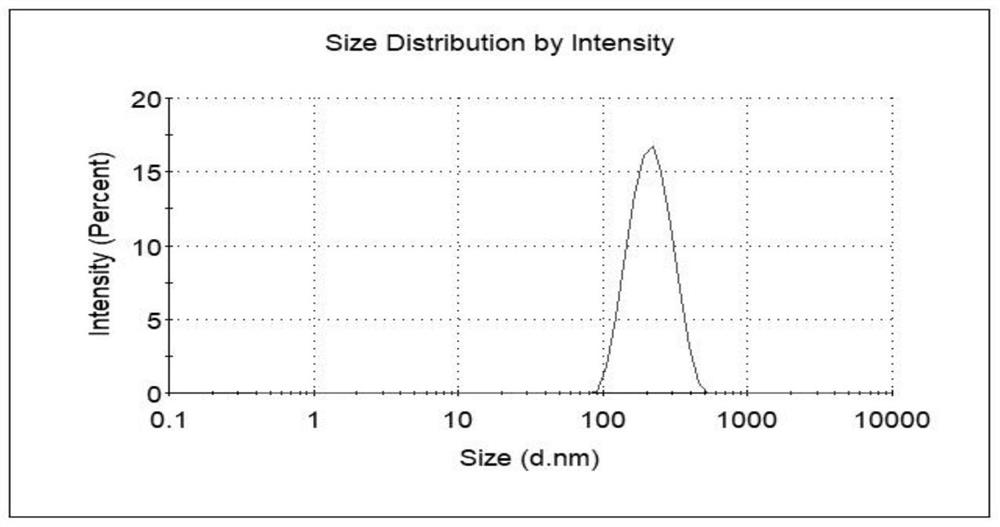
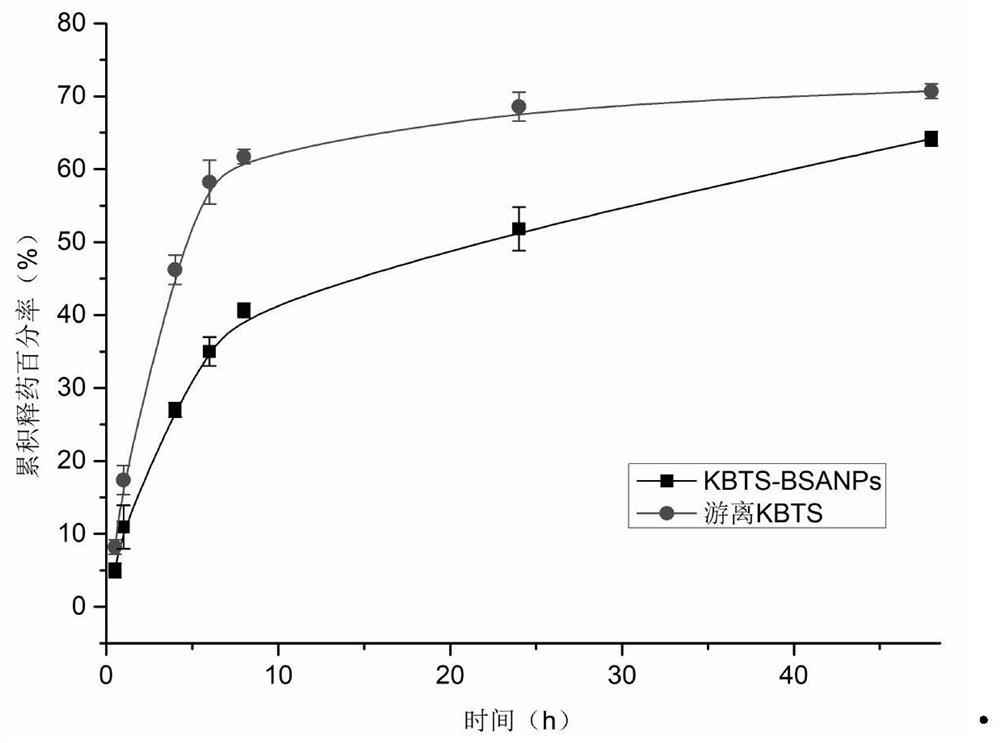
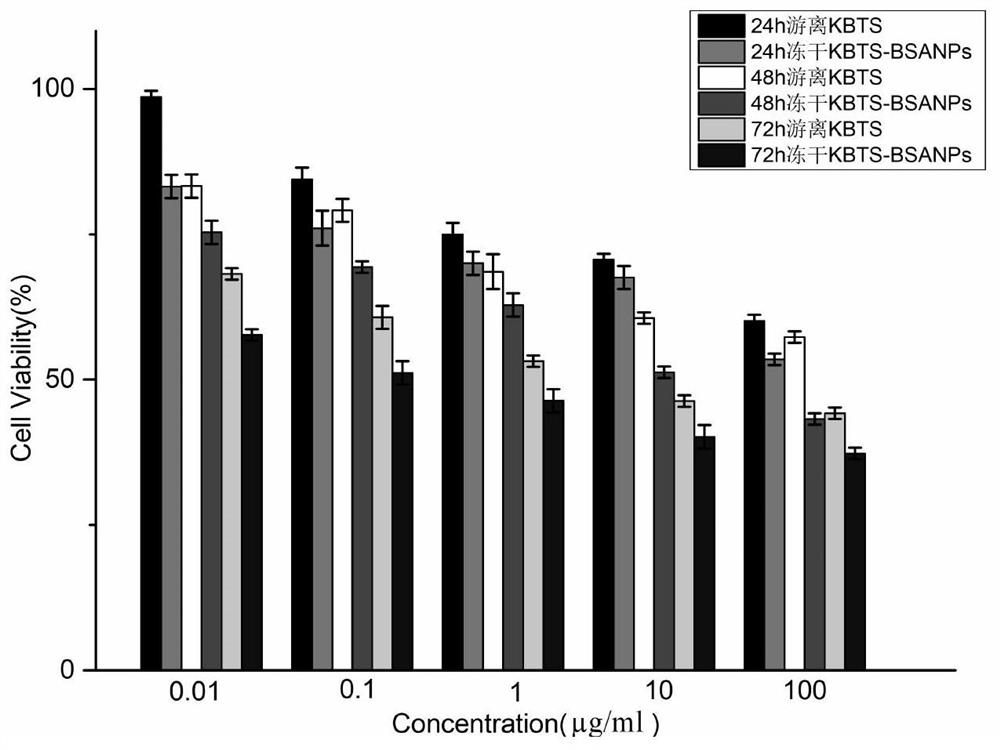
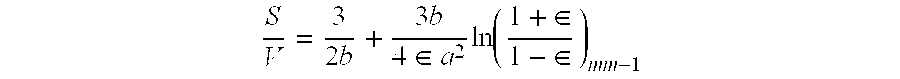Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
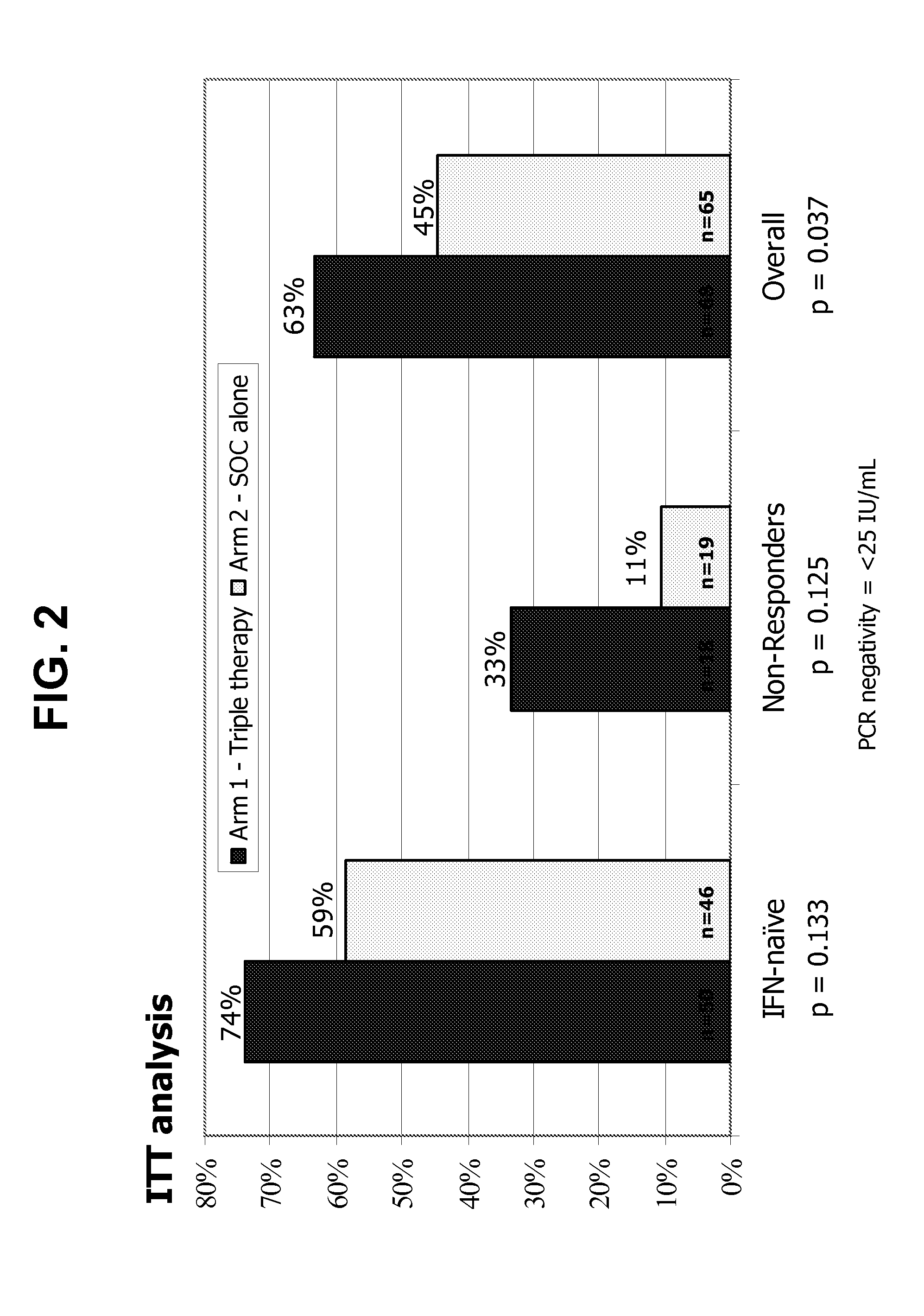
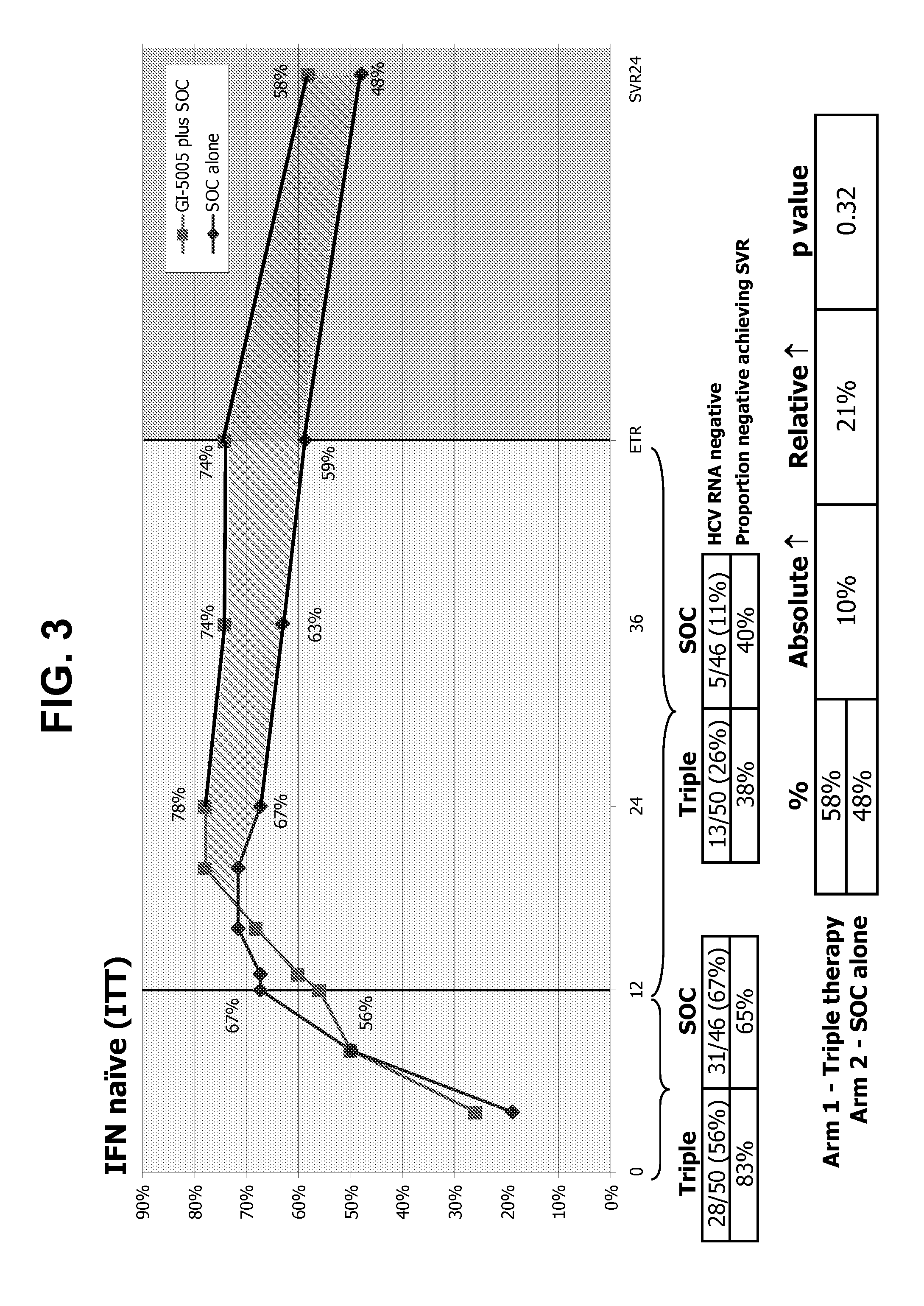
39results about How to "Prolonged dosing time" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for producing a device applicable to biological tissues, particularly a patch for treating damaged tissues, and a device obtained by said method
ActiveUS20120070485A1Slow kineticsEasy to controlPeptide/protein ingredientsMetabolism disorderHaemostatic functionCross-link
The present invention relates to a device consisting of cross-linked nanofibrillary fibrin supported on and rooted to a microporous nonwoven fabric consisting of a biocompatible synthetic polymer material. An active ingredient is advantageously dispersed in the fibrin layer. The fibrin layer does not have a haemostatic function, but is suitable for retaining the active ingredient and releasing it with controlled kinetics. The device forming the object of the invention, preferably in the form of patches, is useful for in vitro cell cultures or for treating tissues damaged by wounds or necrosis, such as cardiac walls bearing the sequelae of infarction, or a tissue damaged by a diabetic ulcer. The patch according to the invention can be manufactured by inducing the polymerisation of the fibrin, under suitable conditions, directly on the support layer, which is suitably impregnated with thrombin (at least in a superficial portion of its thickness), and which has been conveniently prepared by means of a spray phase-inversion technique.
Owner:CONSIGLIO NAT DELLE RICERCHE +1
Packaged particulate detergent composition
ActiveUS8883702B2Lower surfaceHigh trafficInorganic/elemental detergent compounding agentsCapsParticulatesWater soluble
A packaged particulate detergent composition, wherein the composition comprises greater than 40 wt % detergent surfactant, at least 70% by number of the particles comprising a core, comprising mainly surfactant, and around the core, a water soluble coating in an amount of from 10 to 45 wt % based on the coated particle, each coated particle having perpendicular dimensions x, y and z, wherein x is from 0.2 to 2 mm, y is from 2.5 to 8 mm, and z is from 2.5 to 8 mm, the packaged particles being substantially the same shape and size as one another.
Owner:CONOPCO INC D B A UNILEVER
Method for producing a device applicable to biological tissues, particularly a patch for treating damaged tissues, and a device obtained by said method
ActiveUS8628787B2Slow kineticsEasy to controlBiocidePeptide/protein ingredientsHaemostatic functionCross-link
The present invention relates to a device consisting of cross-linked nanofibrillary fibrin supported on and rooted to a microporous nonwoven fabric consisting of a biocompatible synthetic polymer material. An active ingredient is advantageously dispersed in the fibrin layer. The fibrin layer does not have a haemostatic function, but is suitable for retaining the active ingredient and releasing it with controlled kinetics. The device forming the object of the invention, preferably in the form of patches, is useful for in vitro cell cultures or for treating tissues damaged by wounds or necrosis, such as cardiac walls bearing the sequelae of infarction, or a tissue damaged by a diabetic ulcer. The patch according to the invention can be manufactured by inducing the polymerisation of the fibrin, under suitable conditions, directly on the support layer, which is suitably impregnated with thrombin (at least in a superficial portion of its thickness), and which has been conveniently prepared by means of a spray phase-inversion technique.
Owner:CONSIGLIO NAT DELLE RICERCHE +1
Preparation method of ivermectin sustained-release microspheres
ActiveCN102302457AMicrosphere surface roundingImprove uniformityOrganic active ingredientsPharmaceutical non-active ingredientsMass ratioMicrosphere
The invention relates to ivermectin sustained-release microspheres used as an animal medicine for animal injection, and a preparation method thereof. The invention belongs to an interdisciplinary field of biomedical polymer materials and controlled release preparations. The invention discloses ivermectin sustained-release microspheres, which are characterized in that: the ivermectin sustained-release microspheres have particle sizes of 0.1 to 10mum, a medicine loading capacity of 20% to 35%, and an entrapment rate of above 80%. The preparation method of the ivermectin sustained-release microspheres comprises steps that: a carrier material and the medicine ivermectin with a mass ratio of 1:1-10 are dissolved in an organic solvent; the solution is processed through supersonic wave or mechanical stirring, such that the solution is emulsified into an oil phase, wherein a volume ratio of oil phase to water phase is 1:5-10; under a temperature of -10 DEG C to 30 DEG C, the oil phase is injected into a water phase disperse medium solution step-by-step; constant-temperature magnetic stirring is carried out with a rotation speed of 400 to 10000rpm, the mixture is sufficiently emulsified, such that an S / O / W type emulsion is obtained; the emulsion is stirred under room temperature for 3 to 4 hours, such that the organic solvent is sufficiently volatilized; the obtained product is centrifuged, washed, collected, and is vacuum-dried with a pressure of 0.01 to 0.04MPa under room temperature for 10 to 12 hours or is lyophilized under a temperature of -0 DEG C to -20 DEG C. With the processes, ivermectin sustained-release microspheres with particle sizes of 0.1 to 10mum are obtained.
Owner:INST OF MODERN PHYSICS CHINESE ACADEMY OF SCI
Long-acting slow release preparation for treating keratomycosis as well as preparation method and application thereof
ActiveCN104940936AHigh drug loadingFormulation stabilityOrganic active ingredientsSenses disorderAntifungalSide effect
The invention relates to a preparation for treating treating keratomycosis, and particularly relates to a long-acting slow release preparation for treating keratomycosis as well as a preparation method and application thereof. The substrate of the long-acting slow release preparation is formed through electrostatic bonding of a graphene material and a drug-carried chitosan material, drugs are carried on the sheet structure of the graphene material, the structure is stable, and the loading rate is high. The preparation provided by the invention has excellent bacteriostatic activity on fungus and bacterium, and is excellent in cytocompatibility, toughness, and tensile strength. The preparation can be simply and conveniently applied onto cornea, and has no toxic effects on normal tissue while achieving excellent bacteriostatic activity. The long-acting slow release preparation is taken as a dosage form in ophthalmology, can be adhered onto a cornea of a patient for long-acting slow release, so as to achieve effective drug concentration, and the preparation has the advantages that the preparation is simple and convenient, the drug-carried material self is excellent in bacteriostatic activity and mechanical property, stimulation and toxic or side effects on orbital tissue can be avoided, and the use is convenient.
Owner:SOUTH CHINA AGRI UNIV
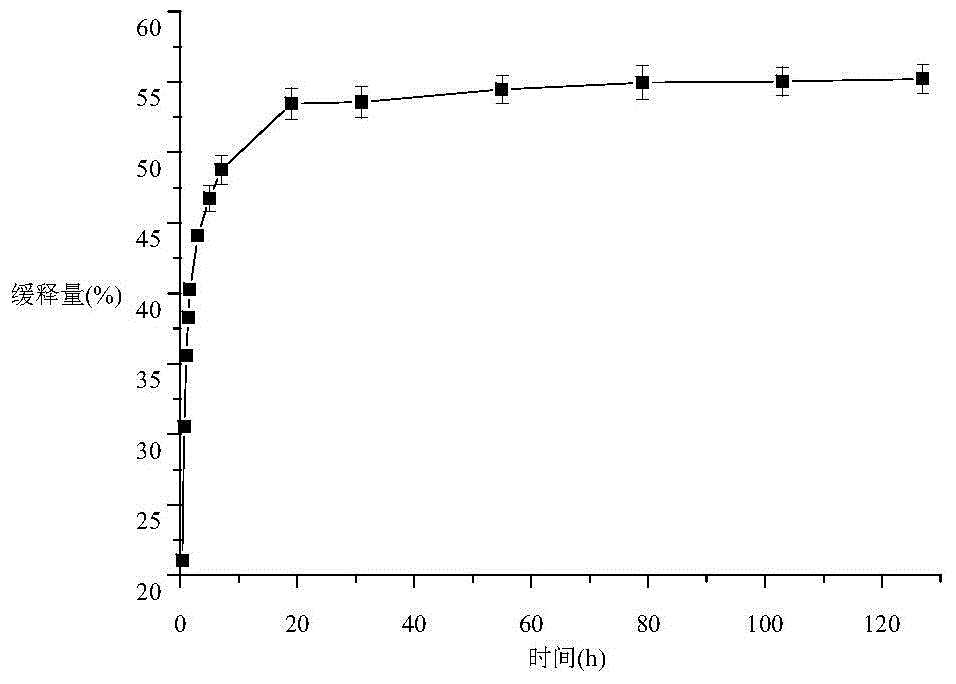
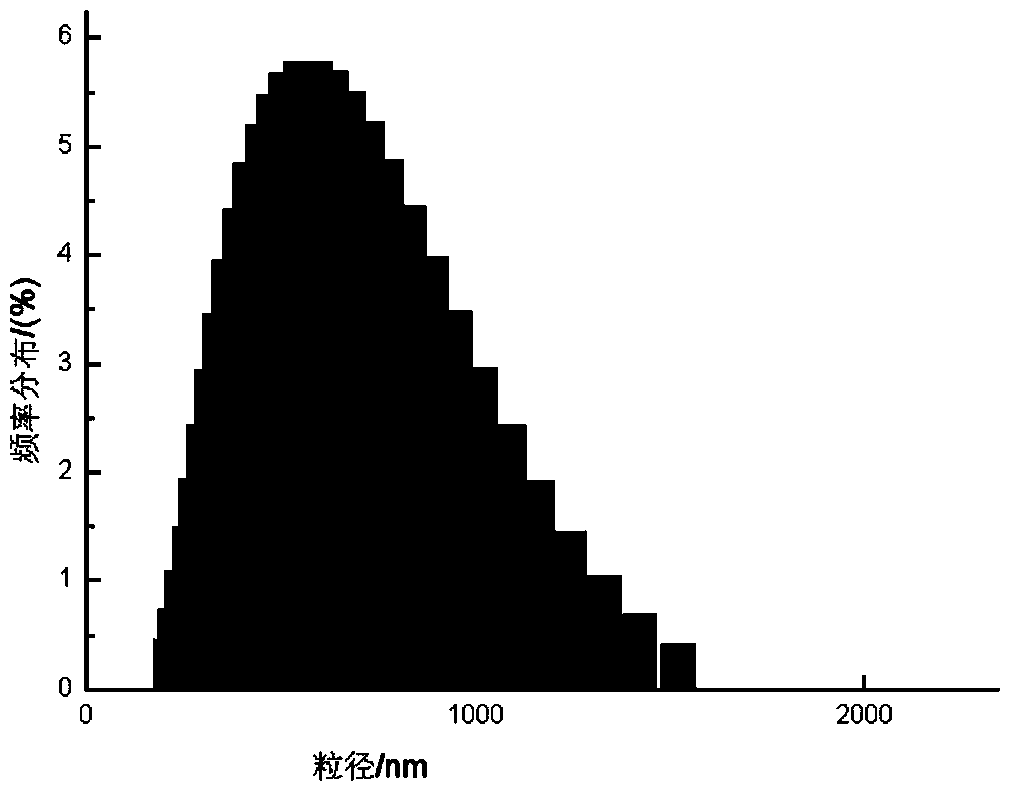
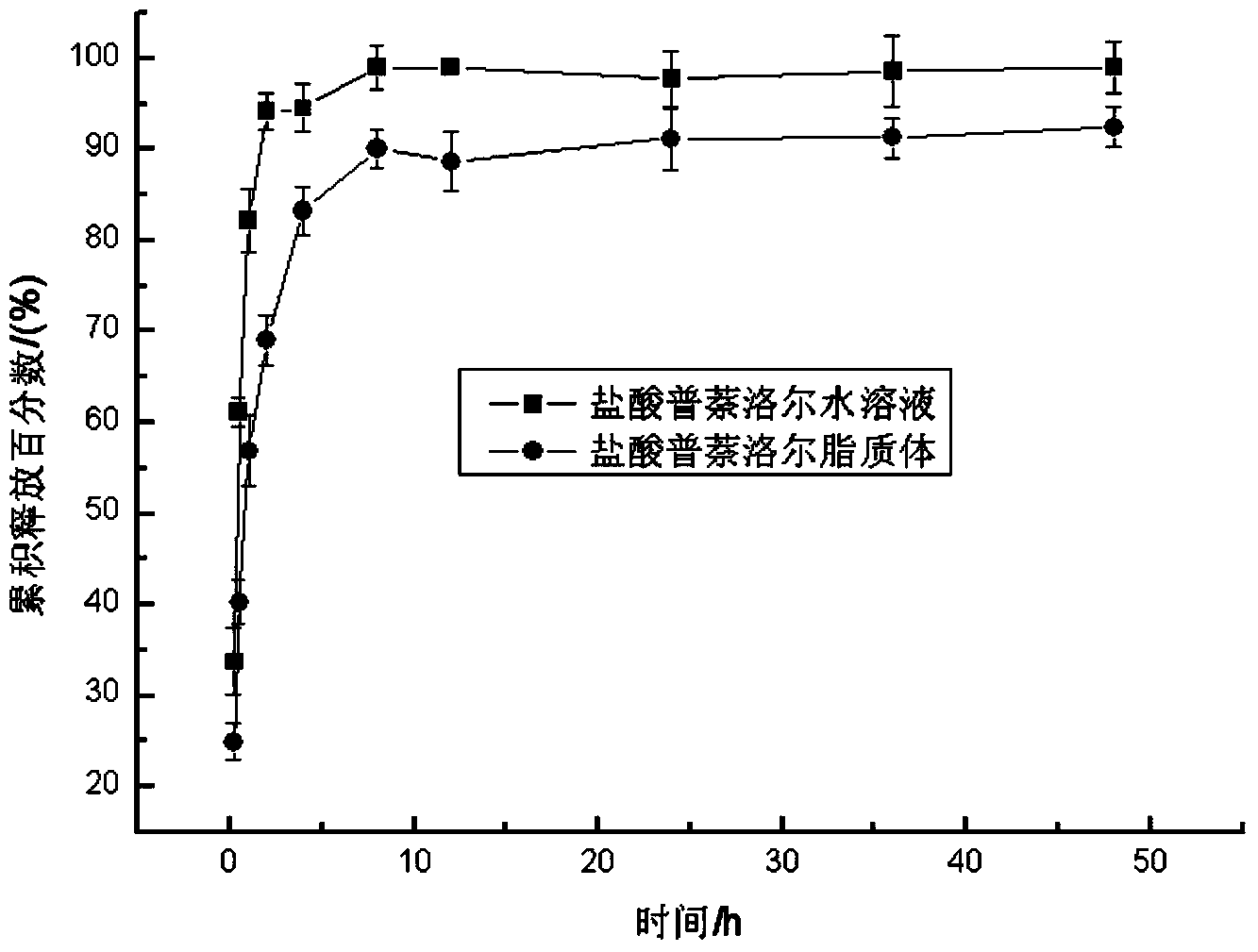
Propranolol hydrochloride lipidosome gel and preparation method thereof
ActiveCN103622903AImprove stabilityProlonged dosing timeOrganic active ingredientsAerosol deliveryWhole bodyCholesterol
The invention discloses a propranolol hydrochloride lipidosome gel, which is prepared by the following bulk drugs and auxiliary materials by weight percent: 0.012-0.075% of propranolol hydrochloride, 0.037-0.150% of phosphatidyl ethanolamine, 0.012-0.075% of cholesterol, 2.5-5% of triethanolamine, 1-2% of carbopol, and the balance of water. According to the propranolol hydrochloride lipidosome gel, during the preparation, the lipidosome is uniformly dispersed in the gel, so that the stability of the lipidosome is improved; the water-soluble gel carbopol has excellent biocompatibility, can be well adhered to skin and cannot stimulate skin; drug administration time is prolonged, the toxic and side effects on the whole body are reduced, the adaptability of patients is improved, and the propranolol hydrochloride lipidosome gel has excellent application prospect.
Owner:SHANDONG UNIV
Method and device for soft capsule micro-jet type micro-needle transdermal delivery
InactiveCN106110490AInhibitory activityGood treatment effectMicroneedlesMedical devicesMicro-needleMembrane configuration
The invention relates to the technical field of micro-needle transdermal delivery, in particular to a method and a device for soft capsule micro-jet type micro-needle transdermal delivery. The drug deliver device comprises a soft capsule which is used for storing medicine liquid, a micro-jet micro-needle unit which is capable of undergoing mechanical reciprocating vibration, a fixing unit and a controller, wherein one side of the soft capsule is bonded with and connected to a membrane of the micro-jet micro-needle unit and the other side of the soft capsule is bonded with and connected to the fixing unit; the micro-jet micro-needle unit is connected to the controller by virtue of a signal line; and the controller is used for controlling a delivery dosage of the medicine liquid and for controlling the micro-jet micro-needle unit to do mechanical reciprocating vibration before each drug delivery. With the implementation of the method disclosed by the invention, by precisely controlling the drug delivery dosage and by controlling the micro-jet micro-needle unit to do mechanical reciprocating vibration before each drug delivery, various pores can generate in skin cuticula, which is conducive to the medicine liquid to permeate skin epidermis; and in addition, the device disclosed by the invention can achieve change the medicine liquid safely without pollution, and the device is simple and convenient to operate and use.
Owner:唐晨
Cabazitaxel protein nanomaterial and preparation method thereof
InactiveCN110123786AUniform particle sizeHigh encapsulation efficiencyOrganic active ingredientsMacromolecular non-active ingredientsCrystallographyCabazitaxel
The invention is applicable to the technical field of pharmaceutical preparations, and provides a cabazitaxel protein nanomaterial and a preparation method thereof. The cabazitaxel protein nanomaterial comprises, by weight, 50-150 parts of cabazitaxel, 400-4000 parts of protein carrier materials and 50-300 parts of protein nano modifying materials. The cabazitaxel protein nanomaterial and the preparation method thereof have the advantages that the cabazitaxel is encapsulated in a spherical or ellipsoidal nano composed of the protein carrier materials and the protein nano modifying materials, or adhered to the surface of the nano, and the obtained cabazitaxel protein nanomaterial has uniform particle size, high encapsulation rate, and good sustained release effect and anti-tumor effect, sothat hypersensitive reactions caused by the use of co-solvents such as surfactants are effectively avoided, the toxicity of the preparations is reduced, the bioavailability is improved, the dosage isdecreased, and the medication time is prolonged.
Owner:深圳市健开医药有限公司 +1
Cabazitaxel protein nanometer injection and preparation method thereof
ActiveCN110075073AGood sustained release effectImprove anti-tumor effectOrganic active ingredientsPowder deliveryCabazitaxelFreeze-drying
The invention is applicable to the technical field of medicinal preparations, and provides a cabazitaxel protein nanometer injection and a preparation method thereof. The cabazitaxel protein nanometerinjection is prepared from the following raw materials in parts by weight: 50-150 parts of cabazitaxel, 400-4000 parts of a protein carrier material, 50-300 parts of a protein nanometer modified material, and 12-100 parts of a freeze-drying protective agent. According to the cabazitaxel protein nanometer injection and the preparation method thereof, the cabazitaxel is encapsulated into a spherical or ellipsoidal nanometer material formed by the protein carrier material and the protein nanometer modified material or is attached to the surface of the spherical or ellipsoidal nanometer material,and freeze-drying treatment is performed by using the freeze-drying protective agent; the obtained cabazitaxel protein nanometer injection has a relatively good slow-release effect, and has a relatively good anti-tumor effect at the same time, hypersensitivity caused by the use of cosolvents such as surfactants is effectively avoided, toxicity of the preparations is reduced, the bioavailability is improved, administration dosage is reduced, and administration time is prolonged.
Owner:深圳市健开医药有限公司 +1
Packaged particulate detergent composition
ActiveUS20130196892A1Lower surfaceHigh trafficInorganic/elemental detergent compounding agentsClosure capsParticulatesWater soluble
A packaged particulate detergent composition, wherein the composition comprises greater than 40 wt % detergent surfactant, at least 70% by number of the particles comprising a core, comprising mainly surfactant, and around the core, a water soluble coating in an amount of from 10 to 45 wt % based on the coated particle, each coated particle having perpendicular dimensions x, y and z, wherein x is from 0.2 to 2 mm, y is from 2.5 to 8 mm, and z is from 2.5 to 8 mm, the packaged particles being substantially the same shape and size as one another.
Owner:CONOPCO INC D B A UNILEVER
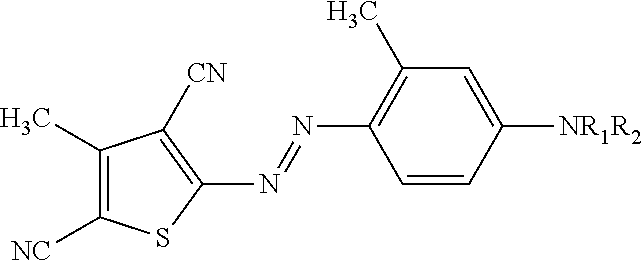
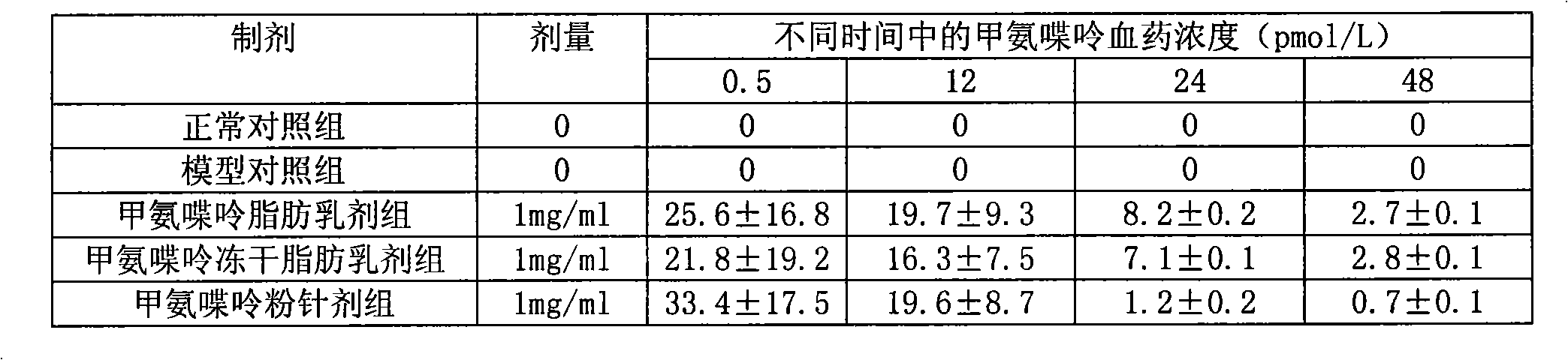
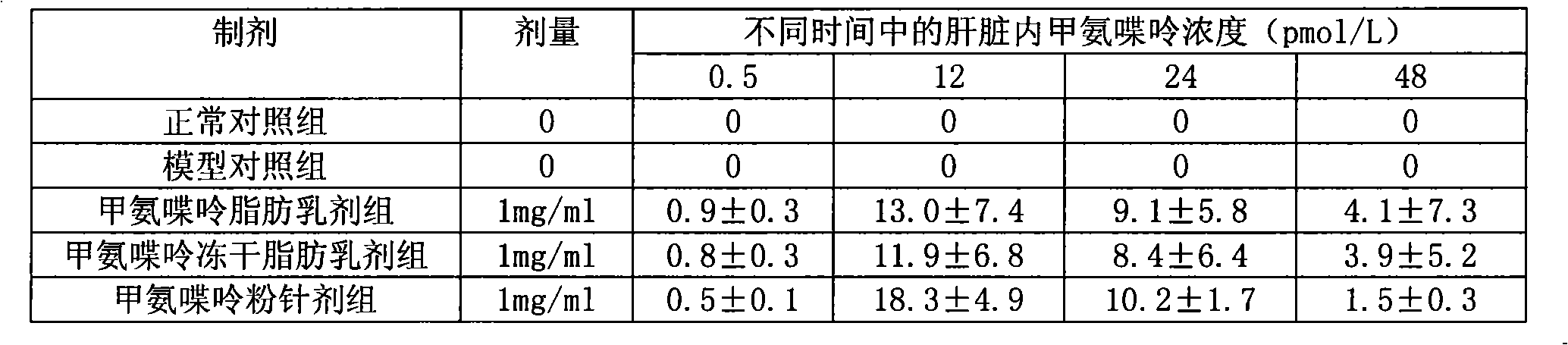
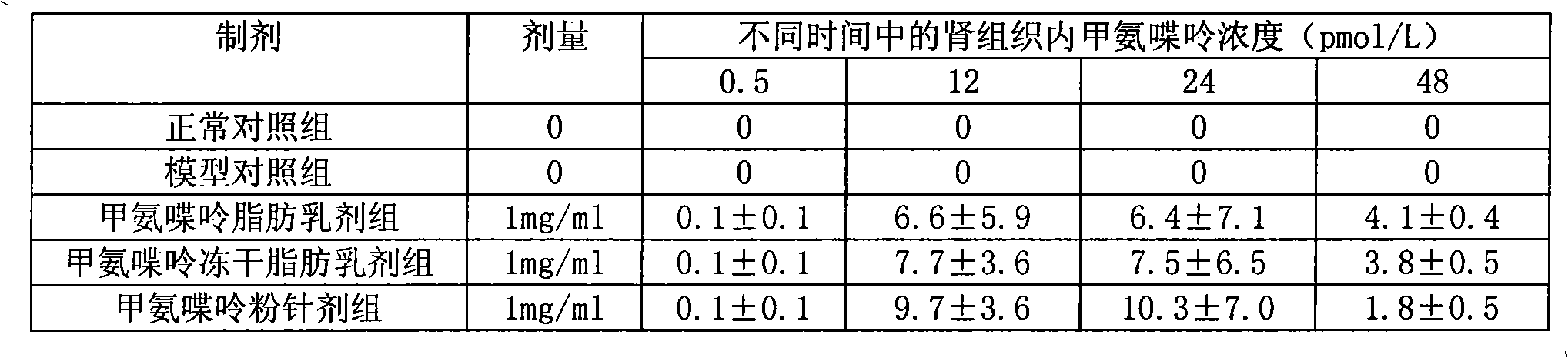
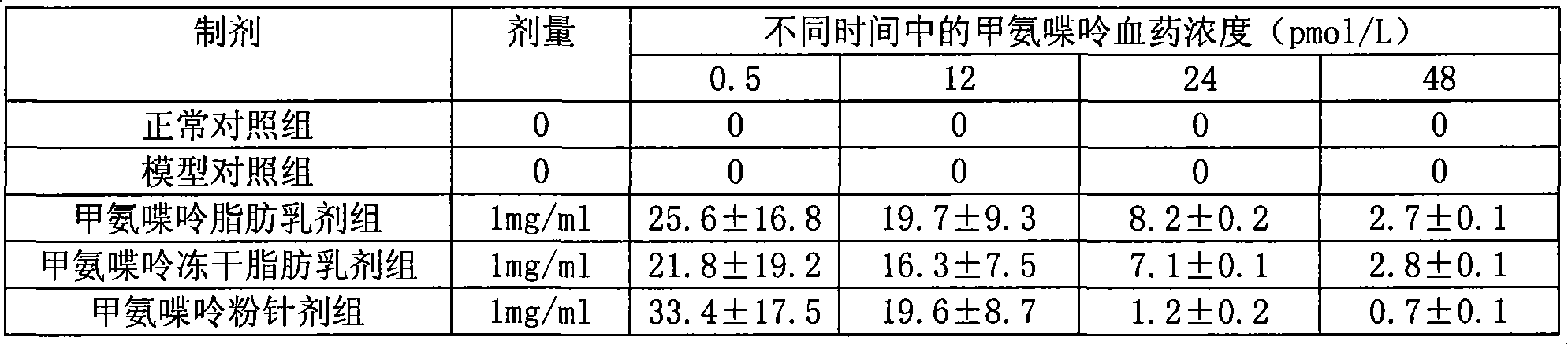
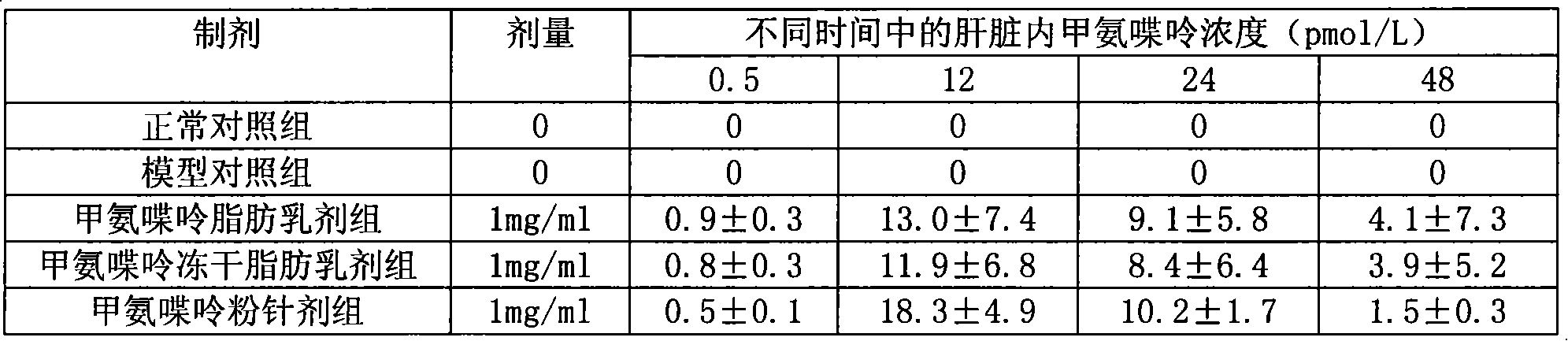
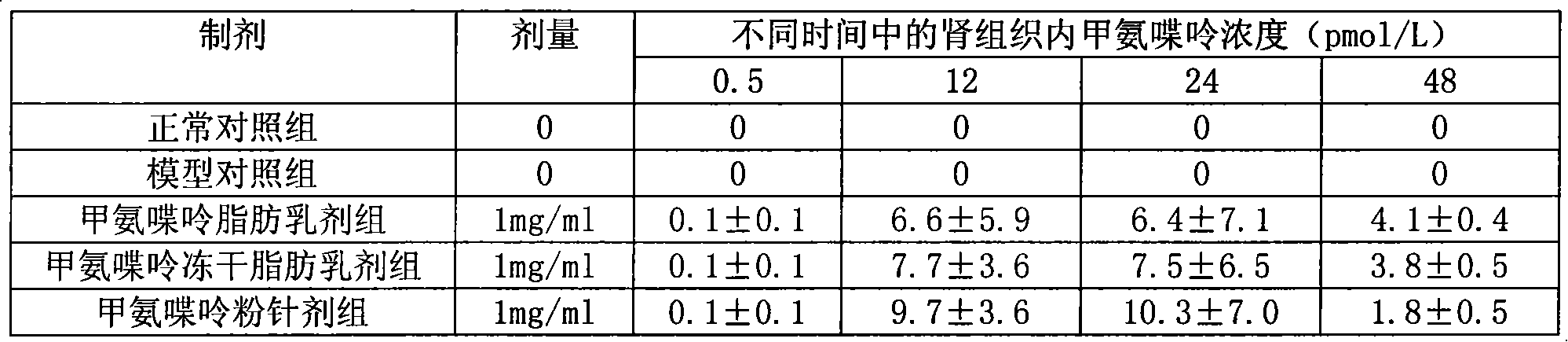
Methopterin intralipid, its freeze drying agent, preparation method and application thereof
ActiveCN101317818AReduce adverse reactionsReduce the single doseOrganic active ingredientsAntipyreticDiseaseAntioxidant
The invention relates to methotrexate intralipid, a freeze-dried agent, a preparation method and an application thereof. The methotrexate intralipid consists of methotrexate active ingredient, oil for injection, emulsifying agent, stabilizing agent, isotonic adjustment agent, antioxidant, amylose, water for injection and pH modifying agent. The methotrexate intralipid of the invention is freeze-dried to obtain the freeze-dried agent of the methotrexate intralipid. The methotrexate intralipid and the freeze-dried agent of methotrexate intralipid can be applied to the medicines for treating a tumor and rheumatoid diseases.
Owner:叶志中 +3
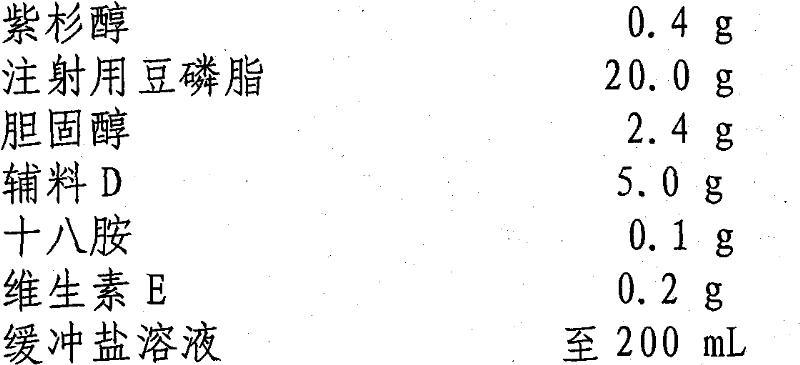
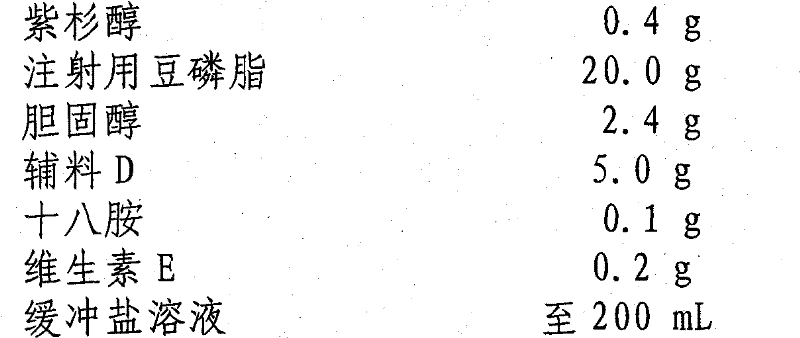
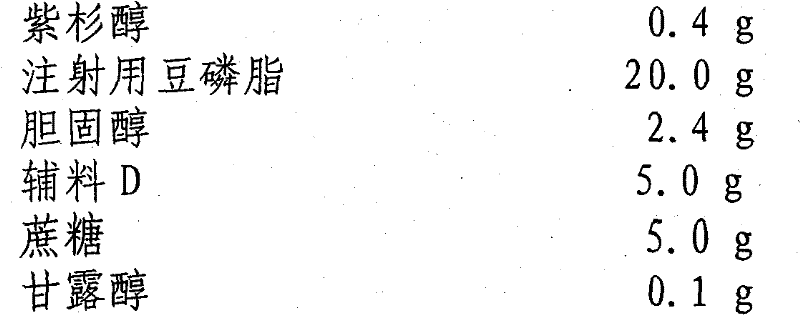
Paclitaxel liposome and preparation method thereof
InactiveCN101015525BSolve the disadvantage of water insolubilityImprove complianceOrganic active ingredientsAntineoplastic agentsAdjuvantCholesterol
Owner:SHENYANG PHARMA UNIVERSITY
Ethyl cellulose drug-loading nanofiber membrane and preparation method and application thereof
InactiveCN106048902ASmall toxicityProlonged dosing timePharmaceutical non-active ingredientsNon-woven fabricsFiberNanofiber
The invention relates to an ethyl cellulose drug-loading nanofiber membrane and preparation method and application thereof. The components of the nanofiber membrane include ethyl cellulose and drug of which the mass ratio is 1:10 to 1:20. The preparation method includes the steps of: adding the drug to an ethyl cellulose (EC) solution, and stirring to disperse uniformly, thereby obtaining a spinning solution; and performing electrostatic spinning, and drying an obtained fibrous membrane, thereby obtaining an ethyl cellulose drug-loading nanofiber membrane. The preparation method provided by the invention is simple and feasible; and the prepared nanofiber membrane is a hydrophobic nanometer material having a drug slow-release function and can be used as a biomedical material.
Owner:DONGHUA UNIV
Medicament for easing pain and eliminating tumour and preparation method thereof
InactiveCN101293086AGood adhesionProlonged dosing timeAntipyreticInorganic active ingredientsDrugToxicity
The invention discloses a drug for relieving pain and eliminating tumor and a preparation method thereof. The active ingredients of the drug for relieving pain and eliminating tumor are realgar and ginger, the weight ratio of the realgar and the ginger is (1 to 3): (1 to 3); and the particle size of the realgar and the ginger is 60 to 500nm. The drug further comprises a carrier, and the weight ratio of the realgar, the ginger and the carrier is (1 to 3): (1 to 3): (4 to 10). The drug for relieving pain and eliminating tumor can be prepared into a cream for relieving pain and eliminating tumor after being added the carrier. The drug for relieving pain and eliminating tumor of the invention has insignificant toxicity and side effects, and the plasma-drug concentration is stable.
Owner:UNIV OF SCI & TECH OF CHINA +1
Preparation method and application of medicament for treating insomnia and gel thereof
InactiveCN105641103ABrain bioavailability is highProlonged dosing timeNervous disorderAerosol deliveryPoloxamerPhosphate
The invention discloses a medicament for treating insomnia and a preparation method and application thereof. The medicament for treating insomnia is composed of the following components in mass percent: 2-6% of jujuboside extract, 16-22% of poloxamer P407, 1-6% of poloxamer P188, 6-12% of PEG400, 0.01-0.03% of nipagin and balance of phosphate buffer solution, wherein the sum of the mass percents is 100%. The medicament for treating insomnia disclosed by the invention adopts a temperature-sensitive gel as a dosage form of jujuboside, thus after the medicament is dripped in liquid form into the nasal cavity, the medicament can flow onto mucous membrane in the deeper olfactory region where the brain bioavailability is verified high for administration; the medicament changes into semi-solid with a biological adhesion action in the nasal cavity, thereby continuously attaching to the mucous membrane in the olfactory region, solving the problem that the traditional dosage form is liable to lose and result in lower bioavailability and transient administration, and improving the sleeping quality of patients continuously.
Owner:XIAN MEDICAL UNIV
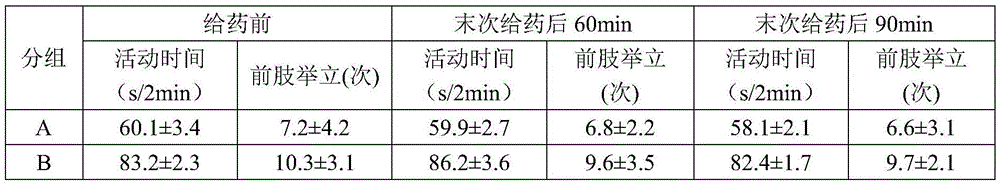
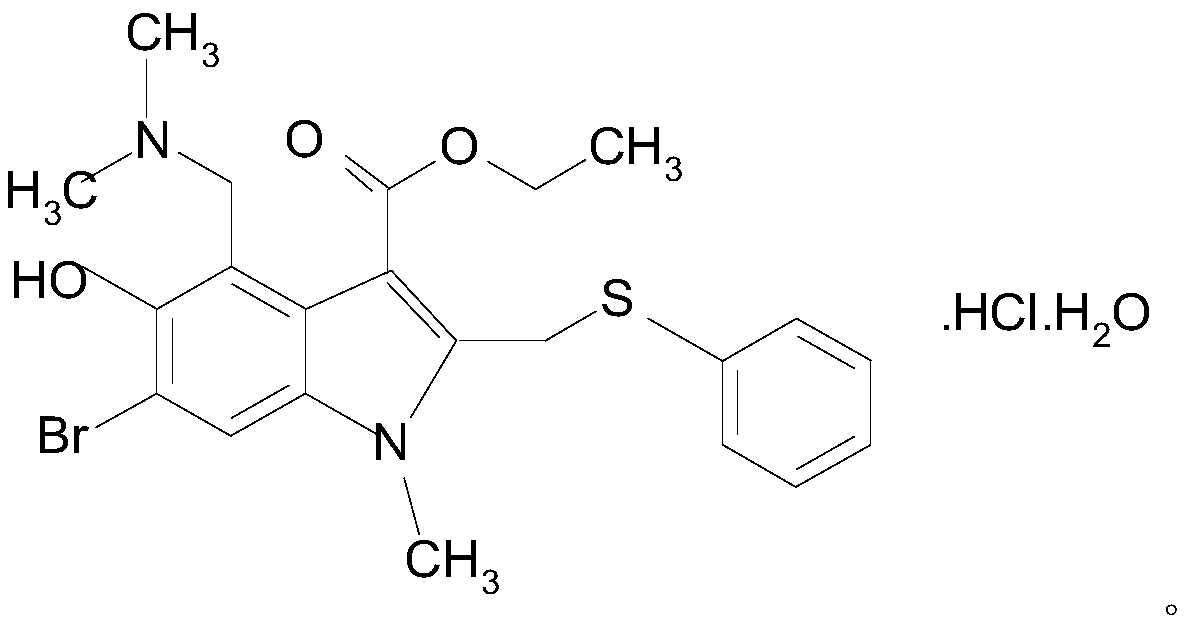
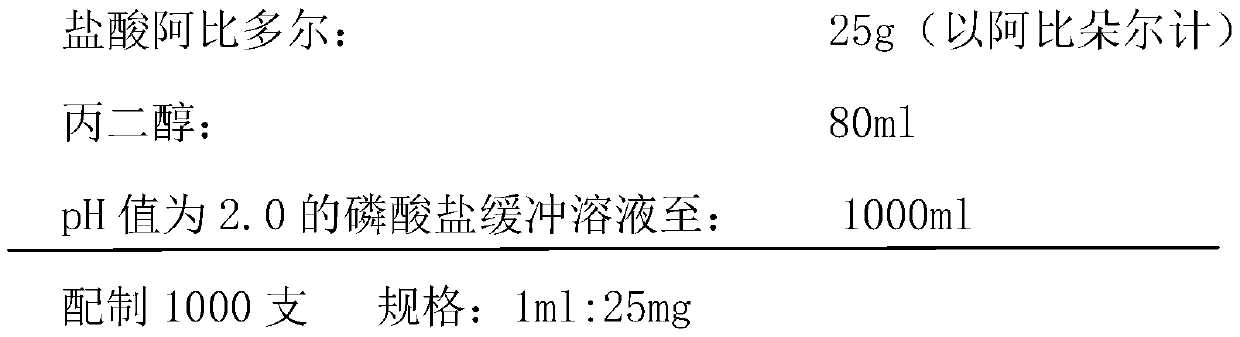
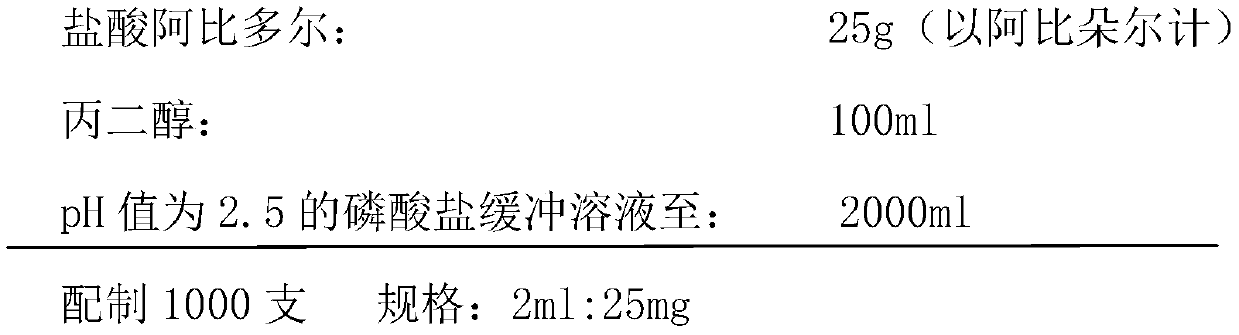
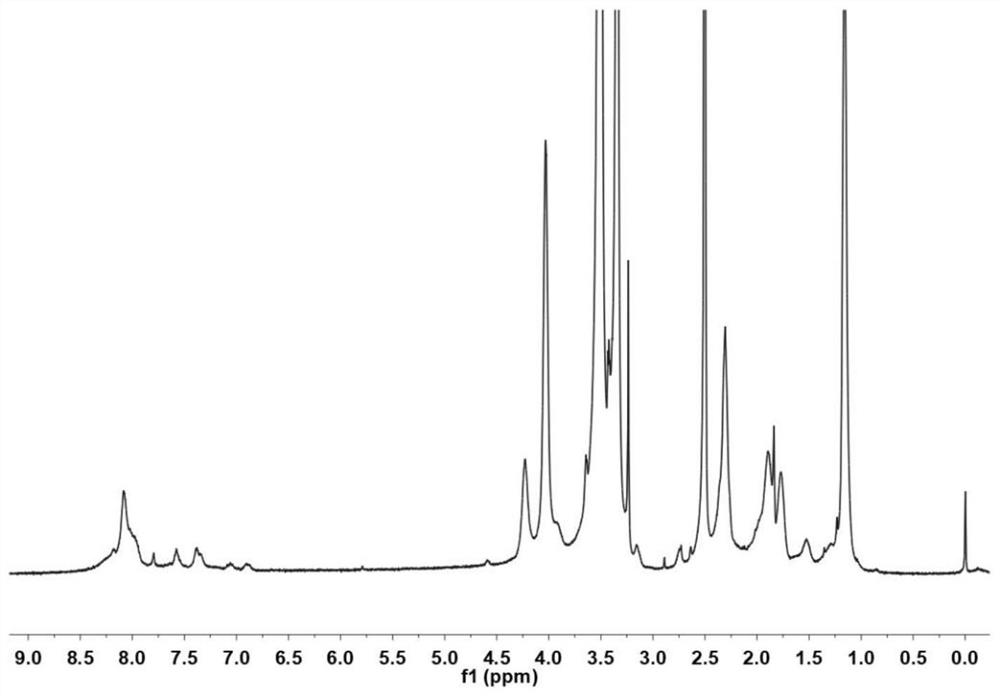
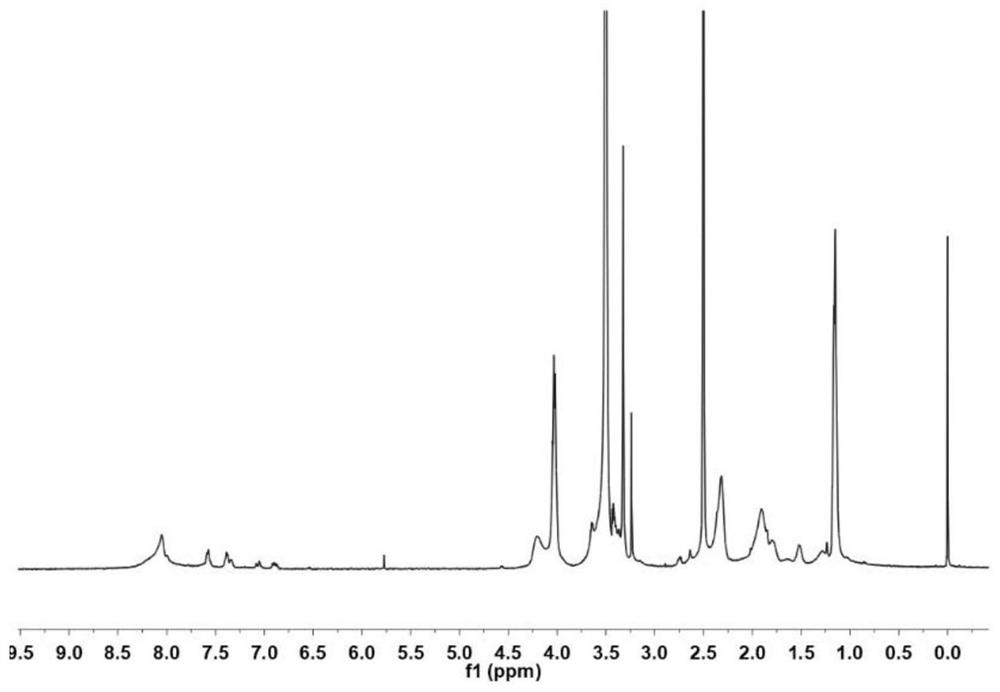
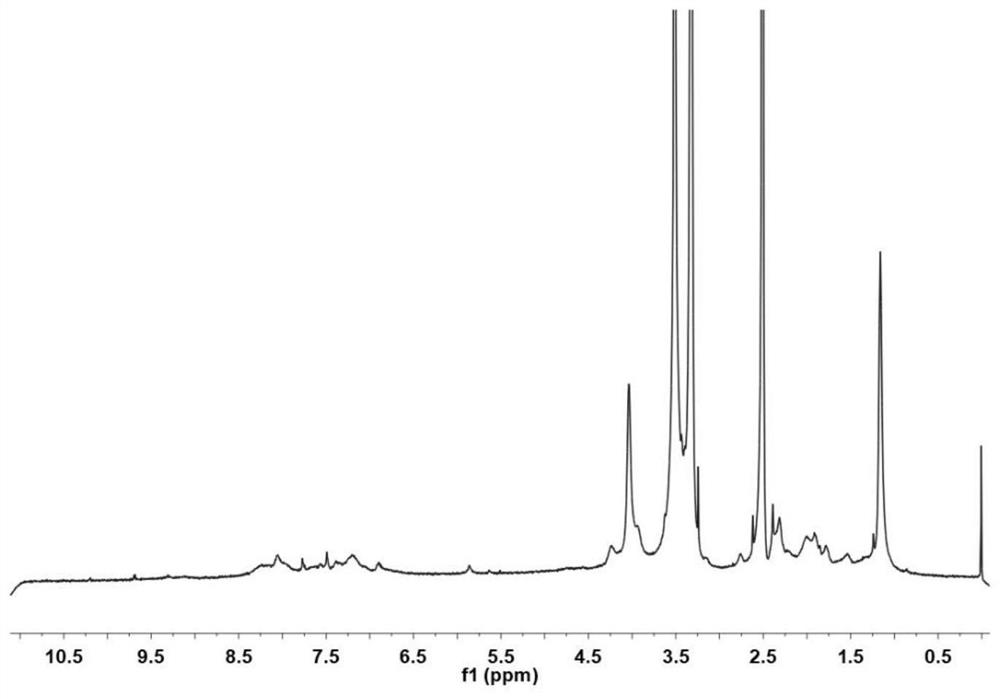
Arbidol hydrochloride injection preparation and preparation method thereof
InactiveCN110664747AImprove bioavailabilityProlonged dosing timeOrganic active ingredientsInorganic non-active ingredientsPharmaceutical medicineBULK ACTIVE INGREDIENT
The invention discloses an arbidol hydrochloride injection preparation. The injection preparation is prepared from an active ingredient arbidol hydrochloride, a pharmaceutically acceptable co-solventand a buffer solution. The invention further discloses a preparation method of the arbidol hydrochloride injection preparation. The preparation method comprises the following steps: adding the buffersolution and the co-solvent, performing uniform stirring, and slowly and uniformly adding arbidol hydrochloride into the mixture to sufficiently dissolve the arbidol hydrochloride in the mixture; adjusting the pH value of the mixture, adding activated carbon into the mixture, and performing uniform stirring; performing decarbonization, filtering filtrate through a microfiltration membrane, fillinga sterilized ampoule with the filtrate, and sealing the sterilized ampolue; and finally, performing water-bath sterilization. The arbidol hydrochloride injection preparation is applicable to intramuscular injection and is mainly used for preventing and treating type A and type B influenza and other acute respiratory virus infections. The injection preparation can be used for improving the bioavailability of the arbidol hydrochloride, prolonging the administration time, effectively reducing cross contamination, improving the stability of the injection preparation and facilitating clinical application.
Owner:河南合智医药科技有限公司
Cinnamyl aldehyde modified polyethylene glycol-polyamino acid block copolymer, preparation method thereof, and hydrogel
ActiveCN112029092AImprove solubilityProlonged dosing timeAntibacterial agentsAntimycoticsCinnamic aldehydeAldehyde
The invention provides a cinnamyl aldehyde modified polyethylene glycol-polyamino acid block copolymer. The cinnamyl aldehyde modified polyethylene glycol-polyamino acid block copolymer comprises a first block with a structure as shown in a formula I or a formula II and a second block with a structure as shown in a formula III. On the basis of the traditional temperature-sensitive polyurethane hydrogel, the temperature-sensitive hydrogel formed by the block copolymer provided by the invention can be used for supporting an aldehyde micromolecular drug cinnamyl aldehyde from a natural source through a chemical bonding effect, regulating the bonding ratio of cinnamyl aldehyde, improving the solubility of cinnamyl aldehyde and prolonging the drug administration time through a sustained and controlled release effect, and the drug administration efficiency is increased. The drug-loaded temperature-sensitive hydrogel provided by the invention has temperature sensitivity and a drug loading effect at the same time, and can be singly used or combined with other drugs by utilizing the pharmacological action of cinnamyl aldehyde which is an aldehyde small molecular drug, so that the further application of the hydrogel is expanded.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
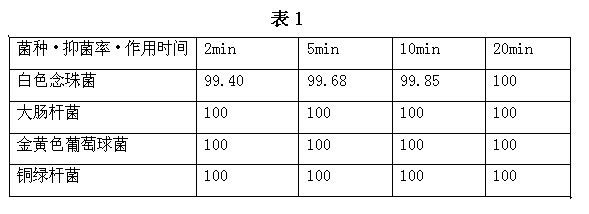
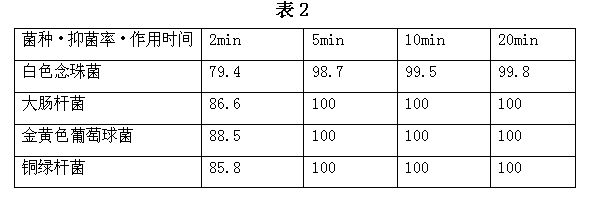
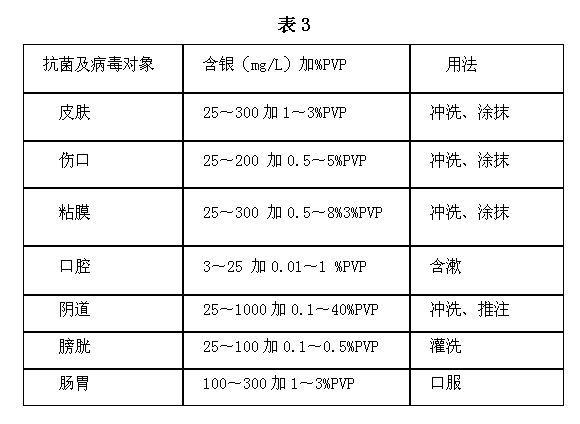
Nanometer silver antibiotic and antiviral compound liquid and its preparation method and products
ActiveCN102499944BImprove antibacterial and antiviral effectsHigh affinityAntibacterial agentsBiocideSilver colloidPolyvinylpyrrolidone
The invention discloses a nanometer silver antibiotic and antiviral compound liquid and its preparation method and products. The compound liquid is prepared by using materials of nanometer silver colloid and polyvinylpyrrolidone, wherein the weight of polyvinylpyrrolidone is 0.01-40% of the weight of the nanometer silver colloid. The invention solves problem that the silver nanometer particles have poor affinity with cells and partially coagulate in long-term preservation when the nanometer silver colloid is in antibiotic and antiviral practical application; the nanometer silver antibiotic and antiviral compound liquid has characteristics of practicability, economy, intoxicity and high efficiency.
Owner:秦社宣
Application of all-cannabinoid in preparation of drug for treating Parkinson's disease
InactiveCN105963358AIncrease vitalityDelay disease progressionNervous disorderPlant ingredientsDynamic balanceCannabinoid
The invention discloses application of all-cannabinoid in preparation of a drug for treating a Parkinson's disease (PD). The all-cannabinoid is prepared by uniformly mixing, by weight, 0.3-99.7 parts of an industrial hemp seed extract with 99.7-0.3 parts of industrial cannabinoids. Experiments prove that the prepared all-cannabinoid has the effects of removing free radicals, inhibiting lipid peroxidation and enabling generation and removal of oxygen radicals to be in dynamic balance to slow disease progress of the PD. The all-cannabinoid has a good application prospect on preparation of the drug for treating the Parkinson's disease.
Owner:云南瑞酚生物科技有限公司
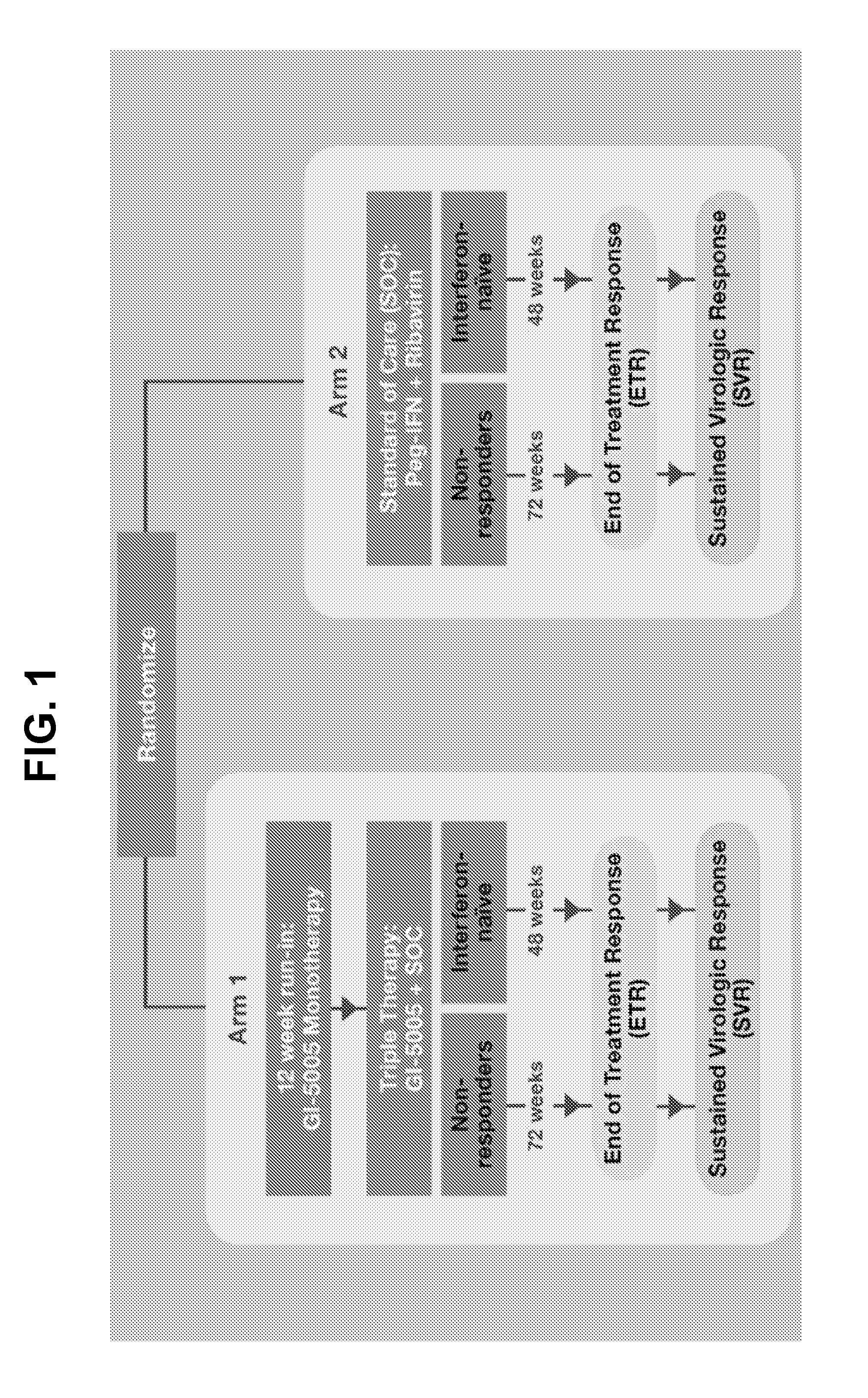
Pharmacogenomic and Response-Guided Treatment of Infectious Disease Using Yeast-Based Immunotherapy
InactiveUS20130121964A1Reducing dosage and duration of administration and frequency of administrationProlonged dosing timeOrganic active ingredientsSsRNA viruses positive-senseGenomicsIl28b genotype
Disclosed are improved methods for treating an infectious disease with yeast-based immunotherapy, including viral disease, such as disease resulting from hepatitis virus infection, using a pharmacogenomic and response-guided approach based on IL28B genotype of the individual.
Owner:GLOBE IMMUNE INC
Chinese medicinal patch for treating polycystic ovary syndrome and preparation method thereof
InactiveCN102430028AEasy to takeImprove complianceSexual disorderOil/fats/waxes non-active ingredientsShiny bugleweedPolycystic ovary
The invention relates to a Chinese medicinal preparation, in particular to a Chinese medicinal patch for treating polycystic ovary syndrome and a preparation method thereof. The patch is prepared from the following components in part by weight: 20 to 40 parts of raw ark shell, 70 to 120 parts of white vinegar, 20 to 40 parts of Chinese angelica, 5 to 15 parts of cassia twig, 10 to 20 parts of nutgrass galingale rhizome (fried with vinegar), 10 to 30 parts of hiraute shiny bugleweed herb, 10 to 20 parts of red paeony root, 15 to 25 parts of raw white paeony root, 10 to 20 parts of liquoric root, 400 to 500 parts of sesame oil, 40 to 50 parts of beeswax and 4 to 8 parts of angelica oil. Animal experiments and clinical observation prove the curative effect of the patch, and prove that the effects of promoting blood circulation and removing blood stasis and eliminating dampness and dispelling phlegm of the patch are enhanced due to the compatibility of the medicines in a prescription.
Owner:SHANXI MEDICAL UNIV
A kind of cabazitaxel protein nano-injection and preparation method thereof
ActiveCN110075073BGood sustained release effectImprove anti-tumor effectOrganic active ingredientsPowder deliveryActive agentSurface-active agents
Owner:深圳市健开医药有限公司 +1
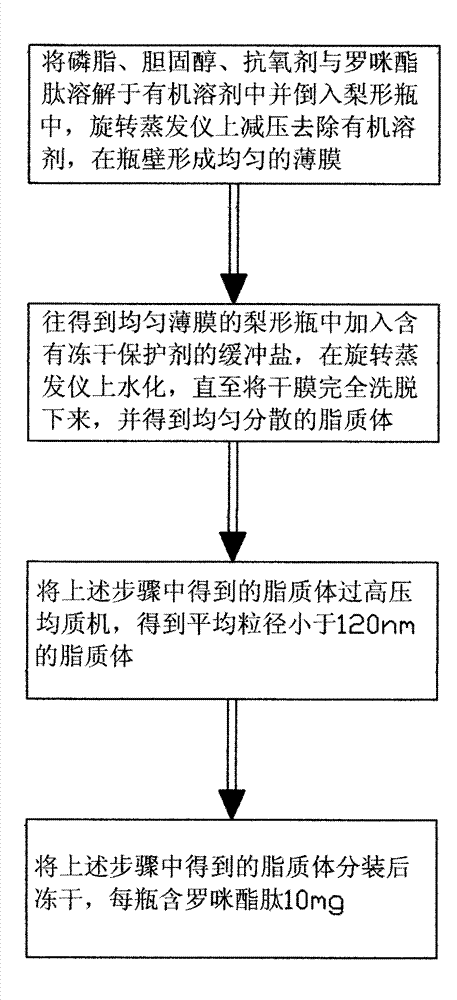
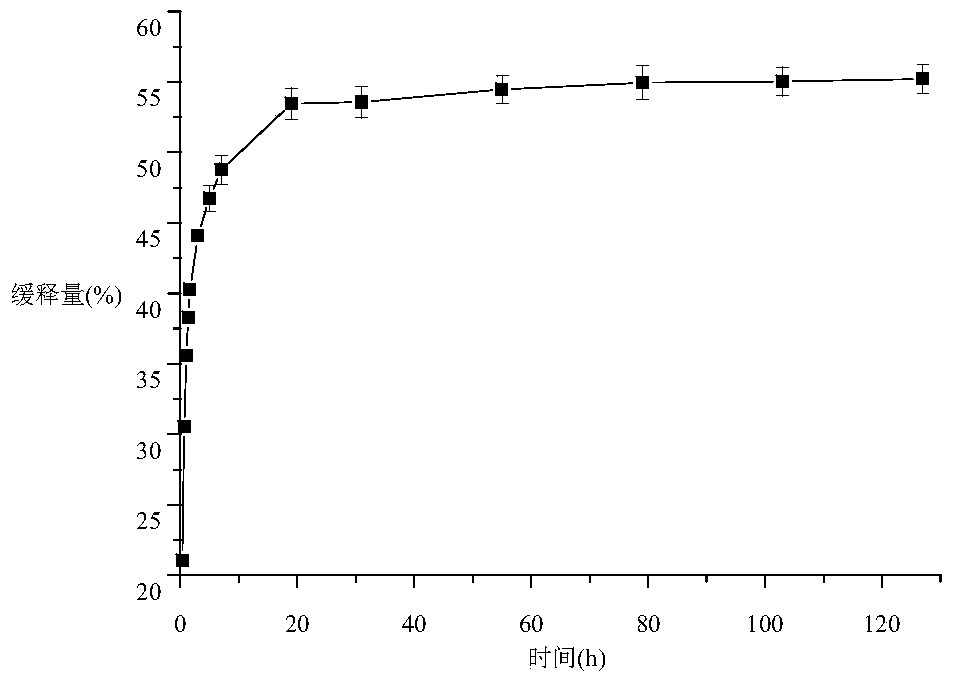
The method for preparing romidolipid liposome
ActiveCN103110931BReduce degradationImprove targetingCyclic peptide ingredientsAntineoplastic agentsSucroseSide effect
The invention discloses a method for preparing romidepsin lipidosome. The romidepsin lipidosome is prepared by using a film dispersion method through regarding cholesterol as a stabilizer, cane sugar as a cryoprotectant and vitamin E as an antioxidant, wherein the particle size of the lipidosome is made to be smaller than 120nm and the encapsulation efficiency is made to be greater than 85% by a high pressure homogenization method at the same time. The romidepsin lipidosome prepared by the method is capable of reducing the degradation of romidepsin, prolonging the administration time, improving the adaptability of patients, improving the targeting of romidepsin, increasing the anti-tumor effect, and reducing the toxic and side effects of drugs as all ingredients in the formula are physically compatible materials with high safety. Moreover, the method for preparing romidepsin lipidosome disclosed by the invention has the advantages of simple process and low cost, and is suitable for industrial production.
Owner:广州迈凯安生物医药研究院有限公司
Medicinal composition for regulating breath and nervous system and application of medicinal composition
ActiveCN106728453ASolve the problem that the price is too expensive and the cost performance is too poorIncrease inhalationNervous disorderPharmaceutical delivery mechanismNervous systemElecampane
The invention discloses a medicinal composition for regulating breath and nervous systems and application of the medicinal composition. The medicinal composition for regulating the breath and nervous systems comprises 100-500 parts of rhizoma atractylodis, 50-250 parts of Chinese violet, 100-500 parts of elecampane, 100-400 parts of folium artemisiae argyi and 20-80 parts of benzoin. The application refers to application of the medicinal composition for regulating the breath and nervous systems in preparing medicines for preventing / treating trachitis and rhinitis. By adopting the medicinal composition, the problems that lung discomfort can be caused as relatively large smoke can be generated because of poor sublimability of medicinal components of medicines for treating trachitis and rhinitis in the current market, and the medicines are complex in formula, high in price of formula materials and very low in cost performance, can be solved. According to the medicinal composition, a fumigant of medicines for treating trachitis and rhinitis is added into a matrix of an electric mosquito-repellent incense piece, when being used, a common electric mosquito-repellent incense box is opened, the mosquito-repellent incense piece with the fumigant of the medicines for treating trachitis and rhinitis is inserted into the common electric mosquito-repellent incense box, after the box is powered on, effective medicinal components can be volatilized into the air through electric heating, and thus the medicinal composition can be breathed in when people sleep.
Owner:CHUXIONG MEDICAL COLLEGE
Methopterin intralipid, its freeze drying agent, preparation method and application thereof
ActiveCN101317818BReduce adverse reactionsReduce the single doseOrganic active ingredientsAntipyreticDiseaseAntioxidant
The invention relates to methotrexate intralipid, a freeze-dried agent, a preparation method and an application thereof. The methotrexate intralipid consists of methotrexate active ingredient, oil for injection, emulsifying agent, stabilizing agent, isotonic adjustment agent, antioxidant, amylose, water for injection and pH modifying agent. The methotrexate intralipid of the invention is freeze-dried to obtain the freeze-dried agent of the methotrexate intralipid. The methotrexate intralipid and the freeze-dried agent of methotrexate intralipid can be applied to the medicines for treating a tumor and rheumatoid diseases.
Owner:叶志中 +3
Long-acting sustained-release preparation for treating fungal keratitis, preparation method and application thereof
ActiveCN104940936BHigh drug loadingFormulation stabilityOrganic active ingredientsSenses disorderSide effectAntifungal drug
The invention relates to a preparation for treating antifungal drugs, in particular to a long-acting slow-release preparation for treating fungal keratitis and its preparation method and application. The matrix of the long-acting sustained-release preparation for the treatment of fungal keratitis according to the present invention is composed of graphene-like materials electrostatically combined with drug-loaded chitosan substances, and the drug is loaded on the sheet-like structure of graphene-like materials, and the structure is stable. Large load. The preparation has good antibacterial activity against fungi and bacteria, and has good cell compatibility, flexibility and tensile strength. The preparation can be easily and conveniently applied to the cornea, and has no toxic effect on normal tissues while achieving excellent antibacterial activity. As an ophthalmic dosage form, it can adhere to the patient's cornea for long-term sustained release to achieve an effective drug concentration, and the preparation is simple, the drug-loaded material itself has excellent antibacterial activity, good mechanical properties, and has no irritation to eye tissues. And toxic and side effects, easy to use and so on.
Owner:SOUTH CHINA AGRI UNIV
A pharmaceutical composition for regulating breathing and nervous system and its application
ActiveCN106728453BTargeted drug deliveryProlonged dosing timeNervous disorderPharmaceutical delivery mechanismNervous systemPharmaceutical drug
Owner:CHUXIONG MEDICAL COLLEGE
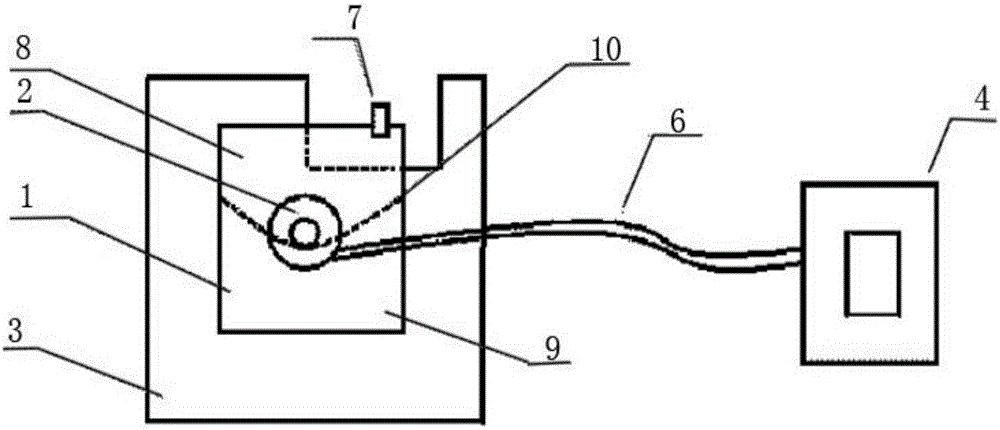
Vestibular administration device for nasal cavity
InactiveCN102125723BProlonged dosing timeAccurate dosageMedical devicesNasal cavityAdministration time
The invention discloses a vestibular administration device for a nasal cavity, which comprises an air bag and a catheter communicated with the air bag; the catheter is used for filling gas or liquid into the air bag; and an administration surface is arranged on the air bag. Compared with the conventional atomizing administration mode or cotton ball administration mode, the vestibular administration device has the advantages that: 1, the administration time is long, and the administration dose is accurate; 2, the administration device is prevented from being fallen off, is convenient to operate and does not influence aeration; 3, the application range is wide in clinical medical treatment; and 4, the vestibular administration device has a structure and low manufacturing cost, and is convenient to popularize and use.
Owner:上海白塔医药科技有限公司
An inductive vagina reconstruction device
The invention discloses an inductive vagina remanufacturing device. The existing moulds are hard rod bodies made of wood, glass, rubber and the like, the perineum is not comfortable after the moulds are placed into the perineum, the new wound face is easy to damage due to repeated taking out of the moulds for dressing change, and the remanufactured vagina is prone to infection. The device comprises an elastic ball bag, an aerating guide tube, a medicine injection tube, a one-way valve and a pressure sensor. The elastic ball bag is of an integrated structure of a cylinder and a hemisphere after being aerated, a spiral groove is formed in the outer lateral face of the cylinder, and the elastic ball bag is of a hollow structure. Through holes are formed in the lower bottom face of the cylinder of the elastic ball bag and the spherical face of the hemisphere, the medicine injection tube penetrates the through holes, and the medicine injection tube and the elastic ball bag form a cavity. The aerating guide tube is arranged on the lower bottom face of the cylinder of the elastic ball bag, the one-way valve is arranged on the tail portion of the aerating guide tube, and the pressure sensor is installed on one side of the one-way valve. The device is simple in structure. The size can be adjusted through aerating, in situ drainage and dressing change are achieved, damage to the new wound face caused by repeated taking out of the device is avoided, and operation success rate is improved.
Owner:张帆
Treatment equipment for improving efficacy of traditional Chinese medicines to resist novel coronavirus
PendingCN113813160ASolve the problem of the weak and sickly not adapting to a lot of sweatingProlonged dosing timeMedical devicesBathing devicesOral medicationDisease
The invention provides treatment equipment for improving the efficacy of traditional Chinese medicines to resist novel coronavirus. Modern medical research shows that the drug effect of nasal and lung administration is 16 times that of oral administration, which is proved by the appearance of atomized vaccines, traditional Chinese herbal medicine fumigation is nasal and lung administration, the difference between traditional Chinese herbal medicine fumigation and western medicine atomized administration is hot body perspiration and detoxification and drug efficacy strengthening and synergy, the drug efficacy can reach the deep part of a human body, pestilence is known as the cold-dampness epidemic cold category in traditional Chinese medicine, a method for coping with cold-dampness diseases is characterized in that heat is used for overcoming cold, the problem is that herbal medicine fumigation obstacles are that steam is difficult to tolerate, so that the administration time is restricted, and the treatment equipment disclosed by the invention is an extension innovation of the original invention patent CN201510111310.5, low-temperature herbal fumigation device, which can greatly improve the efficacy and solve the problem that oral traditional Chinese medicine can not treat severe diseases.
Owner:宫本海
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com