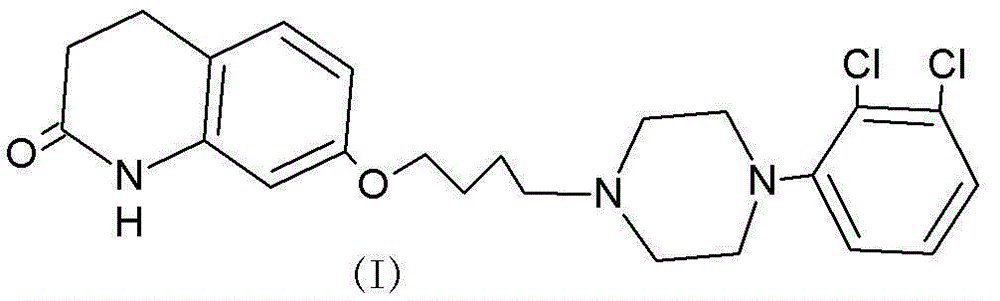
A kind of method for efficiently preparing aripiprazole intermediate
A technology for aripiprazole and intermediates, which is applied in the field of efficient preparation of aripiprazole intermediates, which can solve the problems of unfriendly environment, lack of affinity, and failure to achieve results, so as to reduce production costs and simplify post-processing steps , Reduce the effect of the generation of secondary substitutes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: Use ammonium phosphotungstate-tetrabutylammonium bromide as catalyst to prepare aripiprazole intermediate BBQ (7-(4-bromobutoxy)-3,4-dihydroquinolone)
[0024]In a 1L clean reaction bottle, add 30g of 7-hydroxy-3,4-dihydroquinolone, 360ml of 2-butanone-water, 300g of 1,4-dibromobutane, 0.2g of ammonium phosphotungstate-tetrabutylammonium bromide, After adding 9g of potassium carbonate, heat up to reflux. Reflux for 4-6 hours. Cool down, filter, evaporate the filtrate to remove 2-butanone, add 300ml of water and 900ml of cyclohexane, stir and crystallize, filter with suction, and recrystallize to obtain 50.44g of aripiprazole intermediate etherate. Yield: 92%, purity (HPLC): 97.76%. The filtrate was left to stand and separated, and cyclohexane was recovered after sufficient water washing, and the remaining liquid was 1,4-dibromobutane , the recovery rate is 95%, and it can be directly put into the next batch of reactions. The cyclohexane recovered at the sa...
Embodiment 2
[0025] Embodiment 2: Use ammonium phosphomolybdate-tetrabutylammonium bromide as catalyst to prepare aripiprazole intermediate BBQ (7-(4-bromobutoxy)-3,4-dihydroquinolone)
[0026] In a 1L clean reaction flask, add 30g 7-hydroxyl-3,4-dihydroquinolone, 360ml 2-butanone-water, 300g 1,4-dibromobutane, 0.2g ammonium phosphomolybdate-tetrabutylammonium bromide, After adding 9g of potassium carbonate, heat up to reflux. Reflux for 4-6 hours. Cool down, filter, evaporate the filtrate to remove 2-butanone, add 300ml of water and 900ml of cyclohexane, stir and crystallize, filter with suction, and recrystallize to obtain 49.89g of aripiprazole intermediate etherate. Yield 91%, purity (HPLC): 97.25%. The filtrate was left to separate and separated, and cyclohexane was recovered after sufficient water washing, and the remaining liquid was 1,4-dibromobutane , the recovery rate is 95%, and it can be directly put into the next batch of reactions. The cyclohexane recovered at the same time c...
Embodiment 3
[0027] Embodiment 3: Use ammonium phosphotungstate-trioctylmethyl ammonium chloride as catalyst to prepare aripiprazole intermediate BBQ (7-(4-bromobutoxy)-3,4-dihydroquinolone)
[0028] In a 1L clean reaction bottle, add 30g of 7-hydroxy-3,4-dihydroquinolone, 360ml of 2-acetone-water, 300g of 1,4-dibromobutane, 0.2g of ammonium phosphotungstate-trioctylmethyl ammonium chloride , 9g of potassium carbonate, after the addition, heat up to reflux. Reflux for 4-6 hours. Cool down, filter, evaporate the filtrate to remove 2-butylacetone, add 300ml of water and 900ml of cyclohexane, stir and crystallize, filter with suction, and recrystallize 49.5g of aripiprazole intermediate etherate was obtained. The yield was 90.2%, and the purity (HPLC): 98.05%. Alkanes, the recovery rate is 95%, can be directly put into the next batch of reaction. The cyclohexane recovered at the same time can also be put into the post-treatment of the next batch.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com