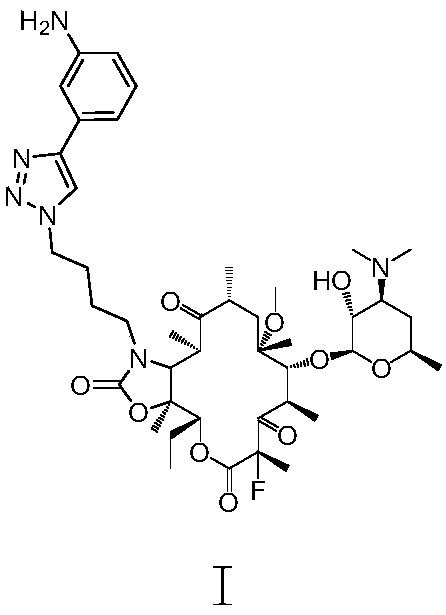
Method for preparing solithromycin
A technology of solithromycin and compounds, applied in the field of preparation of macrolide drug solithromycin, capable of solving problems such as unsuitable for industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
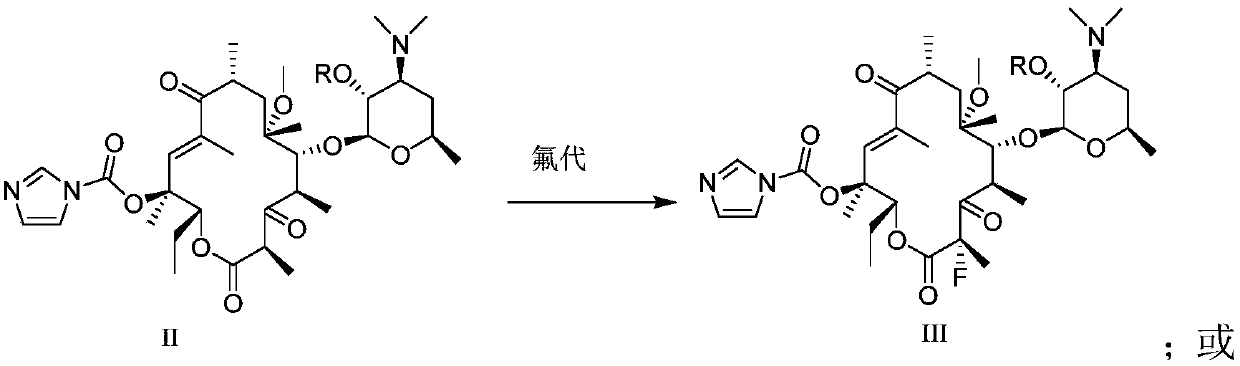
[0082] Preparation of Intermediate II:
[0083] ①Preparation of Compound A
[0084]
[0085]Add clarithromycin (50 g, 0.067 mol), triethylamine (18.75 mL, 0.135 mol, 2 equivalents), and ethyl acetate (350 mL) into a 500 mL reaction flask and stir to mix. Benzoic anhydride (22.5 g, 0.1 mol, 1.5 equiv) was added in portions. After the addition, stir at room temperature (20-25°C) for 24h. After detecting that the clarithromycin reaction was complete, the solvent was distilled off under reduced pressure (temperature<45° C.). Add 500 mL ice methanol to the residue, stir in ice bath (0-5° C.) for 0.5 h, and filter with suction. The filter cake was rinsed with ice-methanol (100 mL×2) and dried under vacuum at 50° C. to obtain 56 g of compound A as a white solid.
[0086] ESI[M+1]:852
[0087] ②Preparation of Compound B
[0088]
[0089] Add compound A (56 g, 0.0657 mol), ethanol (300 mL), and water (300 mL) obtained in the previous step into a 1000 mL reaction flask and m...
Embodiment 2
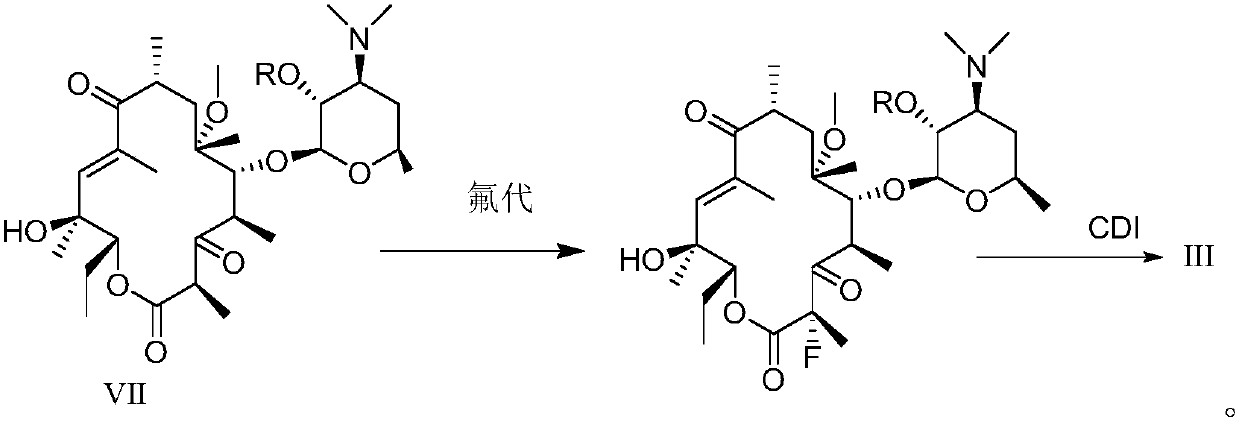
[0117] Preparation of compound III by fluorination reaction
[0118] The sugar hydroxyl protecting group is acetyl
[0119]
[0120] 2.05 g of acetyl-protected compound II (2.90 mmol) was dissolved in 20 ml of DMF / THF mixed solution (9:1), and at -20°C, 0.39 g of potassium tert-butoxide (3.48 mmol) was added in batches, After the addition was completed, the mixture was stirred at -20°C for 0.5 hours, then 1.01 g of NFSI (N-fluorobisbenzenesulfonamide) (3.19 mmol) was added, and the reaction was continued at -20°C for two hours. Sampling HPLC detects that the reaction is complete, adding a small amount of water to quench the reaction, diluting the reaction solution with 50 milliliters of ethyl acetate, then washing (6*50 milliliters) with saturated brine several times, drying over anhydrous sodium sulfate, filtering and spinning to obtain the crude product ( can be used directly in the next step). The crude product was purified by column chromatography (silica gel 200-300 ...
Embodiment 3
[0122] Preparation of compound V by docking reaction
[0123]
[0124] 1.33 g of compound III (1.84 mmol) and 2.44 g of I (7.36 mmol) were dissolved in 20 ml of acetonitrile / water mixture (10:1), and heated to reflux overnight. The next day, the reaction solution was cooled to room temperature, spin-dried directly, and the residue was subjected to column chromatography (silica gel 200-300 mesh, Qingdao Huanghai) (dichloromethane:methanol=30:1) to obtain V crude product (directly used in the following step).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com