Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Spinal Meninges" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
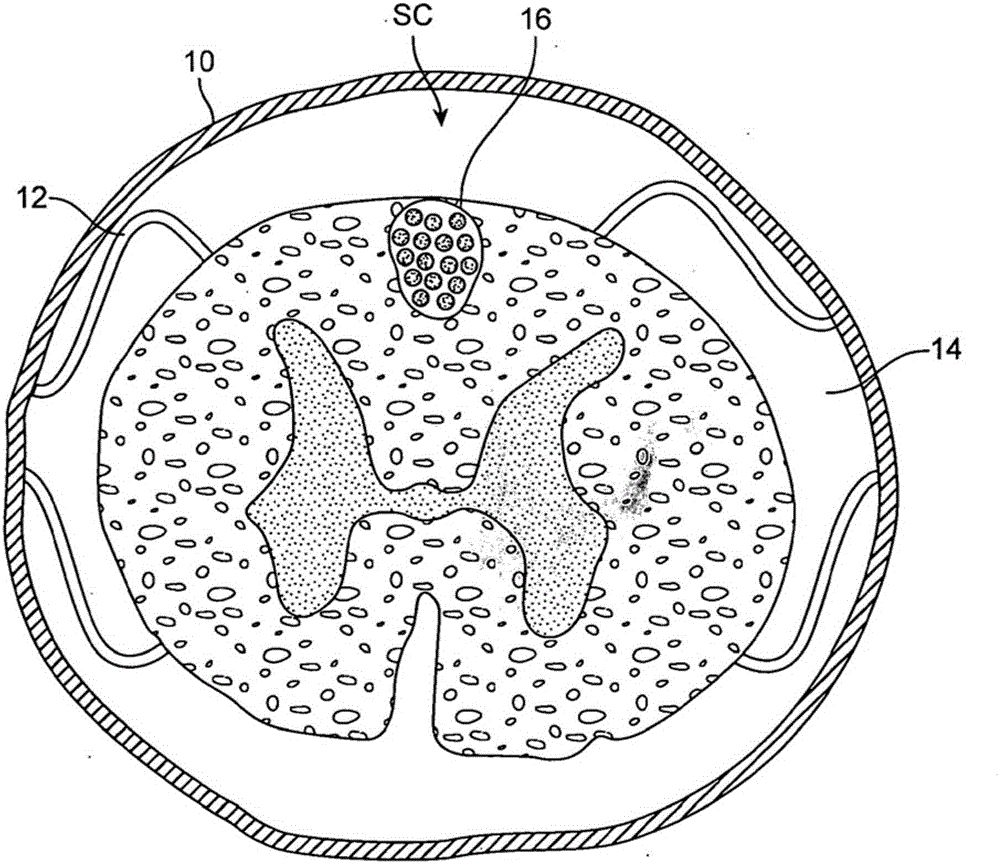
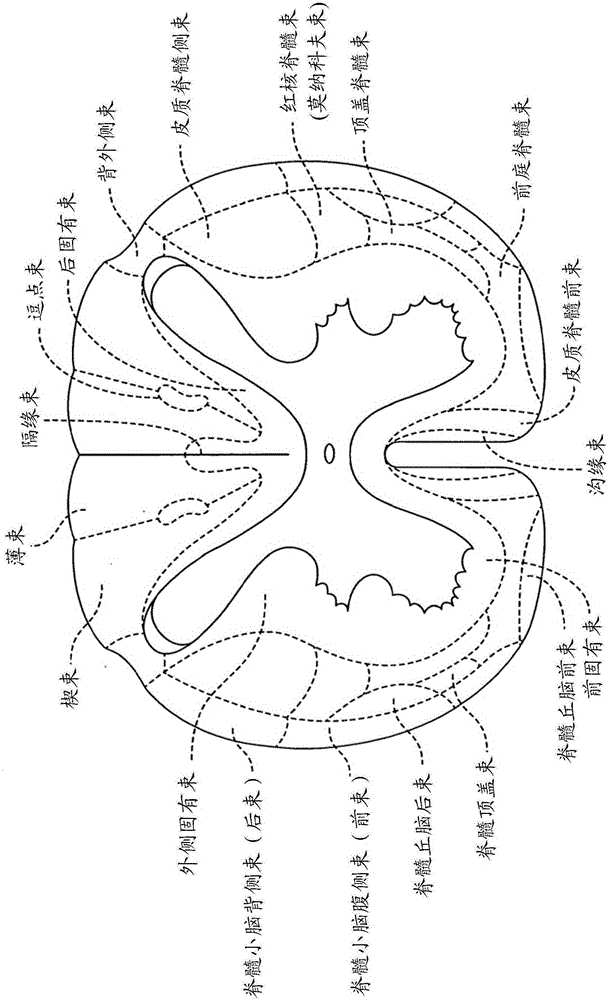
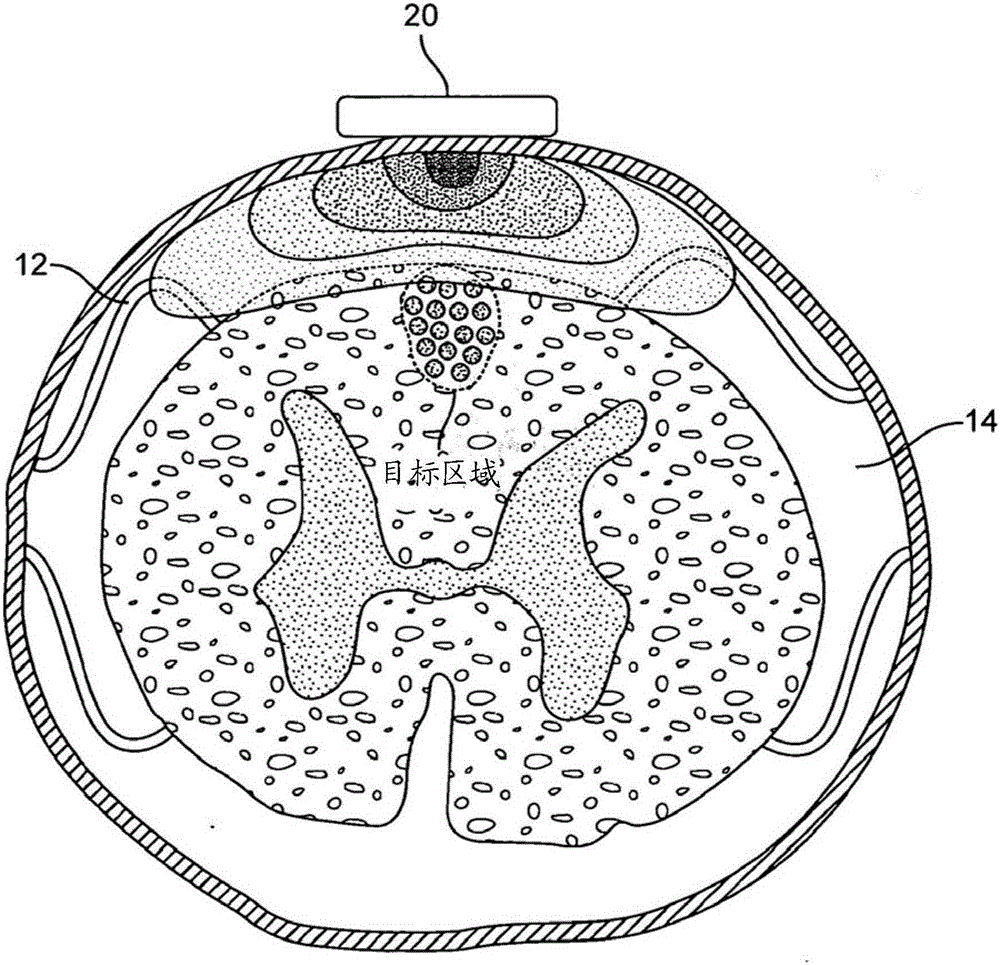
Connective tissue membranes that surround and support the spinal cord and cauda equina. They are continuous with cranial meninges, which surround and support the brain.
Tissue repair support and its preparation method and use
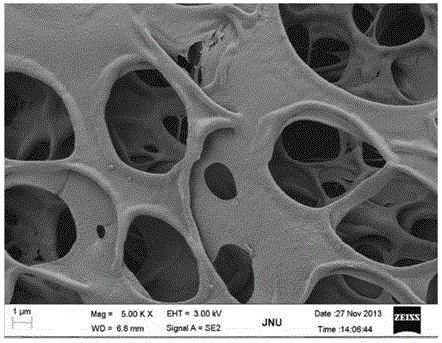
ActiveCN105194737AUnique pore structureHigh porositySurgeryAbsorbent padsTissue repairReticular formation
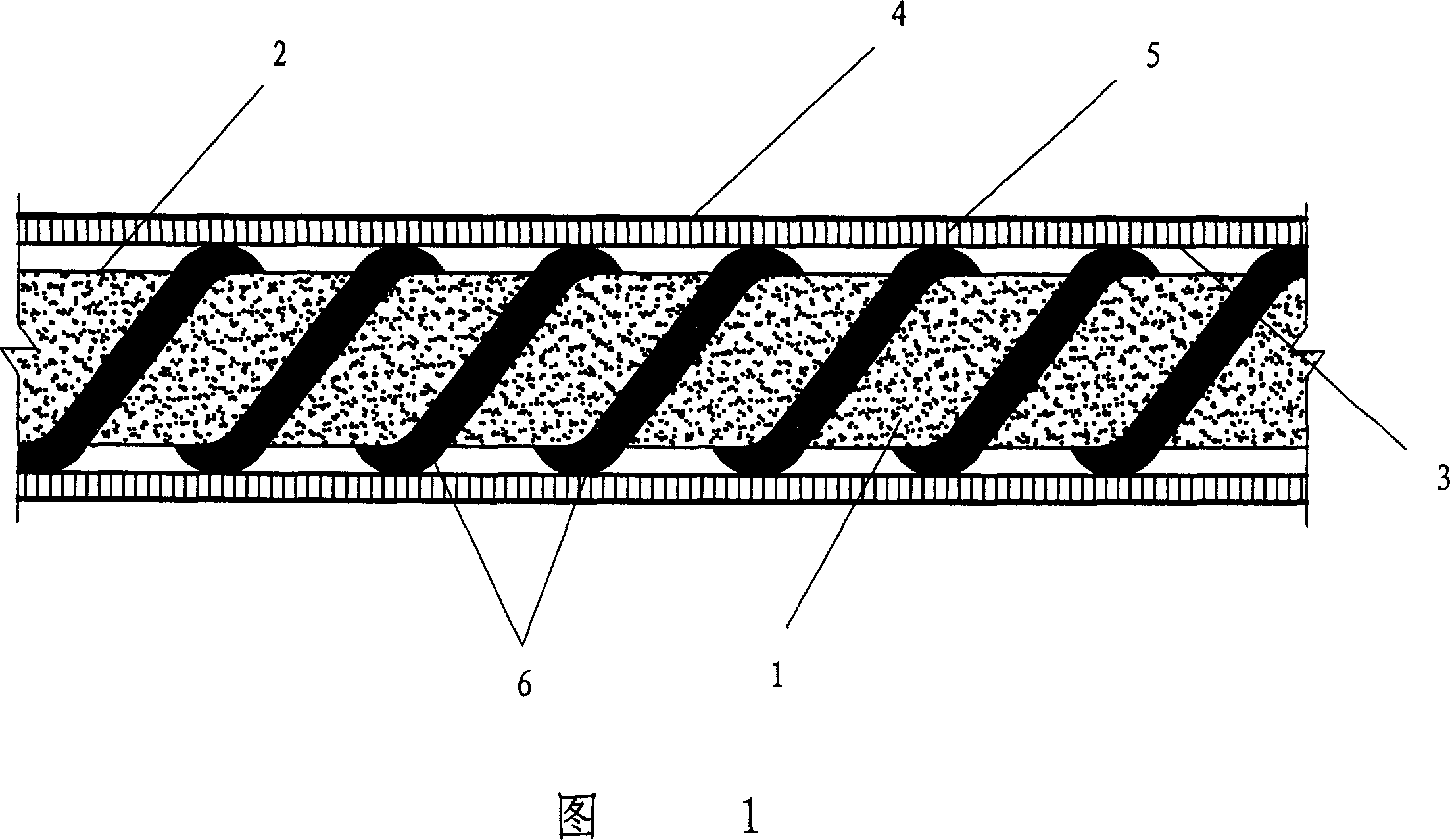
The invention provides a tissue repair support and its preparation method and use. The tissue repair support comprises a porous tridimensional network structure composed of a composite fiber layer and a hydrophilic material. The composite fiber layer comprises composite fibers composed of an adhesion factor and a hydrophobic synthesis material. The tissue repair support is used for meninge repair, spinal meninge repair, a tissue engineering support material, artificial skin, a wound accessory material, a biomembrane, a wound cladding material, a haemostasis material, a postoperative antistick material or a beauty treatment material.
Owner:MEDPRIN REGENERATIVE MEDICAL TECH
I-type medical collagen material keeping original specific triple helix structure of collagen, product and application thereof
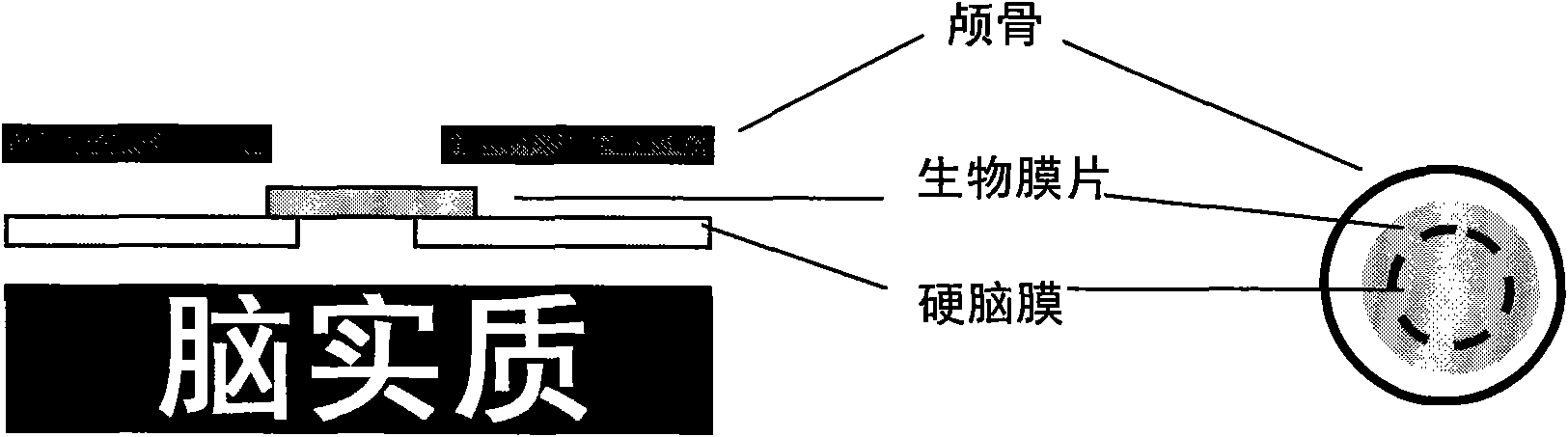
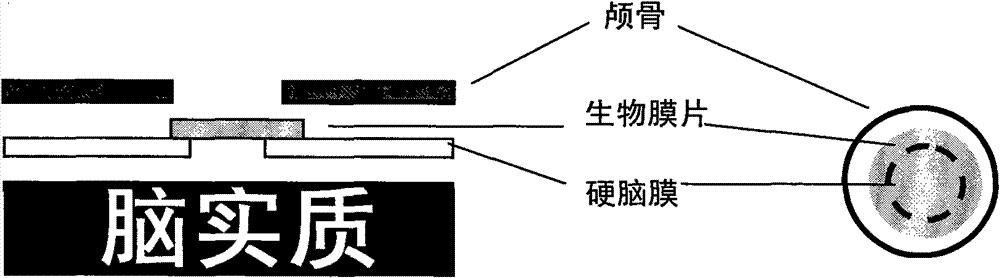
The invention relates to an I-type medical collagen material keeping an original specific triple-helical structure of collagen, and an extraction method thereof, meninges / spinal meninges biomembrane made from the I-type medical collagen material, and a preparation process of the biomembrane, and application of the biomembrane to preparing meninges / spinal meninges tissue repair materials. The I-type medical collagen material keeping the original specific triple helix structure of collagen has the advantages of low immunogenicity, no foreign body reaction, good biocompatibility and controllabledegradation rate, the prepared meninges / spinal meninges biomembrane has certain stretching resistance strength, repairable and regenerative dura mater / spinal meninges tissue and tissue adhesion prevention / reduction, and the biomembrane is applicable to repair and regeneration of injured cerebral dura mater and spinal dura mater.
Owner:许和平
Preparation method and device of duramater/spinal dural transplanting substitute
ActiveCN102727935ASimple Surface Functional StructureWidely sourced and cheapProsthesisAntigenDefect repair
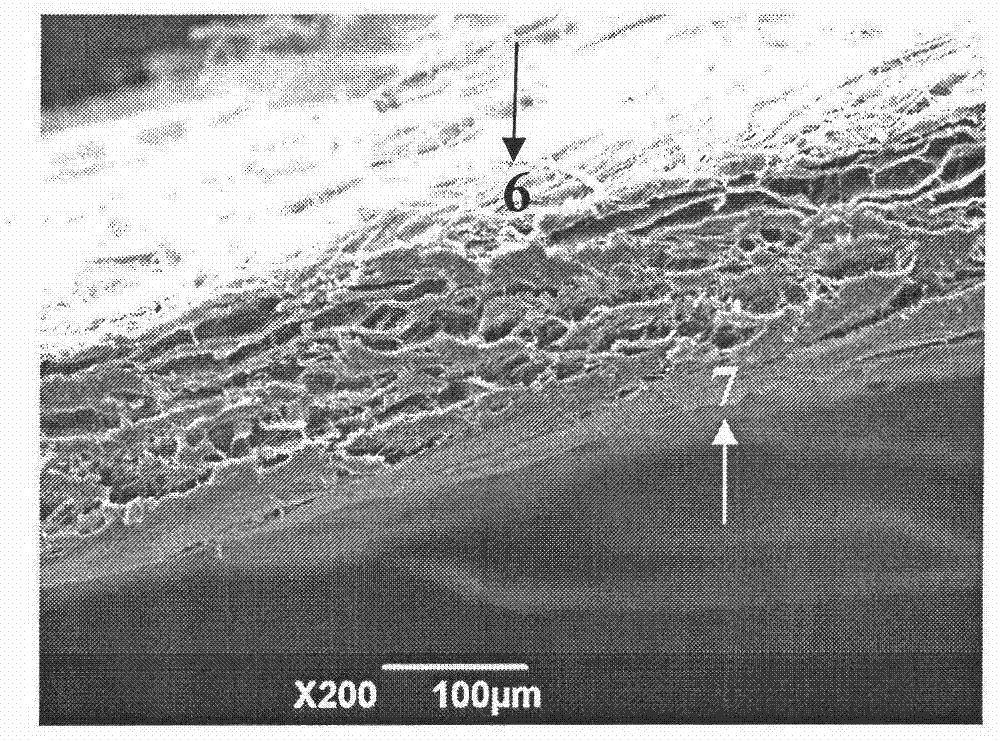
The invention provides a preparation method of a duramater / spinal dural transplanting substitute which is obtained by repeated freezing and thawing of dural tissue, rolling and cracking of cells, crosslinking fixed protection, accellular antigen extraction, dense surface fibrosis modification, packaging and sterilization, and has the advantages of simple method, wide raw material sources, cheap raw materials, and low cost. The prepared dural substitute completely removes components of cells and other antigen components simultaneously when protecting dural tissue natural structure and properties, is good in biocompatibility, free of immune rejection, safe and reliable, good in mechanical performance, and easy in clinical operation, can meet the needs of defect repair, has the function of promoting tissue regeneration as a loose surface is beneficial to the tissue fluid adsorption, active factor enrichment, and growth of blood vessels and cells, and has the advantages of rapidness in fusion with a host, biodegradable absorption, and good repair effect. The animal test shows that the defect can be completely repaired without brain or spinal fluid leakage, or adhesion with brain tissue, and significant rejection is not found.
Owner:SHAANXI BIO REGENERATIVE MEDICINE CO LTD
Tissue repair scaffold and preparation method and purpose thereof
InactiveUS20160175487A1Unique structureHigh porosityElectric discharge heatingPeptide/protein ingredientsTissue repairReticular formation
The present invention provides a tissue repair scaffold and a preparation method and purpose thereof. The tissue repair scaffold comprises at least one composite fiber layer, and the composite fiber layer comprises a composite fiber formed by an adhesion factor and a hydrophobic synthetic material. The composite fiber layer may further form a porous three-dimensional mesh structure with a hydrophilic material. The tissue repair scaffold is applied to brain meninge repair, spinal meninge repair, a tissue engineering scaffold material, artificial skin, a wound assistant, a biological membrane, a wound cladding material, a hemostatic material, a postoperative adhesion prevention material, or a cosmetic material.
Owner:MEDPRIN REGENERATIVE MEDICAL TECH
Absorbable medical biological membrane and preparation method thereof
InactiveCN106390201AGood compatibilityGood flexibilityPharmaceutical delivery mechanismTissue regenerationDiseaseSurgical operation
The invention provides an absorbable medical biological membrane and a preparation method of the absorbable medical biological membrane. The biological membrane is prepared by taking animal pericardium, pleura or intestinal film as an animal source collagen membrane material, and performing a decellularized treatment, adding biological active factors and performing a sterilizing process. Compared with the prior art, the absorbable medical biological membrane and the preparation method thereof, created by the invention, have the advantages that the biological membrane selected from the animal source membrane base material with wide origin and performed with antigen removal treatment and sterilizing treatment is high in histocompatibility, and can degrade in a human body; the addition of growth factors can accelerate the wound healing. The biological membrane prepared by the preparation method is in milky white, and has better flexibility and is closer to natural living tissues, not easy to calcify, and applicable to repair defected soft tissues in surgical operation, such as the repair of cerebral dura mater or spinal meninges defect caused by brain and spinal cord injury, cancer and other brain diseases.
Owner:天津欧尔克医药科技有限公司
Type-I collagen material, meninx and meninge biological membrane and preparation method and application of thereof
The invention relates to a type-I collagen material with a high sewing concentration and a tri-helical structure, a meninx and meninge biological membrane prepared from the type-I collagen material, and a preparation method and application of the meninx and meninge biological membrane, and the meninx and meninge biological membrane can be used for the preparation of dura meninx and meninge tissue repair materials.
Owner:BEIJING YH BIOMAX BIOLOGIC TECH
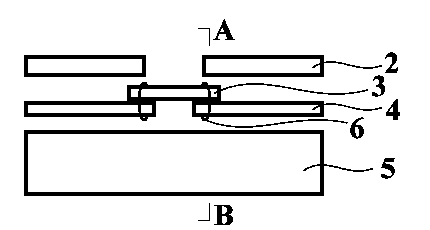
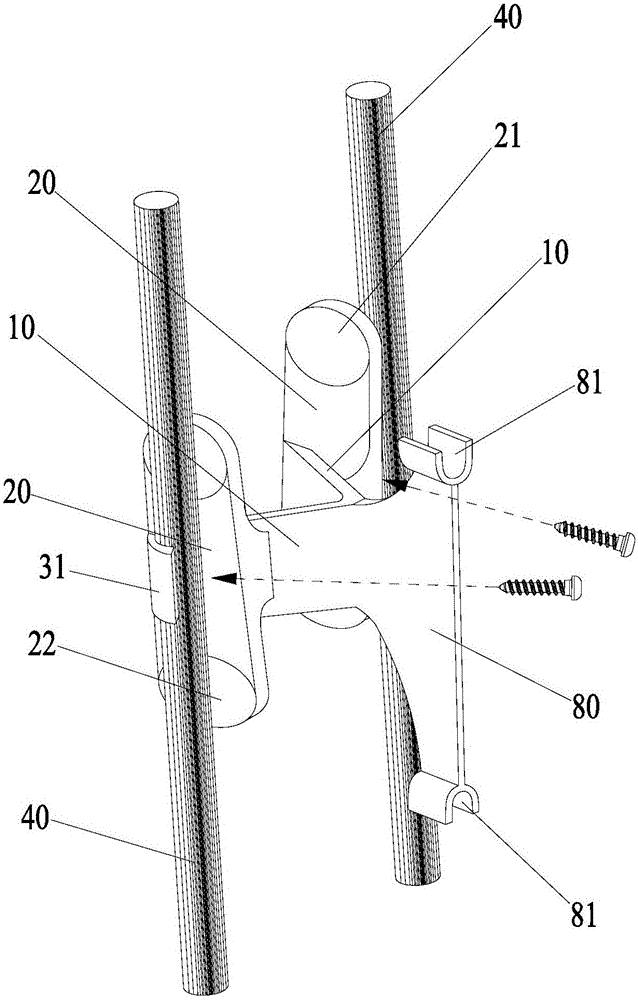
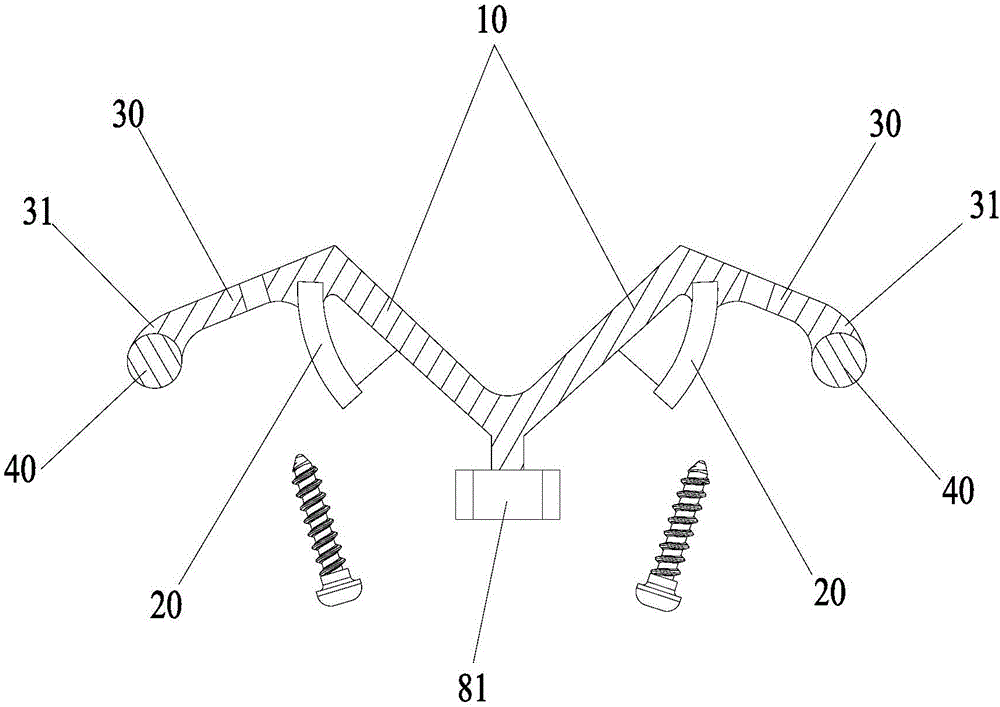
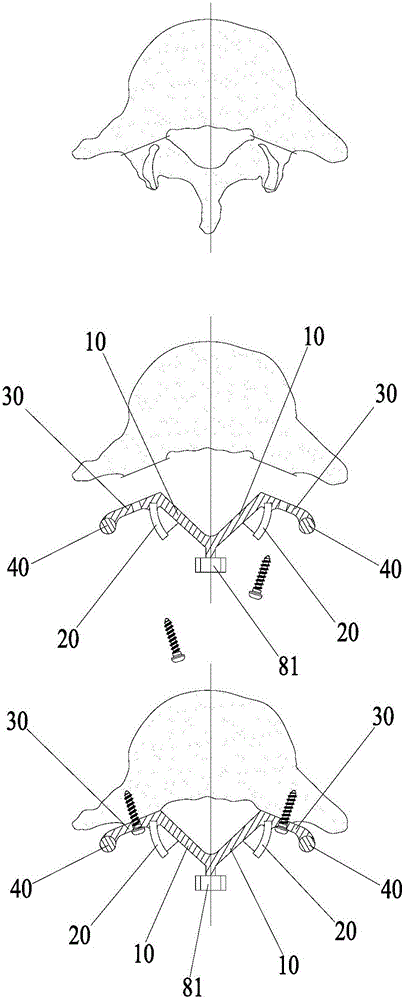
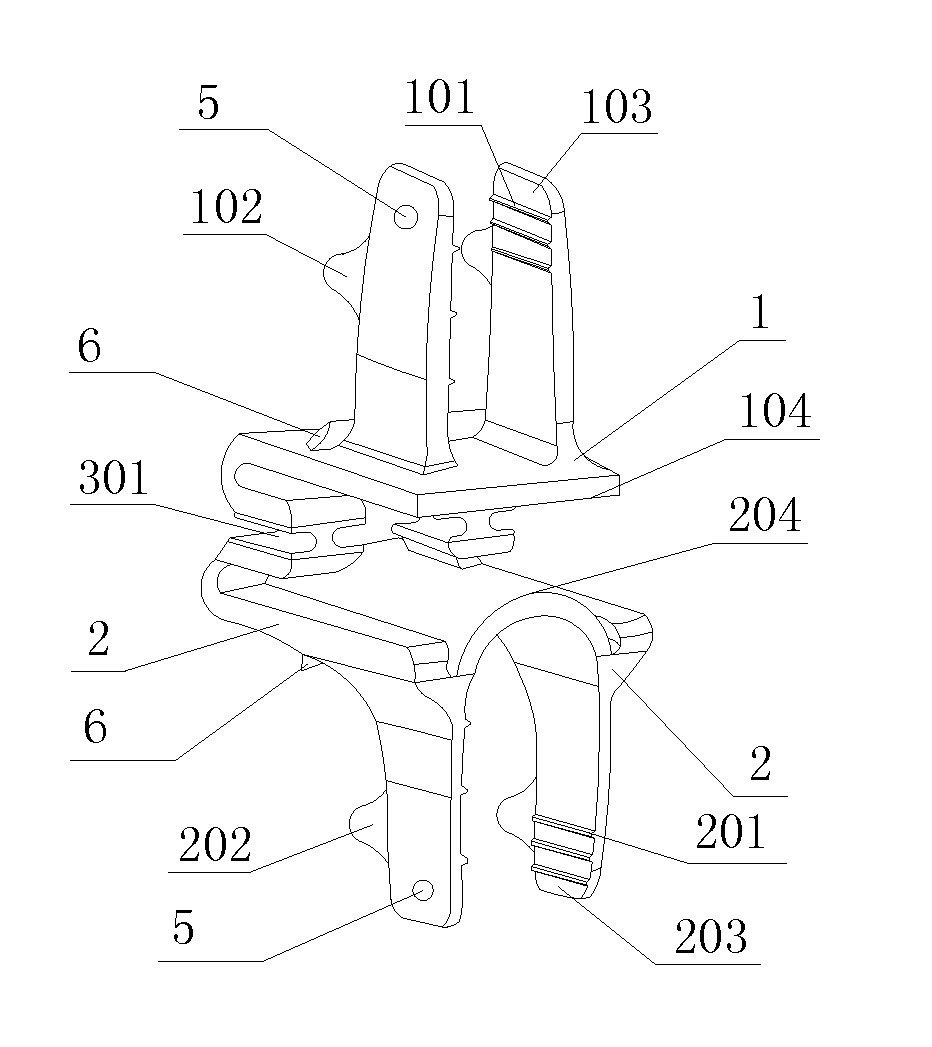
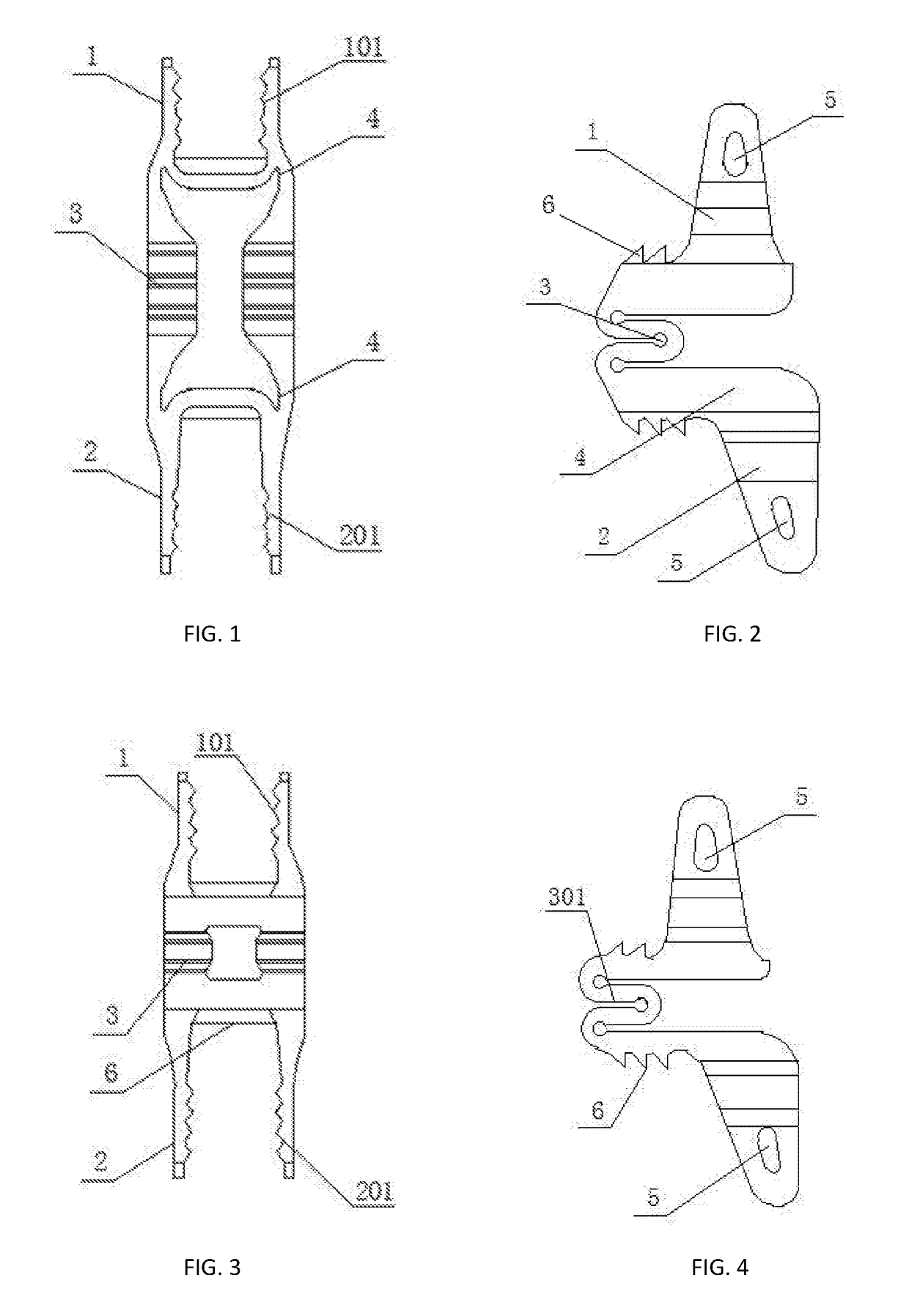
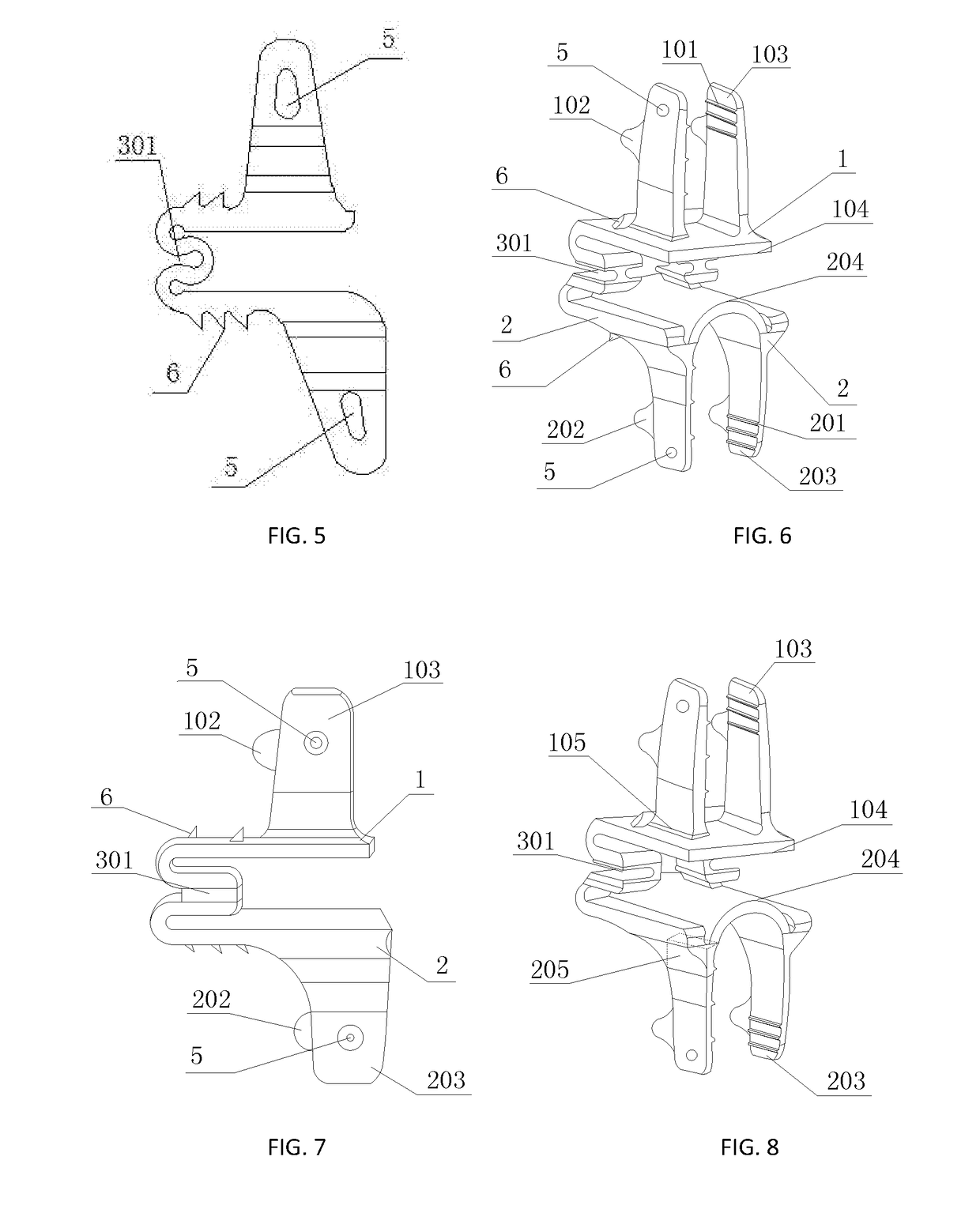
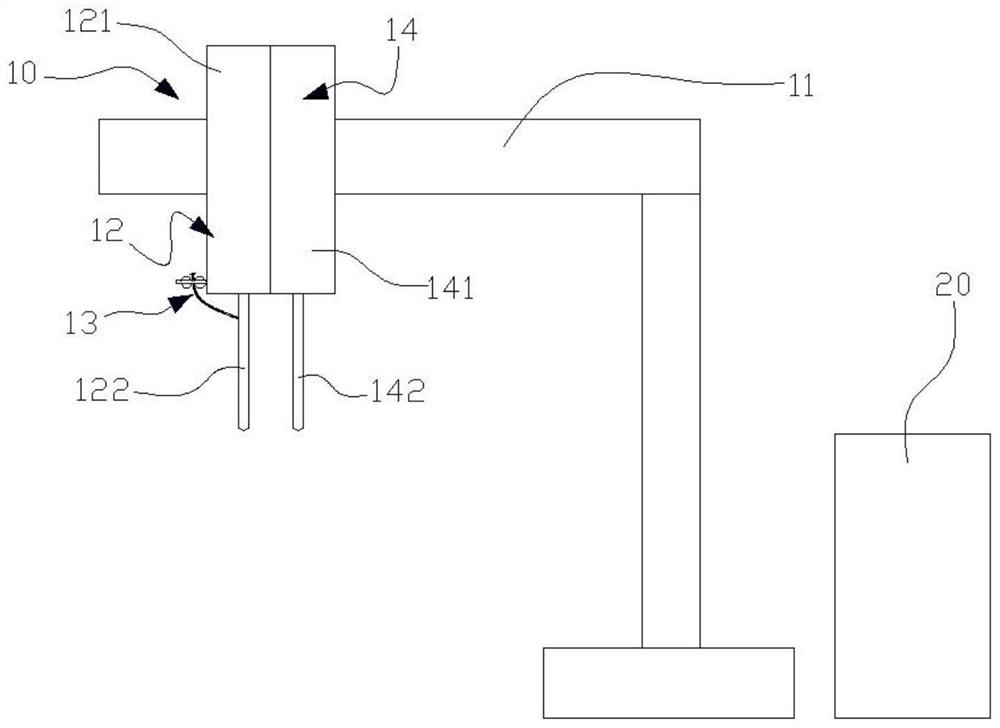
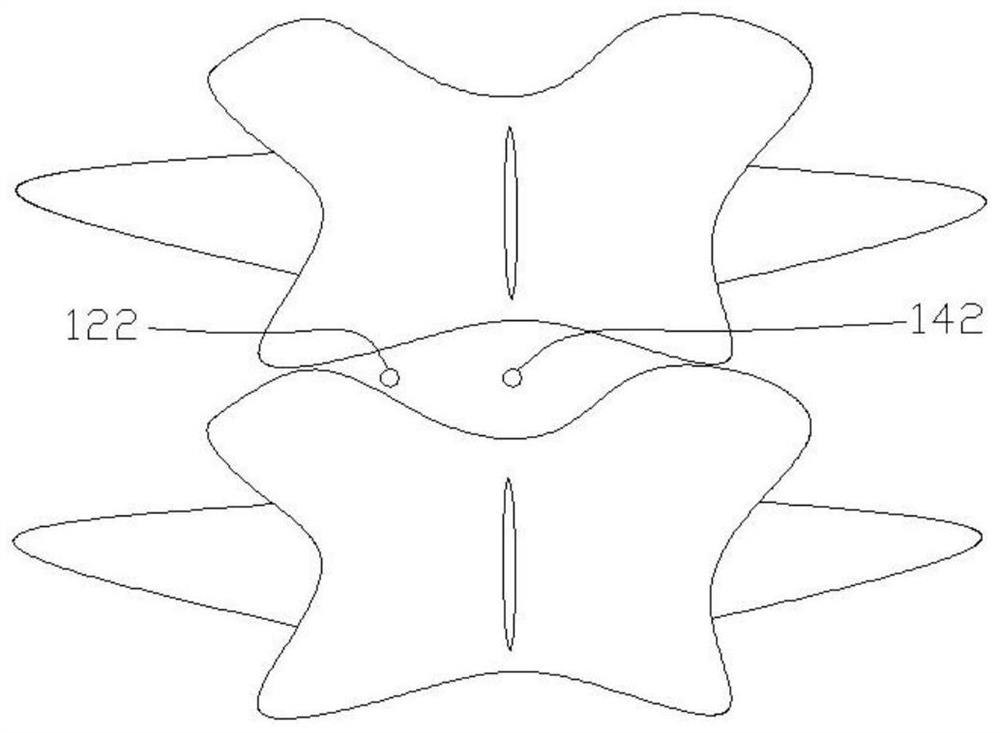
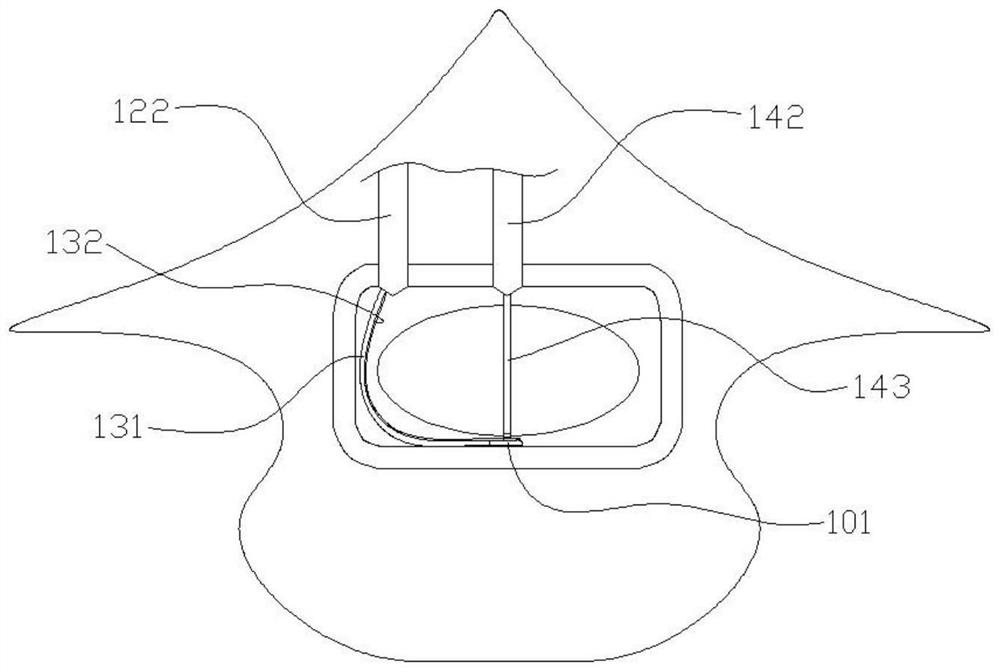
Spine fixing assembly
PendingCN107174325AAchieve protectionAvoid stickingInternal osteosythesisSpinal columnSpinal dura mater
The invention provides a spine fixing assembly which comprises two spinal canal covering plates, wherein the two spinal canal covering plates are connected at an angle to form a first recess between the two spinal canal covering plates; an opening of the first recess faces toward a spinal canal; and a fixed structure is matched with the two spinal canal covering plates to fix the two spinal canal covering plates at the position, corresponding to the centrum of an affected part in the front of the spinal canal, at the rear part of the spinal canal. According to the spine fixing assembly, the problem that spinal dura mater spinalis of the corresponding part is short of bone barrier protection after the spinal centrum is partially excised or integrally removed in the prior art can be effectively solved.
Owner:BEIJING AKEC MEDICAL
Interspinous omnidirectional dynamic stabilization device
ActiveUS20180008429A1Reasonable design structureSmall sizeInternal osteosythesisJoint implantsHuman bodyUltrasound attenuation
The present disclosure relates to an interspinous omnidirectional dynamic stabilization device, including a first fixing part, a second fixing part, a connecting structure and an elastic structure, wherein the first fixing part and the second fixing part are fixedly connected to each other through the connecting structure and elastic structure, the bottoms of the first fixing part and the second fixing part are provided with one or more barbs, the elastic structure is made up of one or more U-shaped structures connected to each other, and the first fixing part and the second fixing part are provided with fixing holes respectively. The device is able to provide the maximum matching for the mobility in all directions, according to the requirements on the physiological activities of the human body, without causing stabilizing structures to be relatively displaced, or loosen and fall off. In addition, the device has a reasonably designed structure, with a small size. The device can be firmly fixed, and have a strong ability of elasticity attenuation resistance. In the device, the prosthesis has strong vertical support force at the bottom of the spinous process after implantation. Moreover, the device is fixed to the spinous processes and lamina, with the elastic structure attached to the spinous processes on either side of an interspinous space, and the bottom of the prosthesis is not forced to be close to the spinal dura mater, to reduce the risk of damaging the spinal dura mate during or after surgery.
Owner:BIODA DIAGNOSTICS WUHAN
Composite biological patch as well as preparation method and application thereof
ActiveCN113663137AHelp slow releaseImprove adhesionPharmaceutical delivery mechanismTissue regenerationRepair tissueBreast repair
The invention provides a composite biological patch as well as a preparation method and an application thereof, and relates to the technical field of medical treatment. The composite biological patch provided by the invention comprises a hydrophobic porous layer, a compact layer and a porous scaffold, the hydrophobic porous layer is opposite to one side of a to-be-repaired tissue and is loaded with an anti-infection drug, the hydrophobicity of the hydrophobic porous layer and the loaded drug can be matched with each other, the drug can be slowly released, and the effects of prevention, anti-infection and anti-adhesion are achieved; the porous scaffold has a porous structure, faces one side of a to-be-repaired tissue, is beneficial to adhesion and growth of cells, and induces membrane tissue regeneration; the hydrophobic porous layer and the porous scaffold spacing compact layer can ensure the dimensional stability and mechanical strength of the product, and also can play a role of a barrier. The composite biological patch provided by the invention can be used for meninx repair, spinal meninx repair, rotator cuff repair, hernia repair, ophthalmology repair, breast repair, hemostasis repair or oral cavity repair.
Owner:BEIJING BONSCI TECH CO LTD
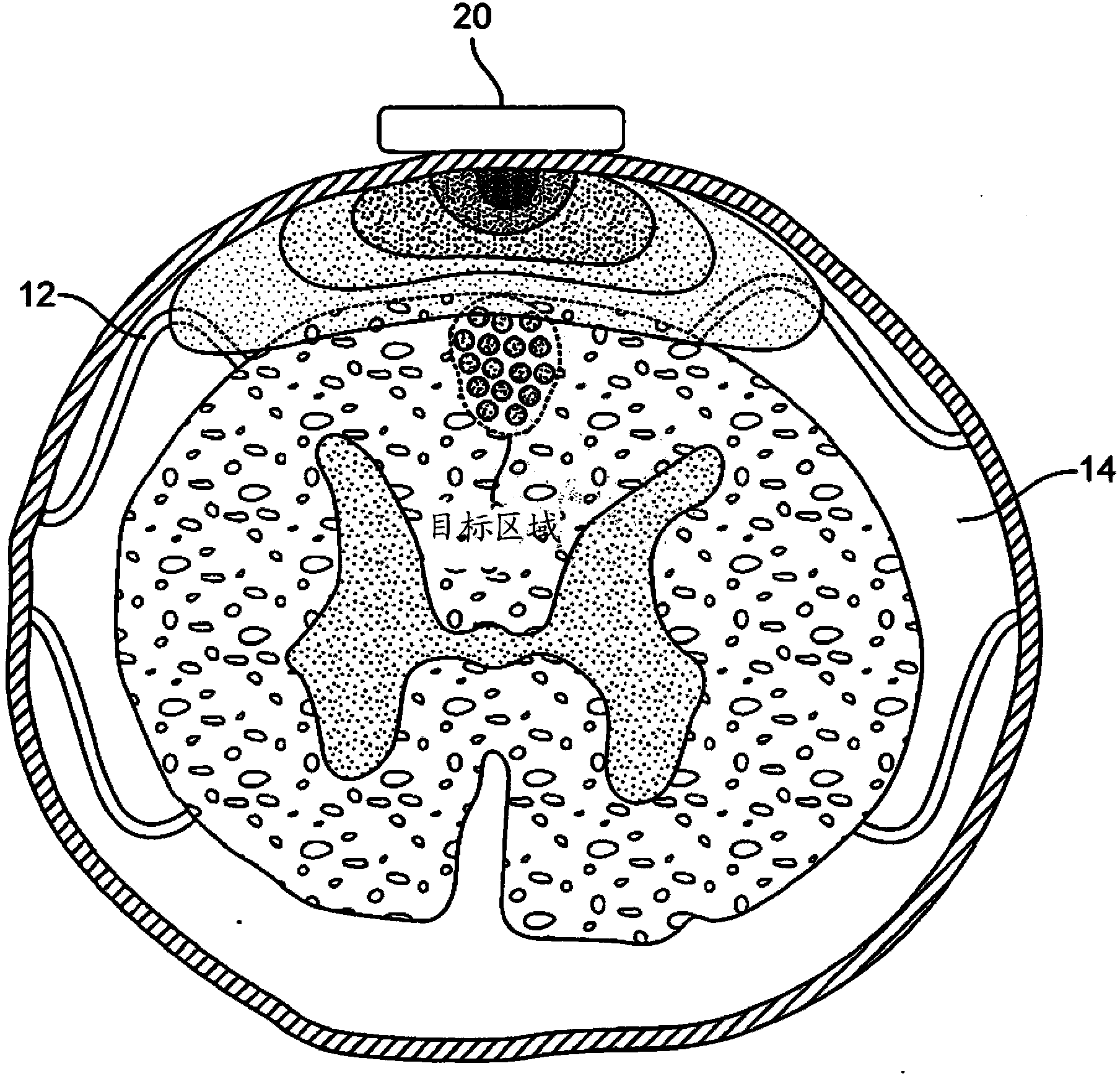
Remotely controlled and/or laterally supported devices for direct spinal cord stimulation
InactiveCN103547310ADoes not affect the natureGood electrical couplingSpinal electrodesElectricityTransceiver
A method for treating intractable pain via electrical stimulation of the spinal cord. Remote, non-contact stimulation of a selected region of spinal cord is achieved by placement of a transceiver patch directly on the surface of that region of spinal cord, with said patch optionally being inductively coupled to a transmitter patch of similar size on either the outer or inner wall of the dura surrounding that region of the spinal cord. By inductively exchanging electrical power and signals between said transmitter and transceiver patches, and by carrying out the necessary electronic and stimulus signal distribution functions on the transceiver patch, the targeted dorsal column axons can be stimulated without the unintended stray stimulation of nearby dorsal rootlets. Novel configurations of a pliable surface-sheath and clamp or dentate ligament attachment features which realize undamaging attachment of the patch to the spinal cord are described.
Owner:UNIV OF IOWA RES FOUND +1
Spinal dura mater sealing hydrogel and preparation method and application thereof
InactiveCN110801528AFast gelationModerate degradation timeSurgical adhesivesPharmaceutical delivery mechanismHydrophilic polymersMedicine
The invention discloses a biodegradable hydrogel sealing agent. The colloid-forming time of the hydrogel is smaller than 20 seconds, the degree of swelling is 50-200%, the bursting strength is not less than 10kPa, and the in vitro degradation time is smaller than 90 days. The preparation raw materials of the hydrogel sealing agent comprise a first component namely albumin, containing nucleophilicfunctional groups, and a second compound of a hydrophilic polymer containing a plurality of electrophilic functional groups, wherein the two components are mixed physically through a dual medicine mixing machine, and in site covalent cross-linking is performed to form the hydrogel. The physical properties and the degradability of the hydrogel can be adjusted through changing the first component orthe second component, so that the biodegradable hydrogel sealing agent can be used for sealing wounded parts of different tissue parts in bodies, such as spinal dura mater damage parts.
Owner:金路平
I-type medical collagen material keeping original specific triple helix structure of collagen, product and application thereof
The invention relates to an I-type medical collagen material keeping an original specific triple-helical structure of collagen, and an extraction method thereof, meninges / spinal meninges biomembrane made from the I-type medical collagen material, and a preparation process of the biomembrane, and application of the biomembrane to preparing meninges / spinal meninges tissue repair materials. The I-type medical collagen material keeping the original specific triple helix structure of collagen has the advantages of low immunogenicity, no foreign body reaction, good biocompatibility and controllabledegradation rate, the prepared meninges / spinal meninges biomembrane has certain stretching resistance strength, repairable and regenerative dura mater / spinal meninges tissue and tissue adhesion prevention / reduction, and the biomembrane is applicable to repair and regeneration of injured cerebral dura mater and spinal dura mater.
Owner:许和平
Artificial biological spinal cord
ActiveCN1985777APromote regenerationRegeneration inducibleSurgeryTissue cultureCross-linkBiocompatibility Testing
The artificial biological spinal cord consists of a medulla, a spinal pia mater coating the medulla, a spiral rack fixed on the spinal pia mater, an arachnoid coating the spiral rack, a collagen gel layer coating arachnoid, and a sinal dura mater coating the collagen gel layer. The medulla, the spinal pia mater, the arachnoid and the sinal dura mater are cross-linked and fixed with no-aldehyde fixing agent, and made of animal spinal cord and membrane tissue with the antigen eliminated by using active reagent and strong hydrogen bond reagent. The present invention is designed through imitating the composition and structure of human spinal cord and made of natural biological material, and possesses the advantages of high biocompatibility, no immunogenicity, capacity of being degraded and absorbed, and capacity of inducing tissue regeneration. The present invention provides excellent matrix and micro environment for repairing spinal cord.
Owner:SUMMIT GD BIOTECH
Method for extracting and culturing embryonic neural stem cells in vitro, and preparation of culture medium
ActiveCN110628706AHigh purityImprove efficiencyCulture processNervous system cellsPenicillinWide mouth
The invention discloses a method for extracting and culturing embryonic neural stem cells in vitro. The method comprises the following steps: newborn mice born within 24 hours are taken, after being killed in a cervical dislocation mode, the newborn mice are soaked into ethyl alcohol to be disinfected, flushing is conducted with a sodium chloride solution, spines are shorn at upper cervical spinesand sacral vertebrae through sterile scissors, and the spine trunks are taken out; spinal cord in the spines are extruded out through wide-mouth tweezers and put into a sodium chloride solution, spinal membranes are stripped under a microscope, and the spinal cord is filtered and then put into a centrifuge tube; and trypsin is added for digestion while blowing and beating, a DMEM / F12 culture medium is added for stopping digestion, after filtering through a cell filter screen, centrifuging is conducted to collect the cells, and an embryonic neural stem cell complete culture medium is added forculture. The formula of the embryonic neural stem cell complete culture medium comprises DMEM / F12, glutamine, lysine, a B-27 additive, an N2 additive, penicillin, streptomycin, bFGF, EGF, insulin anda small molecule inhibitor CHIR99021.
Owner:QINGDAO MUNICIPAL HOSPITAL
Remotely controlled and/or laterally supported devices for direct spinal cord stimulation
A method for treating intractable pain via electrical stimulation of the spinal cord. Remote, non-contact stimulation of a selected region of spinal cord is achieved by placement of a transceiver patch directly on the surface of that region of spinal cord, with said patch optionally being inductively coupled to a transmitter patch of similar size on either the outer or inner wall of the dura surrounding that region of the spinal cord. By inductively exchanging electrical power and signals between said transmitter and transceiver patches, and by carrying out the necessary electronic and stimulus signal distribution functions on the transceiver patch, the targeted dorsal column axons can be stimulated without the unintended stray stimulation of nearby dorsal rootlets. Novel configurations of a pliable surface-sheath and clamp or dentate ligament attachment features which realize undamaging attachment of the patch to the spinal cord are described.
Owner:UNIV OF IOWA RES FOUND +1
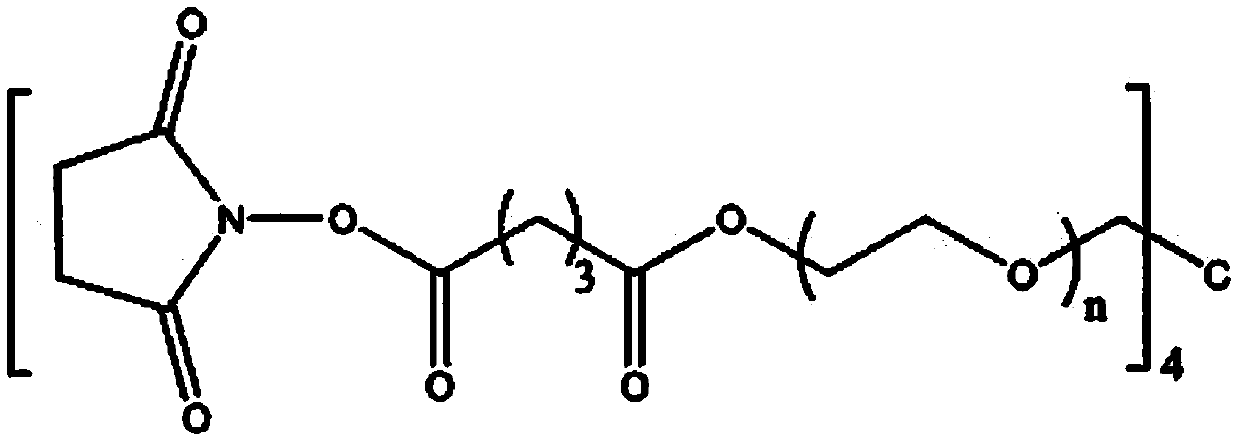
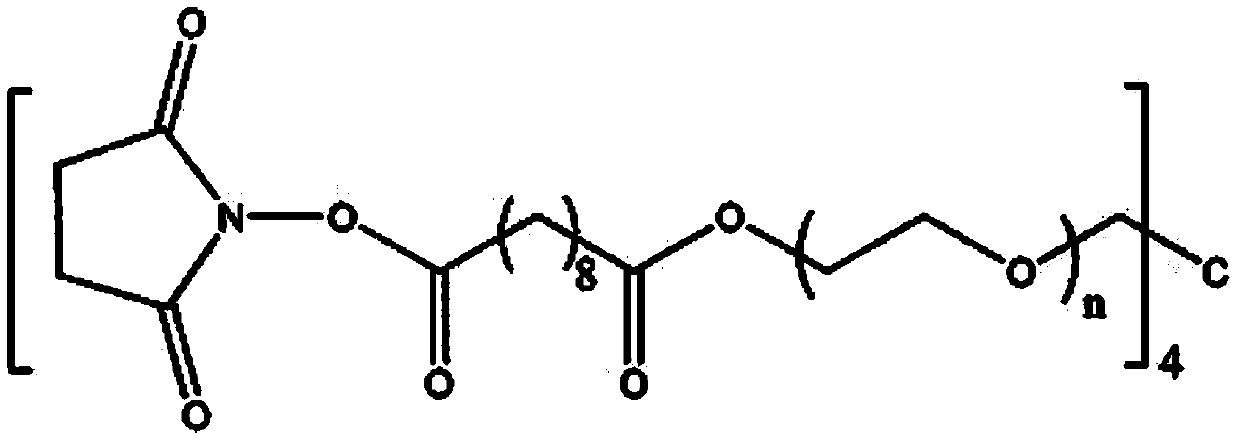
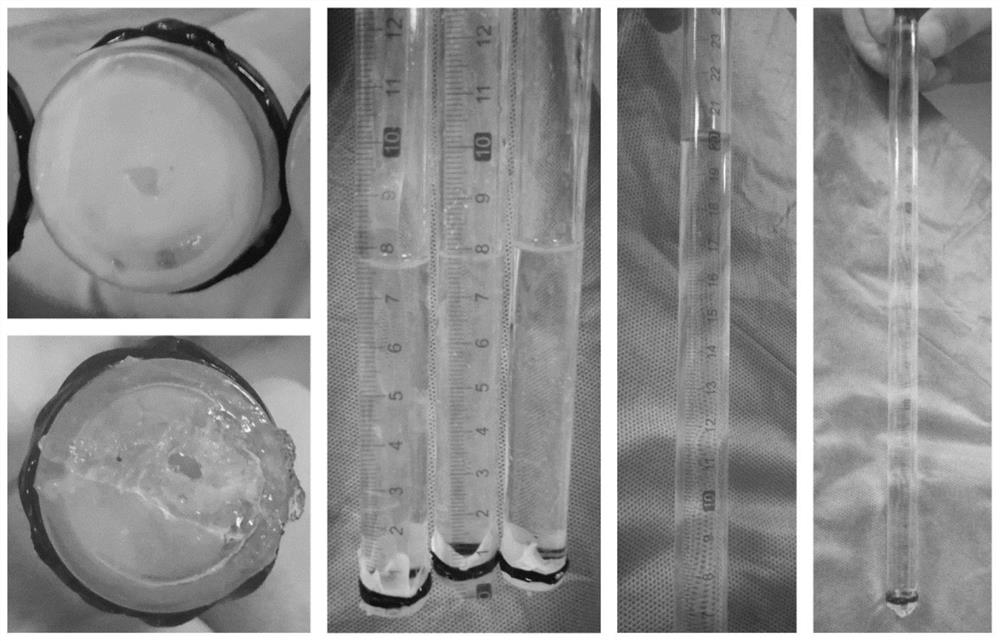
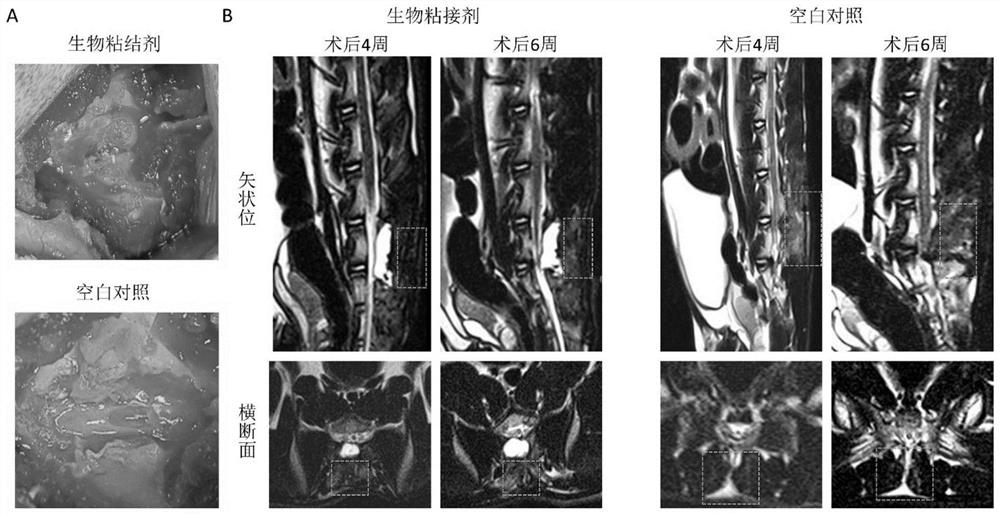
Biological adhesive and preparation method and application thereof
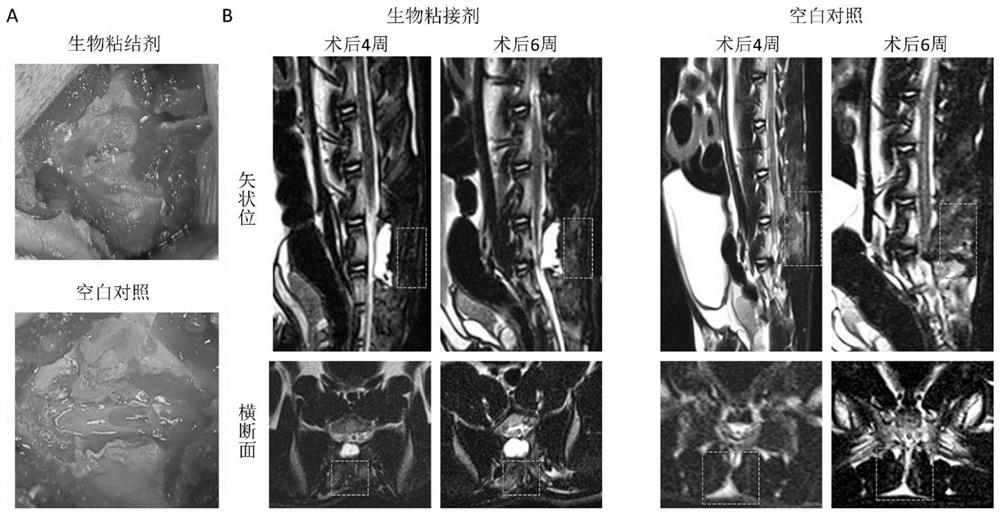
The invention discloses a biological adhesive and a preparation method and application thereof. The biological adhesive comprises 4-arm polyethylene glycol amine and 4-arm polyethylene glycol succinimide succinate according to the mass ratio of 1: (0.1-10). The biological adhesive disclosed by the invention has the advantages of good biocompatibility, degradability and relatively high adhesive capacity, and is enough to tolerate the pressure of cerebrospinal fluid after being adhered to the spinal dura mater. Moreover, the biological adhesive disclosed by the invention is convenient to operatein an operation, can be independently used and does not need to be sutured, so that the operation time is greatly shortened and the operation risk is reduced. The biological adhesive disclosed by theinvention can be used for dura mater injuries of a special part which cannot be effectively sutured by a conventional method, such as injuries in front of and on the side of the dura mater and injuries in the positions of nerve root sleeves, can effectively reduce complications of cerebrospinal fluid leakage after a spinal operation, and improves prognosis of a patient.
Owner:BEIJING NATON INST OF MEDICAL TECH CO LTD
Operation channel system for spine minimally invasive microscope
ActiveCN113081089AReduce difficultyReduced steps for relocating channelsSurgeryLaproscopesSpinal columnDura mater
The invention discloses an operation channel system for a spine minimally invasive microscope, and the system comprises an operation channel which comprises an external tissue channel and an internal tissue channel, the internal tissue channel being fixedly connected with the external tissue channel, and the surface of the external tissue channel being vertically and fixedly provided with a fixed handle; an inner core, comprising a ball head, a ball head handle and a connecting disc, the ball head handle being vertically and fixedly installed on the surface of the connecting disc, and the end, away from the connecting disc, of the ball head handle being fixedly connected with the ball head; and a dura mater pushing device, used for being matched with the operation channel to push and protect dura mater tissues and nerve roots. After skin and deep fascia are cut open, the channel is directly placed for an operation, the step of expanding tissue step by step and then placing the channel is reduced, the operation time is shortened, operation under a microscope can be carried out in a limited space in combination with the application of an epidural pushing device, and the risk of damaging the epidural and nerve roots is avoided.
Owner:遵义医科大学第二附属医院
Quick, safe and accurate united puncture needle
PendingCN107736924AFast, safe and accurate entryPrecise entrySurgical needlesTrocarSubarachnoid spaceAnesthesia needle
The invention relates to quick, safe and accurate united puncture needle. The quick, safe and accurate united puncture needle comprises a spinal epidural puncture needle and an anesthetic needle. By arranging an arc-shaped groove which is axially parallel to a needle stem is formed in the other side, which is opposite to a first opening, of the needle stem of a puncture needle body and designing the arc-shaped groove in a structure of which the cross section is a major arc, the anesthetic needle can be wrapped by the arc-shaped groove which is of the major arc structure when the anesthetic needle slides in the arc-shaped groove, the anesthetic needle cannot deviate from the arc-shaped groove under muscle resistance, and the anesthetic needle can quickly, safely and accurately enter a subarachnoid space; since a wound generated on an arachnoid by the anesthetic needle deviates from the position where the first opening is located, when an epidural anesthetic catheter is inserted in an inner cavity of the puncture needle body, the epidural anesthetic catheter cannot be inserted in the subarachnoid space from the wound by mistake, the safety and the accuracy of anaesthesia are effectively increased, and a medical accident is prevented from occuring.
Owner:戴志福
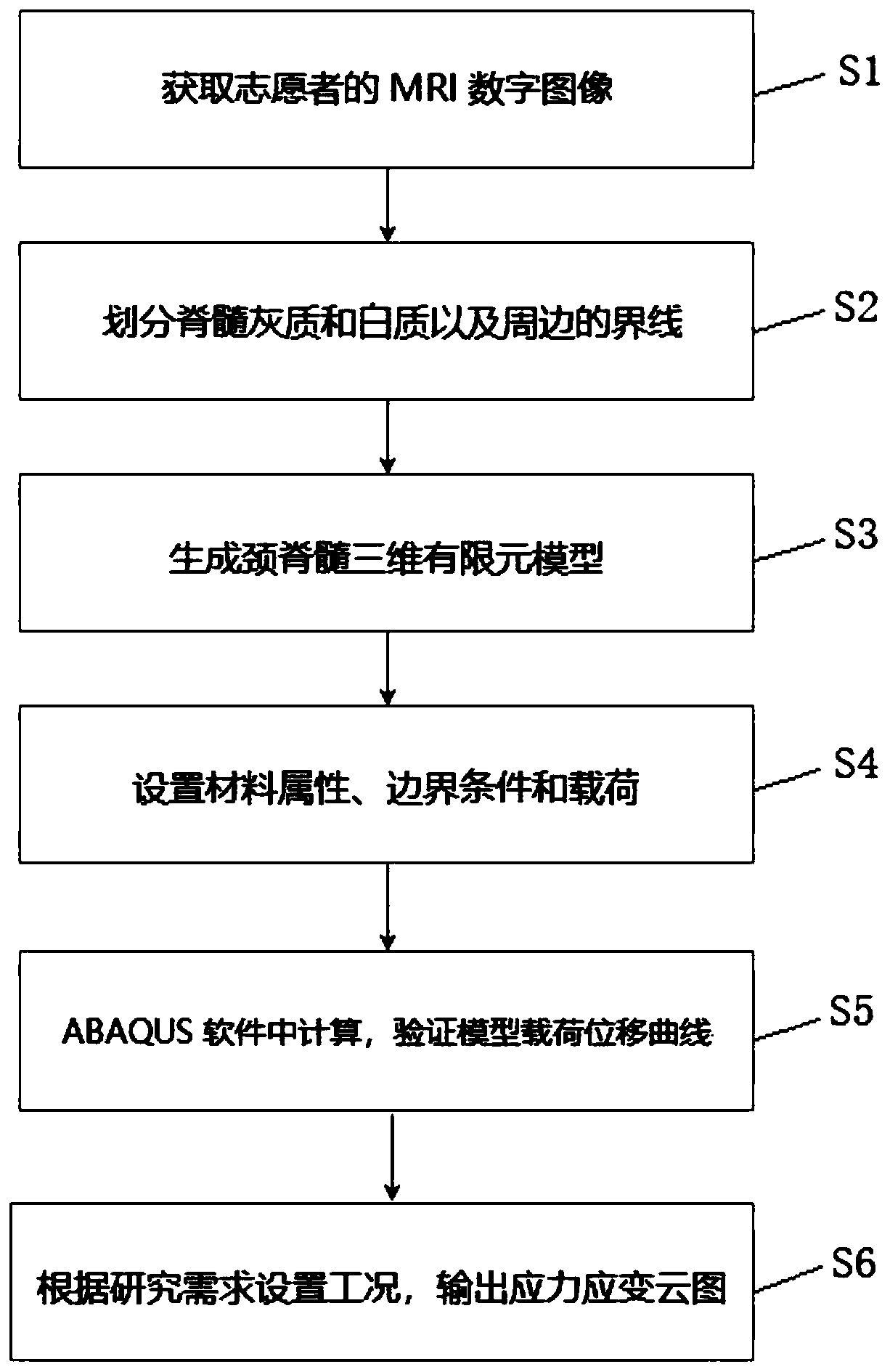
Method and device for establishing cervical spinal cord simulation model
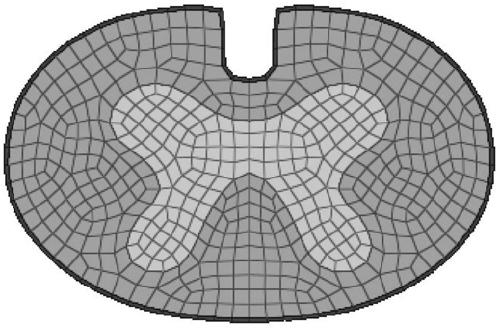
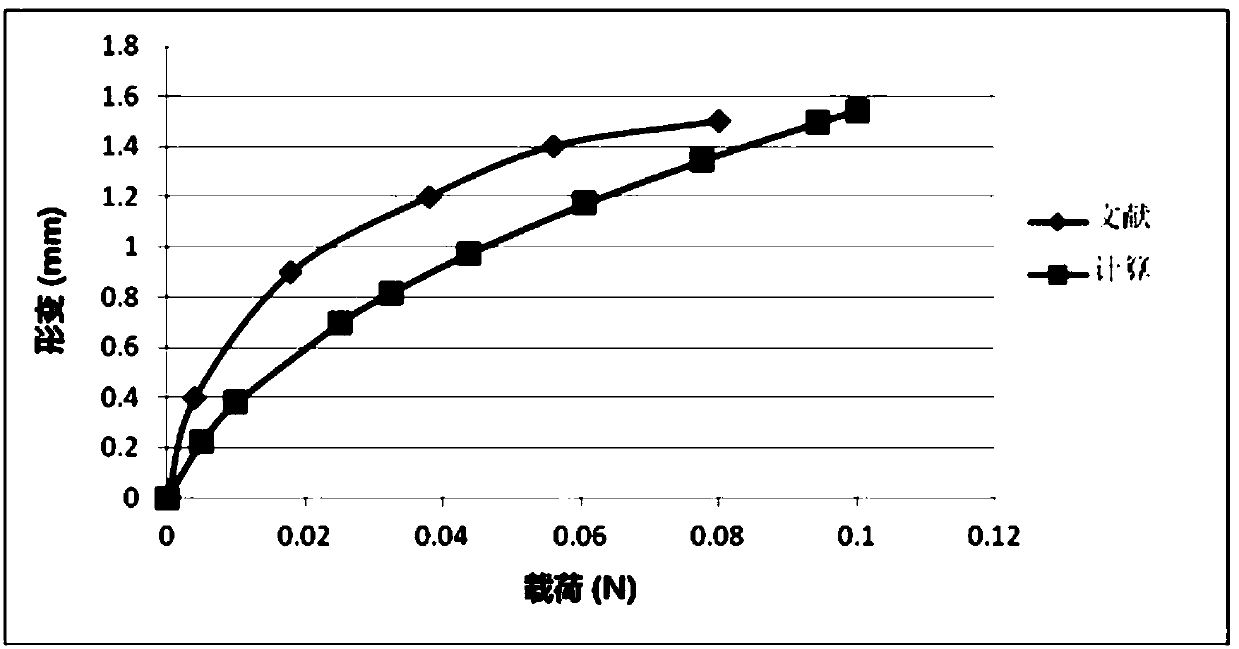
PendingCN111261294AReasonable settingAnatomically intactMedical simulationMedical imagesCervical spinal cord gray matterAnatomical structures
The invention relates to a method and a device for establishing a cervical spinal cord simulation model. The method comprises the following steps that MIR scans a cervical spinal cord image of a volunteer, DICOM data are obtained and imported into medical image processing software, the boundary of cervical spinal cord gray matter and white matter and the boundary of white matter and periphery aredivided, a cervical spinal cord cross section model is generated and imported into finite element modeling analysis software to generate a cervical spinal cord three-dimensional finite element model including gray matter, white matter and soft spinal membrane, and parameter setting is conducted. The device comprises a related unit which is used for establishing the cervical spinal cord simulationmodel. The cervical spinal cord three-dimensional finite element model established by the invention comprises gray matter, white matter and soft spinal membrane; the anatomical structure is complete,and parameters are reasonably set, so that the cervical spinal cord injury condition after collision can be simulated, the stress distribution of the spinal cord can be analyzed, the simulation performance is higher, and the clinical guidance significance is higher when the conditions such as collision test and spinal cord injury mechanism research are simulated.
Owner:SHANGHAI TONGJI HOSPITAL
Method for establishing spinal dura mater leakage arachnoid hernia type sacral canal cyst model
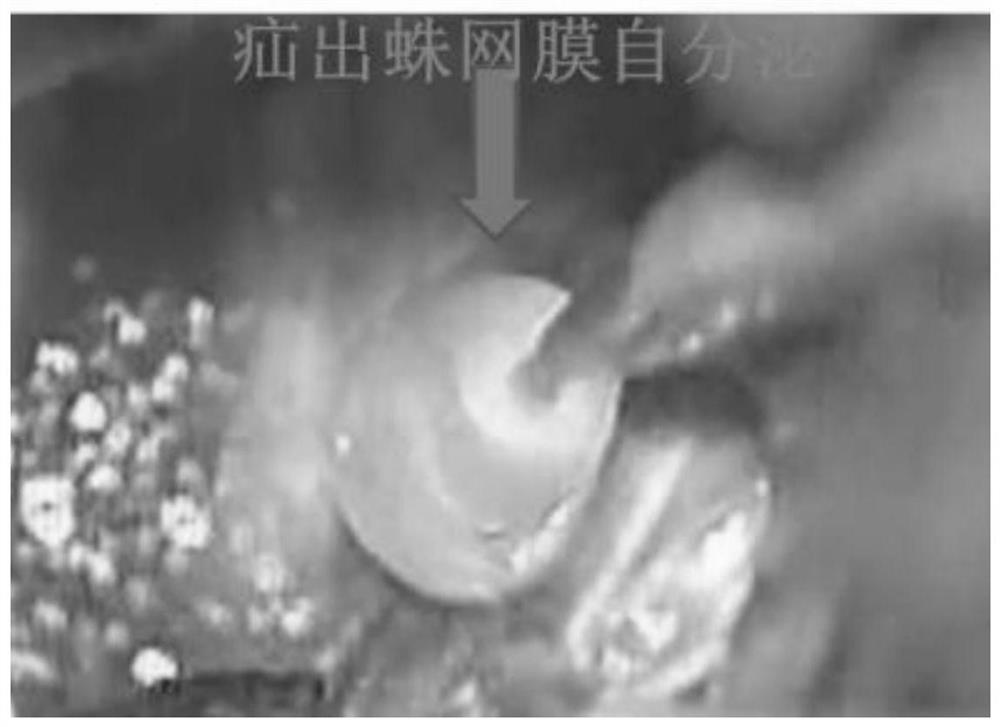
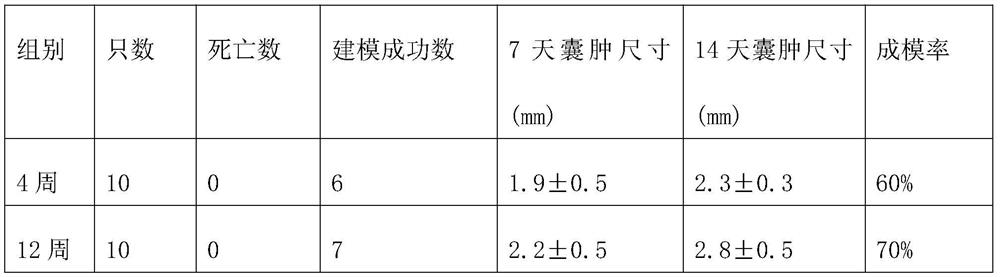
PendingCN112168410AReduce harmEffective preventionSurgical veterinaryAnimal husbandryHerniaSpinal dura mater
The invention relates to a method for establishing a spinal dura mater leakage arachnoid hernia type sacral canal cyst model. The method comprises the following steps: step 1) selecting a model animal; 2) exposing the tail end of the dural sac in the lumbosacral vertebrae of the model animal through an operation; 3) irregularly cutting the tail end of dural sac or thick nerve sleeve adventitia; and 4) completing modeling. By means of the method, the spinal dura mater leakage arachnoid hernia type sacral canal cyst animal model with the survival rate of 100%and the modeling success rate of 60%can be simulated and the cyst form is very similar to that in real cases.
Owner:PEKING UNIV THIRD HOSPITAL
Preparation method and device of duramater/spinal dural transplanting substitute
ActiveCN102727935BSimple Surface Functional StructureWidely sourced and cheapProsthesisCellular componentTissue fluid
The invention provides a preparation method of a duramater / spinal dural transplanting substitute which is obtained by repeated freezing and thawing of dural tissue, rolling and cracking of cells, crosslinking fixed protection, accellular antigen extraction, dense surface fibrosis modification, packaging and sterilization, and has the advantages of simple method, wide raw material sources, cheap raw materials, and low cost. The prepared dural substitute completely removes components of cells and other antigen components simultaneously when protecting dural tissue natural structure and properties, is good in biocompatibility, free of immune rejection, safe and reliable, good in mechanical performance, and easy in clinical operation, can meet the needs of defect repair, has the function of promoting tissue regeneration as a loose surface is beneficial to the tissue fluid adsorption, active factor enrichment, and growth of blood vessels and cells, and has the advantages of rapidness in fusion with a host, biodegradable absorption, and good repair effect. The animal test shows that the defect can be completely repaired without brain or spinal fluid leakage, or adhesion with brain tissue, and significant rejection is not found.
Owner:SHAANXI BIO REGENERATIVE MEDICINE CO LTD
Reversible syringomyelia animal model, and construction method and application thereof
ActiveCN111494054AReversibleWill not proliferateSurgical veterinarySpinal cord lesionSpinal dura mater
The invention discloses a reversible syringomyelia animal model, and a construction method and application thereof. The construction method comprises the following steps: S1, exposing a T13 vertebralplate, and cutting a ligamentum flavum to expose a spinal dura mater; S2, quantitatively oppressing the T13 spinal dura mater to make cerebrospinal fluid extruded to the periphery and the circulationof the cerebrospinal fluid blocked; and S3, removing the vertebral plate and an oppressor to reduce the pressure in order to make the subdural cerebrospinal fluid recovered again. A quantitative cotton sliver is filled into the inner side of the T13 vertebral plate of a rat through microsurgery, cerebrospinal fluid flow is blocked through epidural oppression to make the rat have syringomyelia, andthen an operation relieves the oppression, reverses syringomyelia, and realizes the gradual reduction of rat syringomyelia. According to the method, an animal model of the syringomyelia caused by reversible non-biochemical destruction is established, feasibility verification of MRI dynamic observation of the animal syringomyelia is achieved, the constructed cavity is a reversible cavity, and a model basis is provided for related research of the syringomyelia and spinal cord injury.
Owner:XUANWU HOSPITAL OF CAPITAL UNIV OF MEDICAL SCI
Lumbar puncture training module and manufacturing method thereof
ActiveCN113851033ASmall sizeGood lookingIncreasing energy efficiencyEducational modelsSpinal dura materInterspinous ligament
The invention discloses a lumbar puncture training module which comprises a simulation outer skin, a simulation lumbar vertebra bone, simulation supraspinal ligament and simulation interspinous ligament and a simulation subcutaneous tissue, and is characterized in that a simulation ligamentum flavum and a simulation puncture tube are arranged on the simulation lumbar vertebra bone; the simulation puncture tube is arranged in an entocoele of the simulation lumbar vertebra bone, and the simulation puncture tube comprises a simulation spinal canal and a simulation spinal dura mater; the simulation ligamentum flavum is arranged in an exocoel of the simulation lumbar vertebra bone; the simulation lumbar vertebra bone is wrapped on the simulation supraspinal ligament and interspinous ligament, the simulation supraspinal ligament and interspinous ligament is wrapped in the simulation subcutaneous tissue; and the simulation subcutaneous tissue is wrapped by the simulation outer skin. The manufacturing method of the module is an integrated forming manufacturing method, the problems of needle inserting hand feeling and needle inserting accuracy during lumbar puncture training are solved, and replacement of the puncture module is facilitated.
Owner:TIANJIN TELLYES SCI
A kind of biological adhesive and its preparation method and application
The invention discloses a biological adhesive and a preparation method and application thereof, wherein the biological adhesive comprises four-arm polyethylene glycol amino and four-arm polyethylene glycol succinate with a mass ratio of 1:(0.1-10). imidosuccinate. The bioadhesive of the present disclosure has good biocompatibility, is degradable, and has high adhesion ability, and is sufficient to withstand the pressure of cerebrospinal fluid after being adhered to the dura mater. Moreover, the bioadhesive of the present disclosure is convenient to operate during surgery, can be used alone without suture, greatly shortens the operation time, and reduces the operation risk. The bioadhesive of the present disclosure can be used for dural injuries in special parts that cannot be effectively sutured by conventional methods, such as injuries located in the anterior, lateral and nerve root sleeves of the dura mater, and can effectively reduce the complications of cerebrospinal fluid leakage after spinal surgery disease and improve patient outcomes.
Owner:BEIJING NATON INST OF MEDICAL TECH CO LTD
Spinal cord half-cutting device and half-cutting method for manufacturing animal spinal cord injury model
PendingCN114557792ALower requirementReduce dependenceSurgical veterinaryPhysical medicine and rehabilitationDura mater
The invention discloses a spinal cord semi-cutting device and a semi-cutting method for manufacturing an animal spinal cord injury model.The spinal cord semi-cutting device comprises a spinal cord semi-cutting device body and a main control center, and the main control center is arranged outside the spinal cord semi-cutting device body and used for controlling actions of the spinal cord semi-cutting device body; the spinal cord half-cutting device body comprises a fixing frame, a half-cutting driving mechanism, a spinal cord fixing structure and a cutting assembly, the half-cutting driving mechanism is arranged on the fixing frame, and the cutting assembly is installed on the half-cutting driving mechanism in a lifting mode; after the fixing frame is erected above a non-human primate to be modeled and an epidural opening is cut through an artificial incision operation, the spinal cord fixing structure penetrates into the gap between the spinal cord and the epidural and surrounds the periphery of the spinal cord, the half-cutting driving mechanism is located above an incision and drives the cutting assembly to act, and the spinal cord fixing structure half cuts the spinal cord. Excessive dura mater cannot be damaged, and postoperative maintenance is facilitated.
Owner:JINAN UNIVERSITY
A tissue repair scaffold and its preparation method and application
ActiveCN105194737BUnique pore structureHigh porositySurgeryAbsorbent padsTissue repairNetwork structure
The invention provides a tissue repair support and its preparation method and use. The tissue repair support comprises a porous tridimensional network structure composed of a composite fiber layer and a hydrophilic material. The composite fiber layer comprises composite fibers composed of an adhesion factor and a hydrophobic synthesis material. The tissue repair support is used for meninge repair, spinal meninge repair, a tissue engineering support material, artificial skin, a wound accessory material, a biomembrane, a wound cladding material, a haemostasis material, a postoperative antistick material or a beauty treatment material.
Owner:MEDPRIN REGENERATIVE MEDICAL TECH
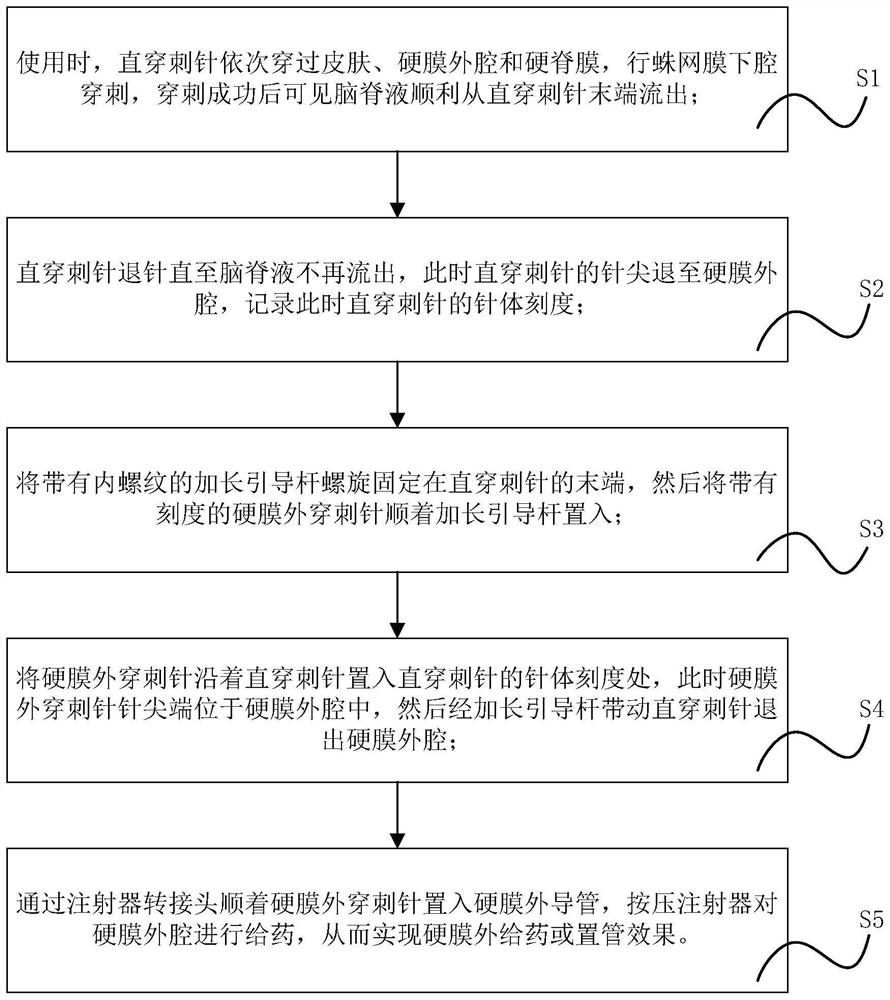
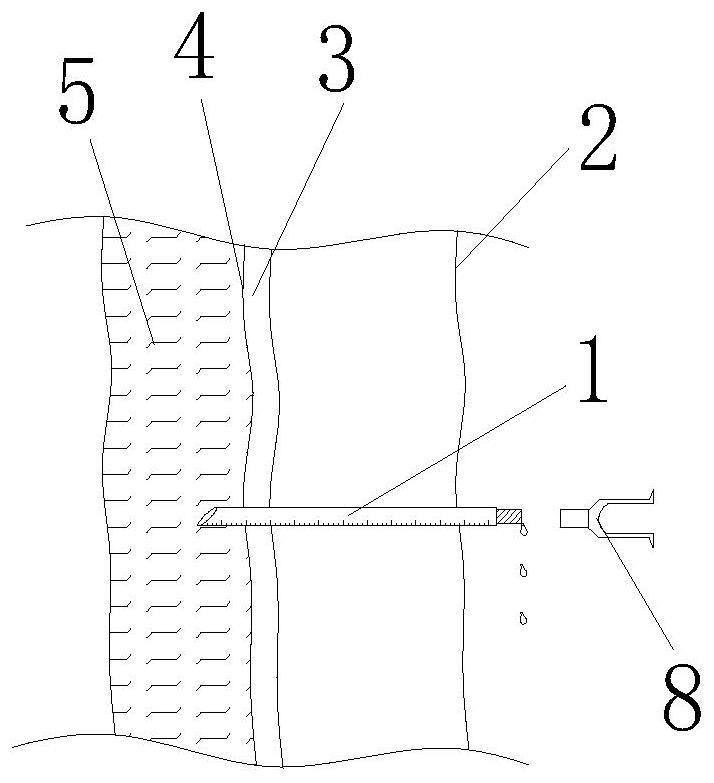
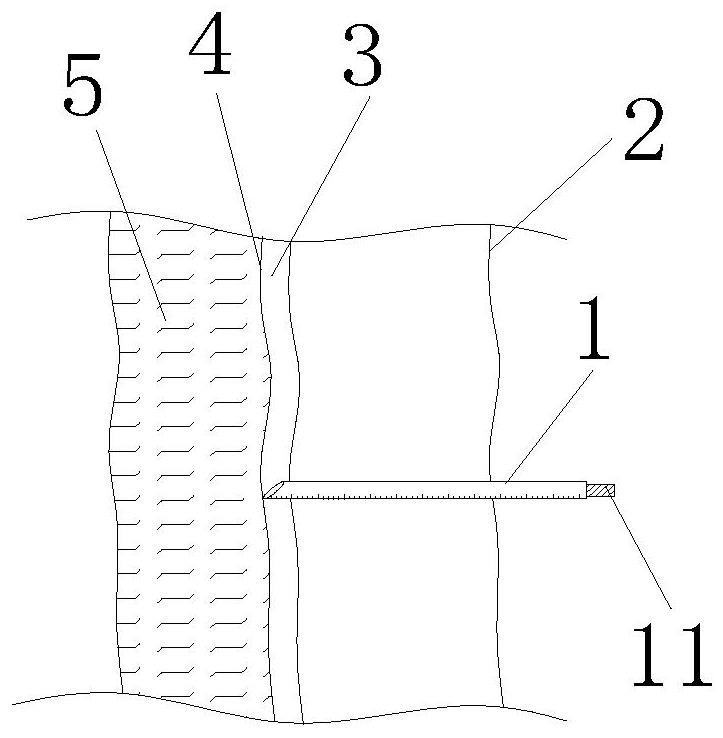
Implementation method of novel combined spinal-epidural anesthesia puncture suite
PendingCN114271904AImprove accuracyIncrease success rateSurgical needlesTrocarSubarachnoid spaceDrug administration
The invention provides an implementation method of a novel combined spinal-epidural anesthesia puncture suite, which comprises the following steps: step S1, during use, a straight puncture needle sequentially penetrates through the skin, an epidural space and an epidural mater to perform subarachnoid space puncture, and after successful puncture, cerebrospinal fluid smoothly flows out from the tail end of the straight puncture needle; s2, withdrawing the straight puncture needle until the cerebrospinal fluid does not flow out, and withdrawing the needle tip of the straight puncture needle to the epidural space; s3, a lengthened guide rod with an internal thread is spirally fixed to the tail end of the straight puncture needle, and then the epidural puncture needle with scales is placed in along the lengthened guide rod; s4, placing the epidural puncture needle into the needle body scale of the straight puncture needle along the straight puncture needle; s5, an injector adapter is placed into the epidural catheter along the epidural puncture needle, and an injector is pressed to conduct drug administration on the epidural space; according to the epidural puncture catheter, the accuracy of epidural puncture catheterization can be improved, and epidural puncture can be effectively carried out.
Owner:FUJIAN PROVINCIAL HOSPITAL
Transparent full-visual U-shaped interbody fusion working channel and manufacturing method thereof
InactiveCN111772697AGood light transmissionSimple structureSurgeryHeatIntervertebral fusionBlood vessel
The invention belongs to the technical field of medical instruments, and discloses a transparent full-visual U-shaped interbody fusion working channel and a manufacturing method thereof. A channel pipe and a handle are integrally formed through injection molding and carving, and the channel pipe and the handle are transparent; a connecting buckle is fixed to the handle, the channel pipe is locatedbetween soft tissues and spines, and an interbody fusion cage is fixed between the spines; and the outer side of the pipe wall of the channel pipe is an accurate position for the compression degree of outer blood vessels, nerves and dura mater, the scraping dynamic state of a soft vertebral plate in the pipe wall and implantation of the interbody fusion cage. A high-light-transmission and high-hardness resin material is adopted, the structure is simple, the cost is low, the basic requirement for serving as a medical-grade material is met, and the compression degree of the tissue outside the pipe wall, the position of the interbody fusion cage in the pipe wall and the scraping state of the soft vertebral plate can be observed in a minimally invasive interbody fusion operation process. Theoperation risk and difficulty are reduced, the operation efficiency is improved, and the health risk of medical staff and patients in the operation process is eliminated.
Owner:青岛钰仁医疗科技有限公司
Externally applied medicine for treating pain caused by cervical disc herniation, lumbar disc herniation and like and preparation method thereof
InactiveCN112933141AReduce edemaGood volatilization effectHydroxy compound active ingredientsAntipyreticMuscle tissueOld injury
The invention belongs to the technical field of Chinese herbal medicines, and particularly relates to an external application medicine for treating pain caused by back inflammation and a use method thereof. When the parts of the intervertebral disc, which protrude, swell and proliferate towards the front side, the left side and the right side, press the spinal sac and indirectly press the nerves, radioactive pain is caused to the pressed part, or the parts of the intervertebral disc, which protrude, swell and proliferate rub with peripheral muscular tissues to cause damage and cause inflammation and edema. During sufficient edema, a spinal sac or a dural sac is pressed, nerves are indirectly pressed, and swelling pain at a focus and radioactive pain of lower limbs occur. Pain or traction pain caused by relapse of new injuries or old injuries of lumbar muscle strain and ligament sprain is often accompanied with symptoms such as inflammation and edema. The invention discloses an external application medicine for treating pain caused by back inflammation. The external application medicine is prepared from 3-20 g of herba ephedrae, 3-20 g of cassia twig, 3-20 g of herba schizonepetae, 3-20 g of radix angelicae, 2-10 g of mint, 5-30 g of stephania tetrandra, 10-30 g of dandelion, 5-20 g of fructus forsythiae, 2-15 g of borneol and 5-20 g of talcum powder.
Owner:陈则均
An Operating Channel System for Spine Minimally Invasive Microscopy
ActiveCN113081089BReduce difficultyReduced steps for relocating channelsSurgeryLaproscopesSpinal columnDura mater
The invention discloses an operating channel system for a spinal minimally invasive microscope, comprising: an operating channel, the operating channel includes an outer tissue channel and an inner tissue channel, the inner tissue channel is fixedly connected with the outer tissue channel, the tissue The surface of the outer channel is vertically fixed with a fixed handle; the inner core includes a ball head, a ball handle and a connecting plate, and the ball handle is fixed vertically on the surface of the connecting plate, and the ball handle is far away from the connecting plate. One end of the disk is fixedly connected with the ball head; the dura mater pusher is used to cooperate with the operation channel to push away the dura mater tissue and nerve root for protection. This channel cuts the skin and deep fascia and directly places the channel for surgery, reducing the steps of gradually expanding the tissue and then placing the channel, reducing the operation time, and combined with the application of the dura mater pusher, it can be performed under a microscope in a limited space. operation without the risk of damage to the dura mater and nerve roots.
Owner:遵义医科大学第二附属医院
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com