Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
124results about "Sodium/potassium compounds" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Titanate and titania nanostructures and nanostructure assemblies, and methods of making same
ActiveUS20130102458A1Improve scalabilityImprove photocatalytic performanceMaterial nanotechnologyHydrogenMicrometer scaleTitanium
The invention relates to nanomaterials and assemblies including, a micrometer-scale spherical aggregate comprising: a plurality of one-dimensional nanostructures comprising titanium and oxygen, wherein the one-dimensional nanostructures radiate from a hollow central core thereby forming a spherical aggregate.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
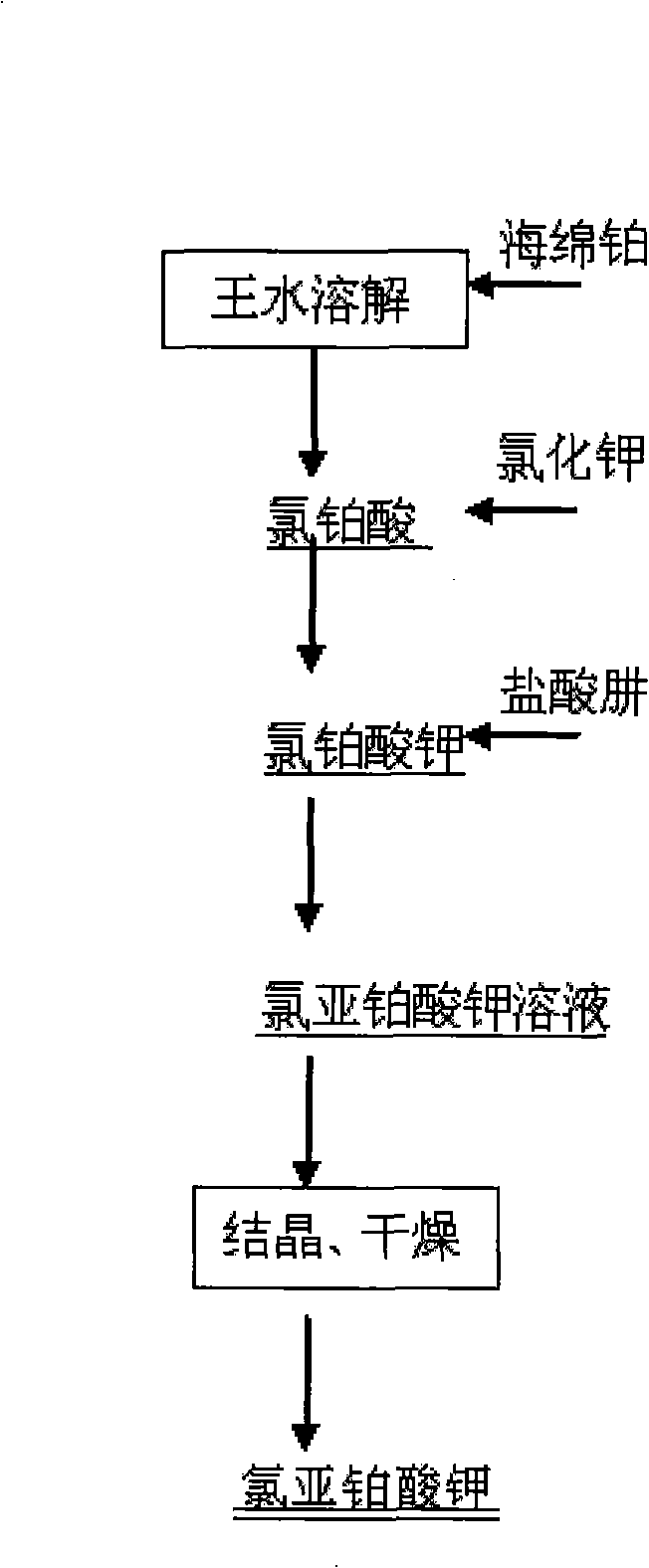
Preparation of potassium platinochloride
ActiveCN101279772AShort preparation processReduce manufacturing costRuthenium/rhodium/palladium/osmium/iridium/platinum compoundsSodium/potassium compoundsPotassium tetrachloroplatinatePotassium
Disclosed is a method for the production of a potassium platinochloride, which relates to a process for preparing a pharmaceutical intermediate potassium platinochloride. The method of preparation is characterized in that the preparation process adopts a spongy platinum as a material to prepare a chloroplatinic acid solution which is reacted with a potassium chloride solution to prepare the potassium chloroplatinite, then a hydrazine hydrochloride is adopted as a reducing agent to reduce the potassium chloroplatinite for preparing the pharmaceutical intermediate potassium platinochloride. The product adopting the method of the invention has a platinum content of 46.9+-0.2 percent and a potassium content of 19.0+-0.2 percent through a total elemental analysis. The total content of metallic impurity is no more than 0.08 percent.
Owner:兰州金川科技园有限公司
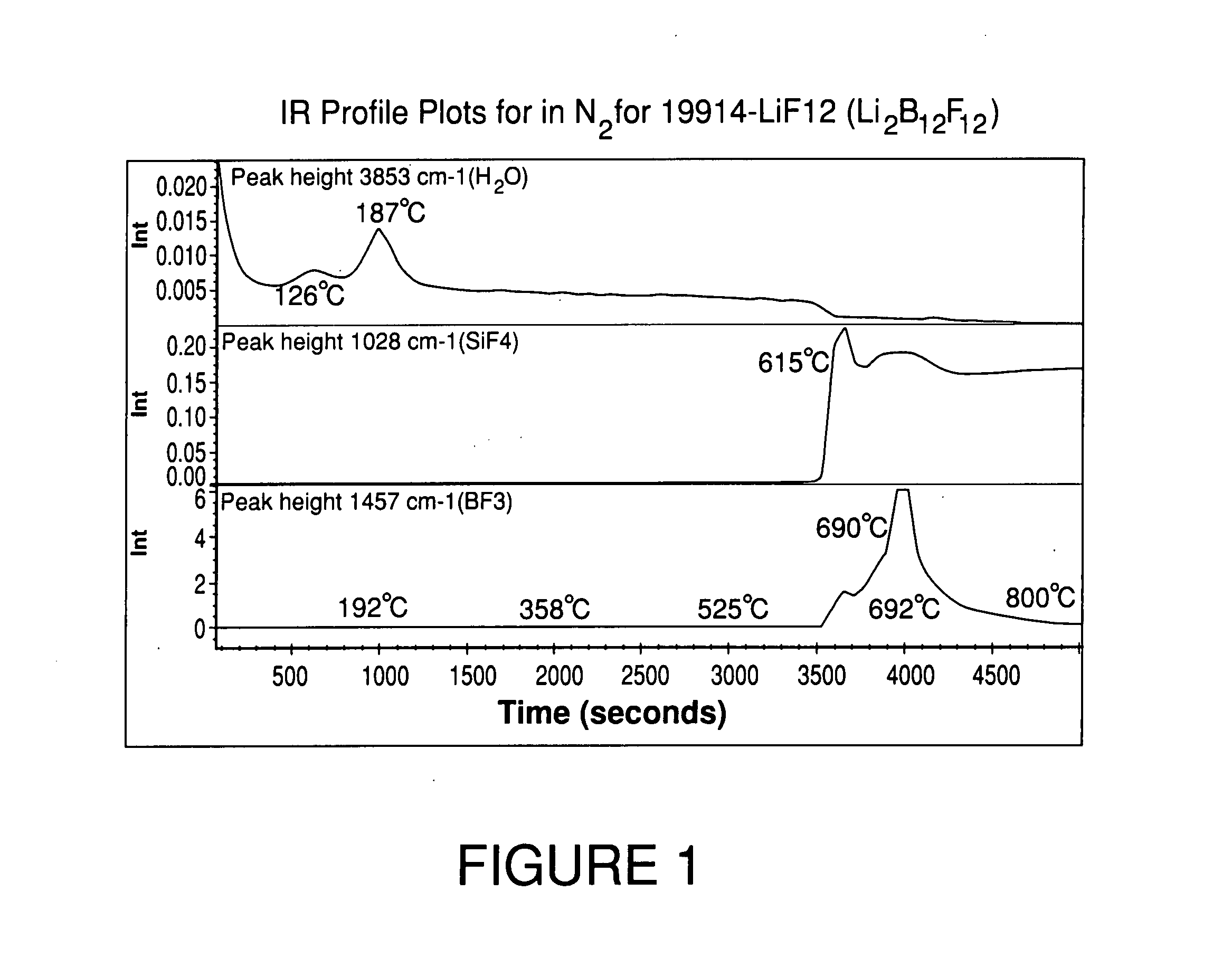
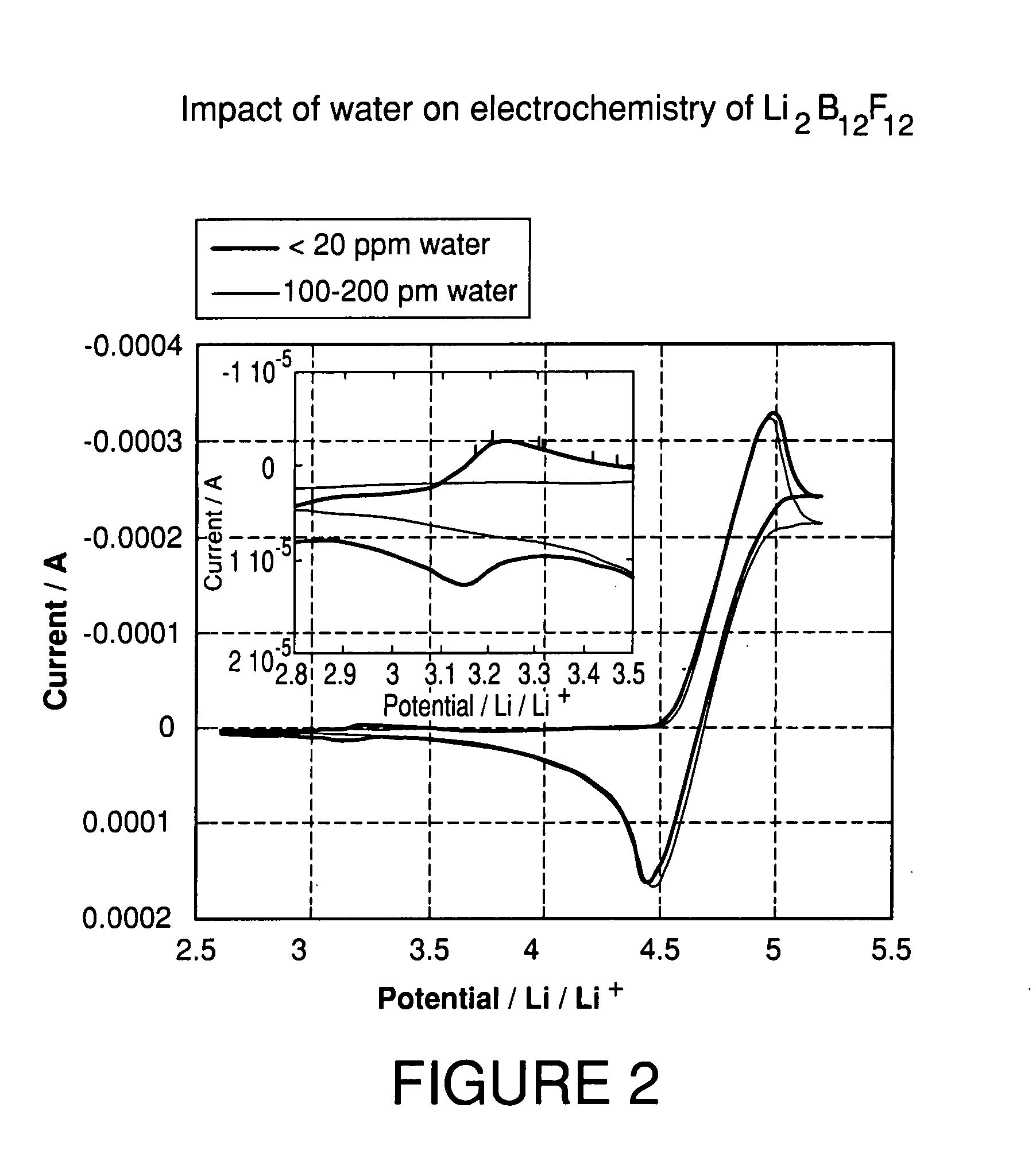
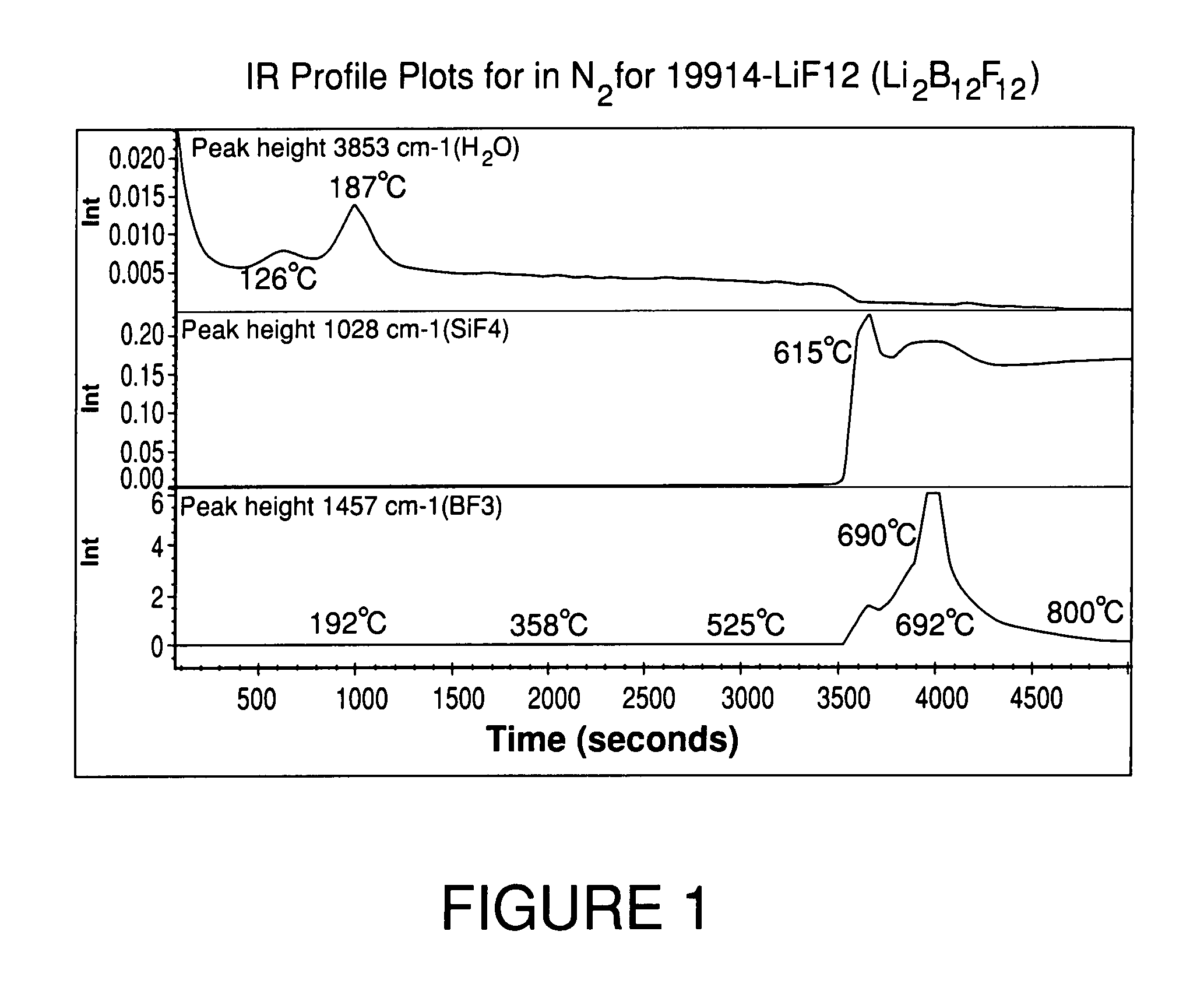
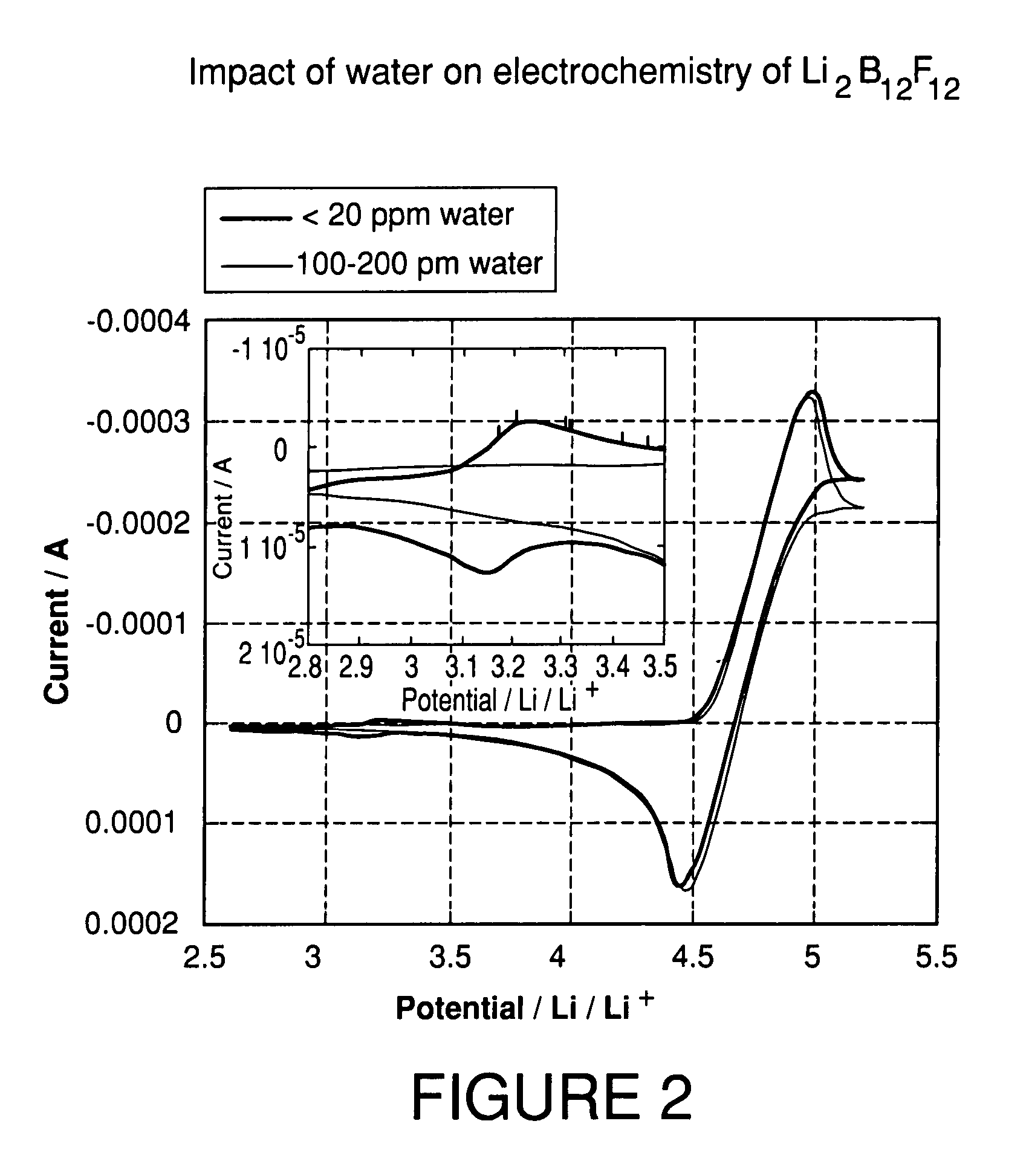
High purity lithium polyhalogenated boron cluster salts useful in lithium batteries
ActiveUS20070189946A1Low viscosityLower impedanceSolvent extractionOther chemical processesBoron clustersPhysical chemistry
The present invention relates to lithium secondary batteries comprising a negative electrode, a positive electrode, a separator and a lithium-based electrolyte carried in an aprotic solvent, and to the electrolyte compositions, and to methods for purifying battery active materials. The electrolyte comprises at least one solvent and a lithium salt of the formula:Li2B12FxH12-x-yZywhere x+y is from 3 to 12, and x and y are independently from 0 to 12, and Z comprises at least one of Cl and Br.
Owner:AIR PROD & CHEM INC
Process for the purification of lithium salts
ActiveUS7981388B2Low impurity contentLower impedanceSolvent extractionCrystallization separationLithiumPhysical chemistry
The present invention relates to lithium secondary batteries comprising a negative electrode, a positive electrode, a separator and a lithium-based electrolyte carried in an aprotic solvent, and to the electrolyte compositions, and to methods for purifying battery active materials. The electrolyte comprises at least one solvent and a lithium salt of the formula:Li2B12FxH12-x-yZy where x+y is from 3 to 12, and x and y are independently from 0 to 12, and Z comprises at least one of Cl and Br.
Owner:AIR PROD & CHEM INC
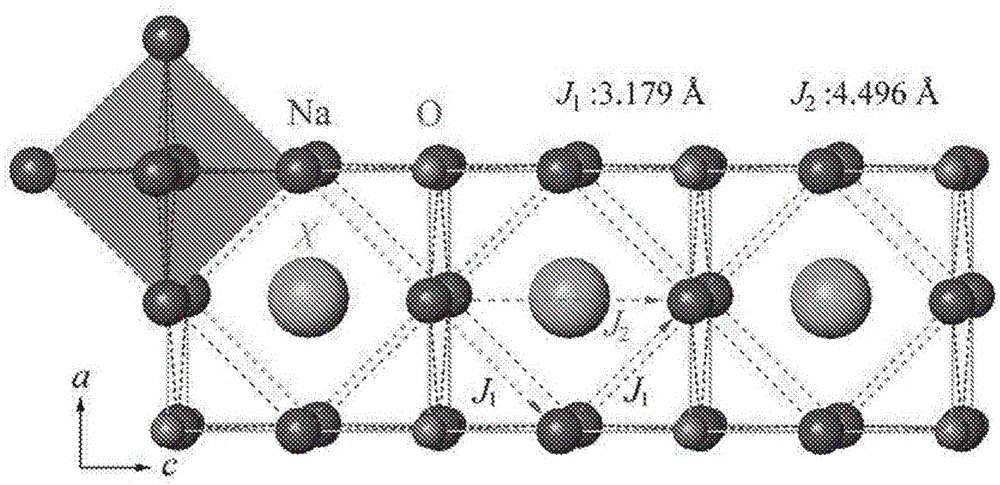
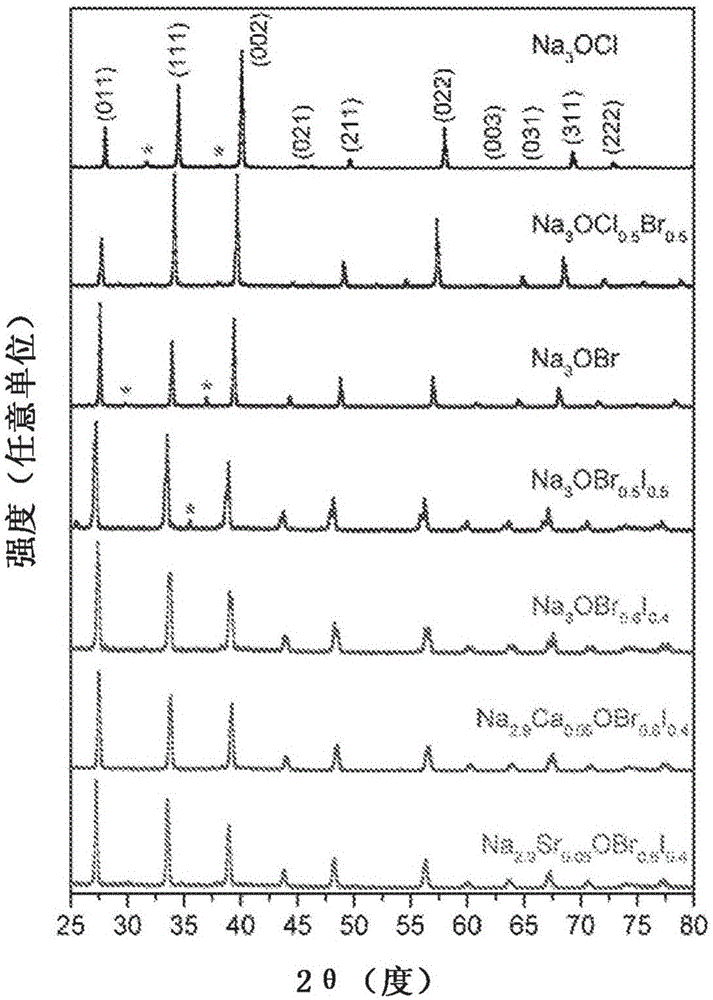
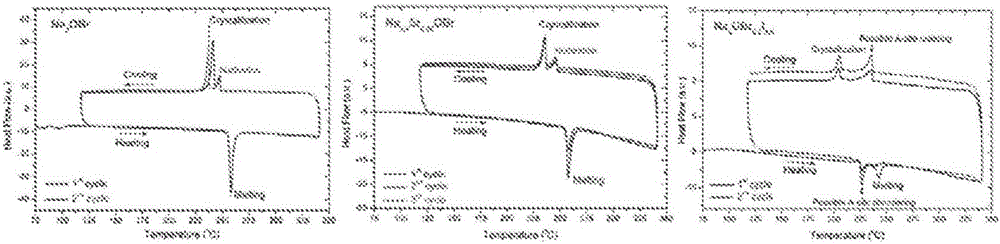
Sodium anti-perovskite solid electrolyte compositions
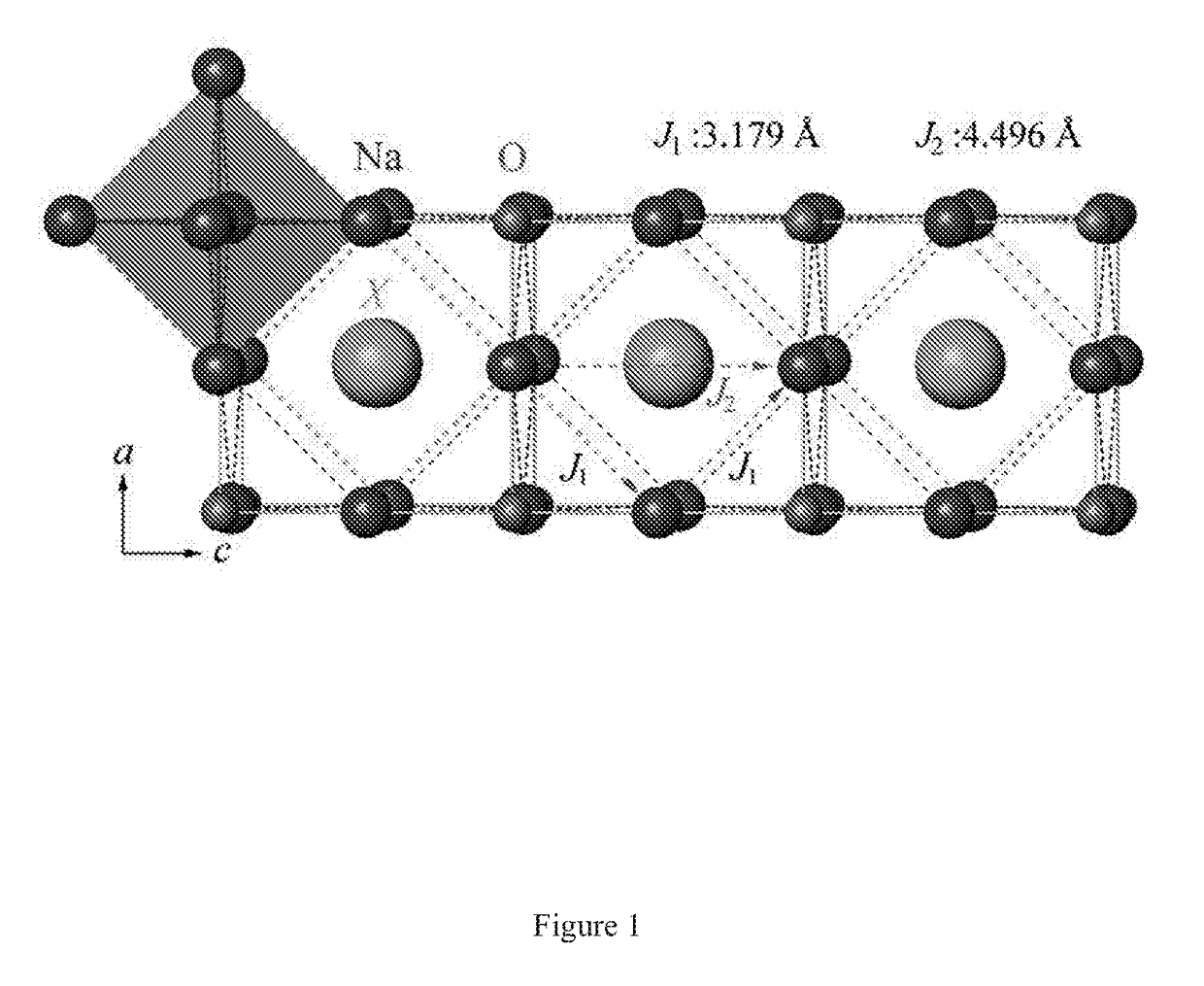
Na-rich electrolyte compositions provided herein can be used in a variety of devices, such as sodium ionic batteries, capacitors and other electrochemical devices. Na-rich electrolyte compositions provided herein can have a chemical formula of Na3OX, Na3SX, Na (3-delta) M delta / 2OX and Na (3-delta) M delta / 2SX wherein 0 ;lt; delta & lt; 0.8, wherein X is a monovalent anion selected from fluoride, chloride, bromide, iodide, H-, CN-, BF4-, BH4-, ClO4-, CH3-, NO2-, NH2- and mixtures thereof, and wherein M is a divalent metal selected from the group consisting of magnesium, calcium, barium, strontium and mixtures thereof. Na-rich electrolyte compositions provided herein can have a chemical formula of Na (3-delta) M delta / 3OX and / or Na (3-delta) M delta / 3SX; wherein 0 & lt;delta & lt; 0.5, wherein M is a trivalent cation M+3, and wherein X is selected from fluoride, chloride, bromide, iodide, H-, CN-, BF4-, BH4-, ClO4-, CH3-, NO2-, NH2- and mixtures thereof. Synthesis and processing methods of NaRAP compositions for battery, capacitor, and other electrochemical applications are also provided.
Owner:BOARD OF RGT NEVADA SYST OF HIGHER EDUCATION ON BEHALF OF THE UNIV OF NEVADA RENO
Rapid synthesis method of drinking water treatment agent (i.e. potassium ferrate)
InactiveCN101597087AGreat tasteEfficient killingIron compoundsSodium/potassium compoundsFerric hydroxideSynthesis methods
The invention discloses a rapid synthesis method of a drinking water treatment agent (i.e. potassium ferrate). The method directly utilizes the industrial pure ferric hydroxide Fe(OH)3, the self-prepared potassium hypochlorite (KClO) saturated solution and the deionized water as raw materials for preparing potassium ferrate. The process comprises the following steps: preparing the potassium ferrate by the potassium hypochlorite (KClO) saturated solution and the industrial pure ferric hydroxide Fe(OH)3, adding the CuCl22H2O stabilizer for solid liquid separation, adding the KOH for recrystallization and purification, and washing and removing impurity by the normal pentane and the inorganic ethanol; and performing vacuum drying and sealing. The method has the advantages of simple process, good quality of the products and low production cost.
Owner:张亮
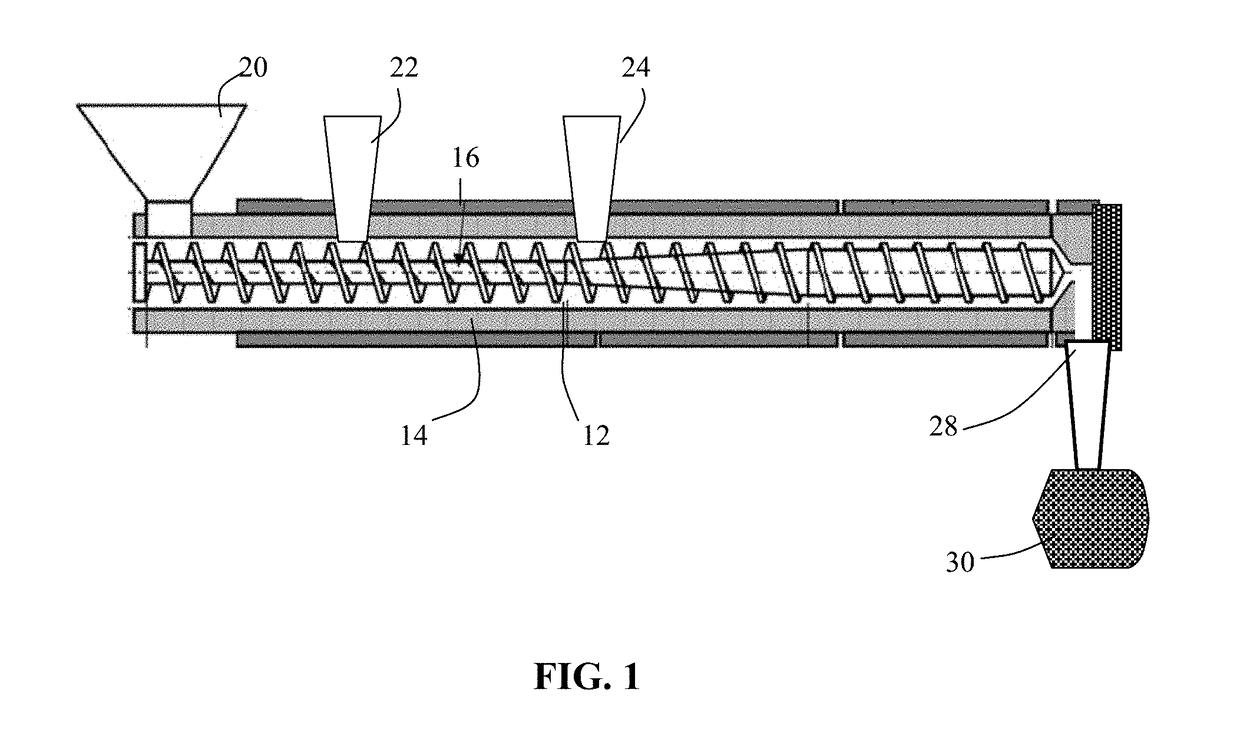
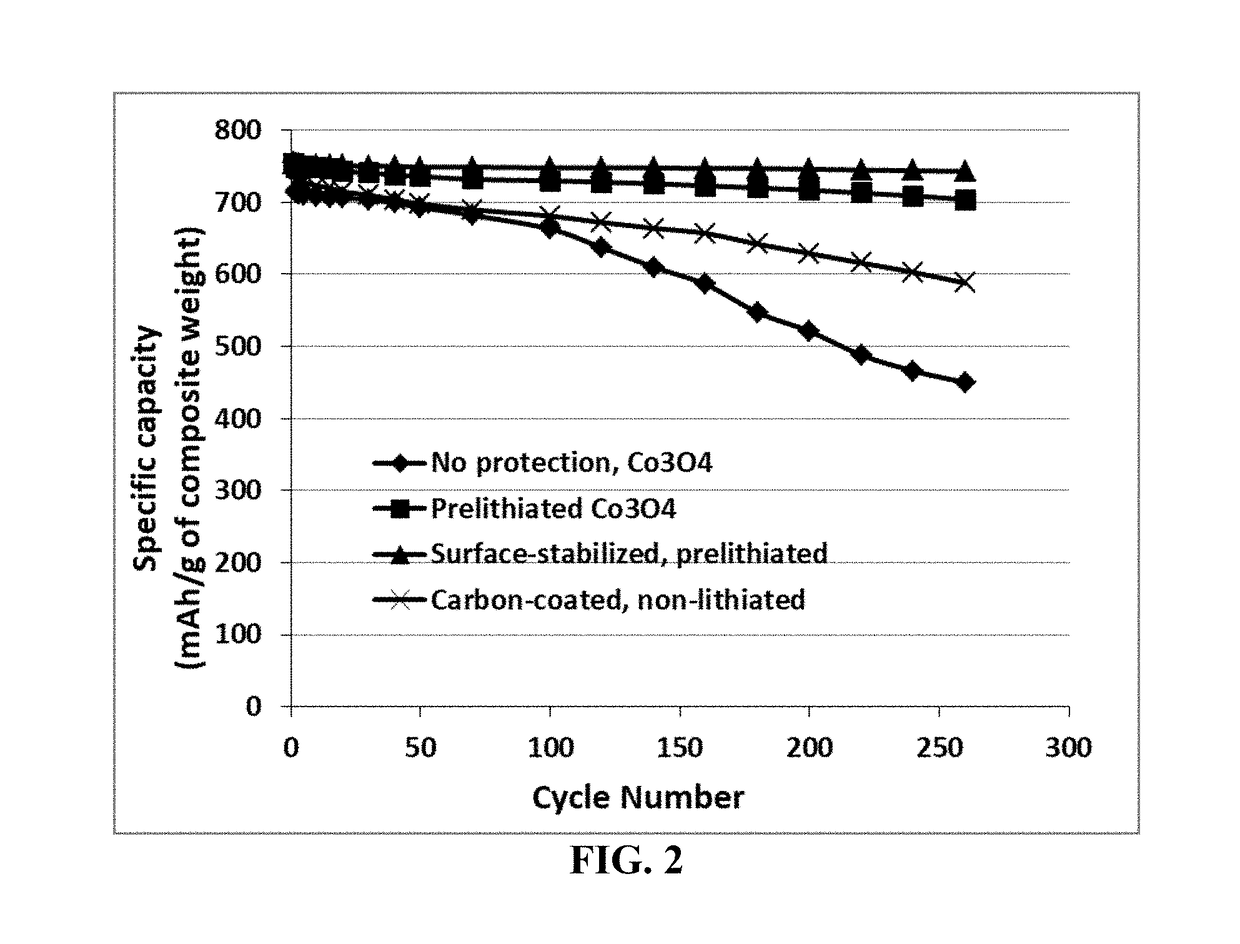
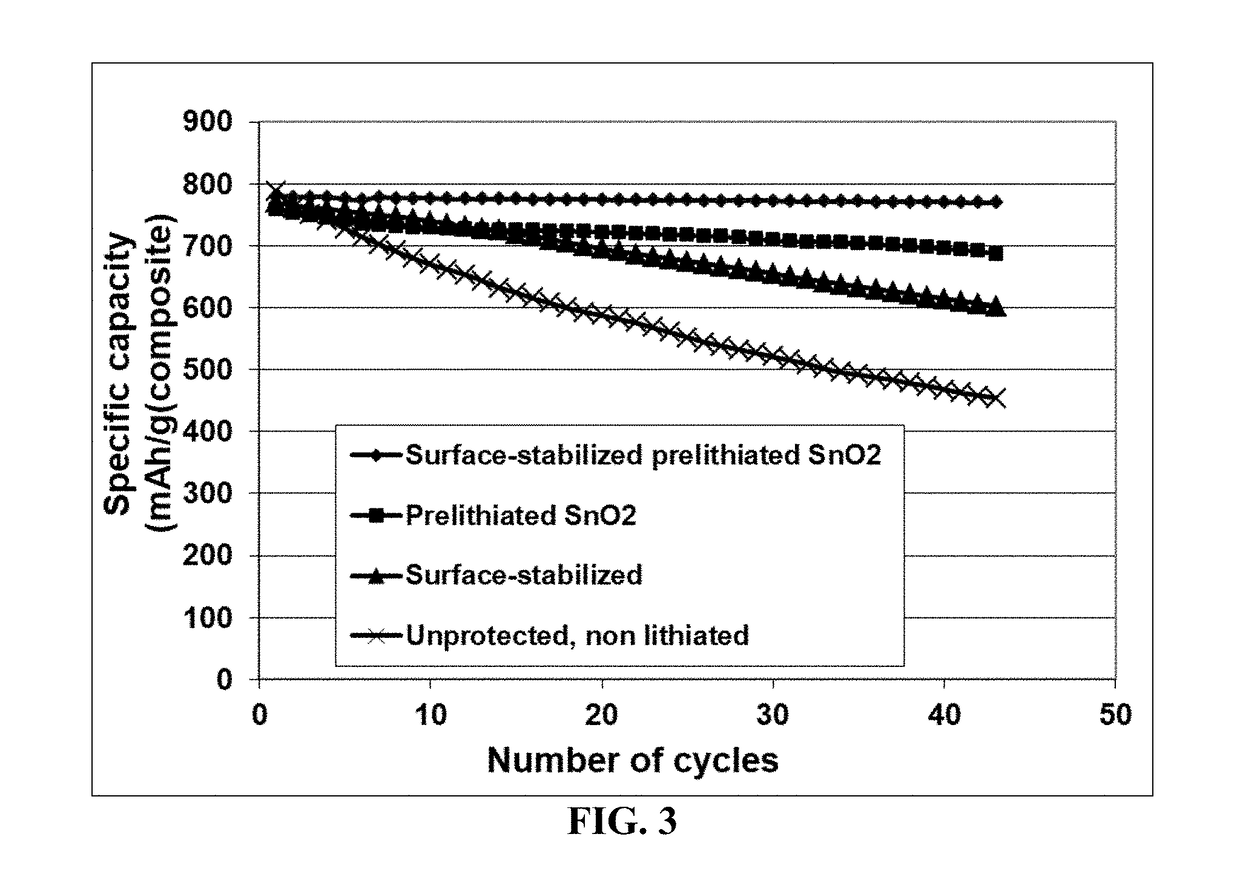
Process for prelithiating an anode active material for a lithium battery
ActiveUS20190088922A1High specific capacityLong charge-discharge cycle lifeElectrode thermal treatmentLithium compoundsLithium metalPhysical chemistry
Provided is a process for producing prelithiated particles of an anode active material for a lithium battery. The process comprises: (a) providing a lithiating chamber having at least one inlet and at least one outlet; (b) feeding a plurality of particles of an anode active material, lithium metal particles, and an electrolyte solution (containing a lithium salt dissolved in a liquid solvent) into the lithiating chamber through at least one inlet, concurrently or sequentially, to form a reacting mixture; (c) moving this reacting mixture toward the outlet at a rate sufficient for inserting a desired amount of lithium into the anode active material particles to form a slurry of prelithiated particles dispersed in the electrolyte solution; and (d) discharging the slurry out of the lithiating chamber through the at least one outlet.
Owner:GLOBAL GRAPHENE GRP INC
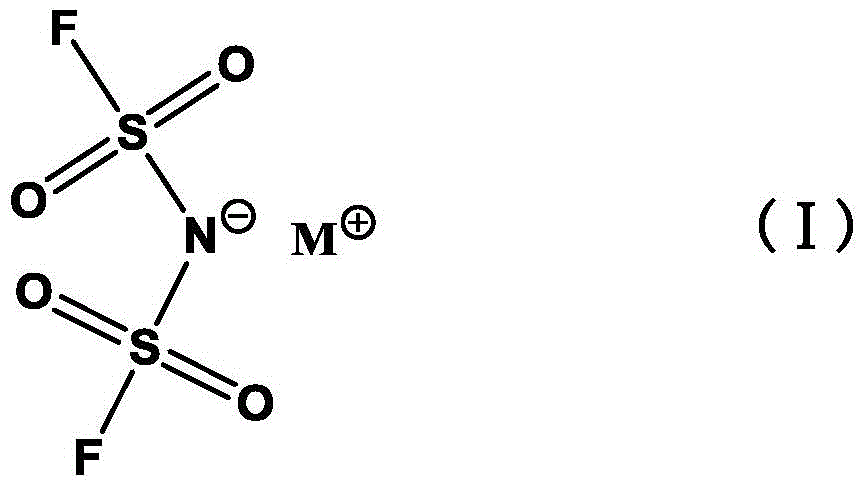
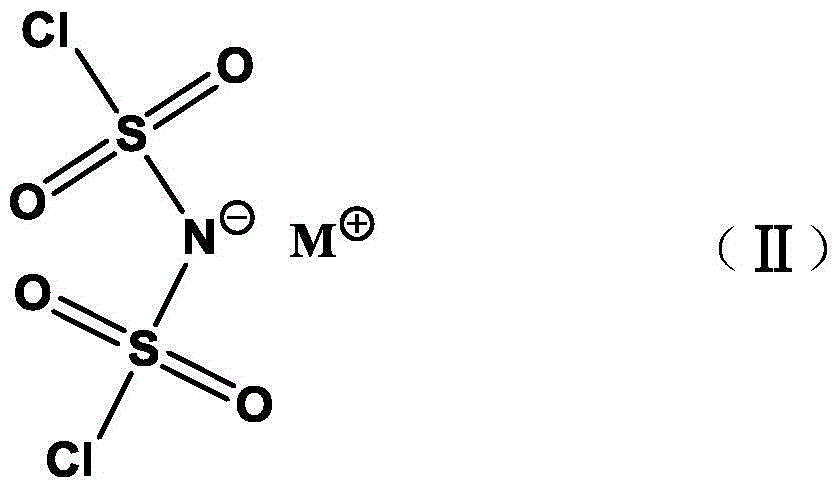
Preparation method of alkali metal salt of bis(fluorosulfonyl)amide
ActiveCN105731398AEasy to separateGood compatibilityNitrosyl chlorideLithium compoundsState of artFluoride
The invention discloses a preparation method of alkali metal salt of bis(fluorosulfonyl)amide. The preparation method comprises the step of carrying out fluoridation on alkali metal salt of bis(chlorosulfonyl)amide in an polar aprotic solvent by taking ion liquid as a phase transfer catalyst and alkali metal fluoride as a fluorinating agent, so as to obtain corresponding alkali metal salt of bis(fluorosulfonyl)amide. Compared with the prior art, the preparation method has the advantages that reaction conditions are mild, the yield and the purity are high, the product is easily separated and purified, the ion liquid catalyst can be recycled and reduced, and the like. The preparation method is applicable to industrial mass production.
Owner:武汉市瑞华新能源科技有限公司
Metal-organic framework electrodes for sodium ion batteries
ActiveUS20180053968A1Improve performanceCell electrodesSmall-sized cells cases/jacketsOrganic solventMetal-organic framework
A sodium ion battery comprises a cathode having a porous redox active metal-organic framework material. The battery can be an organic electrolyte sodium ion battery wherein the electrolyte comprises a sodium salt dissolved in an organic solvent or mixture of organic solvents. Alternatively, the battery can comprise an aqueous sodium ion battery wherein the electrolyte comprises a sodium salt dissolved in an aqueous solvent. Battery performance is especially related to electrolyte and binder selection.
Owner:NAT TECH & ENG SOLUTIONS OF SANDIA LLC
Method for producing imide salt
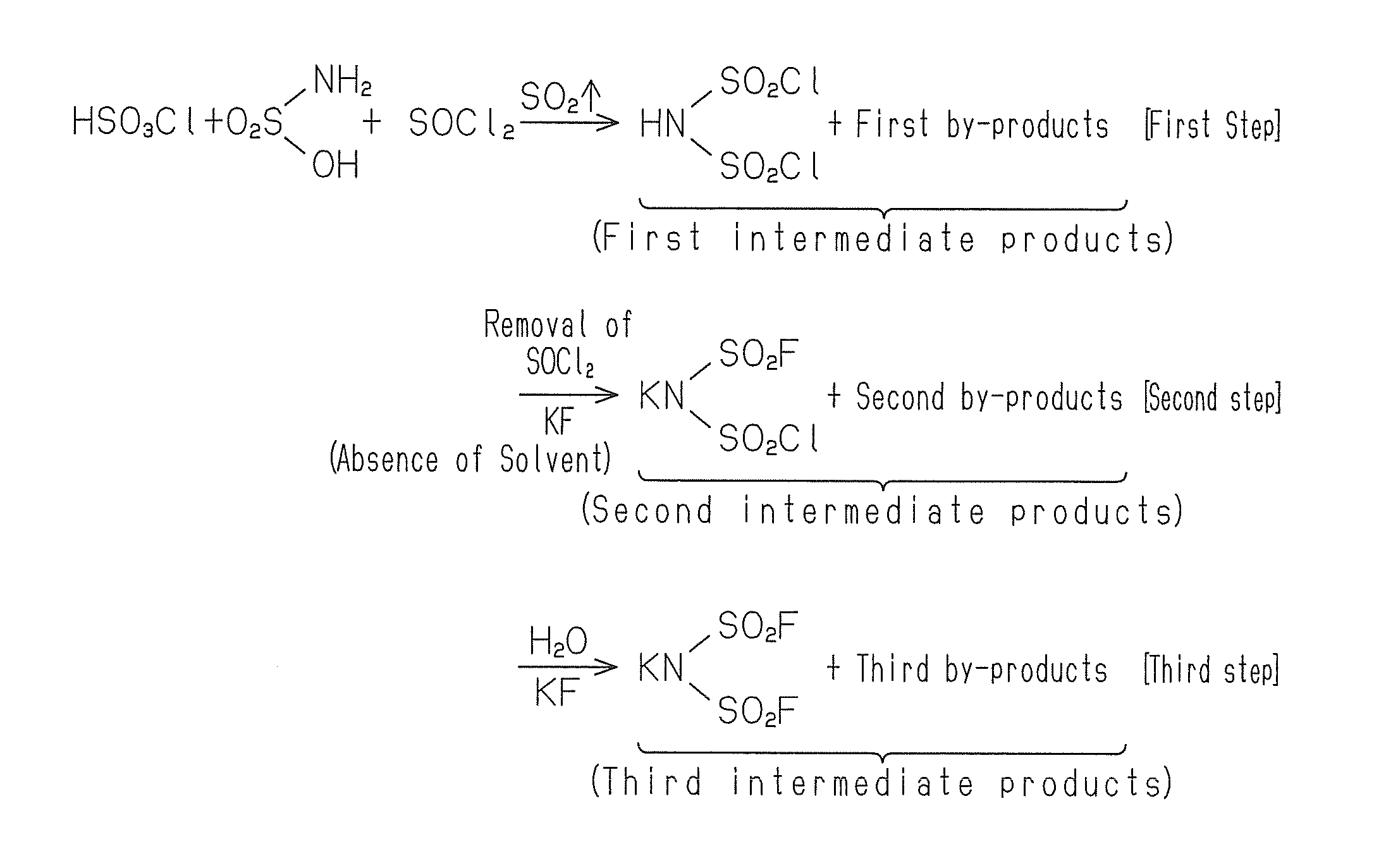
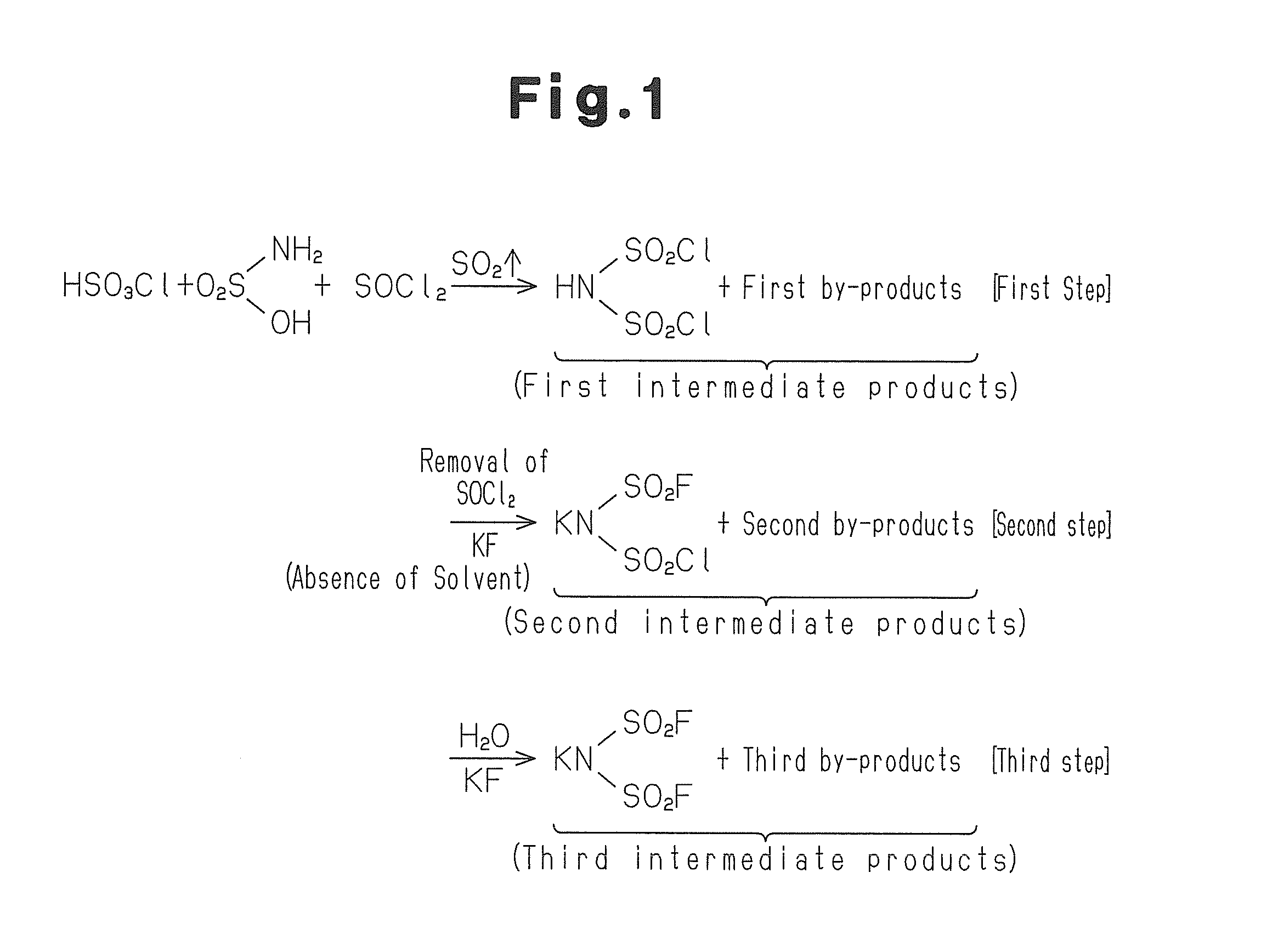
InactiveUS20140241973A1Increase productionReduce the amount requiredNitrosyl chlorideSodium/potassium compoundsImideSulfamic acid
A mixture of sulphamic acid, a halogenated sulphonic acid and thionyl chloride is heated to allow the reaction to proceed, to thereby produce first intermediate products. The first intermediate products are then subjected to reaction with an alkali metal fluoride MF to produce second intermediate products. The second intermediate products is then subjected to reaction with the alkali metal fluoride MF in a polar solvent to obtain a desired product MN(SO2F)2 (where M is an alkali metal).
Owner:SUMITOMO ELECTRIC IND LTD
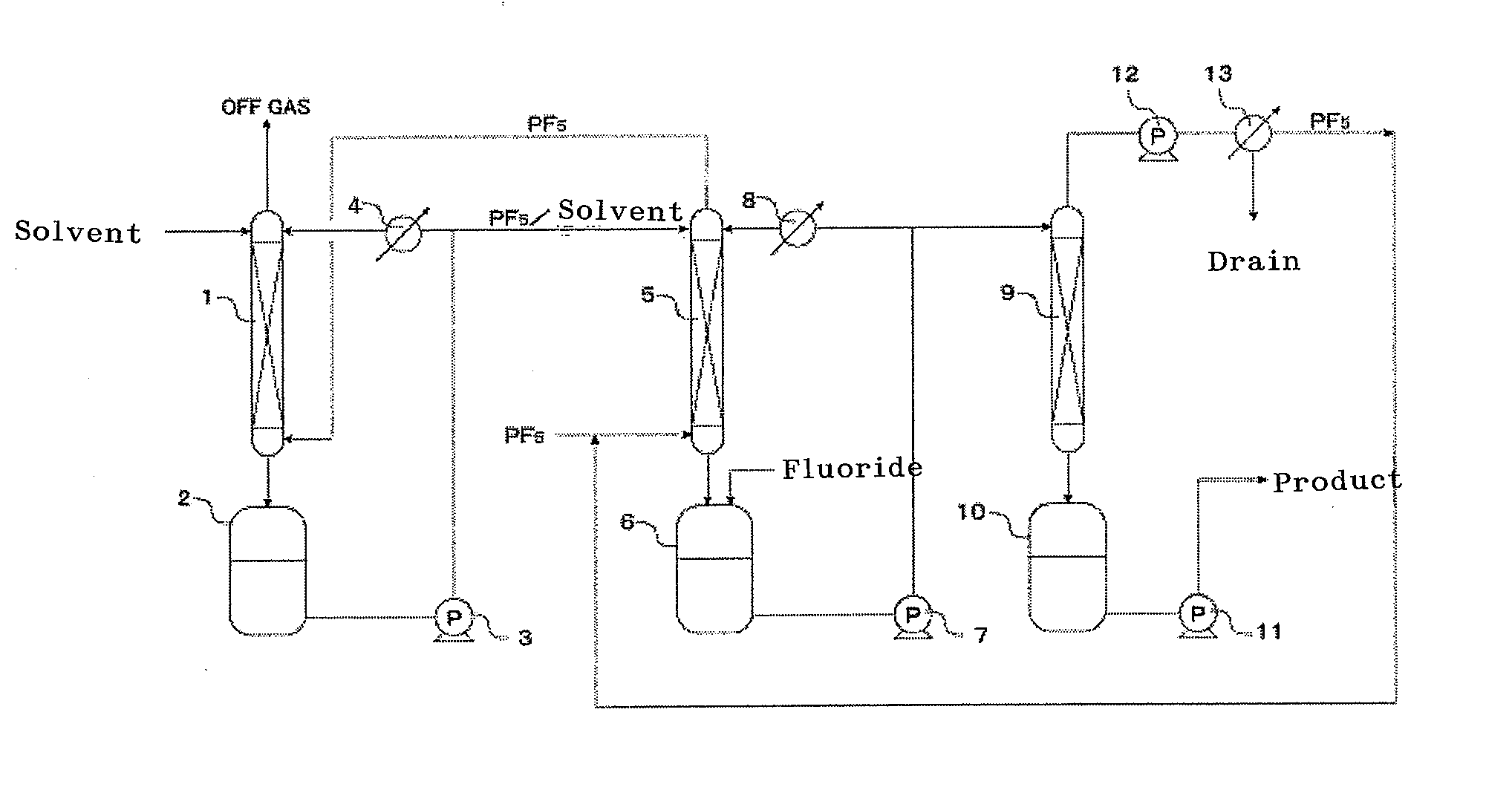
Process for production of hexafluorophosphates
InactiveCN102105395AEasy to manufactureImprove qualitySecondary cellsLithium hexafluorophosphateElectricityFluoride
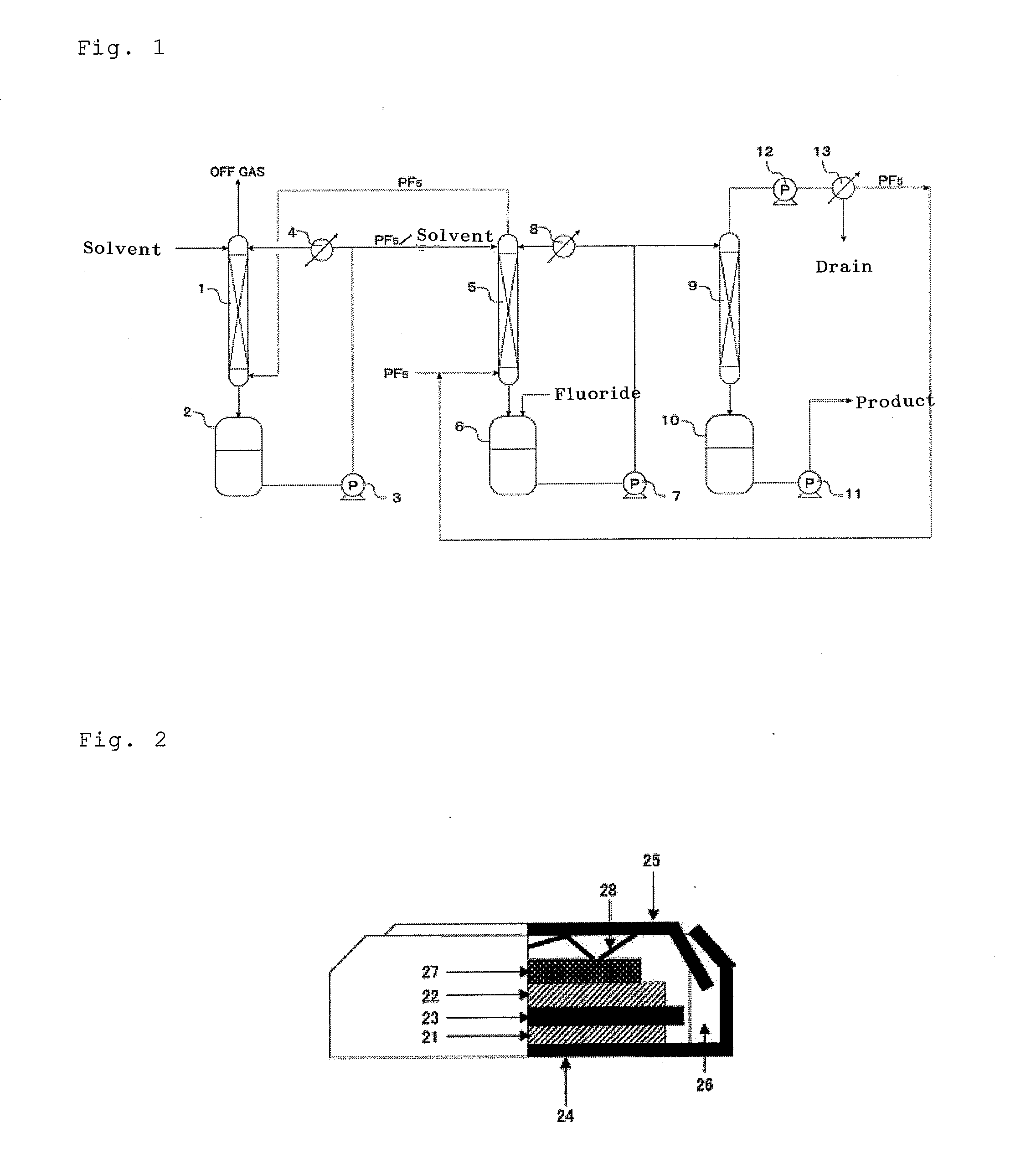
Provided are a process for production of hexafluorophosphates by which inexpensive high-quality hexafluorophosphates can be easily produced with production cost control; an electrolytic solution containing a hexafluorophosphate; and an electricity storage device equipped with the electrolytic solution. The process for production of hexafluorophosphates is characterized by reacting at least a phosphorus compound with a fluoride represented by chemical formula: MFsr(HF) [wherein 0=r=6; 1=s=3; and M is at least one selected from the group consisting of Li, Na, K, Rb, Cs, NH4, Ag, Mg, Ca, Ba, Zn, Cu, Pb, Al and Fe] to form a hexafluorophosphate represented by chemical formula: M(PF6)s.
Owner:STELLA CHEMIFA CORP
Method for producing alkali metal niobate particles, and alkali metal niobate particles
InactiveUS20120064344A1Control shapeMaterial nanotechnologyGlass/slag layered productsFine particulateAlkali metal
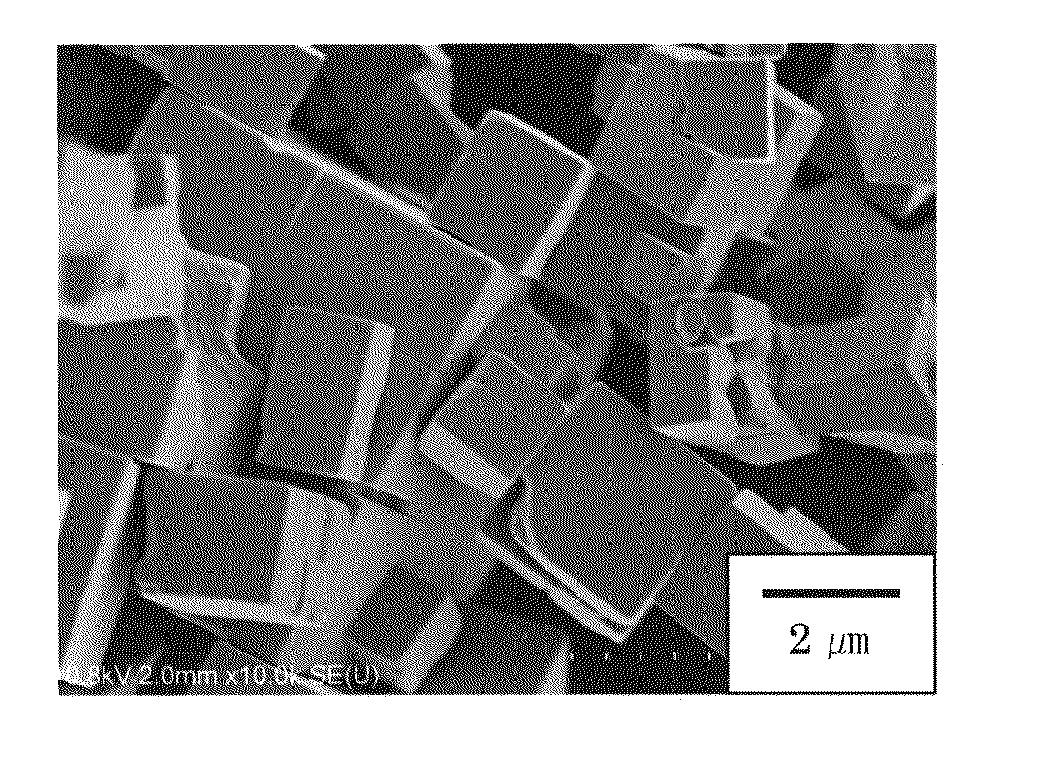
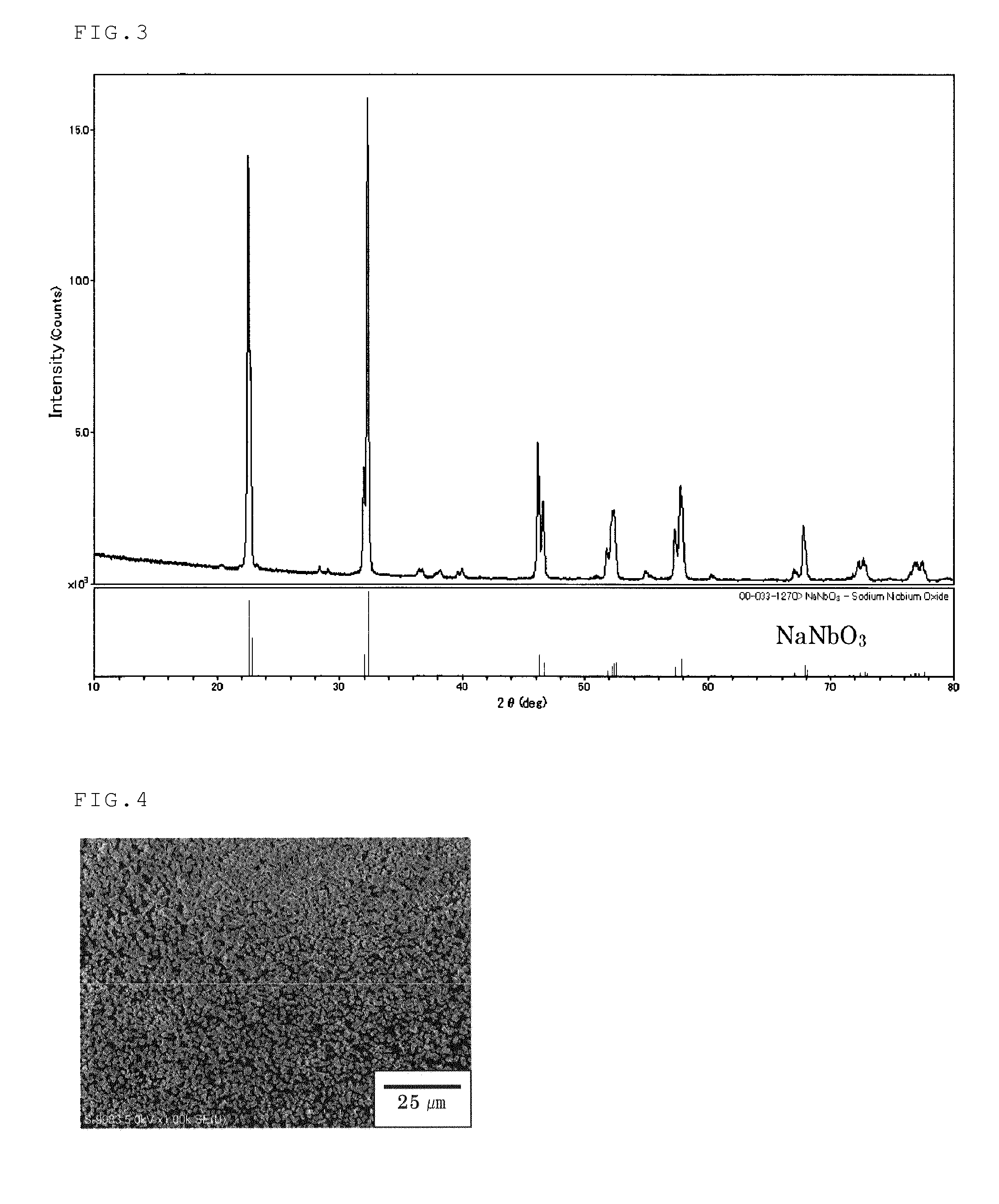
Disclosed are a method of producing fine particulate alkali metal niobate in a liquid phase system, wherein the size and shape of the particulate alkali metal niobate can be controlled; and fine particulate alkali metal niobate having a controlled shape and size. One of specifically disclosed is a method of producing a substantially rectangular cuboid particulate alkali metal niobate represented by MNbO3 (1), wherein M represents one element selected from alkaline metals, including specific four steps. Another one of specifically disclosed is particulate alkali metal niobate represented by the formula (1) having a substantially rectangular cuboid shape, wherein the substantially rectangular cuboid shape has a longest side and a shortest side, the length of the longest side represented by an index Lmax is 0.10 to 25 μm, and the length of the shortest side represented by an index Lmin is 0.050 to 15 μm.
Owner:SAKAI CHEM IND CO LTD +2
Preparation method of sodium hexafluorophosphate
InactiveCN108946769AHigh purityIncrease productionSodium/potassium compoundsProcess equipmentSodium-ion battery
The invention discloses a preparation method of sodium hexafluorophosphate. The preparation method comprises the following steps: (1) introducing phosphorus pentafluoride gas into a reaction kettle containing lithium fluoride and hydrogen fluoride liquid for reaction, so as to obtain a sodium hexafluorophosphate solution; (2) carrying out crystallization under a stirring condition; and (3) drying.The preparation method has the advantages that the reaction conditions are mild, the yield is high, the process equipment is simple, and a high-purity sodium hexafluorophosphate target product can beprepared. Sodium hexafluorophosphate can be used as sodium salt for sodium-ion battery electrolyte.
Owner:MORITA NEW ENERGY MATERIALS ZHANGJIAGANG CO LTD
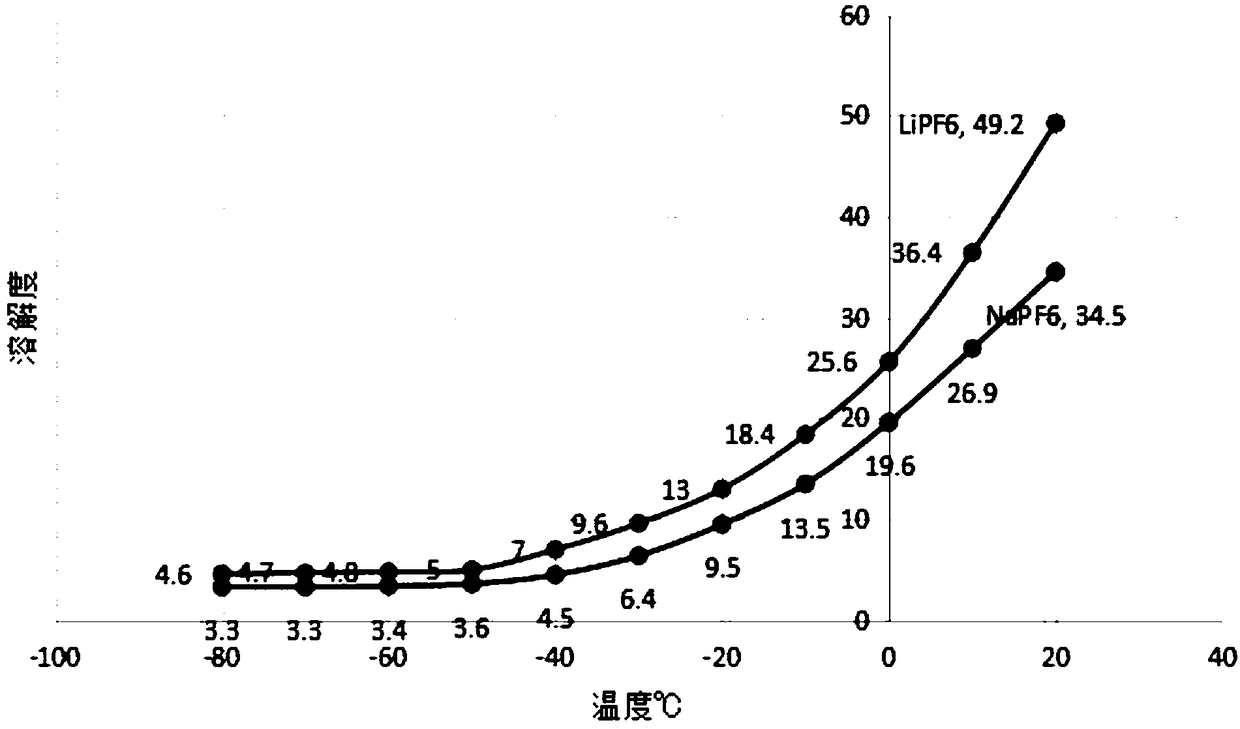
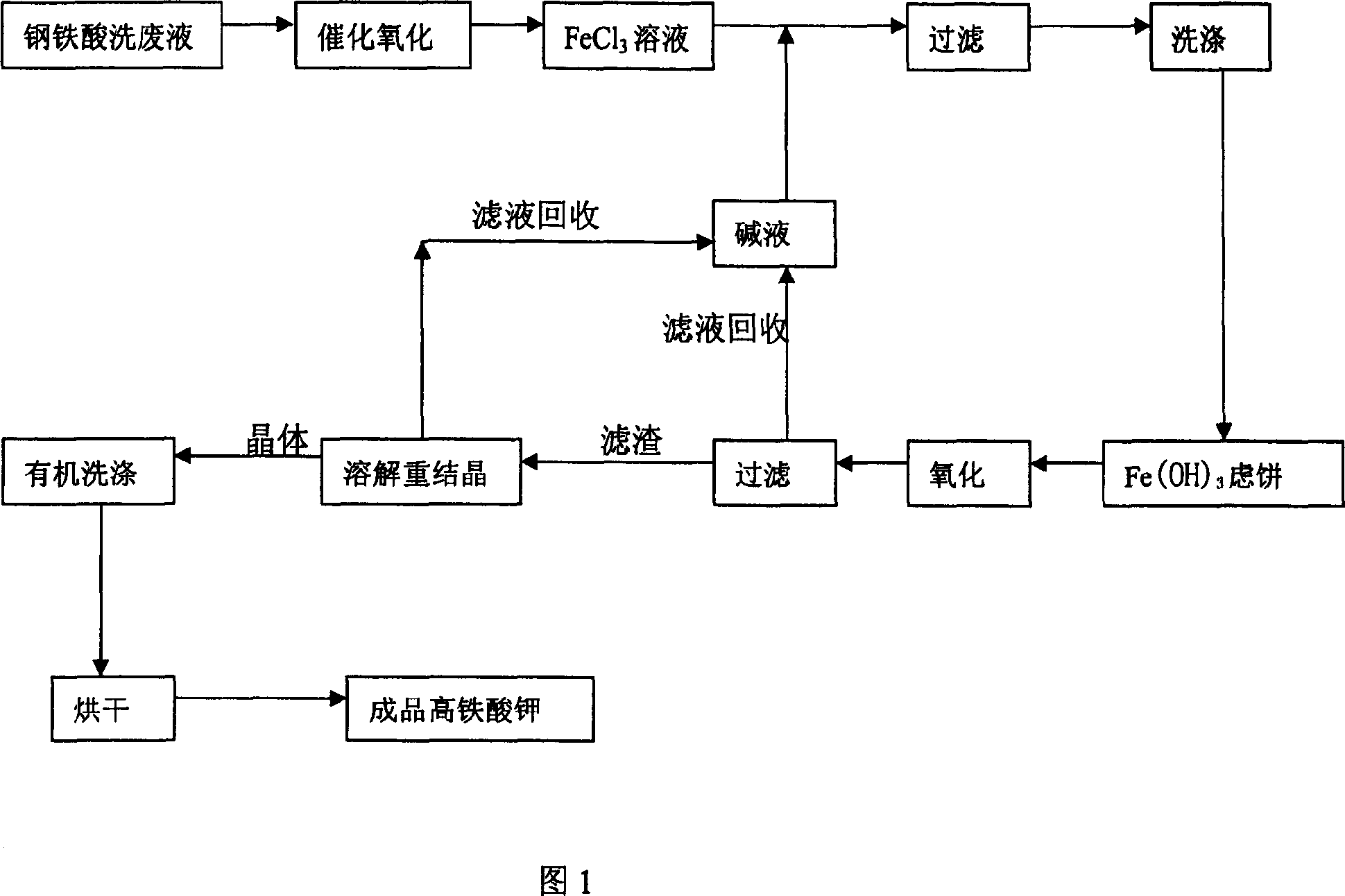
Method for preparing potassium ferrate by using waste liquid from acid washing steel
InactiveCN1958462AAvoid pollutionEasy to operateMultistage water/sewage treatmentIron compoundsOrganic solventAcid washing
This invention relates to a method preparing potassium ferrate from steel acid-washing waste liquid. The method comprises: oxidizing bivalent iron ions in steel acid-washing waste liquid into trivalent iron ions, precipitating trivalent iron ions to obtain Fe (OH) 3, and oxidizing Fe (OH) 3 by KClO in a strong alkaline condition to obtain potassium ferrate. The process mainly comprises: catalytically oxidizing, filtering the precipitate, oxidizing by KClO, recovering the filtrate, recrystallizing for purification, and washing with organic solvent. The method is environmentally friendly.
Owner:WUHAN UNIV OF TECH
Method for producing hexafluorophosphate salt
InactiveCN101605723AEasy to operateLithium hexafluorophosphateSodium/potassium compoundsPhosphateAqueous solution
Disclosed is a method for producing a hexafluorophosphate salt (MPF6: M = Li, Na, K, Rb, Cs, NH4, Ag), which uses at least an aqueous HxPOyFz solution, an aqueous hydrofluoric acid solution and MF r(HF) as raw materials (provided that r >= 0, 0 <= x <= 3, 0 <= y <= 4 and 0 <= z <= 6). This method enables to produce a hexafluorophosphate salt (MPF6: M = Li, Na, K, Rb, Cs, NH4, Ag) having excellent workability at low cost from easily available raw materials, and the reaction can be controlled in this method.
Owner:STELLA CHEMIFA CORP
Process for preparing potassium hydrogen persulfate composite salts
InactiveCN1781846AEasy to operateEase of industrial productionSodium/potassium compoundsAlkali metal sulfites/sulfatesPotassium hydroxidePotassium
The present invention discloses the preparation process of potassium monopersulfate composite salt. The preparation process includes the following steps: the oxidation reaction between hydrogen peroxide and fuming sulfuric acid; the neutralization between the potassium hydroxide aqua and the product of the preceding step to obtain the water solution of the destination product potassium monopersulfate; concentration; adding stabilizer, cooling to crystallize, collecting solid potassium monopersulfate, and drying to obtain the product. The reaction may be performed in a conventional reactor, and the process of the present invention has simple operation, no need of special equipment and high product stability, and is suitable for industrial production.
Owner:宋海鹏
Condensed polyanion electrode
The invention relates to electrodes that contain active materials of the formula: Naa XbMcM'd(condensed polyanion)e(anion)f; where X is one or more of Na+, Li+ and K+; M is one or more transition metals; M' is one or more non-transition metals; and where a>b; c > 0; d >= 0; e >= 1 and f >= 0. Such electrodes are useful in, for example, sodium ion battery applications.
Owner:FARADION LTD
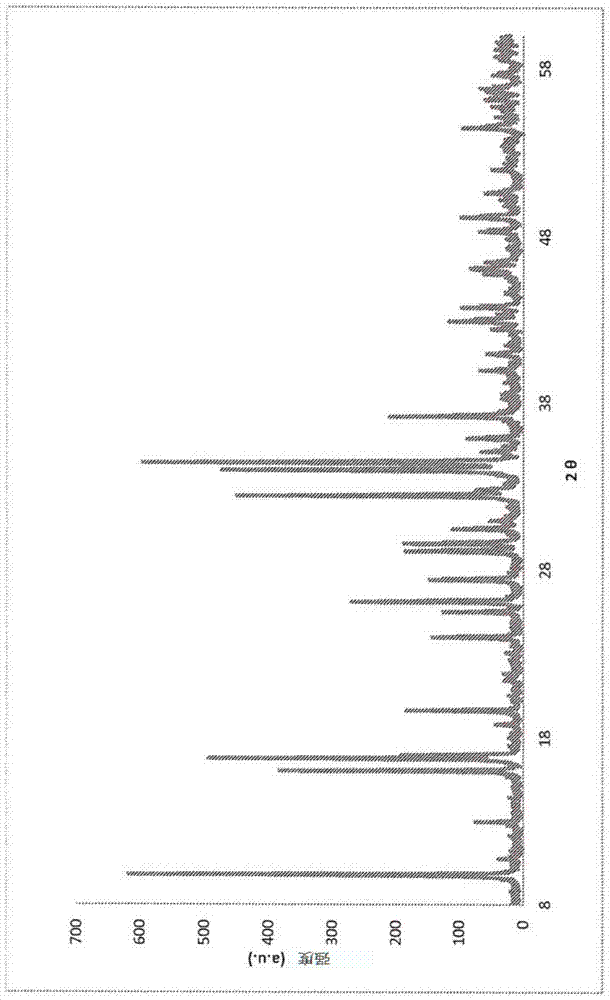
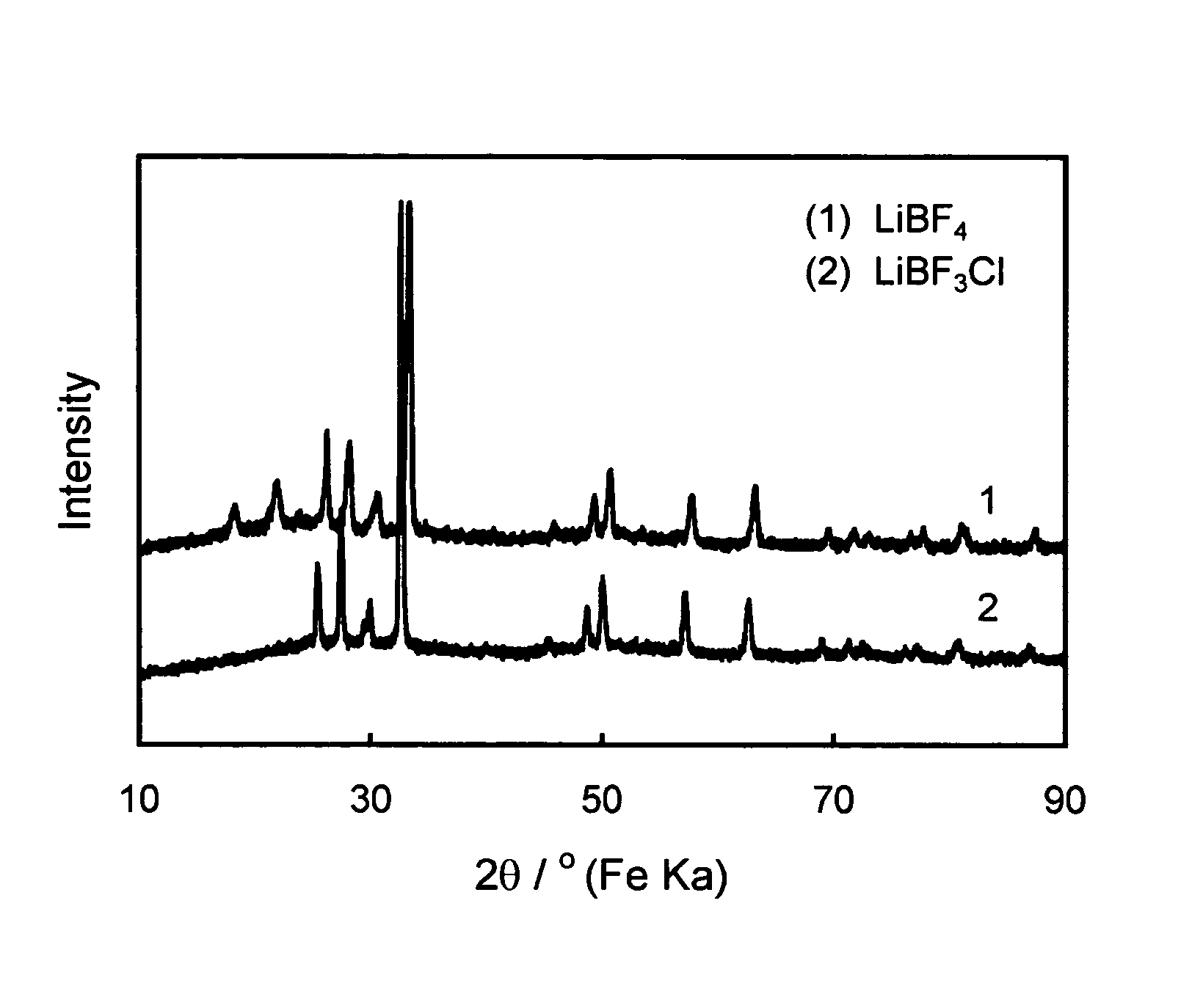
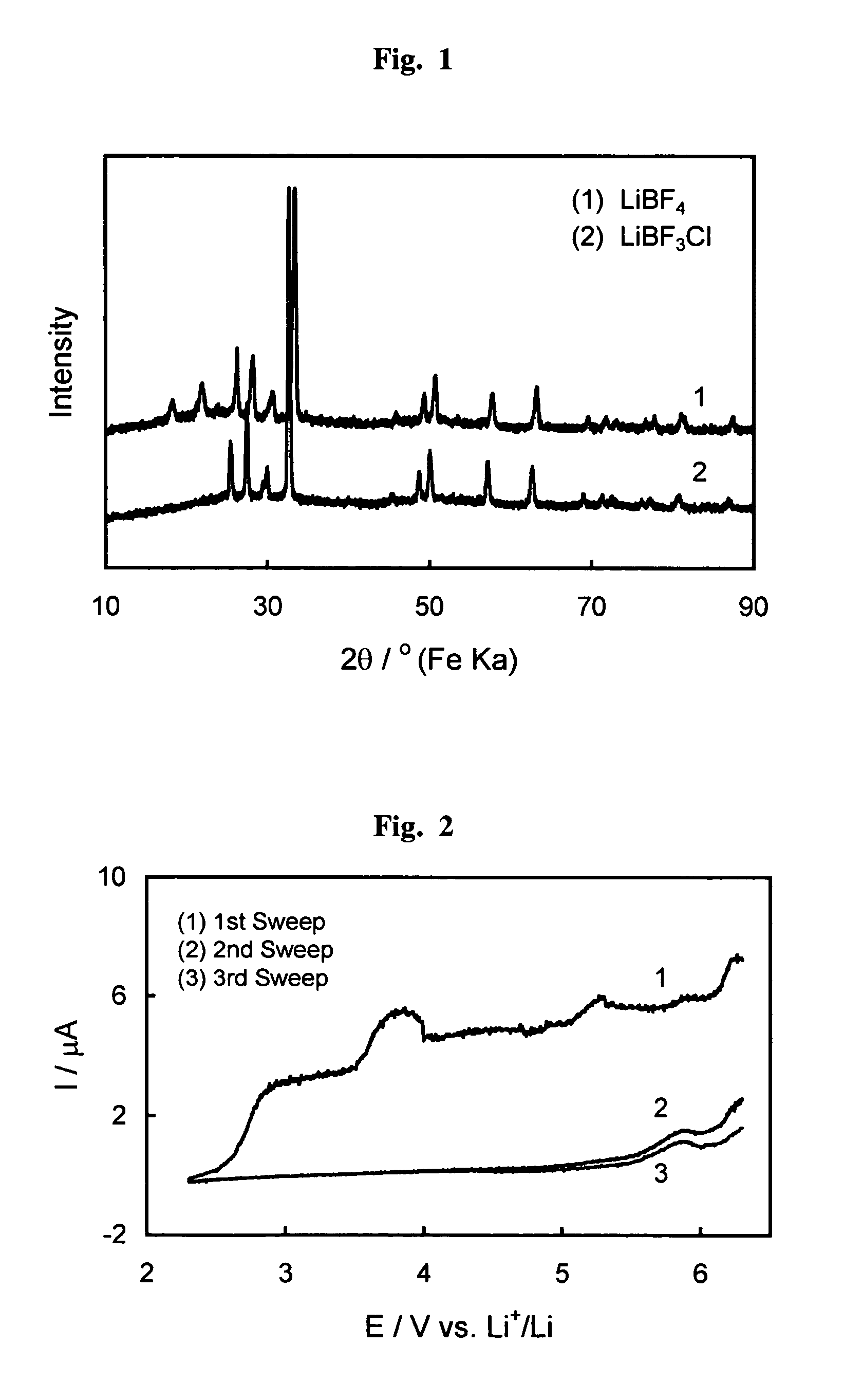
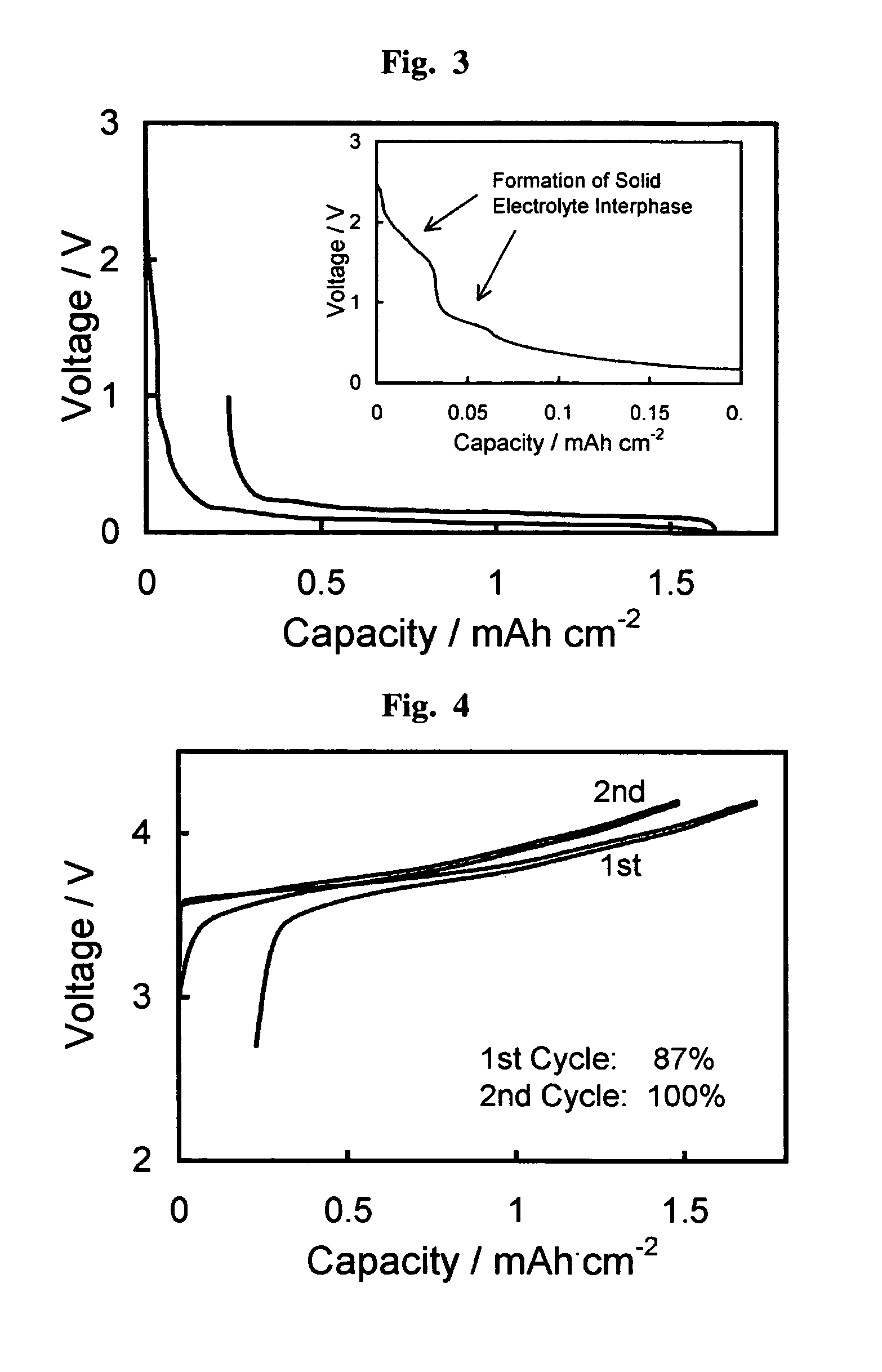
Fluorohaloborate salts, synthesis and use thereof
A composition is provided as a salt having the formula MBF3X where M is an alkali metal cation and X is the halide fluoride, bromide or iodide. A lithium salt has several characteristics making the composition well suited for inclusion within a lithium-ion battery. A process for forming an alkali metal trifluorohaloborate salt includes the preparation of a boron trifluoride etherate in an organic solvent. An alkali metal halide salt where the halide is chloride, bromide or iodide is suspended in the solution and reacted with boron trifluoride etherate to form an alkali metal trifluorohaloborate. The alkali metal trifluorohaloborate so produced is collected as a solid from the solution. The process is simple and yields alkali metal trifluorohaloborate of sufficient purity to be used directly in battery applications.
Owner:ARMY US SEC THE
Sodium ion battery positive electrode material, preparation method thereof and sodium ion battery
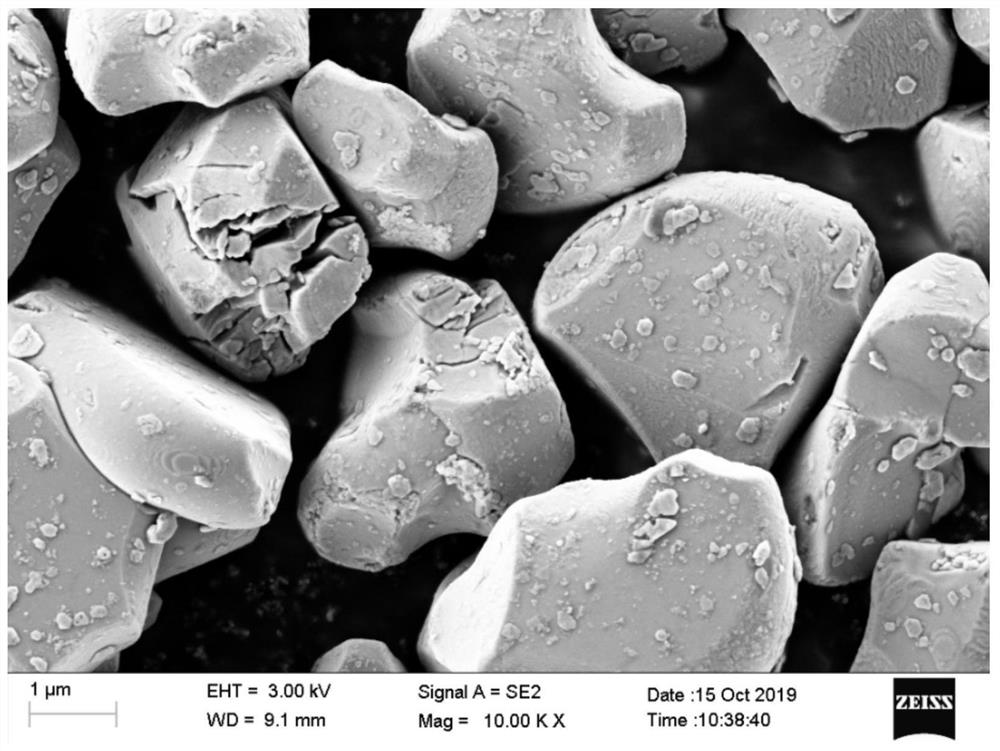
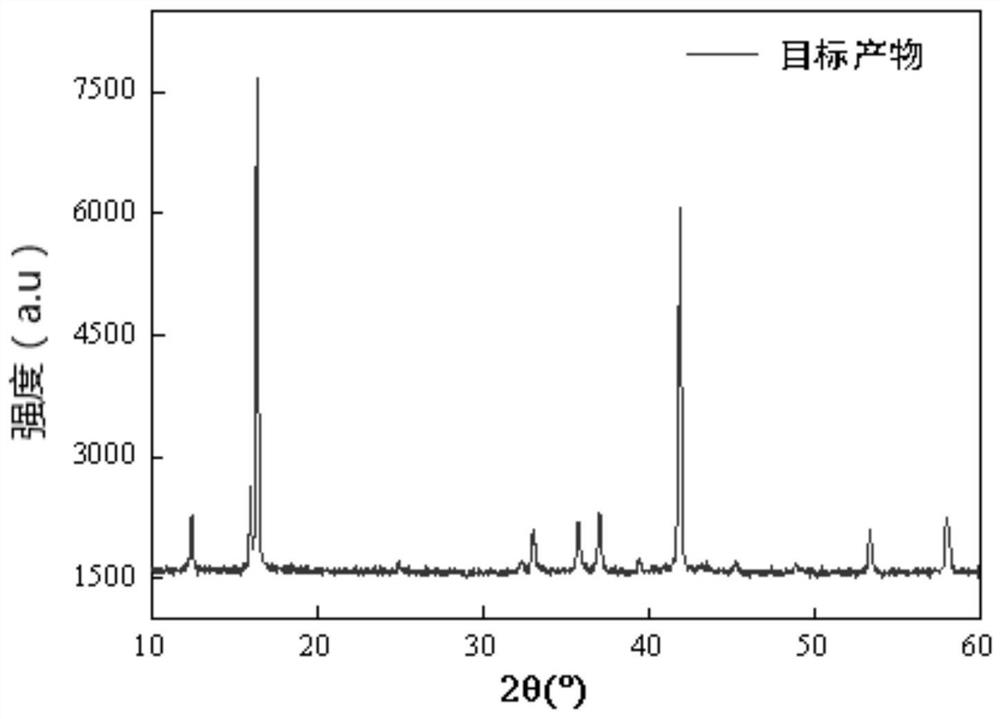
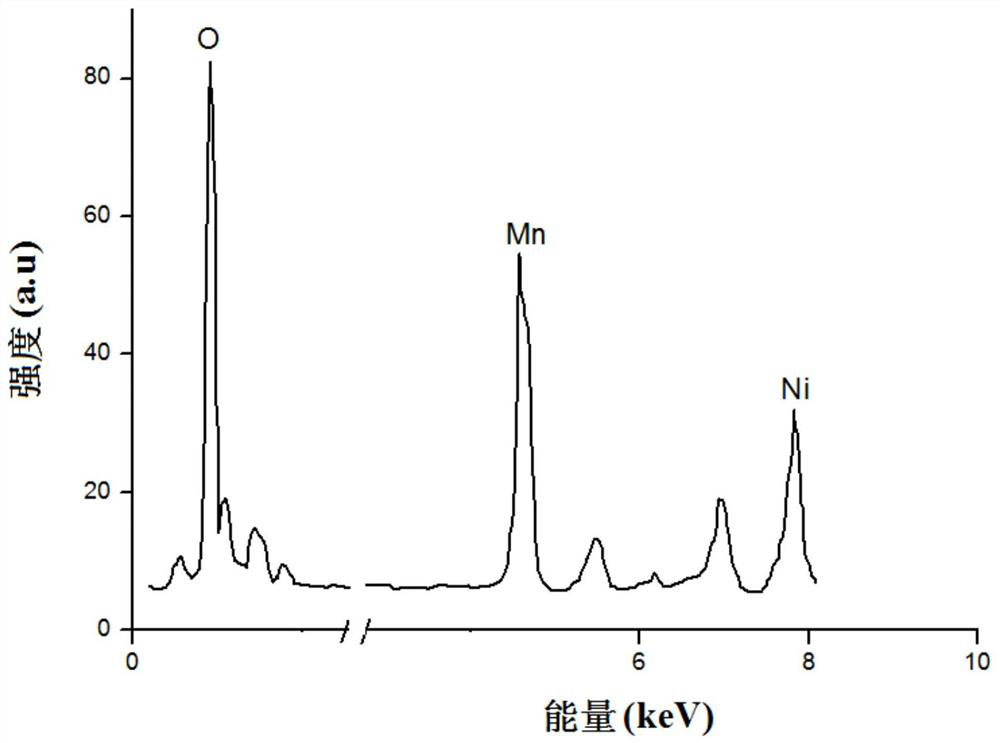
ActiveCN112563484AIncrease the areaHigh tap densitySecondary cellsPositive electrodesPhysical chemistrySodium-ion battery
The invention provides a sodium ion battery positive electrode material, a preparation method thereof and a sodium ion battery. The chemical formula of the sodium ion battery positive electrode material is NaxNiyM<1-y>O2, x is greater than 0.5 and less than 1, y is greater than 0.1 and less than 0.5, and M is selected from at least one of Mn, Fe, Co, V, Cu, Cr and Ti. The sodium ion battery positive electrode material is spherical-like particles, and has a layered structure. The sodium ion battery positive electrode material has good charge-discharge specific capacity and cycle performance. According to the preparation method, the precursor mixed solution is controlled to react under the conditions of high temperature and high pressure, so that the interlayer oxygen content of the layeredstructure of the positive electrode material of the sodium-ion battery is effectively reduced, and the cycle performance of the material is obviously improved. The preparation process is simple in steps, easily available in raw materials, easy to implement and suitable for large-scale production and application.
Owner:SHANDONG YUHUANG NEW ENERGY TECH
Preparation method of solid potassium ferrate
InactiveCN101117240AOptimizationEasy to optimizeIron compoundsSodium/potassium compoundsPotassium hydroxidePotassium hypochlorite
The invention discloses a fabricating method for solid potassium ferrate, which comprises a potassium hypochlorite fabrication procedure, a potassium ferrate synthesizing procedure and a sublimating procedure; the potassium ferrate synthesizing procedure is that a ferric nitrate nonahydrate covering from 4 percent to 10 percent of a total solution weight is added into the newly fabricated saturate potassium hypochlorite under a temperature from 20-25 DEG C and mixed, a solid potassium hydroxide is added slowly to a saturate degree and mixed, and is cooled down to 0 DEG C, and is filtered to get a filter cake which is washed by the potassium hydroxide solution to completely remove purple color, and the washing filtrate is combined and cooled to 0 DEG C, the solid potassium hydroxide is slowly added with mixing process until reaching to a saturate status, after a plurality of violet black solids are separated out, which are filtered by a funnel on a temperature from 0-5 DEG C, the filter cake is washed sequentially by cyclohexane and aether, and is dried by a temperature from 60-70 DEG C; compared with the prior art, the invention optimizes the fabrication process and procedure, the yield is over 85 percent according to the ratio between the actual output and the theoretical output, which can reach to 97 percent maximally.
Owner:HUANGSHAN UNIV
Process for production hexafluorophosphates
InactiveUS20110097626A1InexpensiveImprove securityPhosphorus halides/oxyhalidesLithium hexafluorophosphateFluoridePhosphorus
An object is to provide a method of manufacturing a hexafluorophosphate, that can simply and easily manufacture an inexpensive and high-quality hexafluorophosphate while suppressing the manufacturing cost, an electrolytic solution containing a hexafluorophosphate, and an electricity storage device including the electrolytic solution. The present invention relates to a method of manufacturing a hexafluorophosphate, which comprises reacting at least a phosphorus compound with a fluoride represented by MFs.r(HF) (wherein 0≦r≦6, 1≦s≦3, and M is at least one kind selected from the group consisting of Li, Na, K, Rb, Cs, NH4, Ag, Mg, Ca, Ba, Zn, Cu, Pb, Al and Fe) to produce a hexafluorophosphate represented by the chemical formula M(PF6)s.
Owner:STELLA CHEMIFA CORP
Process for treating alkaline sewage
InactiveCN1369436ANo emissionsEffective treatment processWater/sewage treatment by centrifugal separationSodium/potassium compoundsSodium metasilicateSewage
A process for treating alkaline sewage generated in preparing Zr chemicals includes concentrating, cooling, cystallizing to obtain nonahydrated sodium metasilicate, concentrating residual alkali solution and preparing liquid solution hydroxide or solid alkali.
Owner:柳云珍
Method for preparing nano-tubular sodium titanate/titanci acid
ActiveCN1789141ALarge specific surface areaReduce consumptionTitanium compoundsSodium/potassium compoundsMicrowave ovenStrong acids
A method for preparing nano pipe sodium titanate / metatitanic acid, comprising the following steps: (1) mixing the compound containing titanium with the strong alkaline solution with the wight proportion being 20-80%; (2) heating and backflowing in the microwave oven for 10 minitues-20 hours; (3) cooling and depositing the reacted mixing liquid, washing the deposite to make the pH be 6-8, separating and getting the nano pipe sodium titanate, immersing the product got in the above reaction in strong acid for 3-72 hours, washing, drying and getting the nano pipe metatitanic acid. The invention is characterized by the temperated reacting condition, fast reaction velocity, high performance and cost ratio of the product and being suited to produced industrially.
Owner:HENAN UNIVERSITY
Preparation method for potassium titanate crystal whisker or potassium titanate granule based on disintegration effect
ActiveCN101041906ALow costImprove qualityPolycrystalline material growthTitanium compoundsMicrometerPotassium
The invention discloses a making method of nanometer and micrometer potassium titanate whisker or particle based on disintegration effect, which is characterized by the following: utilizing disintegration effect and series-parallel connection effect to synthesize potassium titanate whisker; adopting normal titanium source compound and potassium source compound as raw material; sintering in the furnace under high temperature; disintegrating directly to synthesize or disperse to obtain nanometer or micrometer graded potassium titanate whisker; reducing sintering temperature; shortening manufacturing period greatly; fitting for large-scale of manufacturing; saving cost effectively.
Owner:唐山纳源微波热工仪器制造有限公司
Method for preparing micro crystalline powder of pure potassium strontium niobite
InactiveCN1686939AUniform particlesGood dispersionSodium/potassium compoundsNiobium compoundsStrontium carbonatePotassium
The present invention relates to a pure strontium potassium niobate microcrystal powder body and its preparation method. Said method includes the following steps: mixing strontium carbonate and columnbium pentoxide according to the ratio of 4:5, grinding potassium chloride, fully mixing said ground potassium chloride and mixture of strontium carbonate and columnbium pentoxide according to 1.2-2:1, roasting for 2-6 hr at 1150 deg.C, repeatedly washing the roasted product, until when the roaste dproduct has not chlorine ion, drying so that the obtained powder body is the strontium potassium niobate microcrystal powder and tetragonal tungsten bronze structure.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Sodium Anti-perovskite solid electrolyte compositions
InactiveUS20170275172A1Increase structural flexibilityEnhanced sodium transport rateAluminium compoundsFrom frozen solutionsIodideSodium-ion battery
Na-rich electrolyte compositions provided herein can be used in a variety of devices, such as sodium ionic batteries, capacitors and other electrochemical devices. Na-rich electrolyte compositions provided herein can have a chemical formula of Na3OX, Na3SX, Na (3-δ) Mδ / 2OX and Na (3-δ) Mδ / 2SX wherein 0<δ<0.8, wherein X is a monovalent anion selected from fluoride, chloride, bromide, iodide, H−, CN−, BF4−, BH4−, ClO4−, CH3−, NO2−, NH2− and mixtures thereof, and wherein M is a divalent metal selected from the group consisting of magnesium, calcium, barium, strontium and mixtures thereof. Na-rich electrolyte compositions provided herein can have a chemical formula of Na (3-δ) Mδ / 3OX and / or Na (3-δ) Mδ / 3SX; wherein 0<δ<0.5, wherein M is a trivalent cation M3, and wherein X is selected from fluoride, chloride, bromide, iodide, H−, CN−, BF4−, BH4−, ClO4−, CH3−, NO2−, NH2− and mixtures thereof. Synthesis and processing methods of NaRAP compositions for battery, capacitor, and other electrochemical applications are also provided.
Owner:BOARD OF RGT NEVADA SYST OF HIGHER EDUCATION ON BEHALF OF THE UNIV OF NEVADA RENO
Method of preparing two kinds of perferrate at the same time
InactiveCN1752002ASolve problems that cannot be exploitedFulfil requirementsCalcium/strontium/barium compoundsIron compoundsIron sulfateBarium dichloride
A process for simultaneously preparing potassium ferrate and barium ferrate used for battery or water-treating material includes such steps as adding potassium iodide to KOH solution, filling Cl gas, adding KOH solid until dissolving, cooling, filtering, collecting the filtrate containing potassium hypochlorite, adding at least one of iron nitrate, iron sulfate and iron chloride, adding it to cooled KOH solution at O deg.C, filtering to obtain coarse potassium ferrate, dissolving it in KOH solution, filter, recrystallizing in KOH solution, filtering, baking the filtered dregs to obtain potassium ferrate, deluting the filtrate, cooling, mixing it with barium chloride (or acetate) in KOH solution, filtering, washing the dregs and baking to obtain barium ferrate.
Owner:XIAMEN UNIV
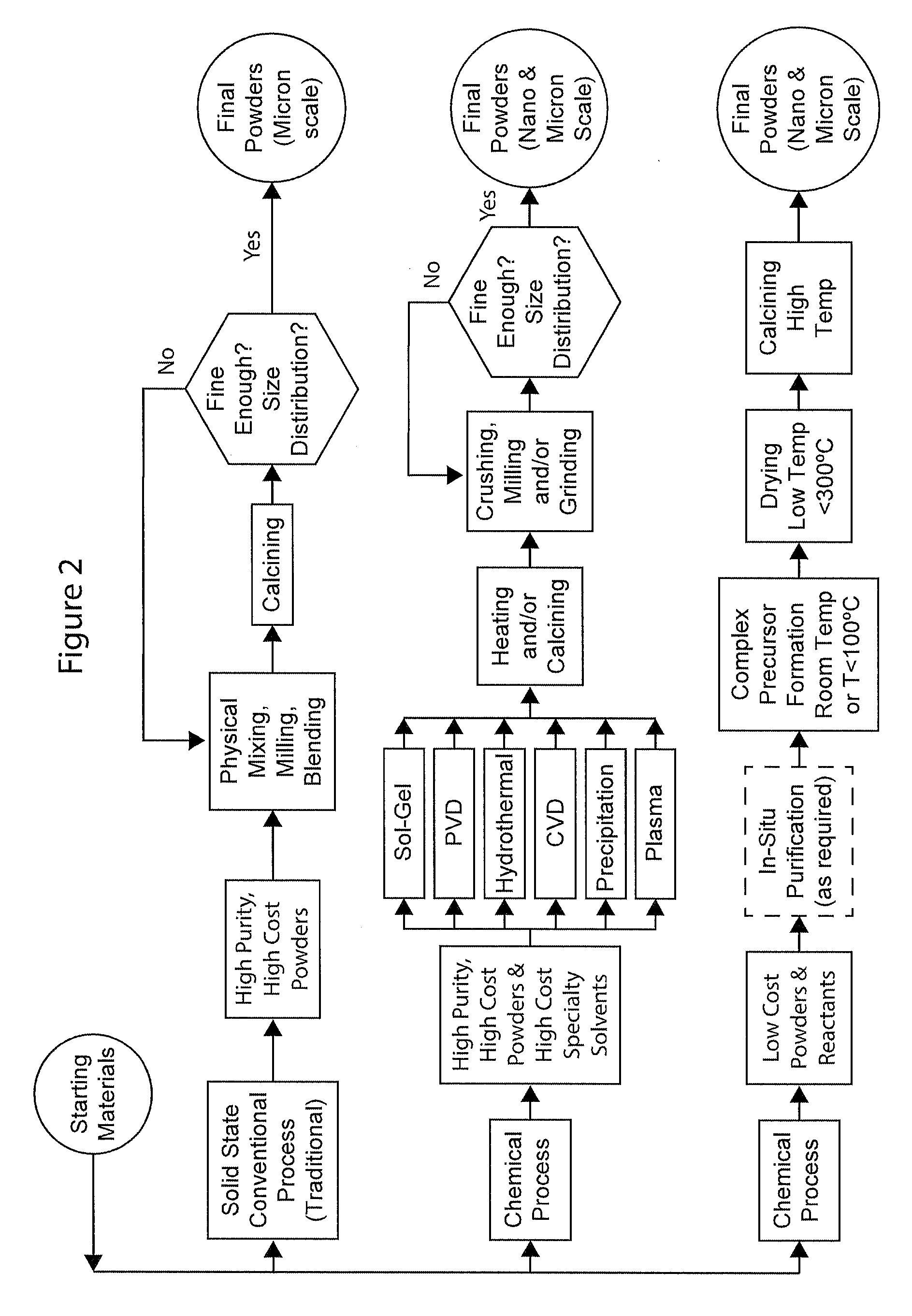
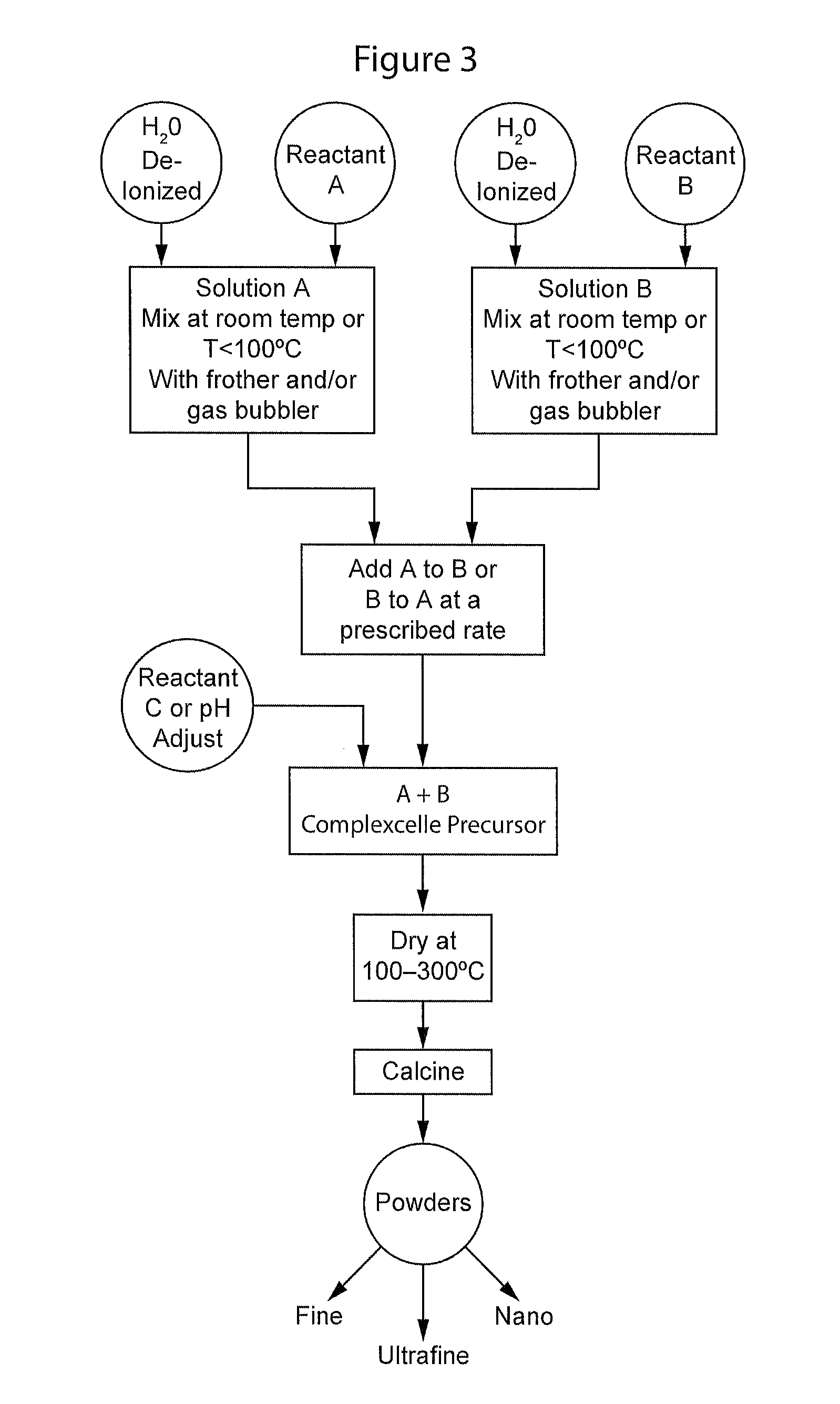
Complexometric precursors formulation methodology for industrial production of high performance fine and ultrafine powders and nanopowders for specialized applications
A method of forming a powder MjXp wherein Mj is a positive ion or several positive ions selected from alkali metal, alkaline earth metal or transition metal; and Xp is a monoatomic or a polyatomic anion selected from Groups IIIA, IVA, VA, VIA or VIIA; called complexometric precursor formulation or CPF. The method includes the steps of:providing a first reactor vessel with a first gas diffuser and an first agitator;providing a second reactor vessel with a second gas diffuser and a second agitator;charging the first reactor vessel with a first solution comprising a first salt of Mj;introducing gas into the first solution through the first gas diffuser,charging the second reactor vessel with a second solution comprising a salt of Mp;adding the second solution to the first solution to form a complexcelle;drying the complexcelle, to obtain a dry powder; andcalcining the dried powder of said MjXp.
Owner:NANO ONE MATERIALS
Preparation of single potassium persulfate
InactiveCN1778669AEasy to operateEase of industrial productionPeroxyhydrates/peroxyacidsSodium/potassium compoundsPotassium persulfatePotassium bisulfate
Production of monoper-potassium bisulfate includes oxidation reacting for hydrogen peroxide sulfuric acid to obtain reaction product, reacting the product with potassium hydrate to obtain monoper-potassium bisulfate solution, cooling, crystallizing, collecting solid monoper-potassium bisulfate, drying and obtaining end product. It is simple and convenient for industrial production.
Owner:JIAYUAN IND SHANGHAI
Process for preparation potassium salt for plant ash
InactiveCN101289199AReliable sourceWell sourcedAlkali metal halidesAlkali metal nitratesFiltrationPotassium ions
The invention relates to a method for preparing sylvite from plant ash, which is characterized in that the plant ash is put into a vessel, water with the weight which is more than twice of the weight of the plant ash is added into the vessel for carrying out fully stirring for 10 minutes to 20 minutes, potassium hydroxide with the weight which is 0.2 percent to 5 percent of the total weight of the plant ash and the water is added for carrying out fully stirring for 10 minutes to 20 minutes, the PH value of the plant ash solution is adjusted to 10 to 14 for sedimentation to remove other impurities, and colorless, transparent and pure solution is obtained after fine filtration; the solution filtered is put into a reaction kettle, 2 percent to 5 percent of selected acid in which the content of the plant ash and the water is 10 percent to 50 percent of the total weight is added according to preparation requirements and the measure value of the potassium ion in the plant ash for carrying out fully stirring for 10 minutes to 20 minutes, and the PH value is adjusted to 4 to 5; heating is carried out for vaporizing the water in the solution, a centrifuge is used for dehydration till the water content is less than 5 percent, and then product obtained is crashed into 50-mesh to 200-mesh fine powder and the sylvite is obtained. The plant ash used as the raw material in the invention is economic and environment-friendly, thereby having great benefit and grand development prospect.
Owner:王玉新
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com