A kind of ruthenium catalysis prepares the method for many substituted 1-naphthoic acid compounds
A compound and multi-substitution technology, which is applied in the field of ruthenium-catalyzed preparation of multi-substituted 1-naphthoic acid compounds, can solve the problems that the product cannot be separated and cannot be used to prepare multi-substituted 1-naphthoic acid, and achieves high yield, The reaction operation is simple and the selectivity is good
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
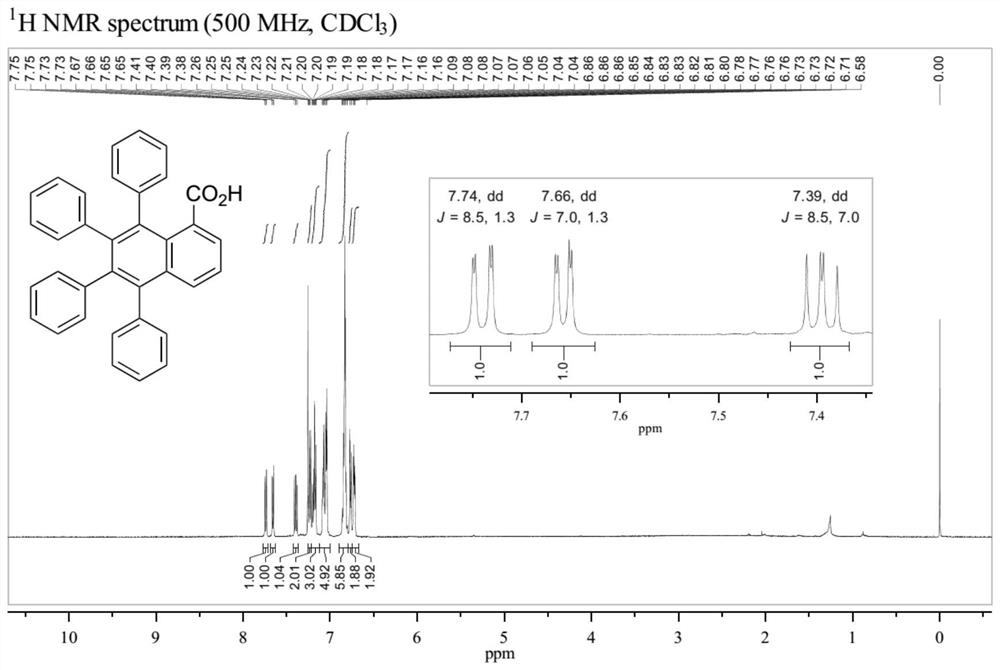
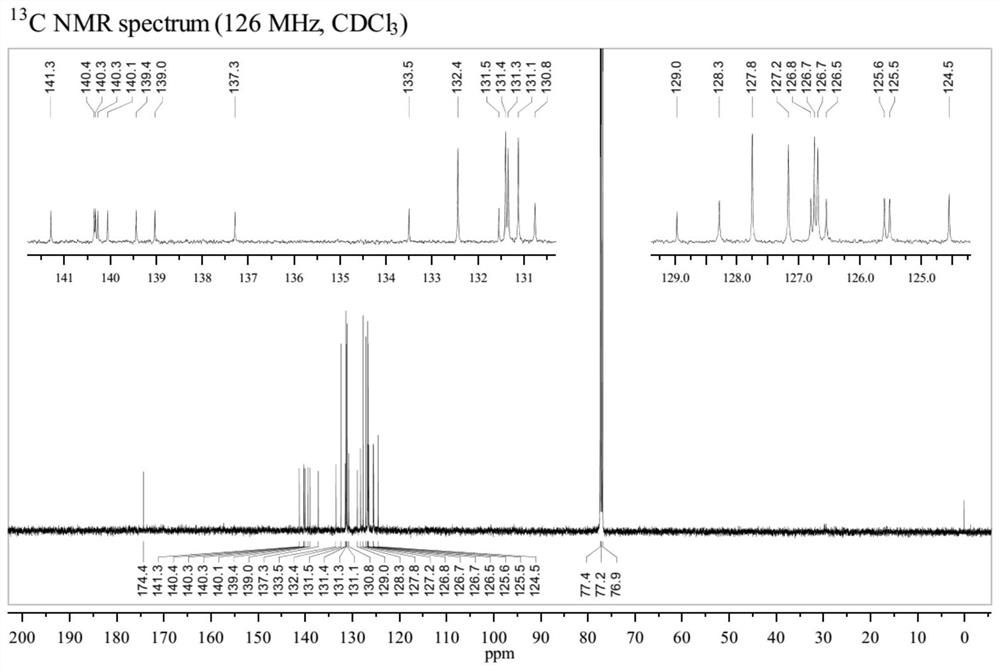
[0043] Add p-cymene dichloride ruthenium dimer (0.01mmol, 6.1mg), toluene (0.4mmol, 71.3mg), phthalic acid (0.3mmol, 49.8 mg), tetrabutylammonium bromide (0.04mmol, 12.9mg) and a magnetic stirrer, add 900μL GVL and 100μL DMF with a pipette gun, cover the upper opening of the reaction tube and leave its branch open to the air, then put Stir in a metal heating block at 100°C for 12 hours. After the reaction was completed, ethyl acetate was added, washed with water, separated, the organic phase was concentrated and then separated by silica gel column chromatography to obtain a light yellow solid which was the product 5,6,7,8-tetraphenyl-1-naphthoic acid, the yield 81%.
[0044] The structure of the obtained product was confirmed by high-resolution mass spectrum, hydrogen nuclear magnetic spectrum and carbon spectrum. HRMS (ESI) calcd for C 35 h 24 o 2 [M–H] – 475.1704, found 475.1712. The H NMR and C NMR spectra of the product are shown as figure 1 and figure 2 shown. ...
Embodiment 2
[0046] Add ruthenium trichloride trihydrate (0.024mmol, 6.3mg), diphenylacetylene (0.4mmol, 71.3mg), phthalic anhydride (0.3mmol, 44.4mg) and tetrabutyl bromide successively into a 10mL reaction tube with a branch Ammonium (0.05mmol, 16.1mg) and a magnetic stirring bar were added to 1mL of GVL, the upper opening of the reaction tube was covered and its branch was connected to the oxygen bulb, and then placed in a metal heating module at 100°C and stirred for 20 hours. After the reaction was completed, ethyl acetate was added, washed with water, separated, the organic phase was concentrated and then separated by silica gel column chromatography to obtain a light yellow solid which was the product 5,6,7,8-tetraphenyl-1-naphthoic acid, the yield 68%.
Embodiment 3
[0048] Add p-cymene dichloride ruthenium dimer (0.01mmol, 6.1mg), toluene (0.4mmol, 71.3mg), 4-methylphthalic anhydride (0.3mmol, 48.6mg), tetrabutylammonium bromide (0.04mmol, 12.9mg) and a magnetic stirrer, add 900μL GVL, 100μL DMF and 20μL water with a pipette gun, cover the upper opening of the reaction tube and leave its branch open to the air , and then placed in a metal heating block at 100°C and stirred for 20 hours. After the reaction was completed, ethyl acetate was added, washed with water, separated, the organic phase was concentrated and separated by silica gel column chromatography to obtain a light yellow solid which was the product 3-methyl-5,6,7,8-tetraphenyl-1- Naphthoic acid, yield 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com