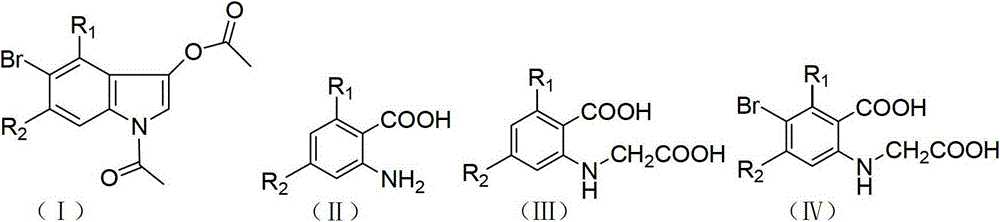
Method for synthesizing 1-acetyl-halo-indolyl-3-acetate
A halogenated indolyl and synthetic method technology, applied in the field of chemical synthesis, can solve the problems of long reaction time, pollutants, post-processing troubles, etc., and achieve the effects of high yield, simple reaction operation, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1) Nucleophilic substitution reaction:
[0023] (1) Synthesis of N-(3-chloro-2-carboxy)phenylglycine
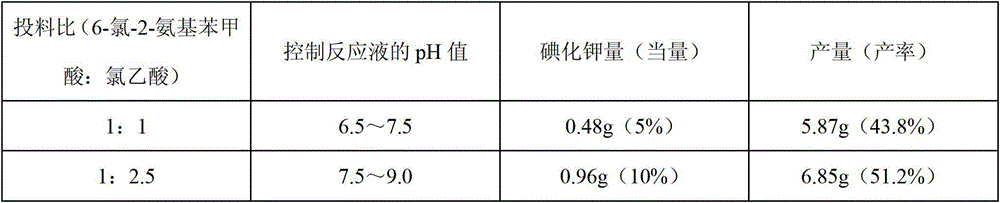
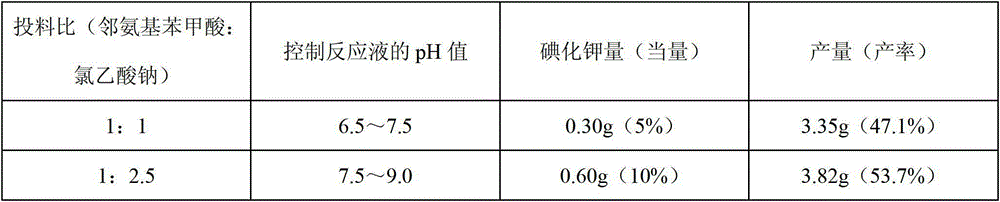
[0024] Weigh 10.00g (0.058mol) of 6-chloro-2-aminobenzoic acid and 8.80g (0.093mol) of chloroacetic acid, stir and mix, slowly add 100g / L sodium hydroxide solution to neutralize, and use 3mol / L carbonic acid Adjust the pH value of the sodium solution to 7.5-8.0, add 0.50g potassium iodide, and react under heating and reflux. During this period, add 3mol / L sodium carbonate solution intermittently to control the pH value of the reaction solution at 7.0-8.0, and react until the pH value remains unchanged within 20 minutes. until. Add 2g of activated carbon, stir for 0.5-1h at the remaining temperature of the oil bath, filter with suction, dilute with an appropriate amount of water, acidify the filtrate with concentrated hydrochloric acid to a pH value of 2.0-2.5, filter with suction and dry it, and then dry it in a sufficient vacuum to obtain Off-white product (N-(3-ch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com