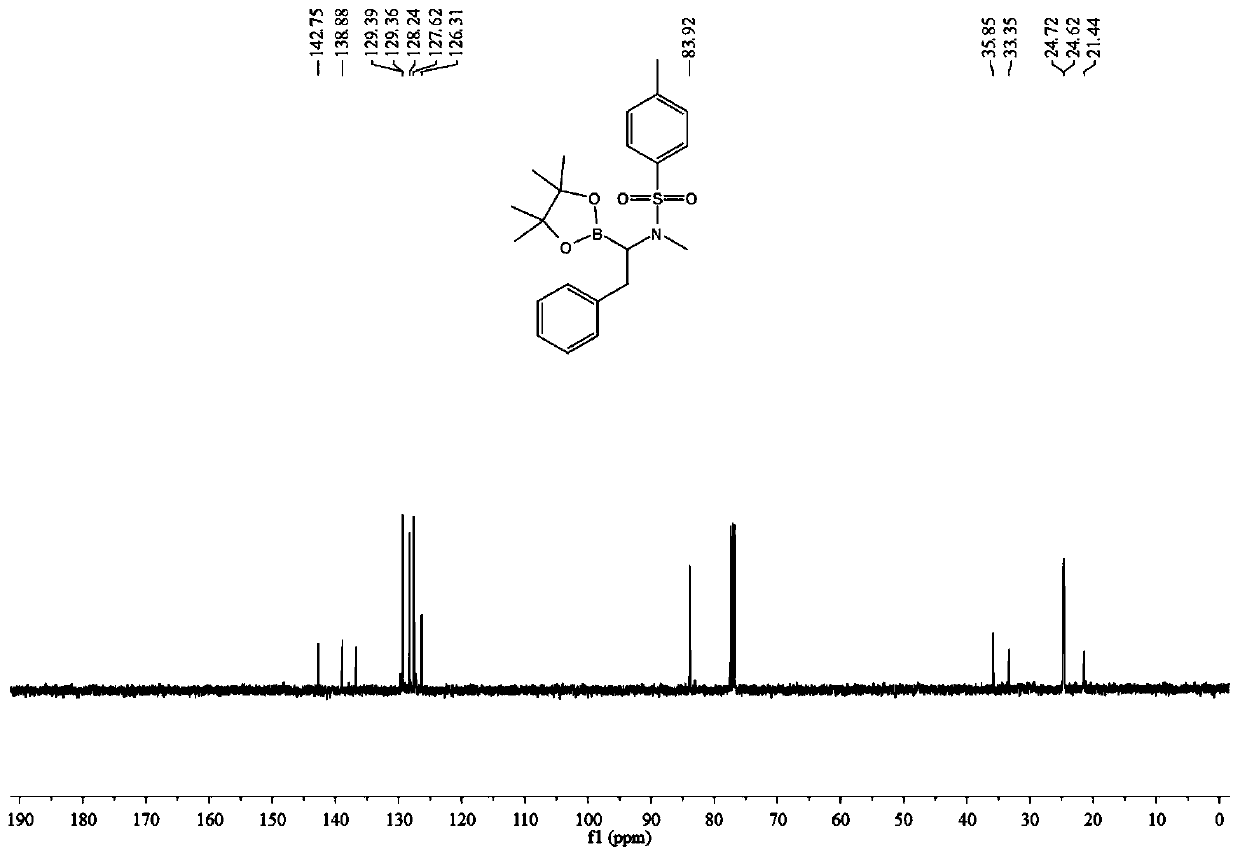
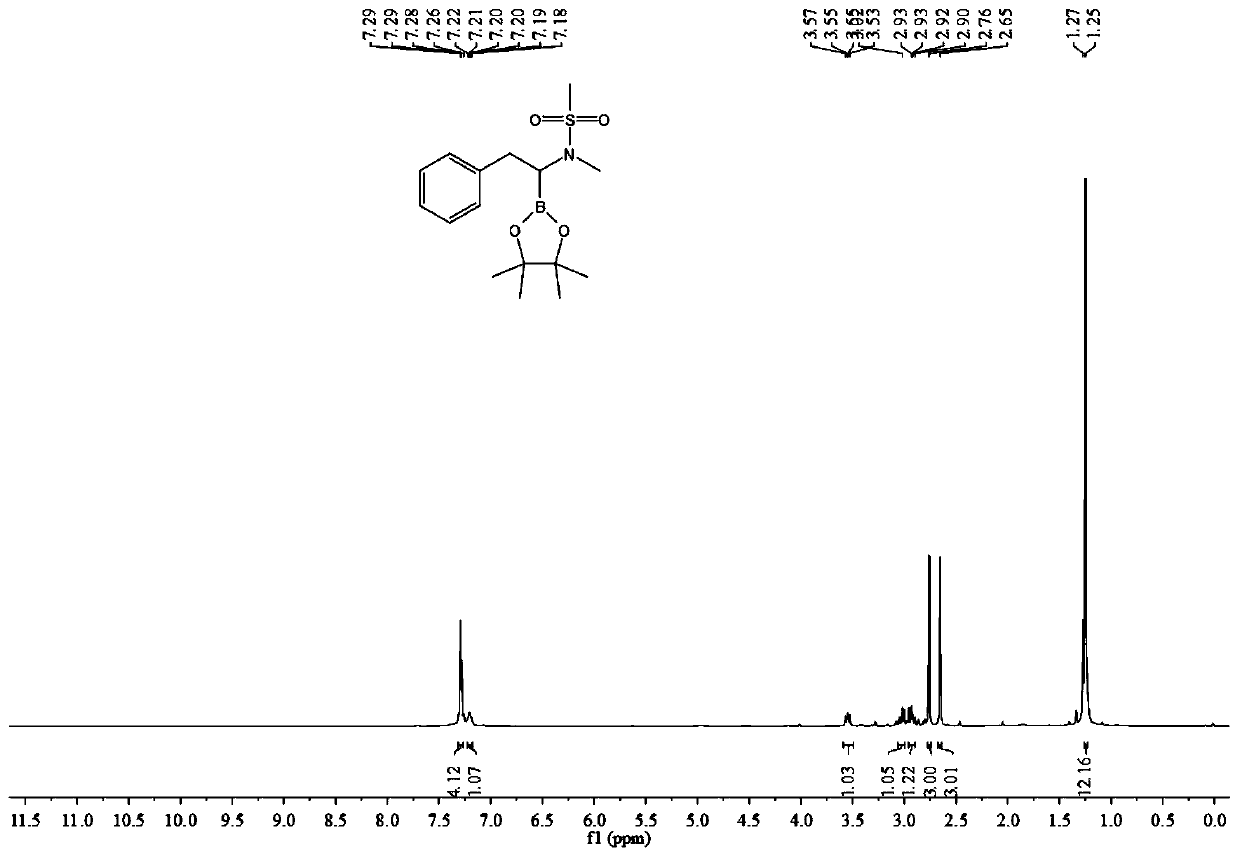
A kind of method of synthesizing α-amino boron compound
ActiveCN108178770BReact Operational SecurityWide substrate applicabilityGroup 3/13 element organic compoundsOrganic solventPtru catalyst
The invention belongs to the technical field of organic synthesis and discloses a method for synthesizing an alpha-amino boron compound. The method comprises the following steps: alkynyl amine, B2Pin2, a base, a ligand, a copper salt catalyst and an organic solvent are added to a reactor and subjected to a stirring reaction at room temperature for 6-12 h, cooling is performed after the reaction, areaction liquid is filtered, the solvent is removed by evaporation, and a crude product is obtained; the obtained crude product is mixed with a reaction solvent, the mixture is fed into a pressure reactor, a catalyst is added, hydrogen under certain pressure is introduced, the substances are subjected to a stirring reaction at 40-80 DEG C for 6-12 h, cooling is performed after the reaction, separation and purification are performed on a product, and the alpha-amino boron compound is obtained. The method has the advantages of simple and easily available raw materials, safe reaction operation,environment-friendly reaction process, high substrate applicability, highly compatible functional groups and good atom economy, and is extensively applied to pesticides and medicines.
Owner:SOUTH CHINA UNIV OF TECH
A kind of method for efficiently preparing aripiprazole intermediate
ActiveCN103833629BReduce generationReduce manufacturing costOrganic chemistryChemical recyclingQuinoloneAlcohol
The invention provides an efficient preparation method of an aripiprazole intermediate, and in particular relates to an efficient preparation method of aripiprazole intermediate BBQ (7-(4-bromobutoxy)-3,4-dihydro-quinolone), and the efficient preparation method of the aripiprazole intermediate comprises the use of a heteropoly acid or heteropoly acid salt and a phase transfer catalyst to form a composite catalyst and the use of a ketone (alcohol)-water azeotropic system. According to the method, in an etherification reaction, a low boiling point lower ketone and the heteropoly acid or the heteropoly acid salt and the phase transfer catalyst are used, the reaction temperature is reduced, at least 6 times molar equivalent of 1, 4-dibromobutane reacts with 7-hydroxy-3, 4-dihydro quinolone, and the formation of disubstituted substances can be reduced, a non-polar solvent is used for precipitation of the aripiprazole intermediate BBQ (7-(4-bromobutoxy)-3,4-dihydro-quinolone), and meanwhile the 1, 4-dibromobutane is recycled. The efficient preparation method of the aripiprazole intermediate has the advantages of high yield, high purity, simple procedure, low cost, and safe and reliable operation, and is friendly to the environment, low in energy consumption, and suitable for industrial mass production.
Owner:HUNAN XIANGZHONG PHARM CO LTD
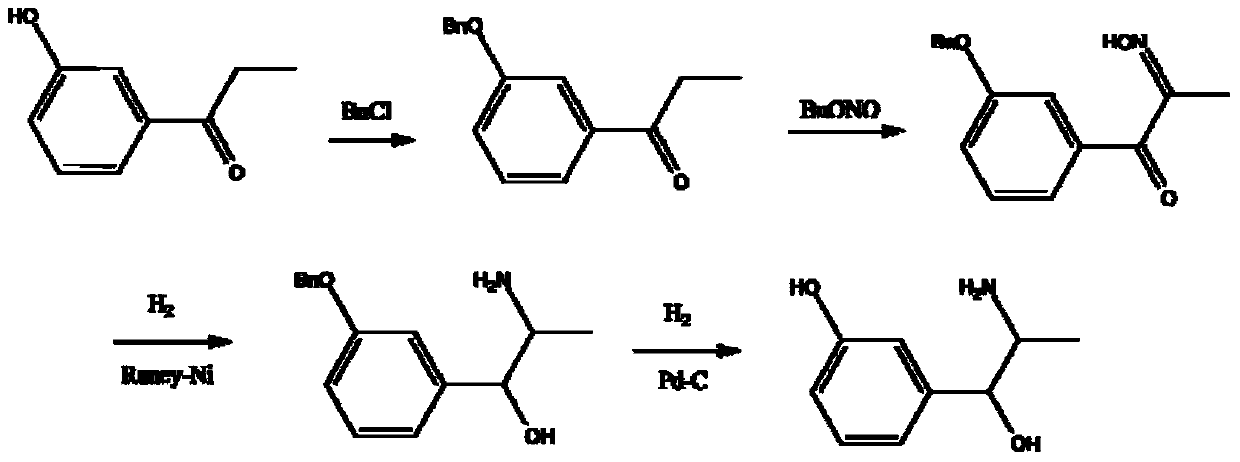
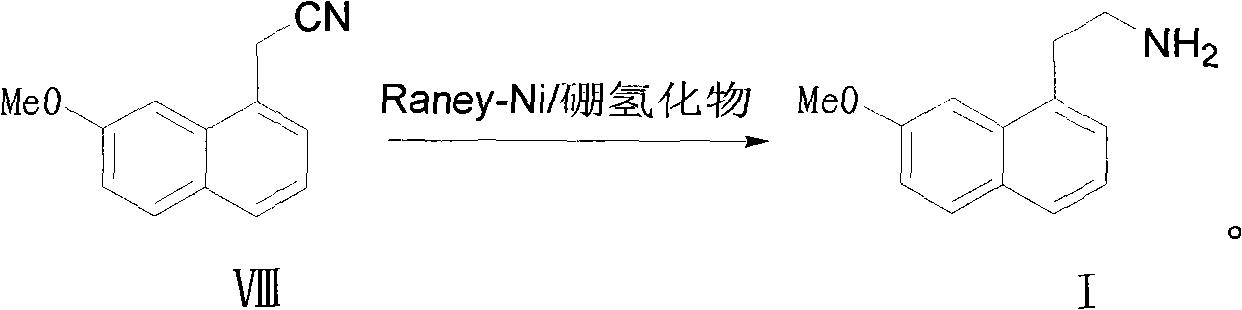
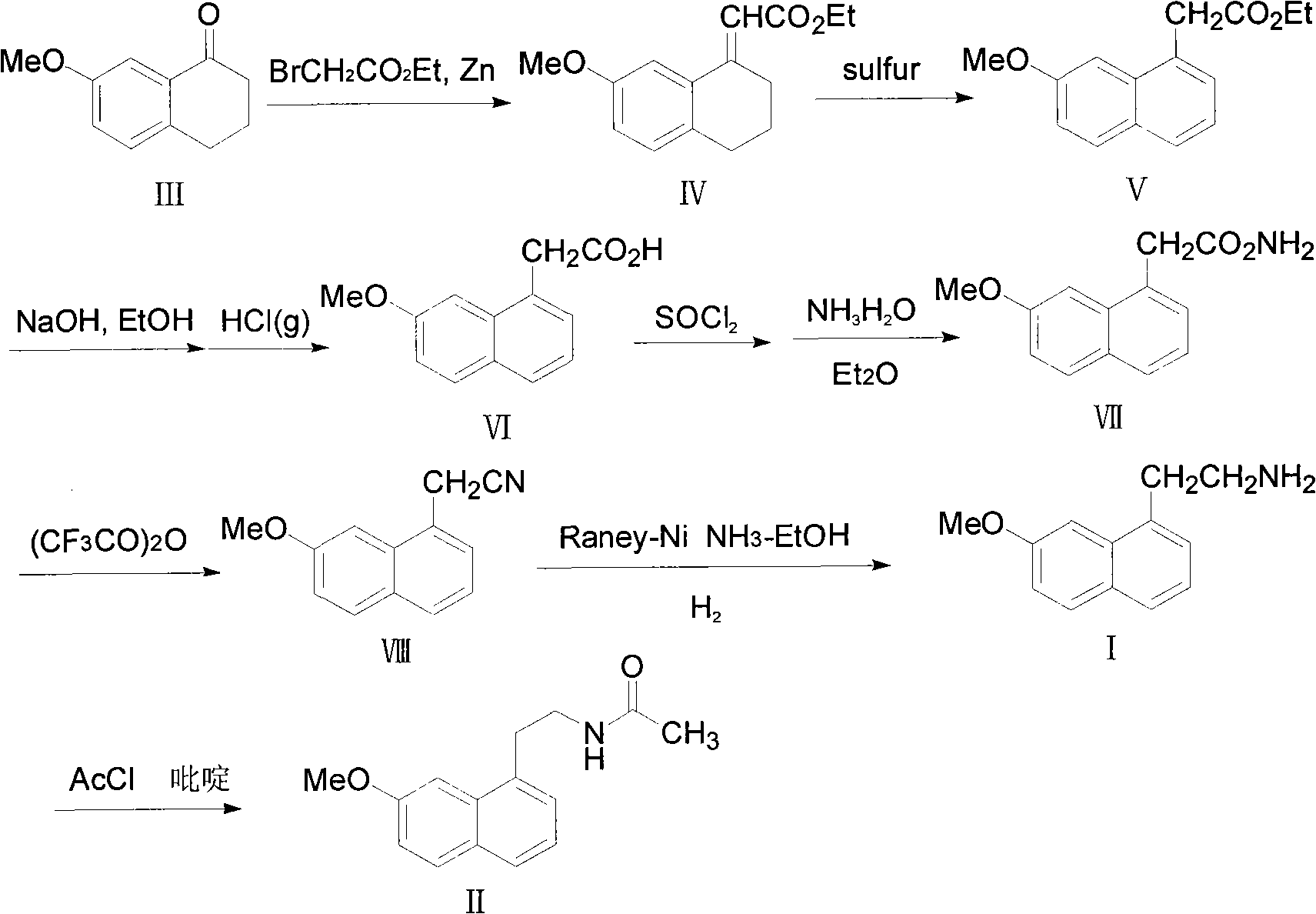
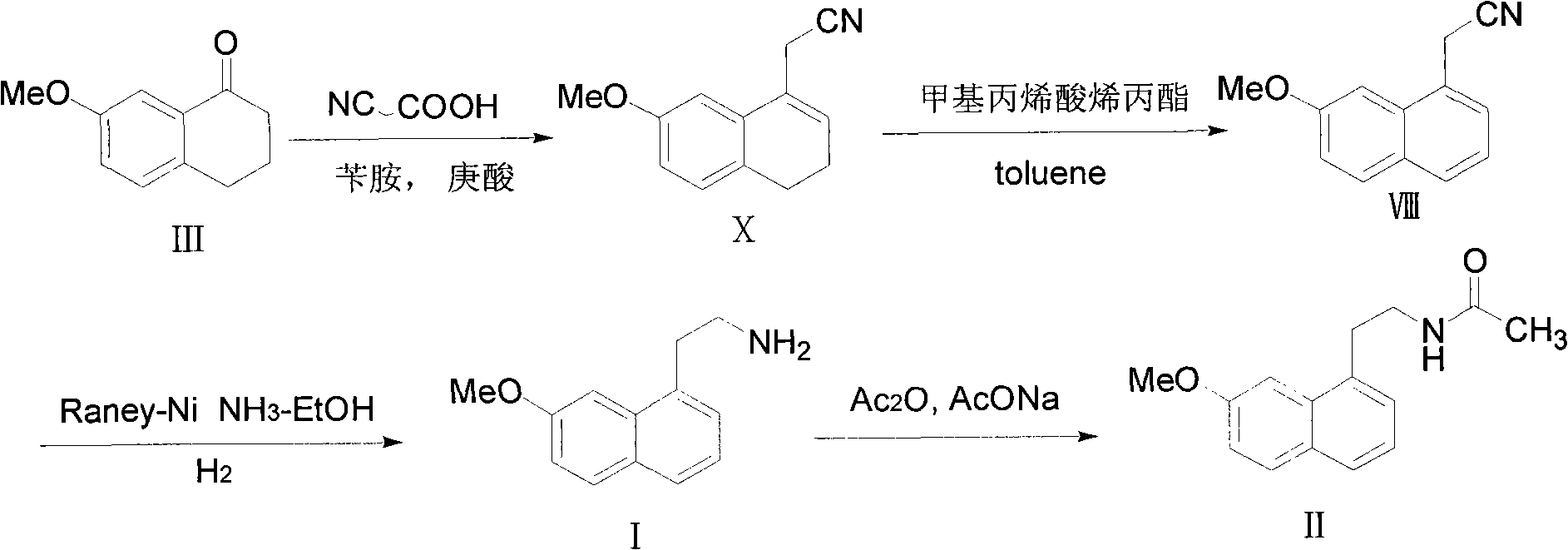
Method for preparing 2-(7-methoxy-1-naphthyl) ethylamine industrially
InactiveCN102234238BHigh purityHigh yieldOrganic compound preparationAmino-hyroxy compound preparationOrganic solventHigh pressure
The invention belongs to the technical field of a preparation method for 2-(7-methoxy-1-naphthyl) ethylamine. In an organic solvent, a compound in a formula (VIII) is reacted under the action of Raney-Ni and hydroboron to form the 2-(7-methoxy-1-naphthyl) ethylamine. The method solves the problem that reaction conditions of high pressure and heating are required in the conventional reports, and a preparation method which has mild reaction conditions, only needs normal temperature and normal pressure and does not have any special requirement on reaction equipment and staff is provided; in addition, by the method, byproducts are absent, the product purifying difficulty is greatly reduced, aftertreatment steps are reduced, and the product yield is improved; and during the whole reaction, the operation is safe, the aftertreatment is simple, the controllability is high, and the purity and yield are high; and compared with the conventional process, the method is more suitable for industrial production.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
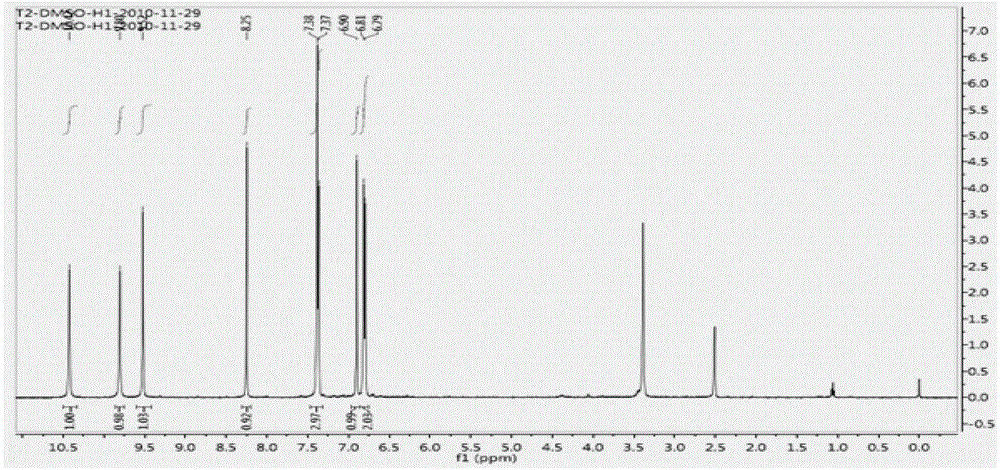
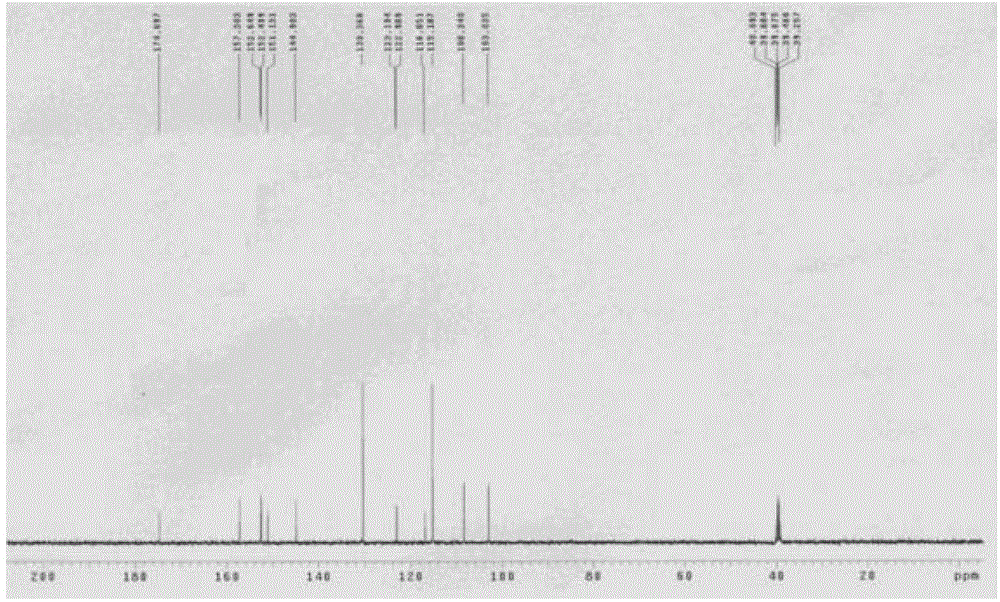
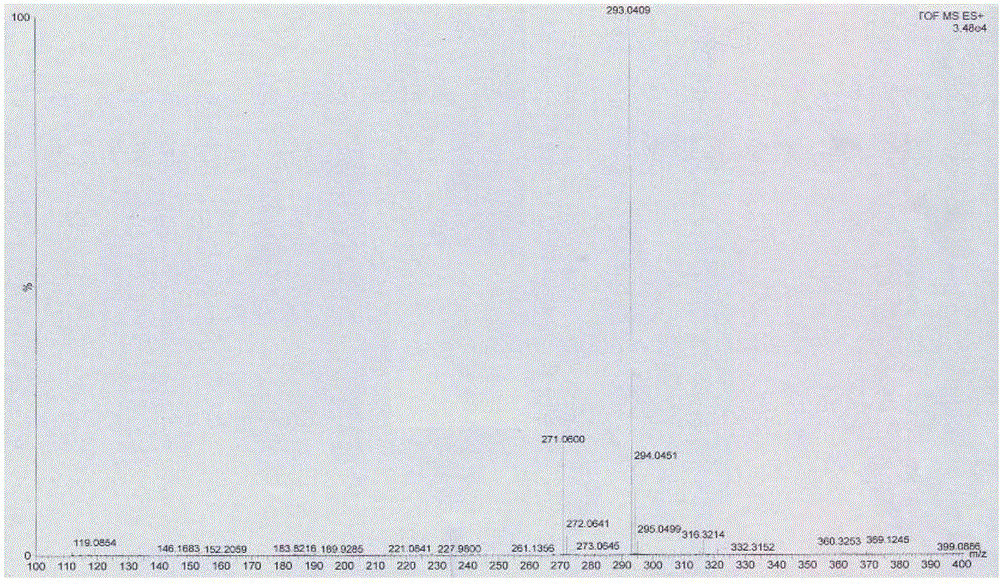
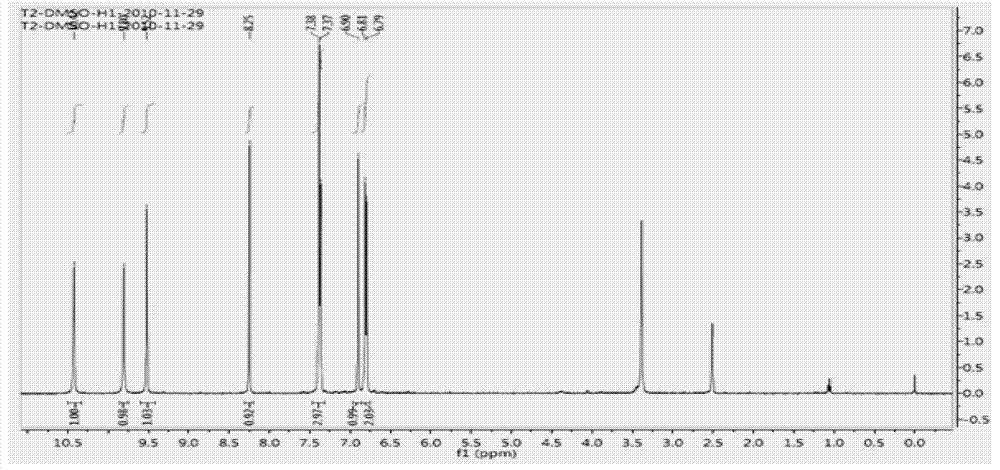
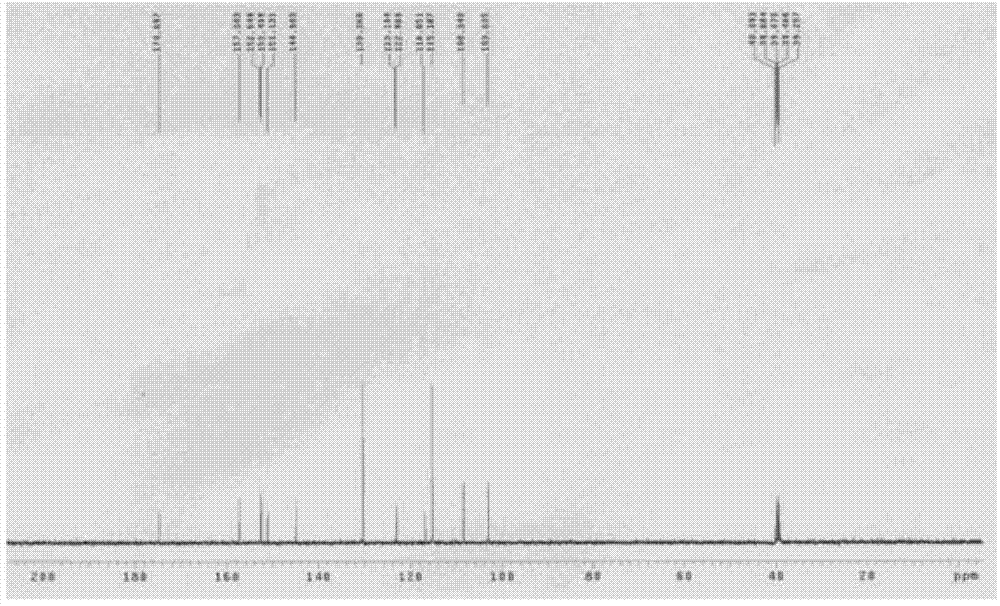
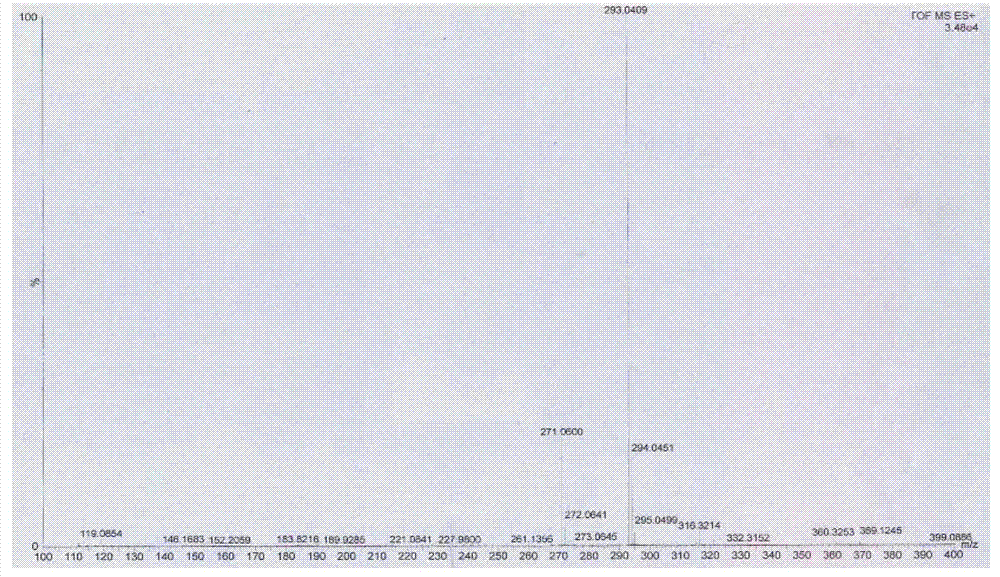
Preparation method of 4-[(4,6-dihydroxyl-2-pyrimidinyl)amino] cyanobenzene
The invention relates to a preparation method of 4-[(4,6-dihydroxyl-2-pyrimidinyl)amino] cyanobenzene. The method comprises the following steps: a, using an aqueous solution of para-cyanoaniline and mono cyanamide as a raw material, water or alcohol solvent as the single solvent or a mixed solvent of water and alcohol solvents as the reaction solvent, and hydrochloric acid as a catalyst to react so as to obtain an N-(4-cyanophenyl)guanidine hydrochloride; and b, using the N-(4-cyanophenyl)guanidine hydrochloride as the raw material, methanol as the reaction solvent, and sodium methoxide and dimethyl malonate as the reaction reagents to react so as to obtain the 4-[(4,6-dihydroxyl-2-pyrimidinyl)amino] cyanobenzene. The method is an improvement of the prior art and has the advantages of short reaction process, high production efficiency, high reaction yield, low cost, low impurity content, complete and stable reaction and the like.
Owner:浙江瑞博制药有限公司
Preparation process of polyhydroxy isoflavone
ActiveCN103087027BHarm reductionAchieve room temperature reactionOrganic chemistryCyanideBenzyl cyanide
The invention relates to a preparation method of polyhydroxy isoflavone. The polyhydroxy isoflavone comprises 4',6,7-trihydroxy isoflavone and 3',4',6,7-tetrahydroxy isoflavone. According to the invention, the large-scale preparation of 4',6,7-trihydroxy isoflavone and 3',4',6,7-tetrahydroxy isoflavone can be realized by treating cheap and easily-acquired chemical raw materials including 3,4-dimethoxy phenol, 3,4-dimethoxy benzyl cyanide and hydroxybenzyl cyanide as starting materials through optimal research on Hoesch reaction, carburization n-cyclohexylmaleimide reaction and demethylation protection. The preparation method of polyhydroxy isoflavone disclosed by the invention is economic, efficient, environment-friendly, safe and easy to industrialize. The 4',6,7-trihydroxy isoflavone and the 3',4',6,7-tetrahydroxy isoflavone can be applied to the research and the development of new medicines in the aspects of medicines, food hygiene and the like.
Owner:中国人民解放军第三军医大学军事预防医学院
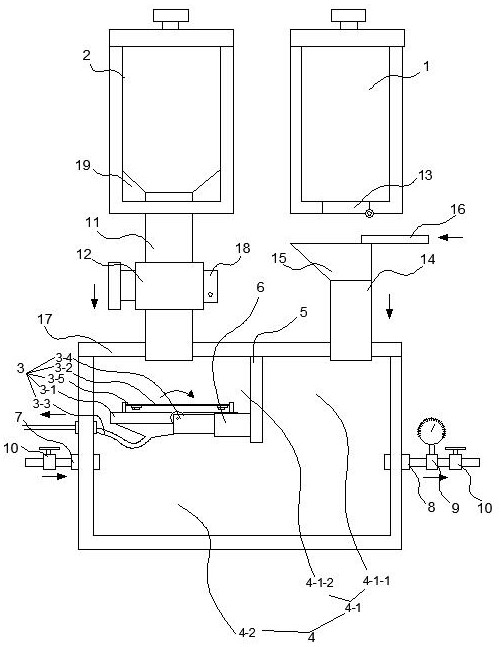
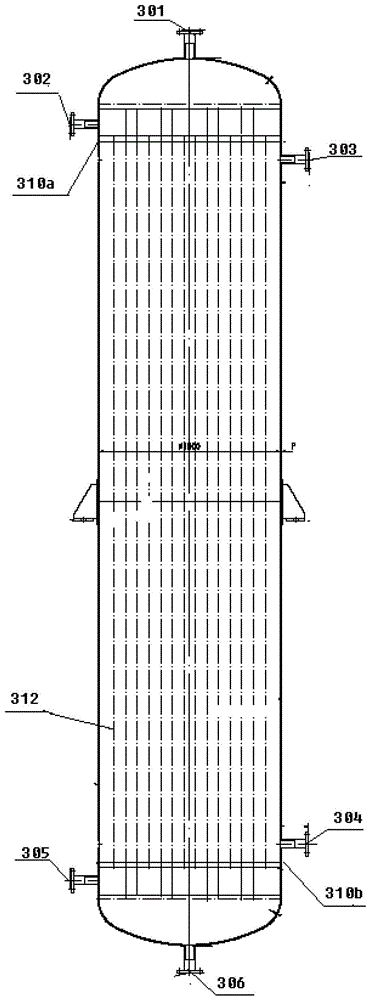
A solid material filtrate feeding structure
InactiveCN108339511BReact Operational SecurityProcess control/regulationChemical/physical/physico-chemical stationary reactorsAnimal scienceElectric machine
The invention provides a feeding structure of solid material filtrate. The feeding structure comprises a dried material storage box, a wet material storage box, a filtering plate and a reaction box, the reaction box comprises an isolated feeding part arranged at the upper part and a reaction tank body part arranged at the lower part, the isolated feeding part is separated into a dried feeding areaand a wet feeding area, the filtering plate is arranged in the wet feeding area, the filtering plate comprises a plate body, a filtering net is arranged on the plate body, a drainage tube is arrangedat the lower portion of the filtering net, the plate body is connected with a rotary motor, and a filtering net vibrator is arranged on the filtering net. The feeding structure has the advantages ofoperational safety of the reaction of dangerous materials.
Owner:相孟成
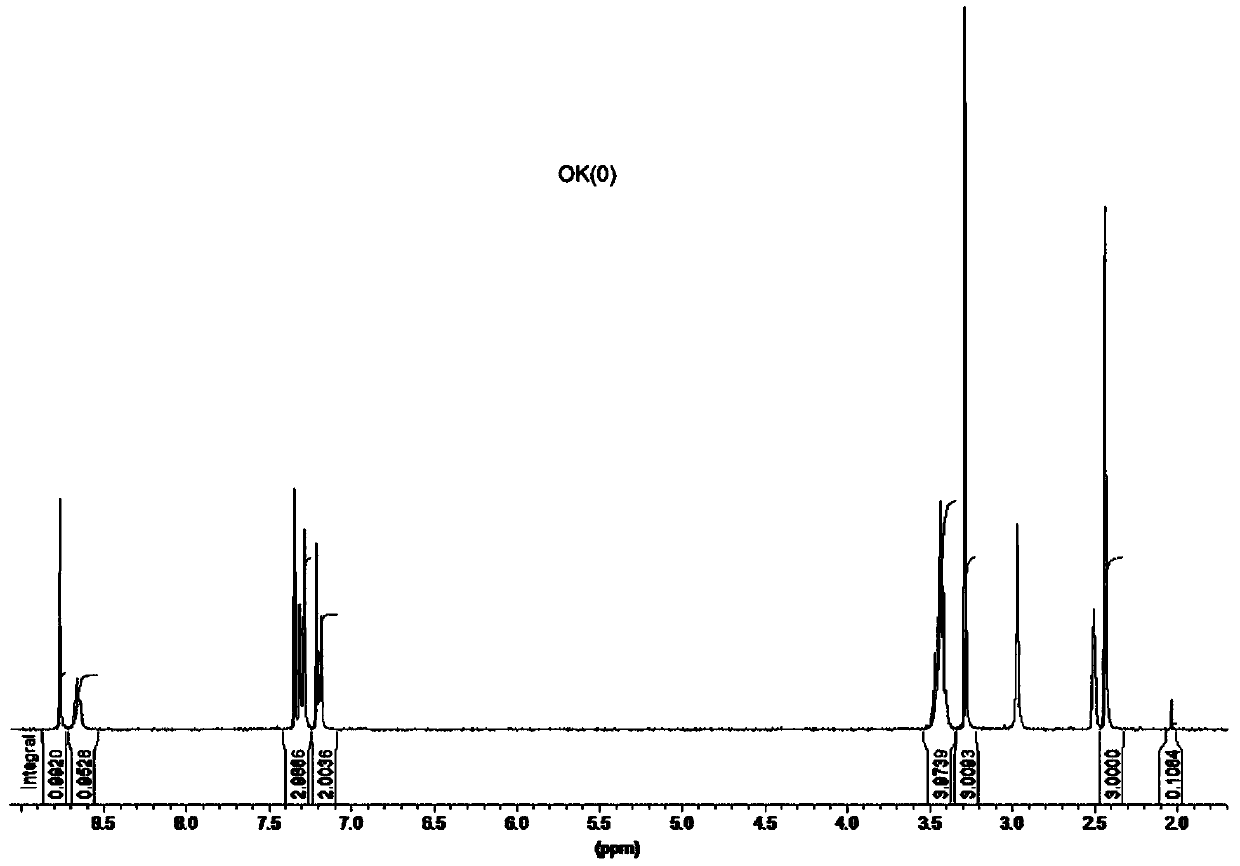
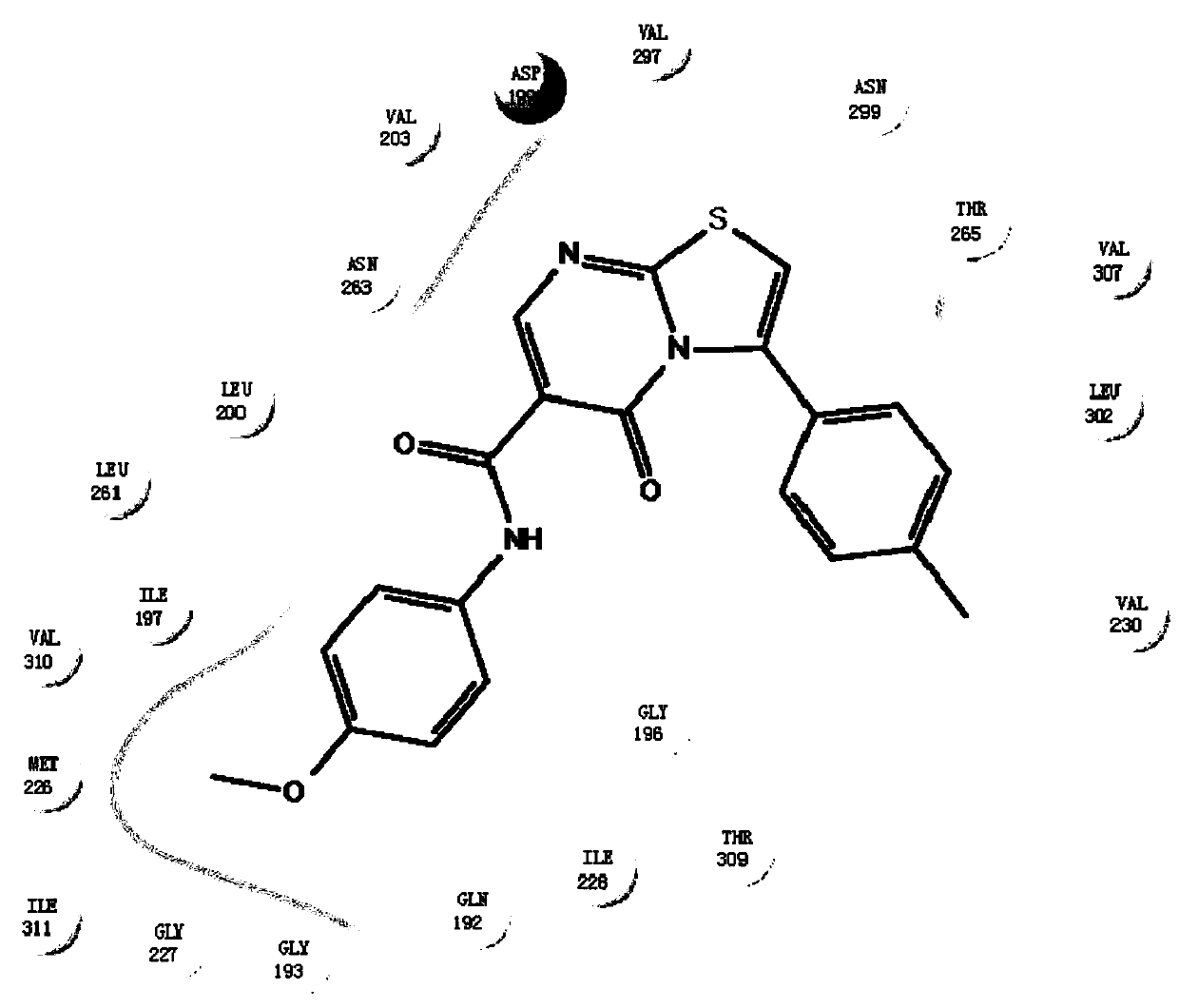
A novel thiazole drug molecule for hospital disinfection and its preparation method
Owner:THE FIRST AFFILIATED HOSPITAL OF HENAN UNIV OF SCI & TECH
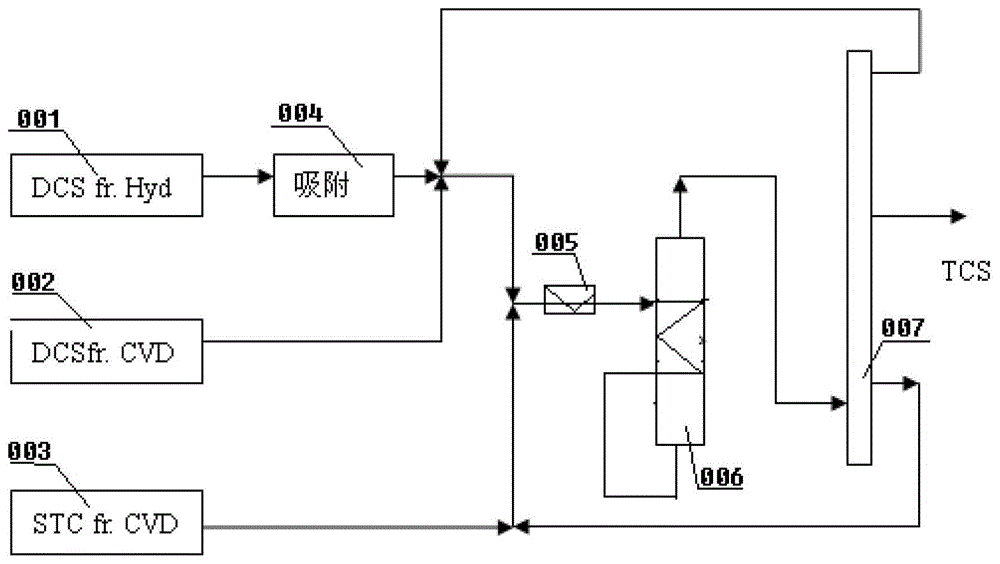
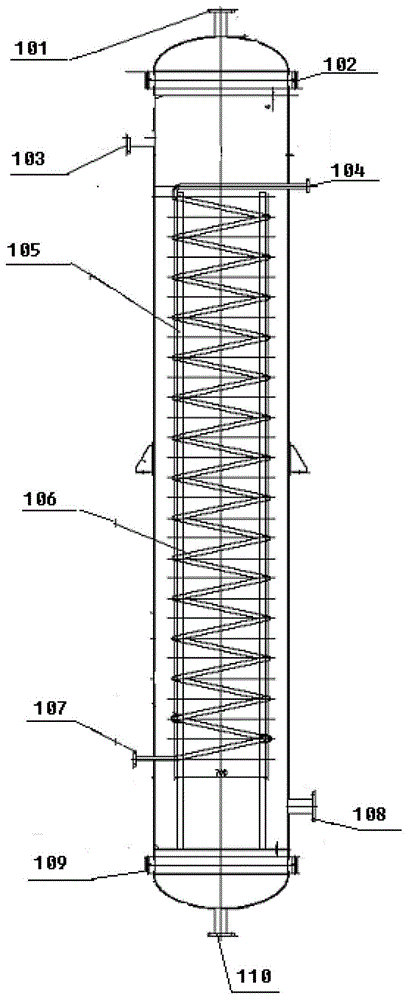
Reactor and method for realizing chlorosilane synthesis
ActiveCN103172072BRealize heat exchangePrevent or reduce localized overheatingHalogenated silanesDisproportionationChlorosilane
The invention provides a reactor and a method for realizing the synthesis of chlorosilane. The reactor for realizing the synthesis of chlorosilane comprises a reactor cavity, the reactor cavity is provided with a catalyst feed inlet, a catalyst loading inlet, a catalyst discharge outlet, a catalyst orifice, a raw material feed inlet and a product discharge outlet; the reactor cavity is provided with an internal or external preheating mechanism; and a heat exchange device is arranged on and / or in the reactor cavity, a heat exchange medium outlet and a heat exchange medium inlet of the heat exchange device are both arranged on the reactor cavity, and the heat exchange medium outlet is communicated with the raw material feed inlet. The invention also discloses a method for carrying out chlorosilane disproportionation and inverse disproportionation synthesis by using the reactor. The reactor and method for realizing synthesis of chlorosilane disclosed by the invention overcome various defects in the prior art, and realize the efficient, energy-saving, long-term, stable, safe and reliable production of chlorosilane.
Owner:储晞
A kind of preparation method of sugammadex sodium
The invention discloses a preparation method of sugammadex sodium. The method uses γ-cyclodextrin as raw material, reacts with 3-mercaptopropionic acid substituted ester under the action of phosphine reagent and azo reagent to generate sugammadex; Hydrolysis in temperature and solvent to obtain the target product, sodium sugammadex. The method for preparing sodium sugammadex has mild reaction, omits the γ-cyclodextrin perhalogenation reaction process, is simple and time-saving in operation, has high yield of the obtained target product, and is suitable for industrial scale-up production.
Owner:LUNAN PHARMA GROUP CORPORATION
A kind of ruthenium catalysis prepares the method for many substituted 1-naphthoic acid compounds
ActiveCN109665958BGood choiceHigh yieldOrganic compound preparationCarboxylic compound preparationPtru catalystOrganic synthesis
The invention belongs to the technical field of organic synthesis and discloses a ruthenium-catalyzed method for preparing multi-substituted 1-naphthoic acid compounds. Heat reaction of phthalic acid (I) or phthalic anhydride compound (II) with diaryl-substituted symmetrical alkyne compound (III), ruthenium catalyst, additive and solvent in air or oxygen environment, and the reaction product is separated and purified , to obtain many substituted 1-naphthoic acid compounds (IV). The method of the present invention uses easy-to-obtain phthalic acid or phthalic anhydride compounds and alkynes as raw materials, and the high catalytic activity of the ruthenium catalyst system used enables the reaction to use air or oxygen as a green oxidant, avoiding the use of toxic, dangerous or expensive The oxidizing agent, the reaction operation is simple and safe, and it is easy to realize industrialization.
Owner:GUANGZHOU UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com








![Preparation method of 4-[(4,6-dihydroxyl-2-pyrimidinyl)amino] cyanobenzene Preparation method of 4-[(4,6-dihydroxyl-2-pyrimidinyl)amino] cyanobenzene](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/48b6ea57-7514-4e3a-bed7-d9cfd869584e/G2009101020073D00011.PNG)
![Preparation method of 4-[(4,6-dihydroxyl-2-pyrimidinyl)amino] cyanobenzene Preparation method of 4-[(4,6-dihydroxyl-2-pyrimidinyl)amino] cyanobenzene](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/48b6ea57-7514-4e3a-bed7-d9cfd869584e/G2009101020073D00012.PNG)
![Preparation method of 4-[(4,6-dihydroxyl-2-pyrimidinyl)amino] cyanobenzene Preparation method of 4-[(4,6-dihydroxyl-2-pyrimidinyl)amino] cyanobenzene](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/48b6ea57-7514-4e3a-bed7-d9cfd869584e/G2009101020073D00013.PNG)