The method for preparing romidolipid liposome
A technology for romide liposide and romide liposome is applied in the field of preparing romide liposome liposomes to achieve the effects of prolonging administration time, reducing degradation and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
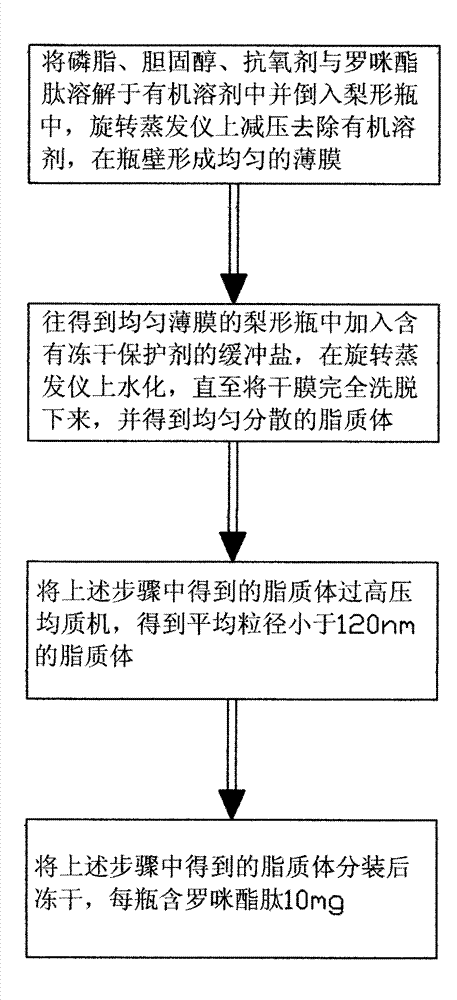
[0035] Example 1: See figure 1 , the present invention discloses a method for preparing romidolipid liposomes, comprising the following process:
[0036] Romiglutide liposome prescription:
[0037] Romiglutide
[0038] Preparation process of romiglutide liposome: Dissolve the prescription amount of soybean lecithin for injection, cholesterol, vitamin E and romiglutide in 1:1 chloroform-methanol and pour it into a pear-shaped bottle. Remove the organic solvent under reduced pressure in a water bath at 40°C, and form a uniform film on the bottle wall; dissolve the prescribed amount of sucrose in 100ml of phosphate buffer with a pH value of 6.8, pour it into a pear-shaped bottle, and use a rotary evaporator at 30rpm, Hydration is complete under 25°C water bath conditions; the obtained liposomes are passed through a high-pressure homogenizer, the pressure is 900 bar, and the number of cycles is 3 times to obtain liposomes with an average particle size of 108nm; the lipo...
Embodiment 2
[0039] Example 2: See figure 1 , the present invention discloses a method for preparing romidolipid liposomes, comprising the following process:
[0040] Romiglutide liposome prescription:
[0041] Romiglutide
[0042] Preparation process of romiglutide liposomes: Dissolve the prescription amount of soybean lecithin for injection, cholesterol, vitamin E and romiglutide in 1:3 chloroform-methanol and pour it into a pear-shaped bottle, and rotate the evaporator at 40rpm, Remove the organic solvent under reduced pressure in a water bath at 45°C, and form a uniform film on the bottle wall; dissolve the prescribed amount of sucrose in 150ml of phosphate buffer with a pH value of 6.8, pour it into a pear-shaped bottle, and use a rotary evaporator at 40rpm, Hydration is complete under 35°C water bath conditions, the obtained liposomes are passed through a high-pressure homogenizer, the pressure is 700bar, and the number of cycles is 5 times to obtain liposomes with an aver...
Embodiment 3
[0043] Example 3: See figure 1 , the present invention discloses a method for preparing romidolipid liposomes, comprising the following process:
[0044] Romiglutide liposome prescription:
[0045] Romiglutide
0.3g
Soybean Lecithin for Injection
5g
cholesterol
1g
2g
Vitamin E
0.3g
Phosphate buffered saline with a pH of 6.8
200ml
[0046] Preparation process of romiglutide liposomes: dissolve the prescription amount of soybean lecithin for injection, cholesterol, vitamin E and romiglutide in 1:5 chloroform-methanol and pour it into a pear-shaped bottle. Remove the organic solvent under reduced pressure in a water bath at 50°C, and form a uniform film on the bottle wall; dissolve the prescribed amount of sucrose in 200ml of phosphate buffer with a pH value of 6.8, pour it into a pear-shaped bottle, and use a rotary evaporator at 50rpm, Hydration is complete under 45°C water bath conditions, the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com