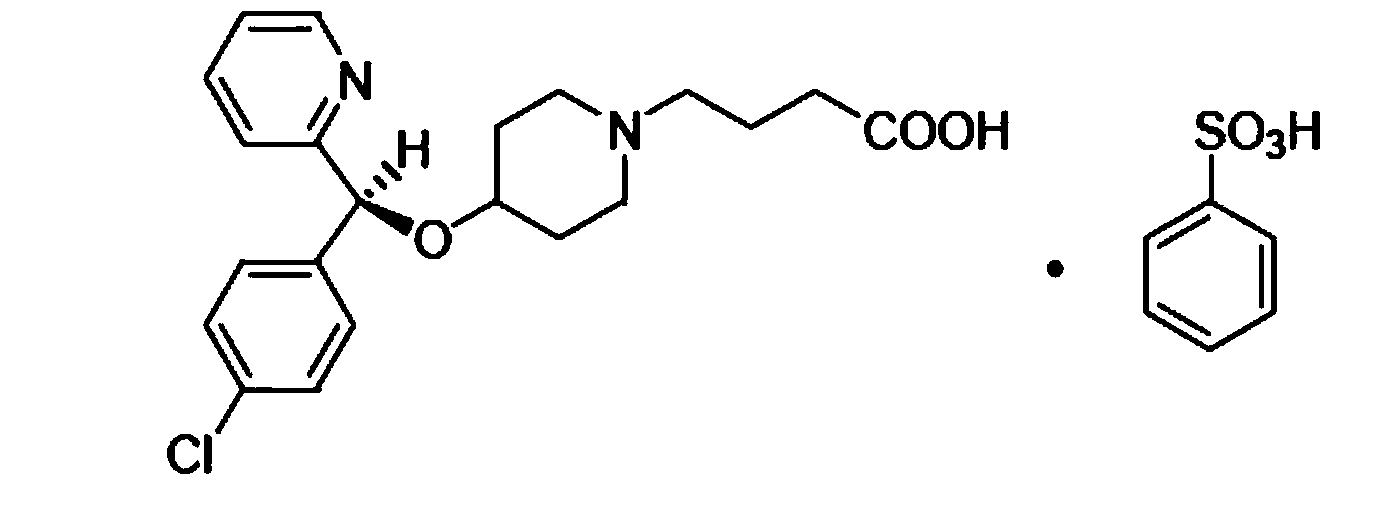
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
37 results about "Macrogol 300" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Water-soluble lutein ester, preparation method thereof and hard candy containing same
ActiveCN103262931AImprove qualityGood water solubilityConfectionerySweetmeatsMonoglycerideDistillation
The invention discloses a preparation method of water-soluble lutein ester. The preparation method comprises the steps of sufficiently and uniformly mixing oil-soluble lutein ester with an emulsifying agent and an antioxidant so as to obtain a mixture, and carrying out vacuum drying on the mixture, wherein the emulsifying agent consists of one to three of molecular distillation monoglyceride, polyglycerol fatty ester, tripolyglycerol monostearate, polyglycerol polyricinoleate, diacetyl tartaric acid mono / di-glyceride, polyethylene glycol 300, polyethylene glycol 400 and polyethylene glycol 600. According to the preparation method, the molecular distillation monoglyceride, the polyglycerol fatty ester and the like serve as the emulsifying agent, so that good emulsifying effect can be achieved, and the water-soluble lutein ester with high quality is obtained finally. The invention further provides the water-soluble lutein ester obtained through the preparation method and hard candy containing the water-soluble lutein ester.
Owner:YUNNAN RAINBOW BIO TECH
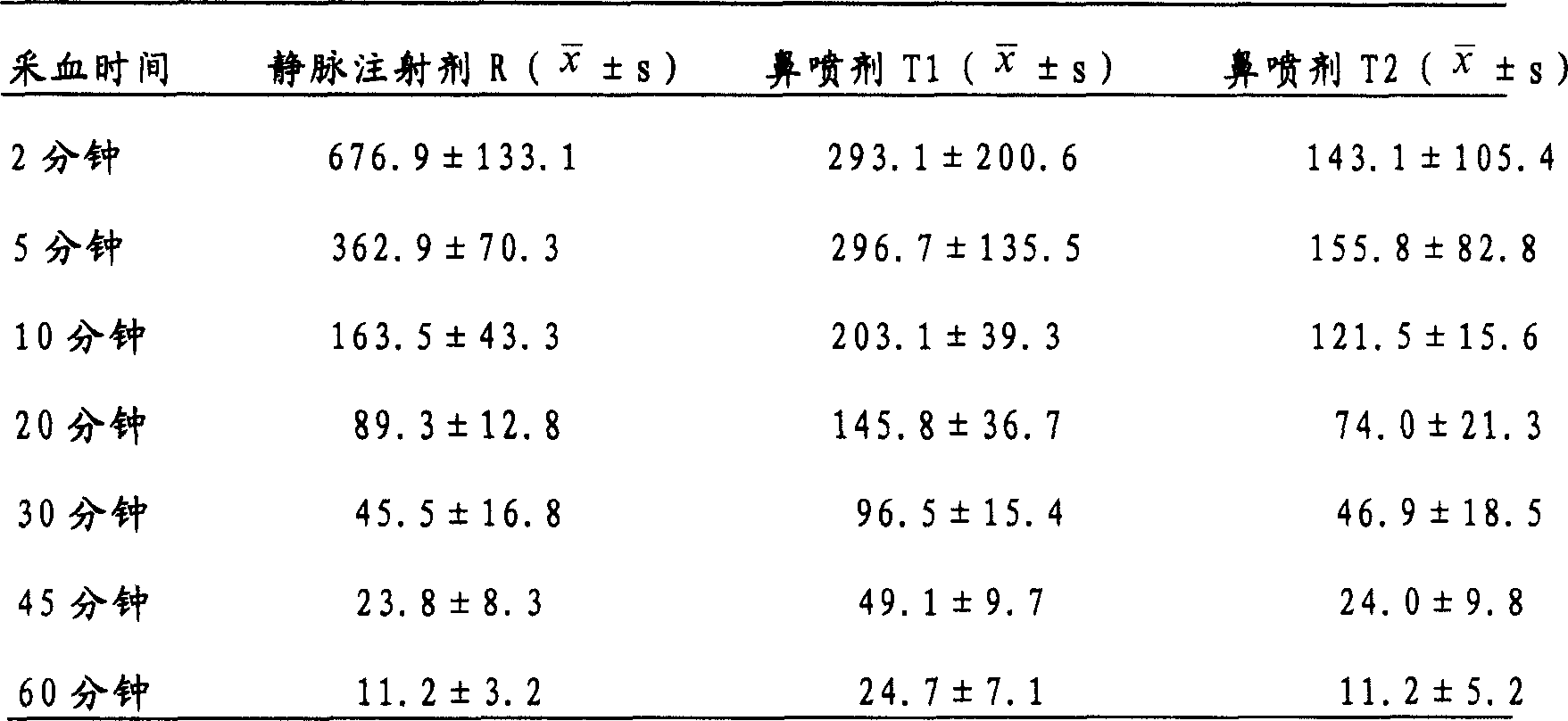
Nasal administered preparation of melatonin
InactiveCN1969814APromote absorptionQuick effectOrganic active ingredientsNervous disorderPEG 400Ethylene glycol
The invention relates to a nasal administration preparation comprising melatonin and at least one kind of polyethylene glycol selected from polyethylene glycol 200, polyethylene glycol 300, polyethylene glycol 400, or polyethylene glycol 600.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Injection containing meclofenoxate hydrochloride and its preparing method
InactiveCN100998585ASolve the problem of hydrolysisImprove stabilityOrganic active ingredientsNervous disorderPEG 400Alcohol
An injection of meclofenoxate hydrochloride and its preparing process which features use of non-water solvent chosen from alcohol, propanediol, glycerin, polyethanediol-300 and polyethanediol-400, and use of filter sterilizing method or wet-heat sterilizing method for high stability are disclosed.
Owner:ZHENDI MEDICINE CONSULTATION NANJING
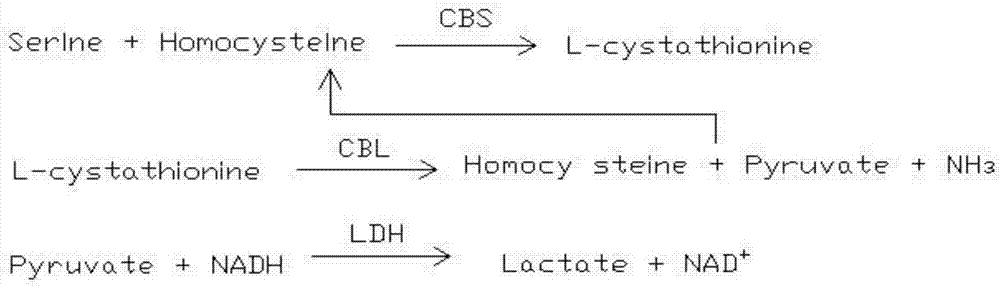
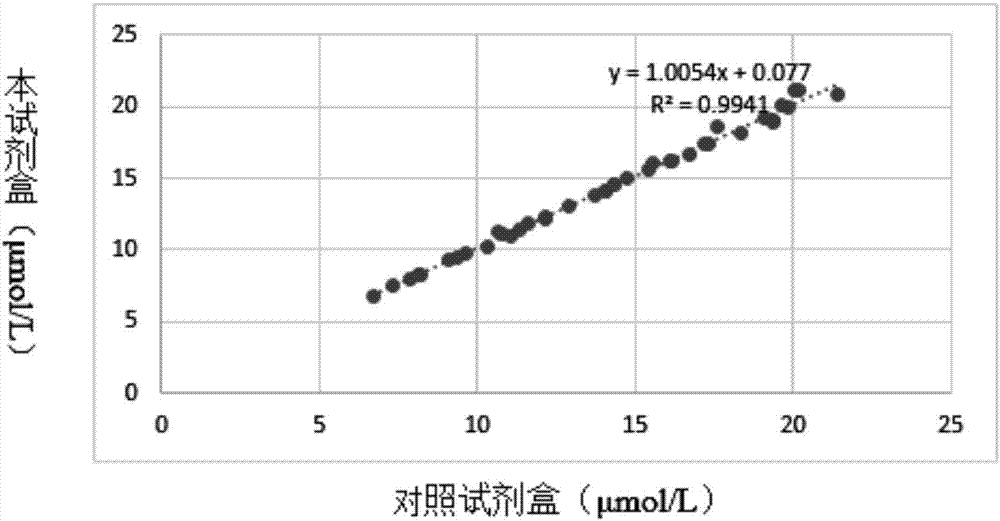
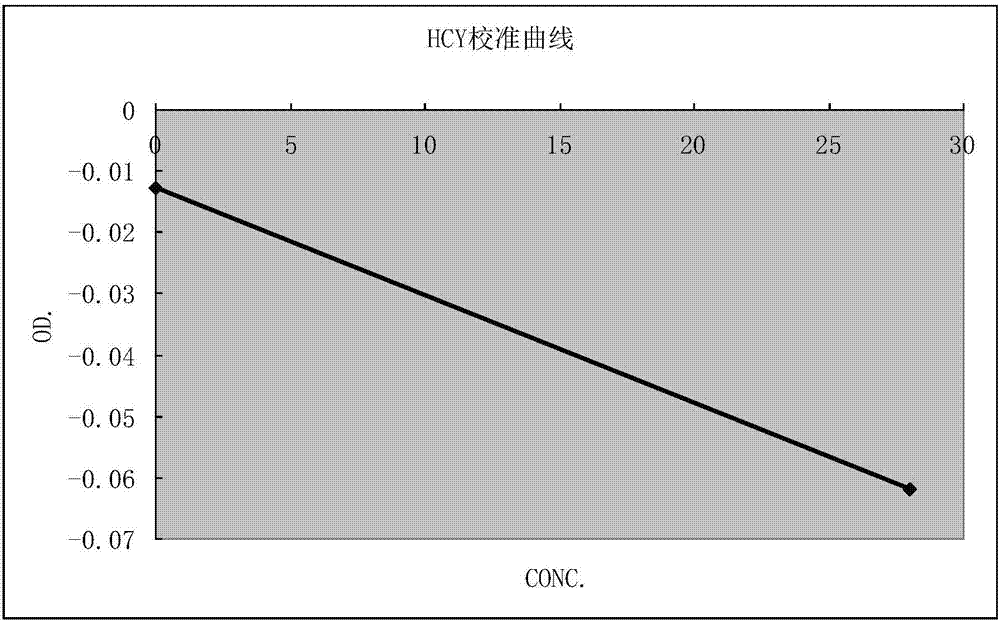
Homocysteine detecting kit and using method thereof
The invention provides a homocysteine detecting kit and a using method thereof, relates to the field of biochemical detection, and aims to provide a homocysteine detecting kit which is high in stability, high in accuracy and high in precision and a using method of the homocysteine detecting kit. The homocysteine detecting kit comprises a reagent 1 and a reagent 2, wherein the reagent 1 comprises a capso buffer solution, lactic dehydrogenase (LDH), L-serine, reduced coenzyme Thio-NADH, tris((2-carboxyethyl)phosphine, mercaptoethanol, an alkylphenol polyoxyethylene series, EDTA, polyethylene glycol 300, ascorbic acid oxidase, a composite stabilizer, and a proclin series preservative; and the reagent 2 comprises a capso buffer solution, cystathionine-beta-synthase, methionine synthase, cystathionine-beta-lyase, methionine-gamma lyase, a surfactant and sodium dodecylbenzene sulfonate. By the various added mixed enzymes and methionine-gamma lyases, the accuracy of the kit on detection of homocysteine can be improved.
Owner:WHITMAN BIOTECH NANJING
Bepotastine besilate nasal spray and preparation method thereof
ActiveCN103816121AOrganic active ingredientsAerosol deliveryMucous membrane inflammationPolyethylene glycol
The invention discloses a bepotastine besilate nasal spray and a preparation method thereof. For the nasal spray, 1-10 g of bepotastine besilate and 3-15 g of solubilizing composition are added in 100 mL of water; the solubilizing composition comprises components in percentage by weight as follows: 80%-95% of propylene glycol, polyethylene glycol 300 or polyethylene glycol 400 and the balance of caprylocaproyl macrogolglycerides; and pH of a nasal spray solution ranges from 6 to 8. In the composition, bepotastine besilate does not exist in a solid particle mode and cannot be crystallized during a long-term storage process, so that not only is rapid medicine absorption facilitated, but also a spray pump port is not blocked easily, and anaphylactic rhinitis and mucous membrane inflammation related to rhinitis can be cured effectively.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
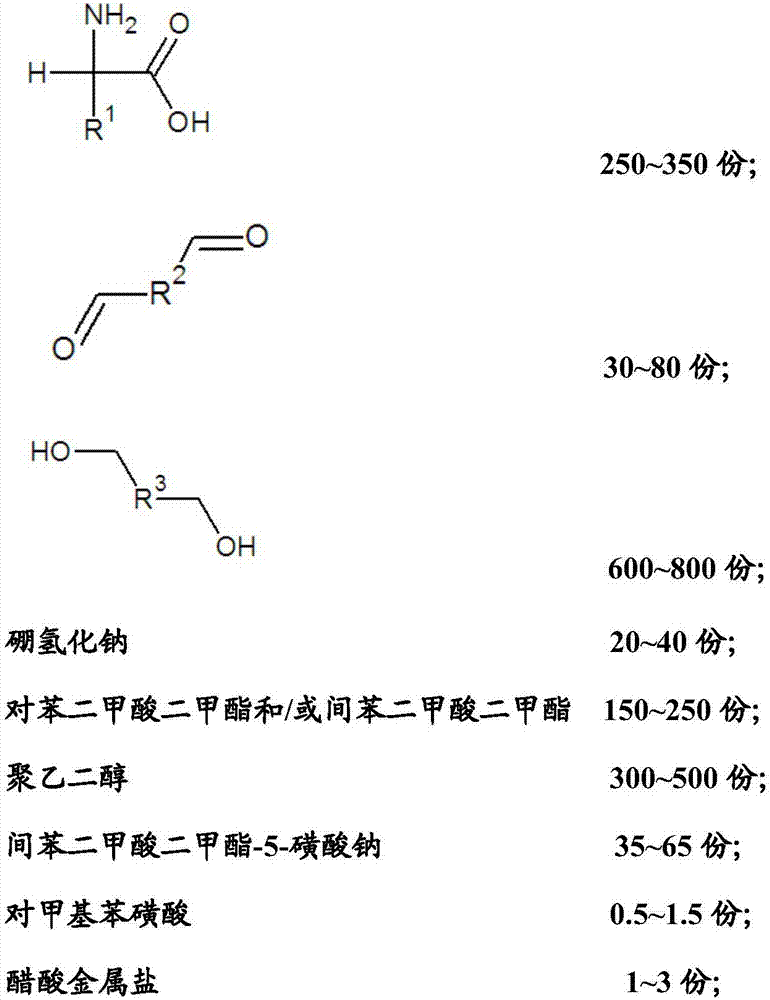
Reductive cleaning agent for synthetic fibers as well as preparation method and application of reductive cleaning agent
ActiveCN105714585AWon't fail quicklyImprove efficiencyDyeing processPolyethylene glycolCleansing Agents
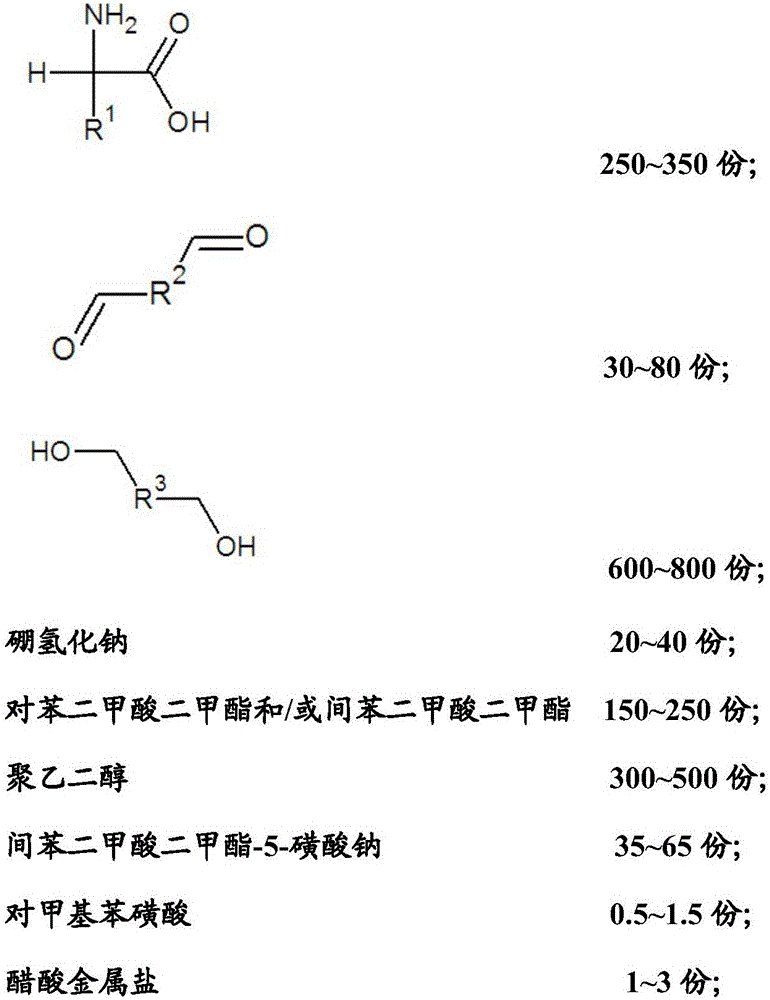
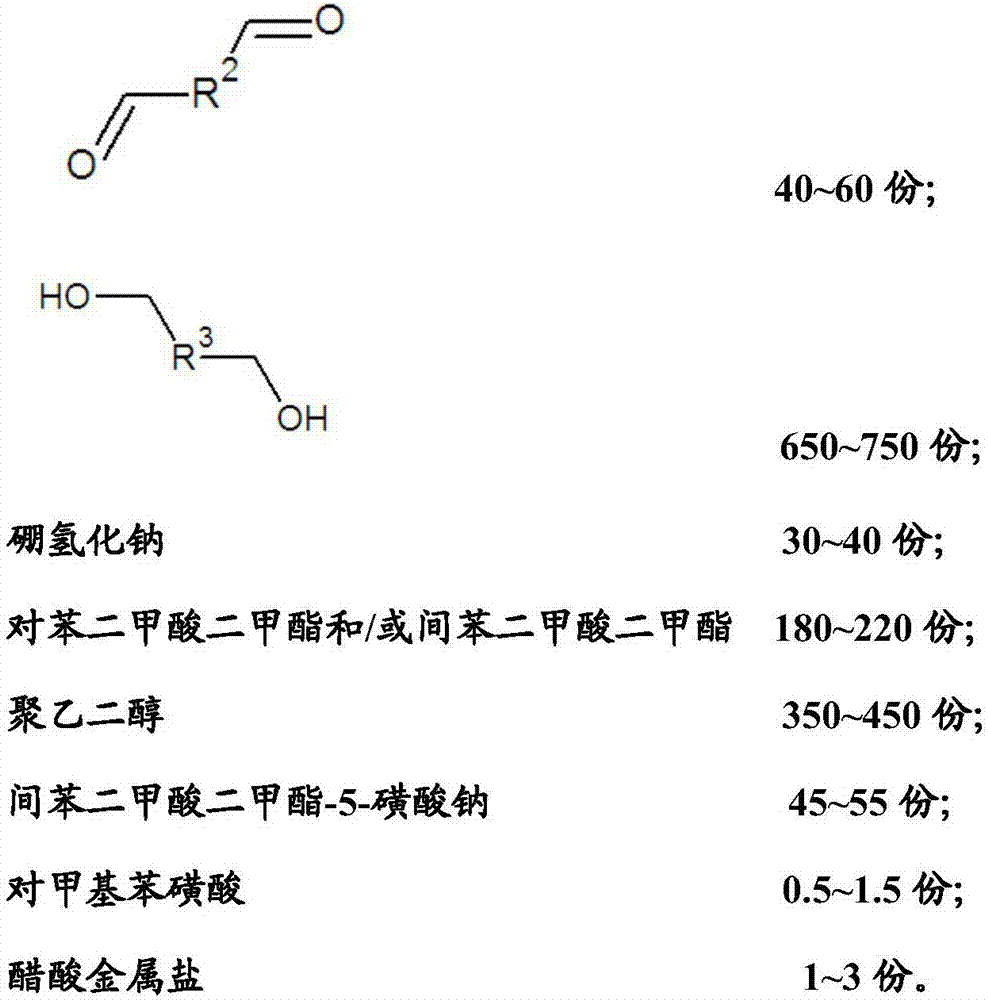
The invention relates to a reductive cleaning agent for synthetic fibers as well as a preparation method and an application of the reductive cleaning agent. The reductive cleaning agent is prepared from raw materials in parts by weight as follows: 250-350 parts of a substance A shown in the specification, 30-80 parts of a substance B shown in the specification, 600-800 parts of a substance C shown in the specification, 20-40 parts of sodium borohydride, 150-250 parts of dimethyl terephthalate / dimethyl isophthalate, 300-500 parts of polyethylene glycol, 35-65 parts of sodium dimethyl isophthalate-5-sulfonate, 0.5-1.5 parts of p-methylbenzene sulfonic acid and 1-3 parts of acetic metal salt. The reductive cleaning agent has a good cleaning effect, a durable effect and a sufficient cleaning effect when used, cannot lose efficacy at high temperature, is stable after meeting with water or absorbing moisture, cannot decompose or release combustible gas or corrosive gas, does not contain carcinogenic aromatic amine or environmental hormone, cannot be influenced by poor storage environment and transportation environment, requires lower transportation cost, has few transportation risks and is a real environment-friendly and efficient printing and dyeing auxiliary.
Owner:DUPLUS CHEM OF ZHANGJIAGANG CITY
Frozen section embedding agent
InactiveCN106018050AGood water solubilityHigh transparencyPreparing sample for investigationSolubilityPolyvinyl alcohol
The invention discloses a frozen section embedding agent, which comprises the following components in parts by mass: 1000 parts of purified water, 80-100 parts of polyvinyl alcohol, 30-50 parts of polyvinylpyrrolidone, sodium carboxymethyl cellulose 10-20 parts and polyethylene glycol 300 50-80 parts. The embedding agent designed in the present invention has good thermal conductivity, transparency, viscosity, water solubility and ductility, and its hardness is suitable. The frozen section embedding agent of the present invention can be applied to the embedding of tissue for pathological diagnosis in surgery, and can also be used for conventional pathological diagnosis, immunohistochemical diagnosis technology and molecular biology diagnosis technology.
Owner:北京九州柏林生物科技有限公司
Polyacrylic acid film forming compsn.
InactiveCN1480496AImprove color uniformityDoes not inhibit dissolutionPharmaceutical product form changePharmaceutical non-active ingredientsPolymer sciencePEG 400
Water soluble, gelatin-free dip coatings for substrates comprising an acrylic film former; a paraben plasticizer; and a secondary plasticizer such as polyvinylpyrrolidone, polyethylene glycol 300, polyethylene glycol 400 or mixtures thereof.
Owner:MCNEIL PPC INC
Liquid formulations of compounds active at sulfonylurea receptors
ActiveUS20170216321A1Rapid and readily controlled increase in circulating drug concentrationsQuick cureBiocideMetabolism disorderMeglitinideWater based
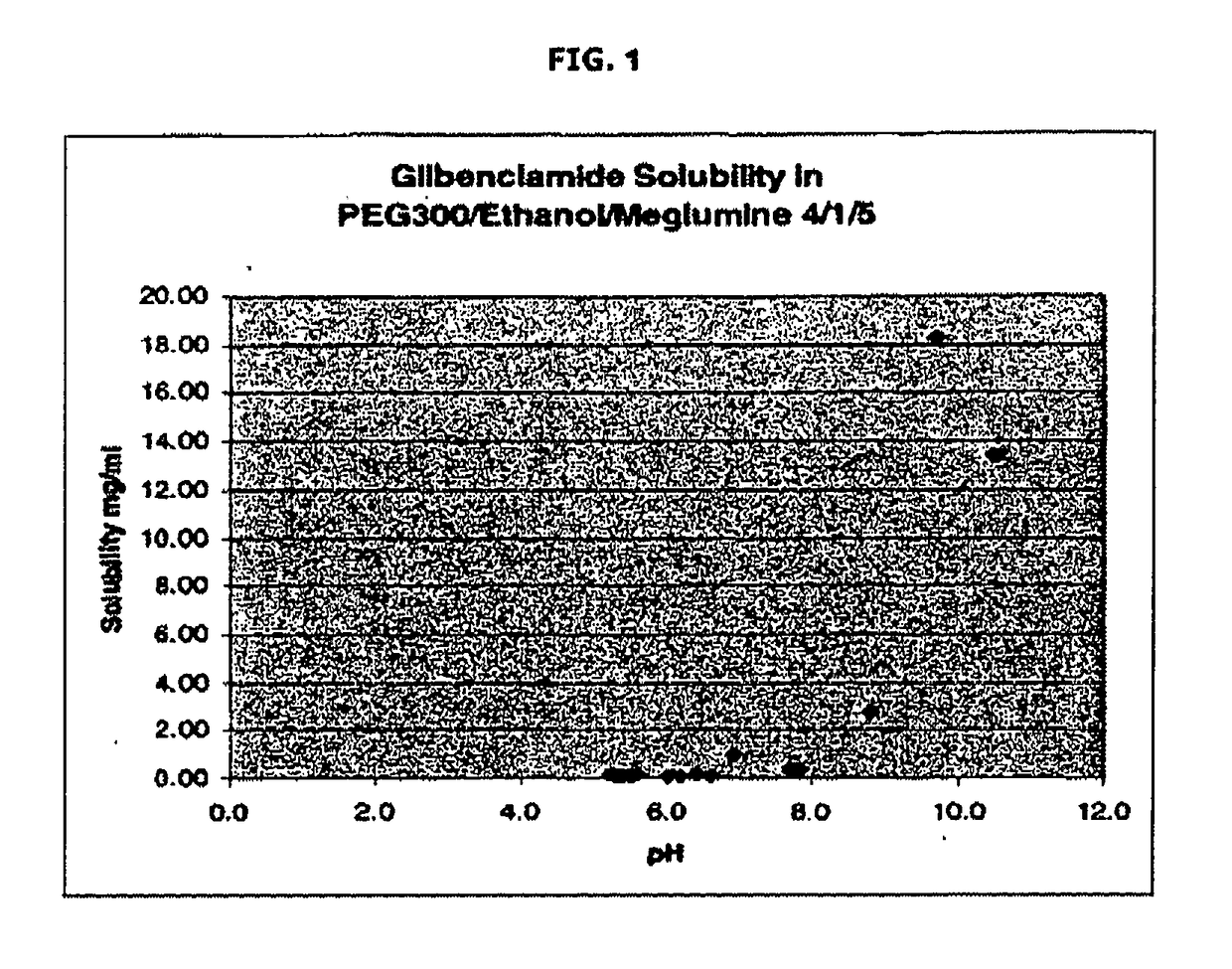
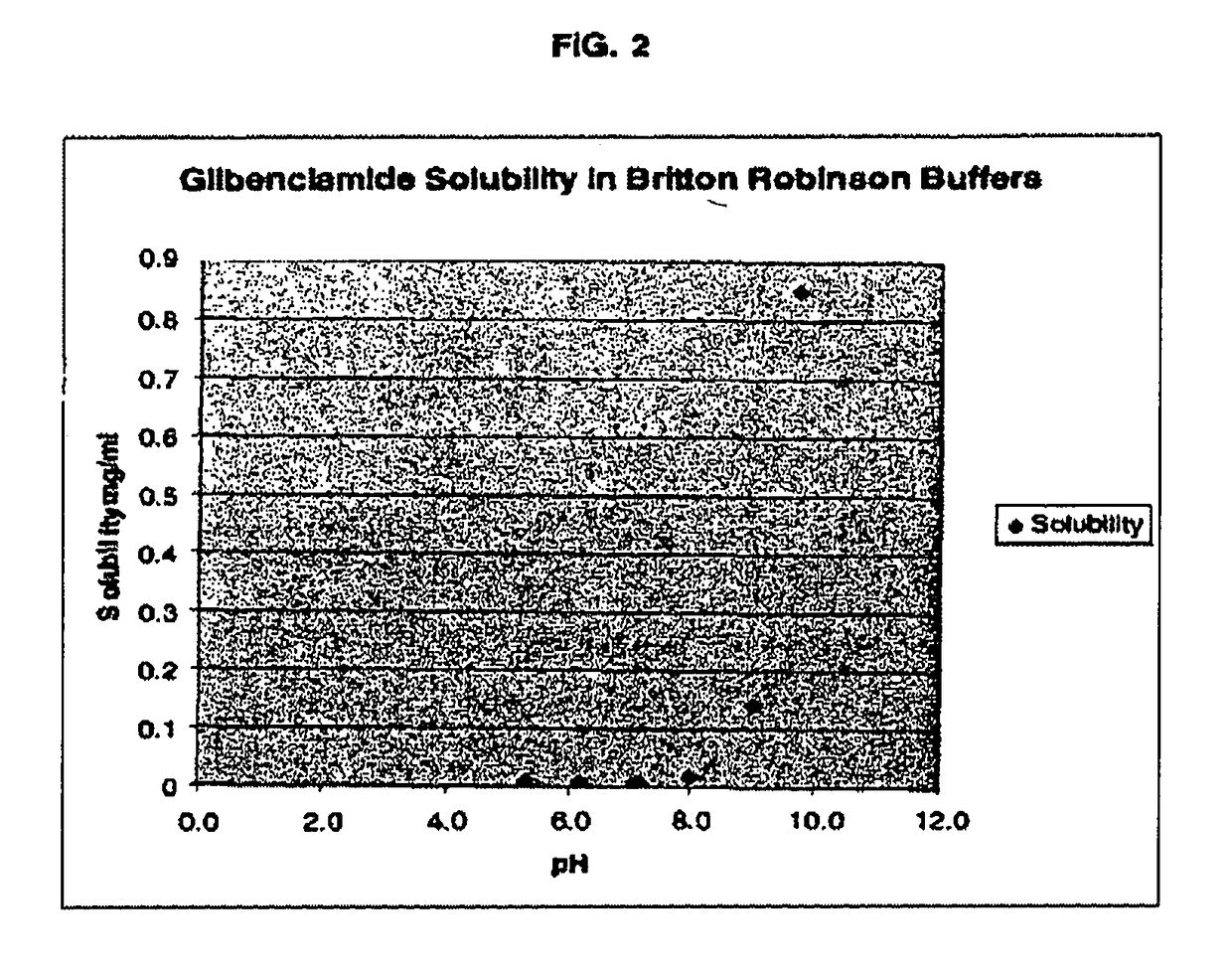
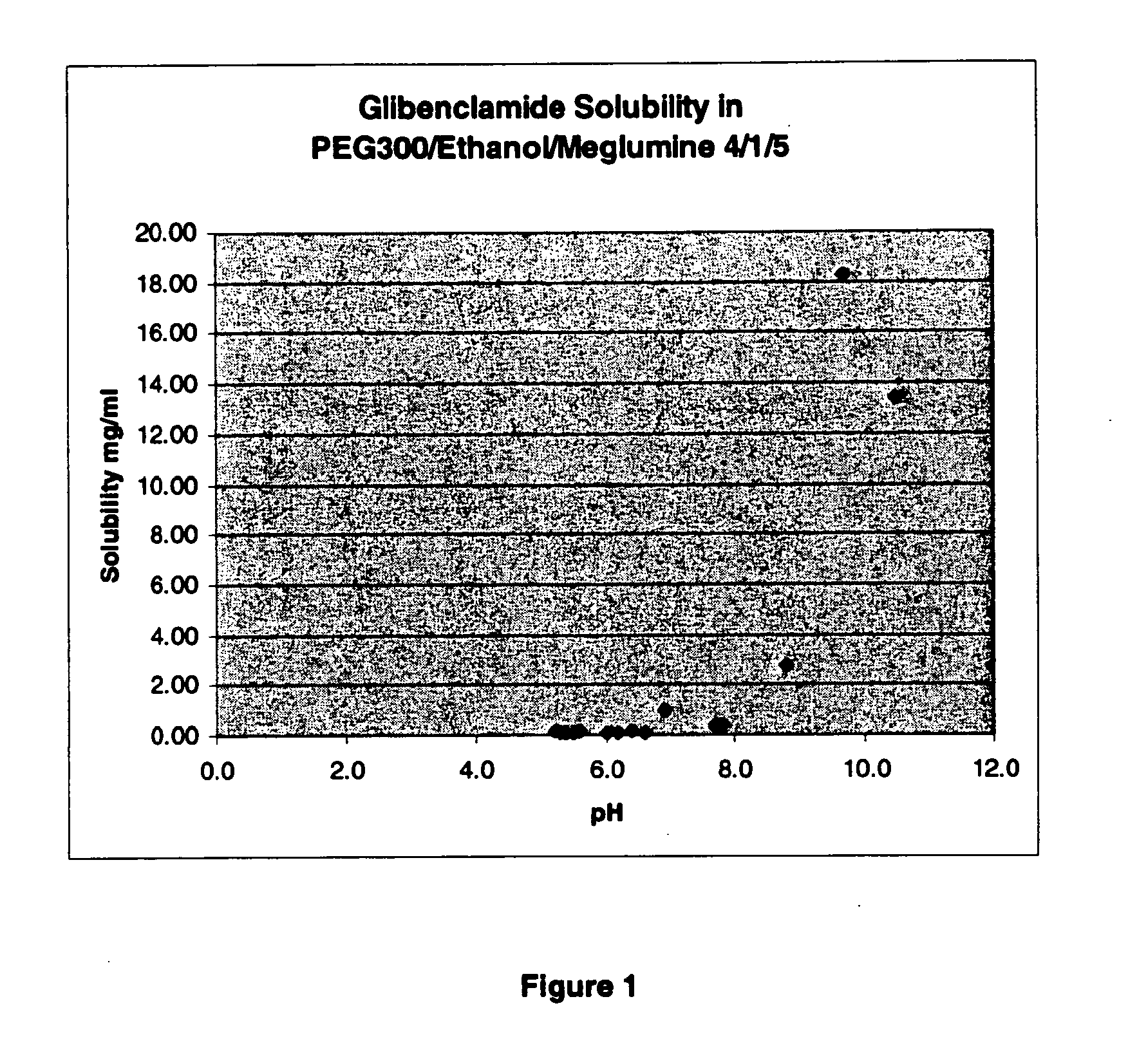
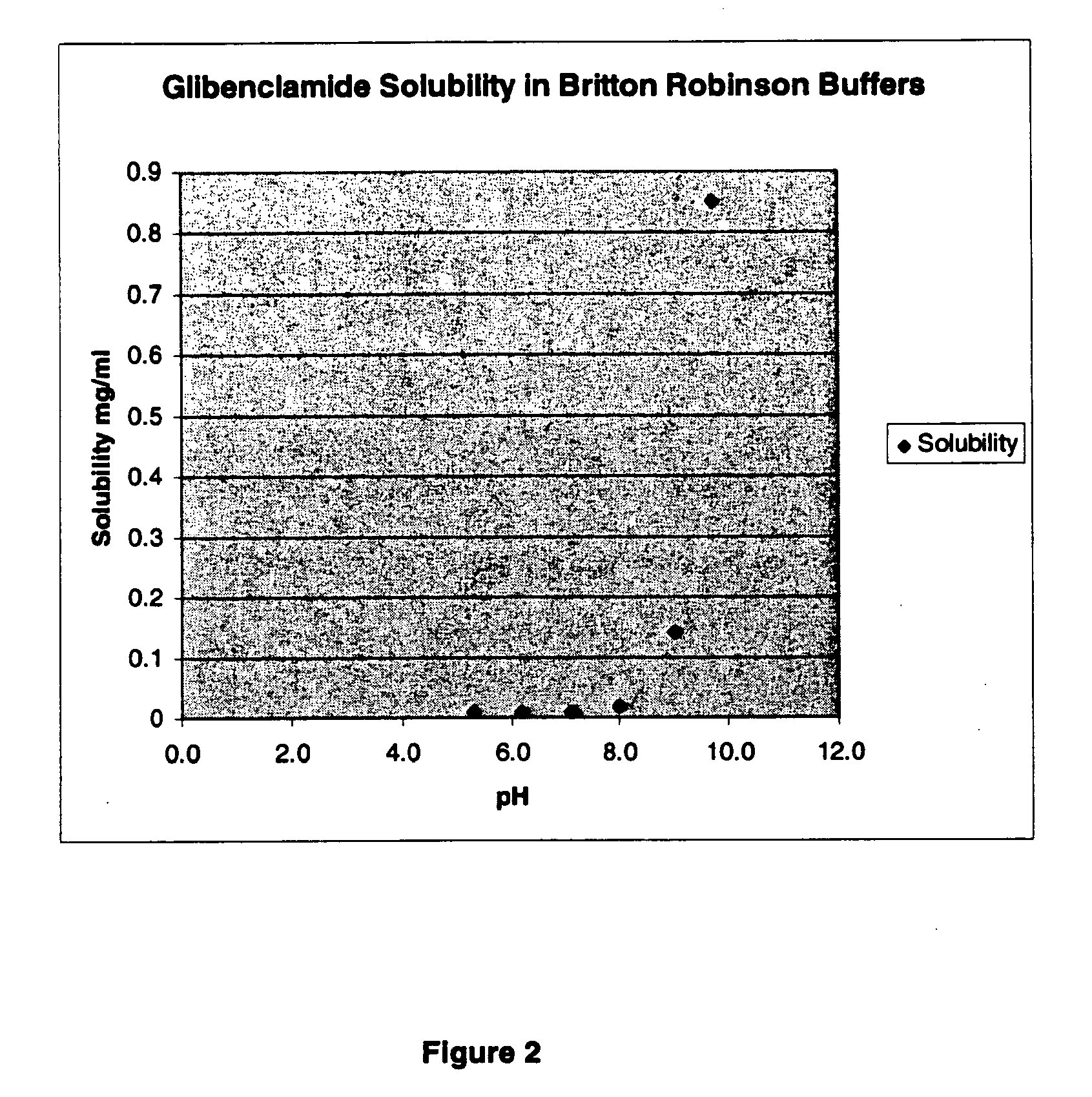
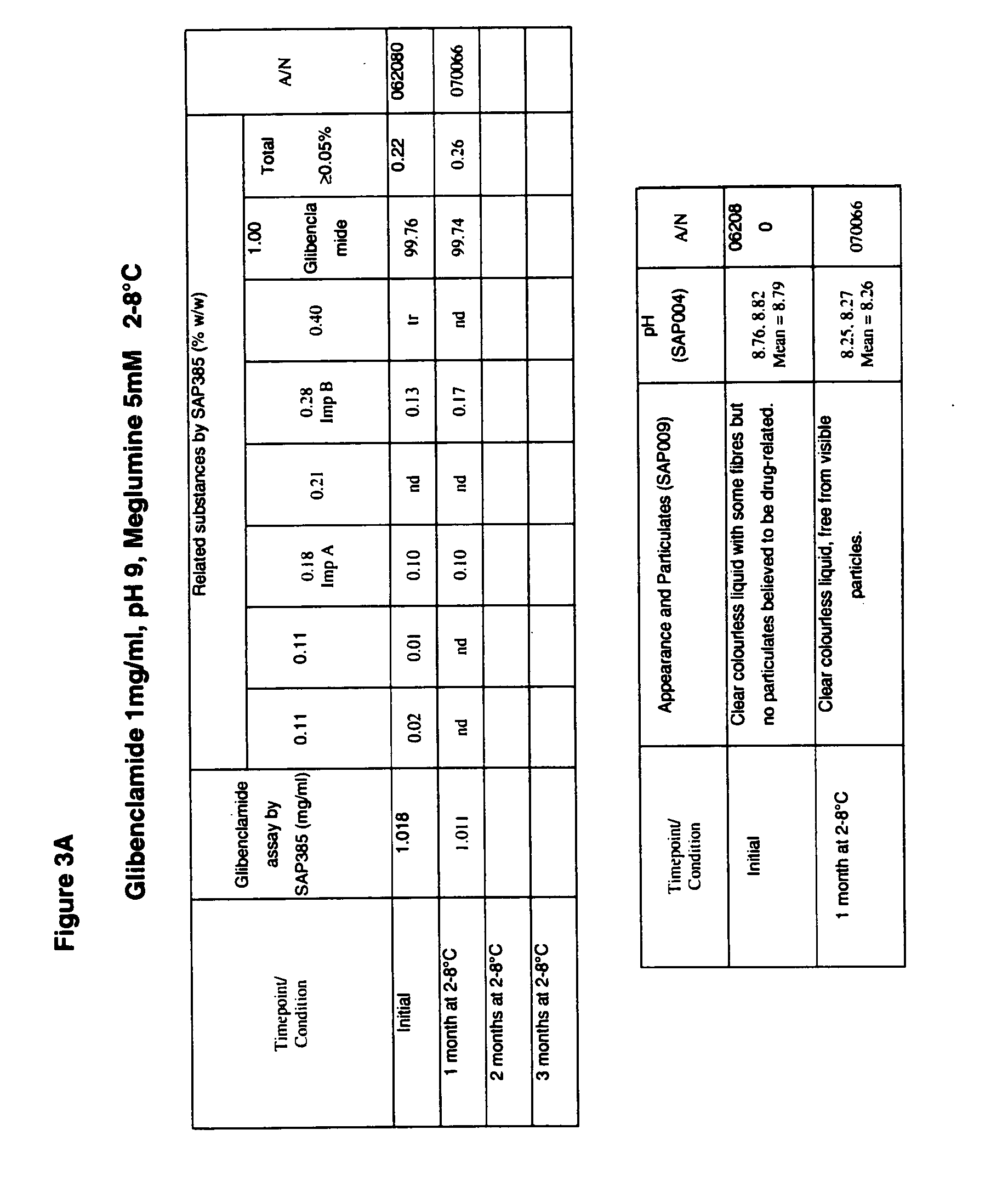
The invention provides liquid formulations of compounds that act at sulfonylurea receptors that are suitable for intravenous and intra-arterial infusion. Compounds active at a sulfonylurea receptor include glibenclamide, tolbutamide, repaglinide, nateglinide, meglitinide, midaglizole, LY397364, LY389382, glyclazide, and glimepiride. Liquid formulations may be concentrated solutions suitable for storage; may be diluted (e.g., dilution of 1:1 or 1:1.2) suitable for bolus injections, and may be further diluted (e.g., dilution of 1:10 or 1:20 or more) for intravenous and intra-arterial infusion over an extended period of time. For example, a liquid formulation may include at least about 0.05 mg / ml glibenclamide in a water-based solution including 40% polyethylene glycol 300, 10% Ethanol, 50% water, at about pH 9. The solution may include a buffer, and is suitable for storage in refrigerator or at room temperature. This solution may be diluted 1:1, or more (e.g., 1:20) without precipitation of the glibenclamide.
Owner:BIOGEN CHESAPEAKE LLC
Fabric softening finishing agent and preparation method thereof
ActiveCN108486899ASimple manufacturing methodRaw materials are easy to getLight resistant fibresGrip property fibresOrganic solventPolyethylene glycol
The invention discloses a fabric softening finishing agent and a preparation method thereof. The preparation method comprises the following steps: dissolving 3-[3-(2-H-benzotriazole-2-yl)-4-hydroxyl-5-tert-butylphenyl]-acrylic-polyethylene glycol 300 ester in an organic solvent to form a solution, then adding epoxy silicone oil and a catalyst into the solution, stirring and reacting for 8 to 10 hours at 70 to 80 DEG C in a nitrogen or inertial gas atmosphere, removing the organic solvent in a rotary steaming manner, and obtaining the fabric softening finishing agent. The fabric softening finishing agent disclosed in the invention has the characteristics of good hydrophilic property, more firmness in combination in a form of chemical bonds when acting on the fabric, more washability, excellent softening effect, no yellowing, smoothness, and good antistatic performance.
Owner:上海鑫妙纺织助剂有限公司
Smokeless electronic cigarette tobacco tar
The invention belongs to the technical field of electronic cigarette tobacco tar and discloses smokeless electronic cigarette tobacco tar; the smokeless electronic cigarette tobacco tar is composed ofthe following components: 30-70% of medicinal propylene glycol, 10-30% of propylene glycol flavor essence, 0-10% of nicotine, nicotine salt crystals or nicotine salt propylene glycol, 0.5-5% of distilled water, 0-10% of polyethylene glycol 400, 0-10% of polyethylene glycol 300 and 0.5-5% of medical vegetable glycerol. A user can select smoke or smokeless electronic cigarette in smoking actively,so that the use occasion of the electronic cigarette is expanded.
Owner:钟加川
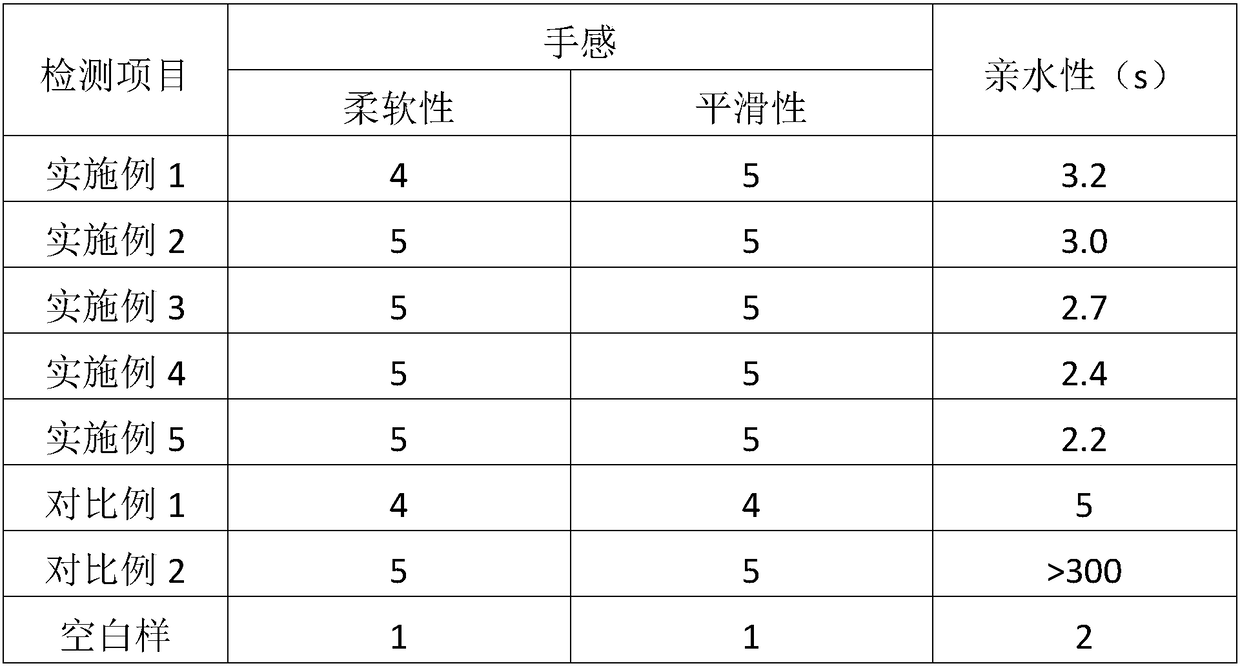
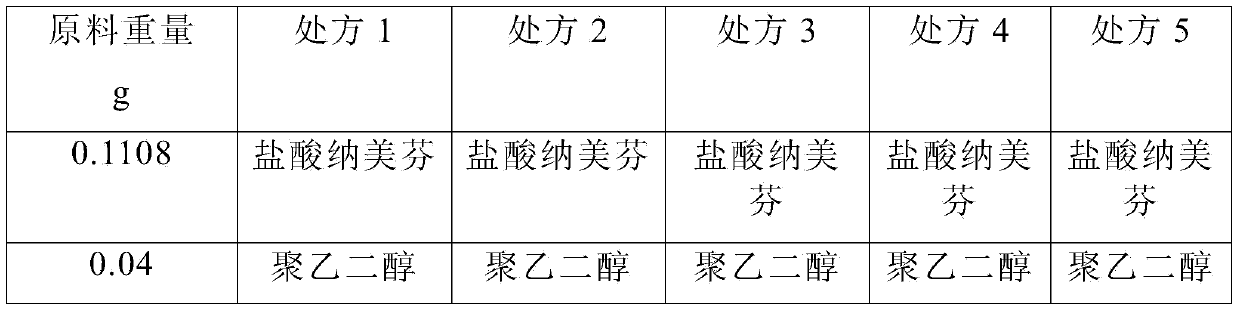
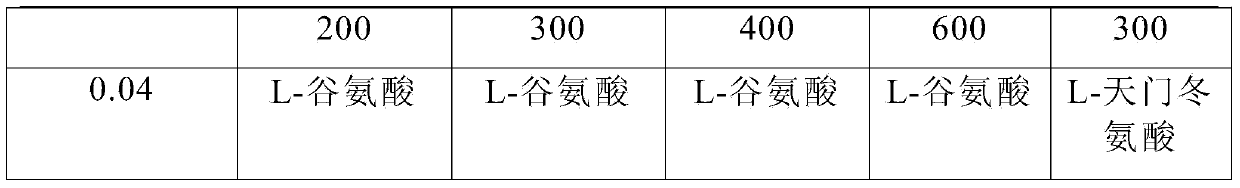
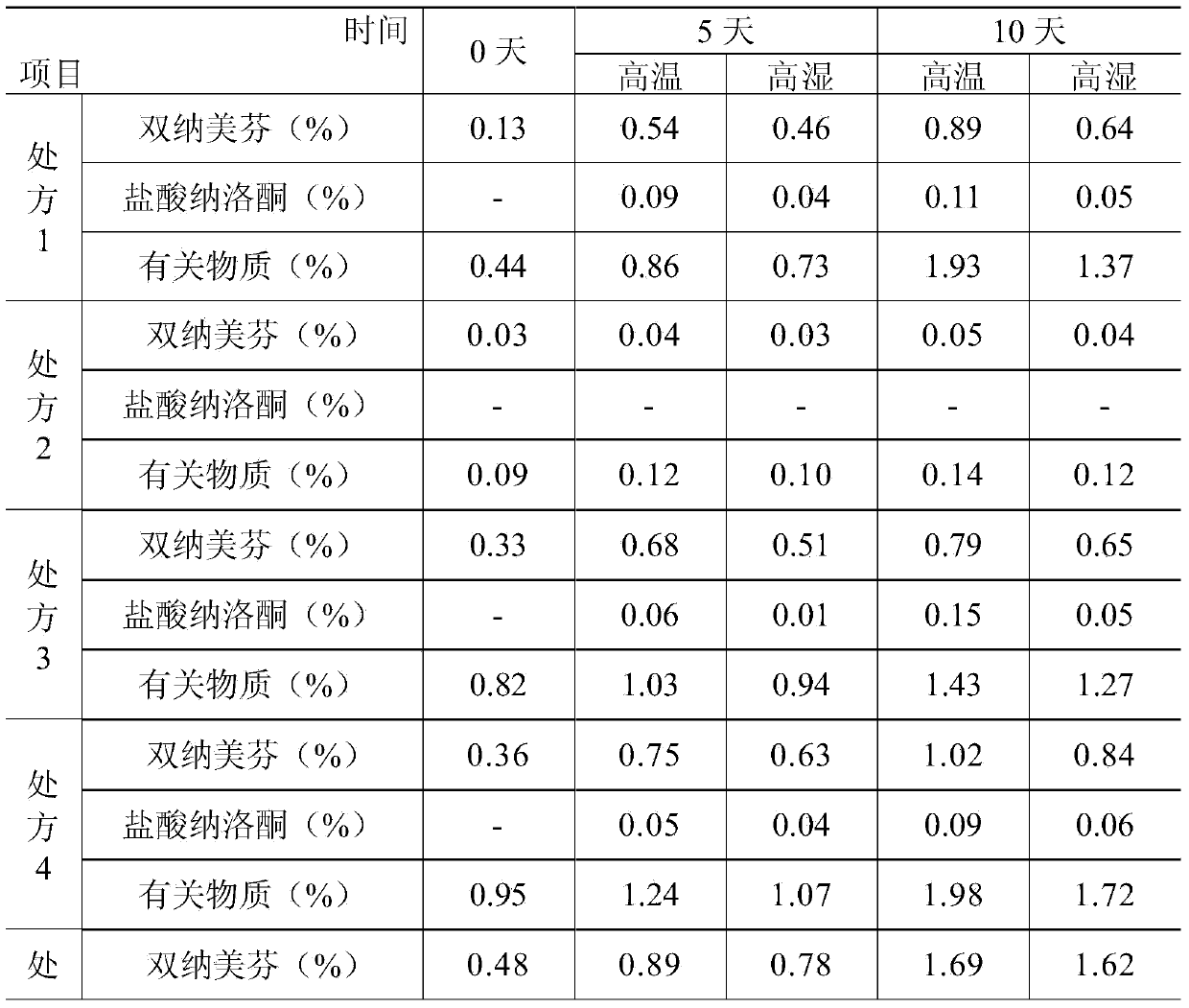
Pharmaceutical composition containing active component, namely nalmefene hydrochloride
ActiveCN104000827AGuaranteed contentOrganic active ingredientsNervous disorderActive componentPolyethylene glycol
The invention belongs to the technical field of medicines, and in particular relates to a pharmaceutical composition containing an active component nalmefene hydrochloride. The pharmaceutical composition disclosed by the invention contains nalmefene hydrochloride, polyethylene glycol 300 and L-glutamic acid. Injection prepared from the pharmaceutical composition disclosed by the invention is good in stability; after placing for 24 months, the content of bis-nalmefene is less than 0.1%; furthermore, the content of naloxone hydrochloride is less than 0.01%; the injection prepared from the pharmaceutical composition disclosed by the invention is convenient to use and beneficial to storing and transporting; the pharmaceutical composition disclosed by the invention is simple in preparation method, easy for industrialization production and low in production cost.
Owner:西藏易明西雅医药科技股份有限公司
Liquid formulations of compounds active at sulfonylurea receptors
InactiveUS20110034560A1Rapid and readily controlled increaseRapid adjustment and ready maintenance of circulating drug concentrationBiocideMetabolism disorderWater basedMeglitinide
The invention provides liquid formulations of compounds that act at sulfonylurea receptors that are suitable for intra-venous and intra-arterial infusion. Compounds active at a sulfonylurea receptor include glibenclamide, tolbutamide, repaglinide, nateglinide, meglitinide, midaglizole, LY397364, LY389382, glyclazide, and glimepiride. Liquid formulations may be concentrated solutions suitable for storage; may be diluted (e.g., dilution of 1:1 or 1:1.2) suitable for bolus injections, and may be further diluted (e.g., dilution of 1:10 or 1:20 or more) for intravenous and intra-arterial infusion over an extended period of time. For example, a liquid formulation may include at least about 0.05 mg / ml glibenclamide in a water-based solution including 40% polyethylene glycol 300, 10% Ethanol, 50% water, at about pH 9. The solution may include a buffer, and is suitable for storage in refrigerator or at room temperature. This solution may be diluted 1:1, or more (e.g., 1:20) without precipitation of the glibenclamide.
Owner:BIOGEN CHESAPEAKE LLC
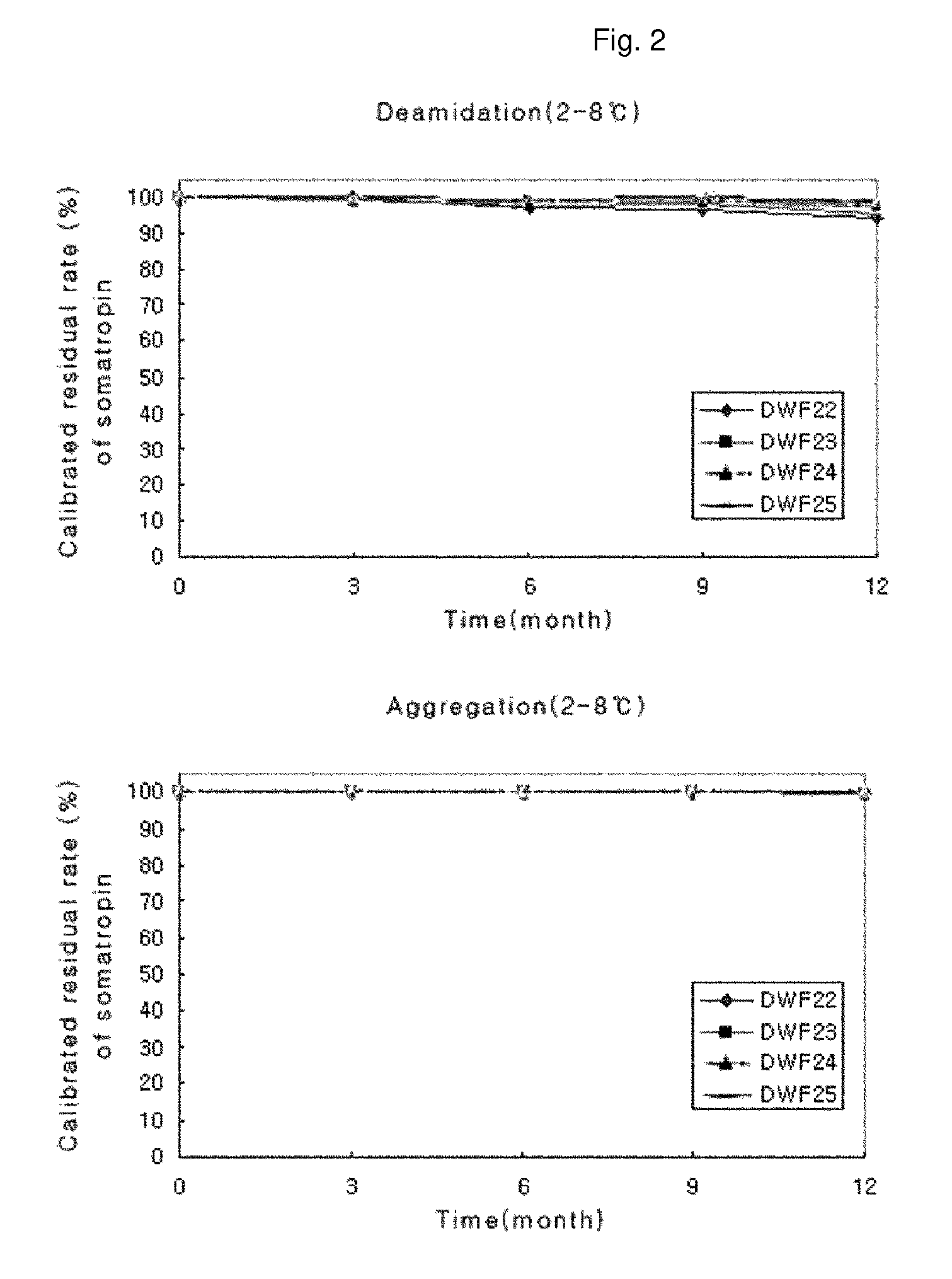
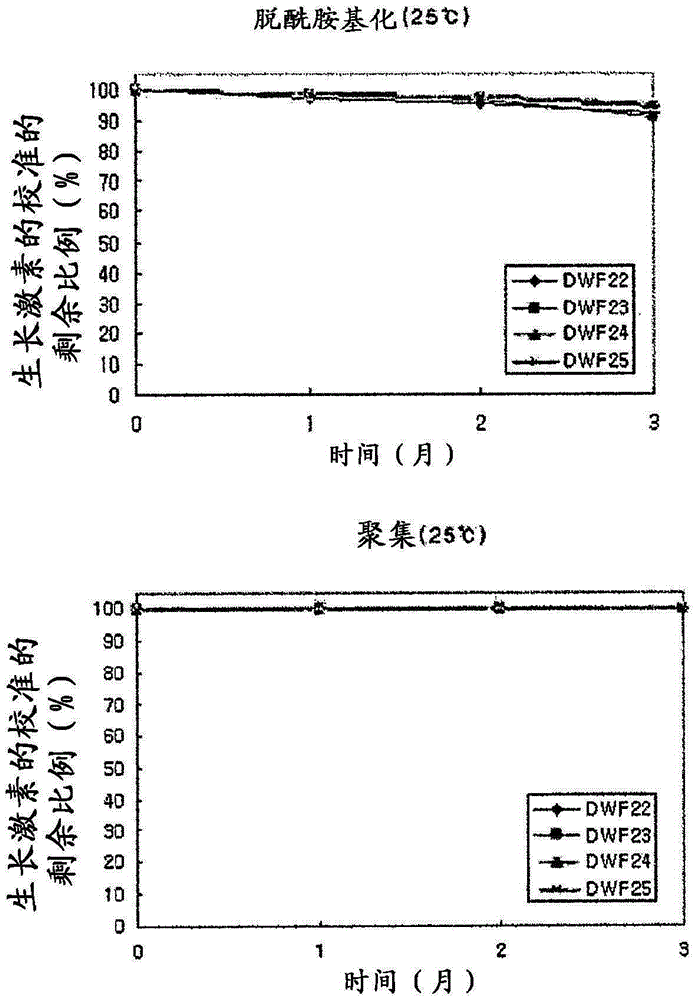
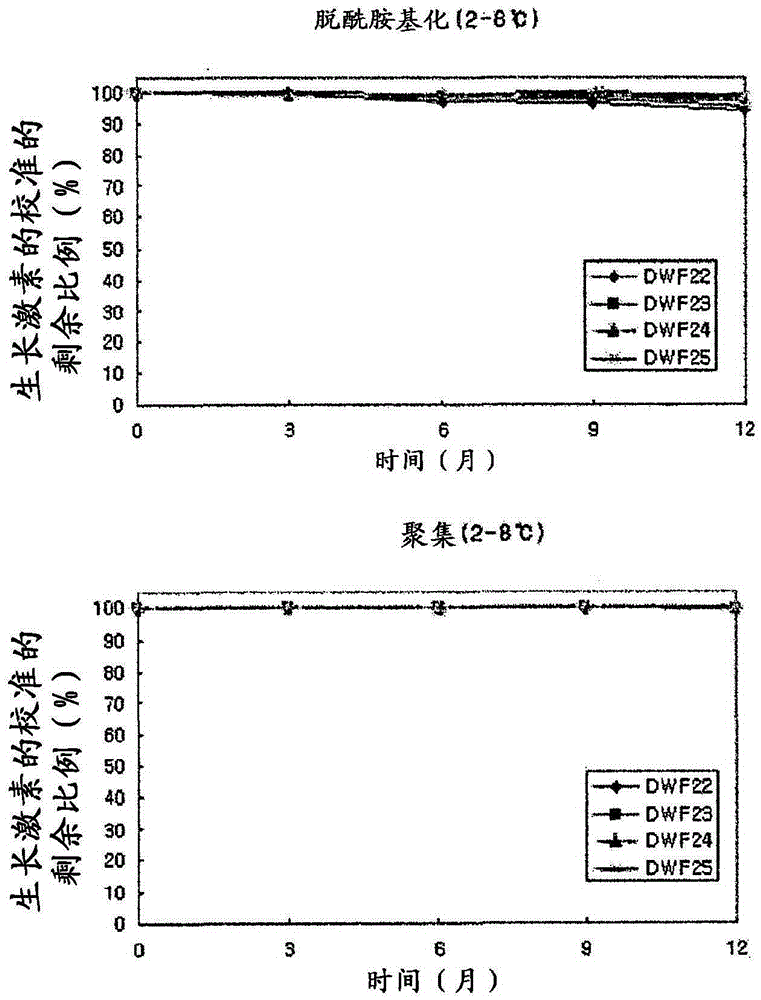
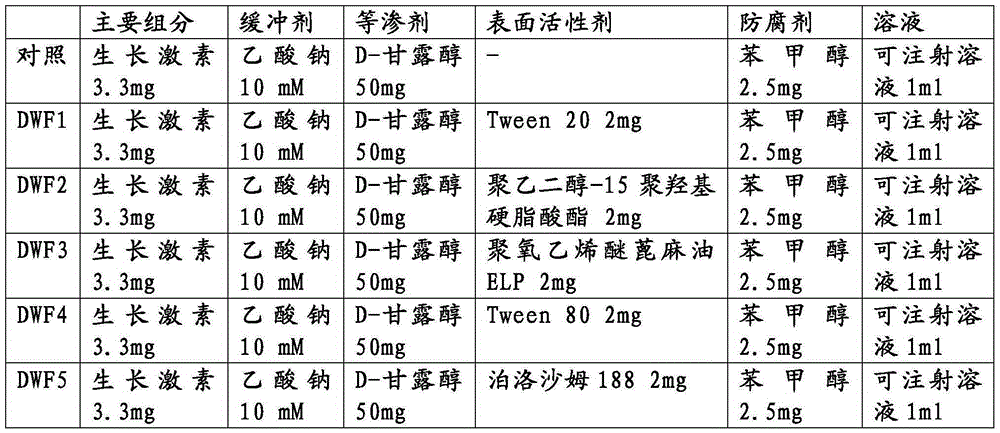
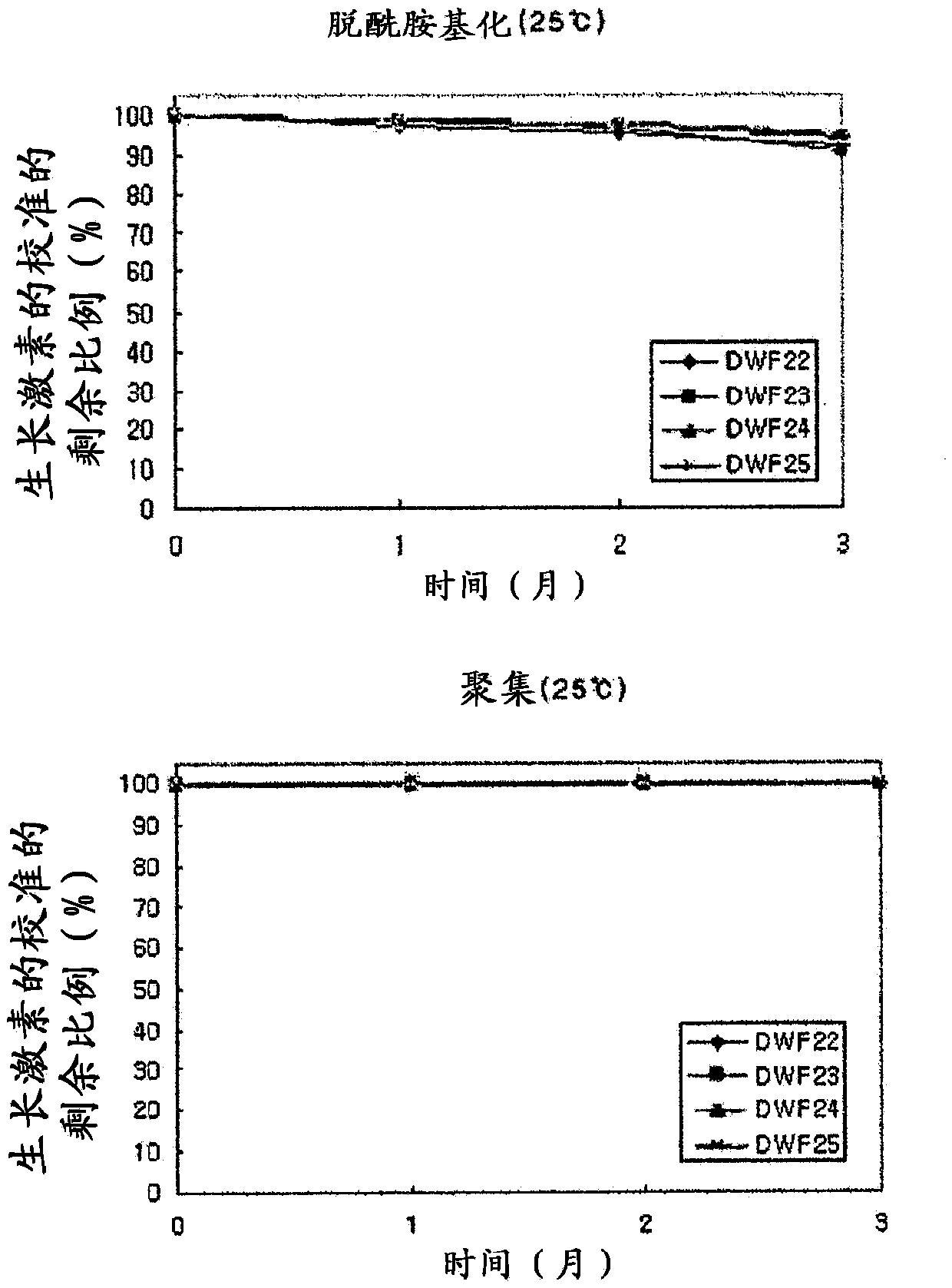
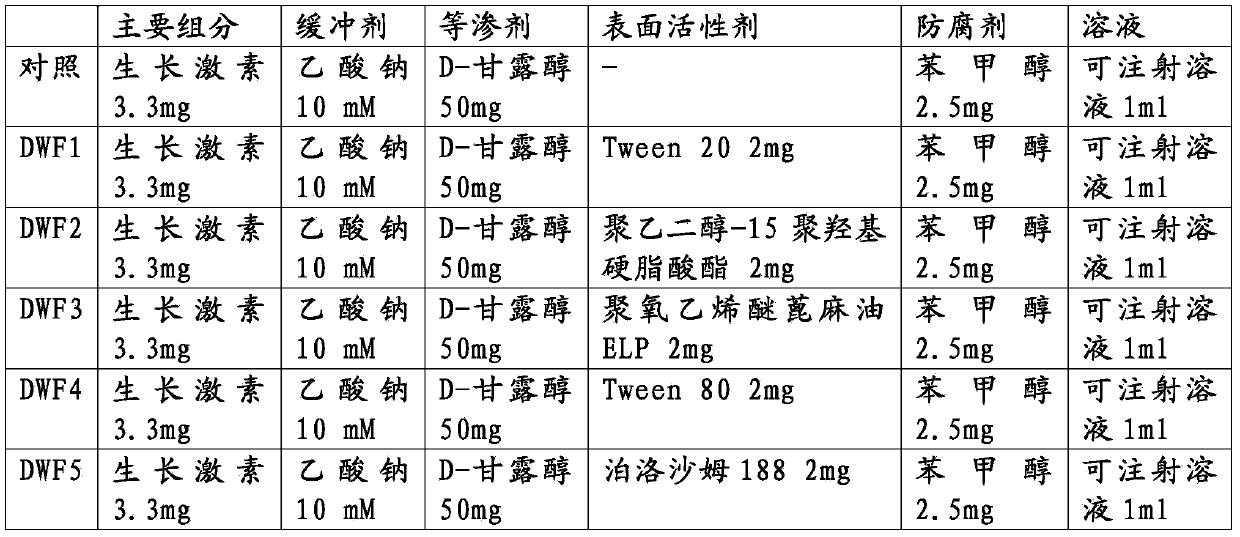
Stable liquid formulation of human growth hormone
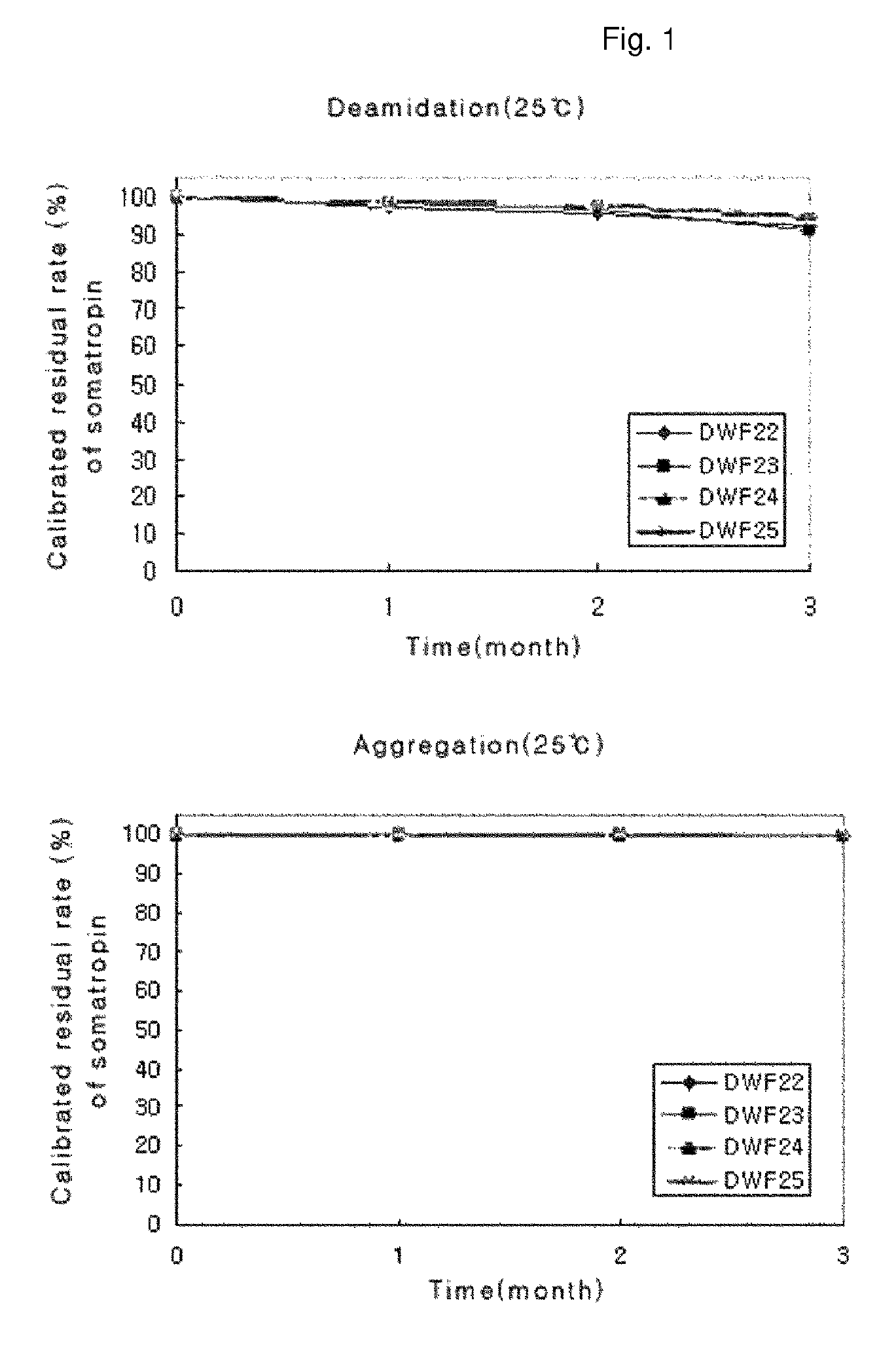
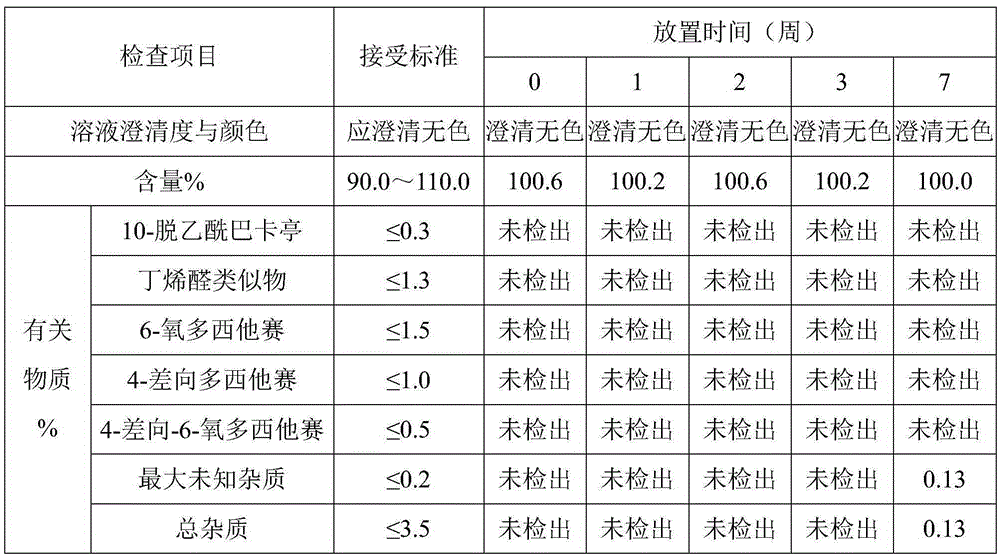
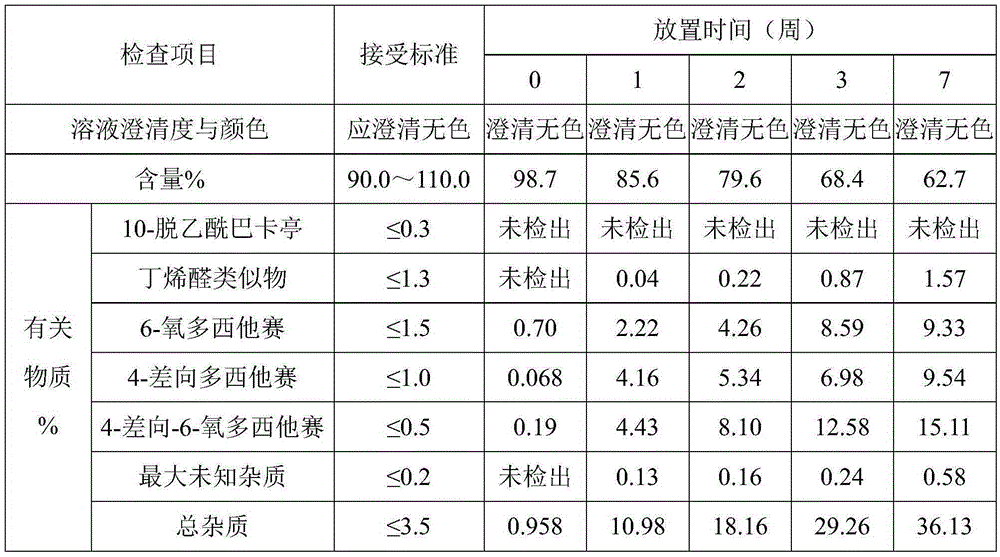
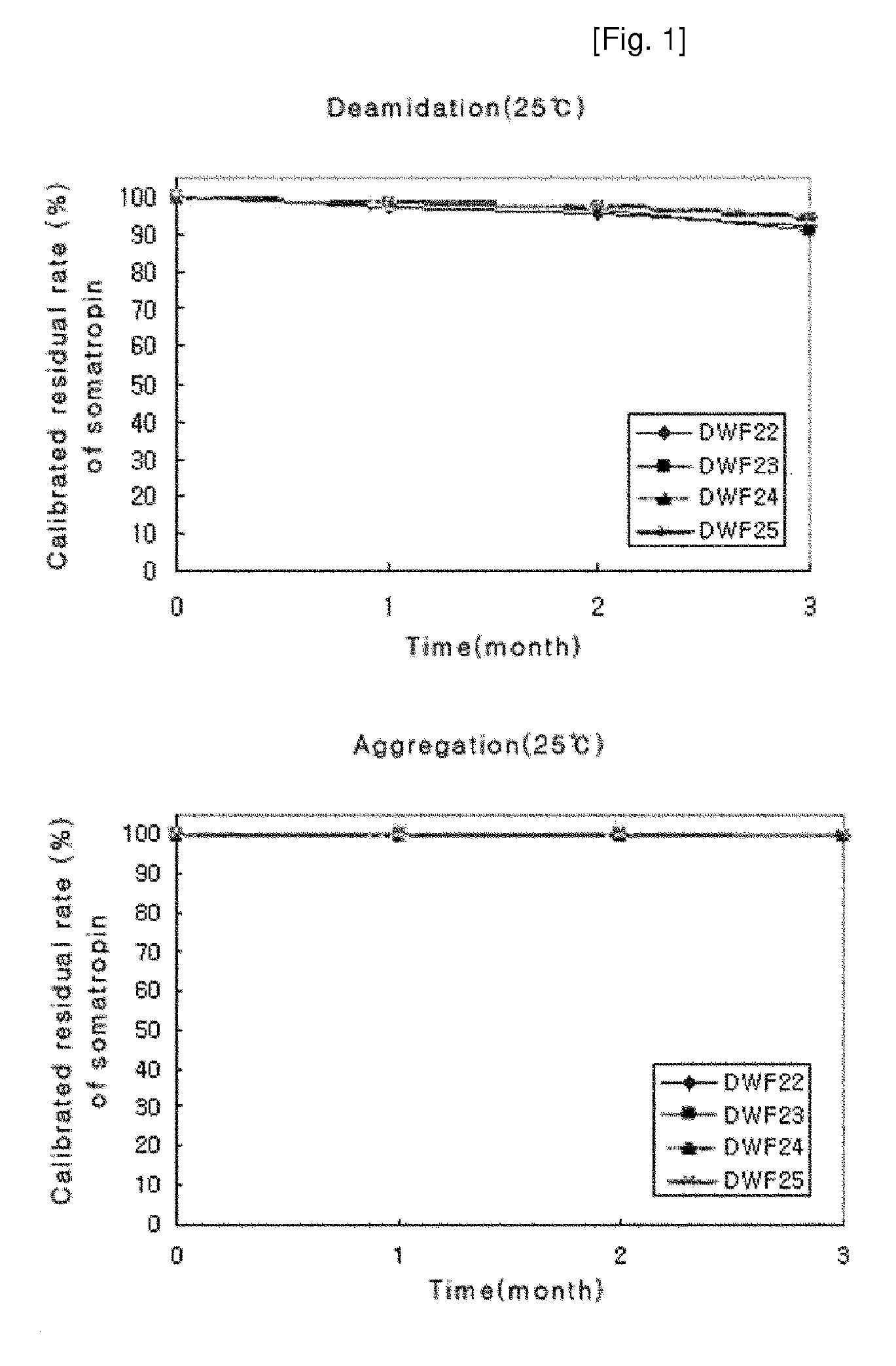
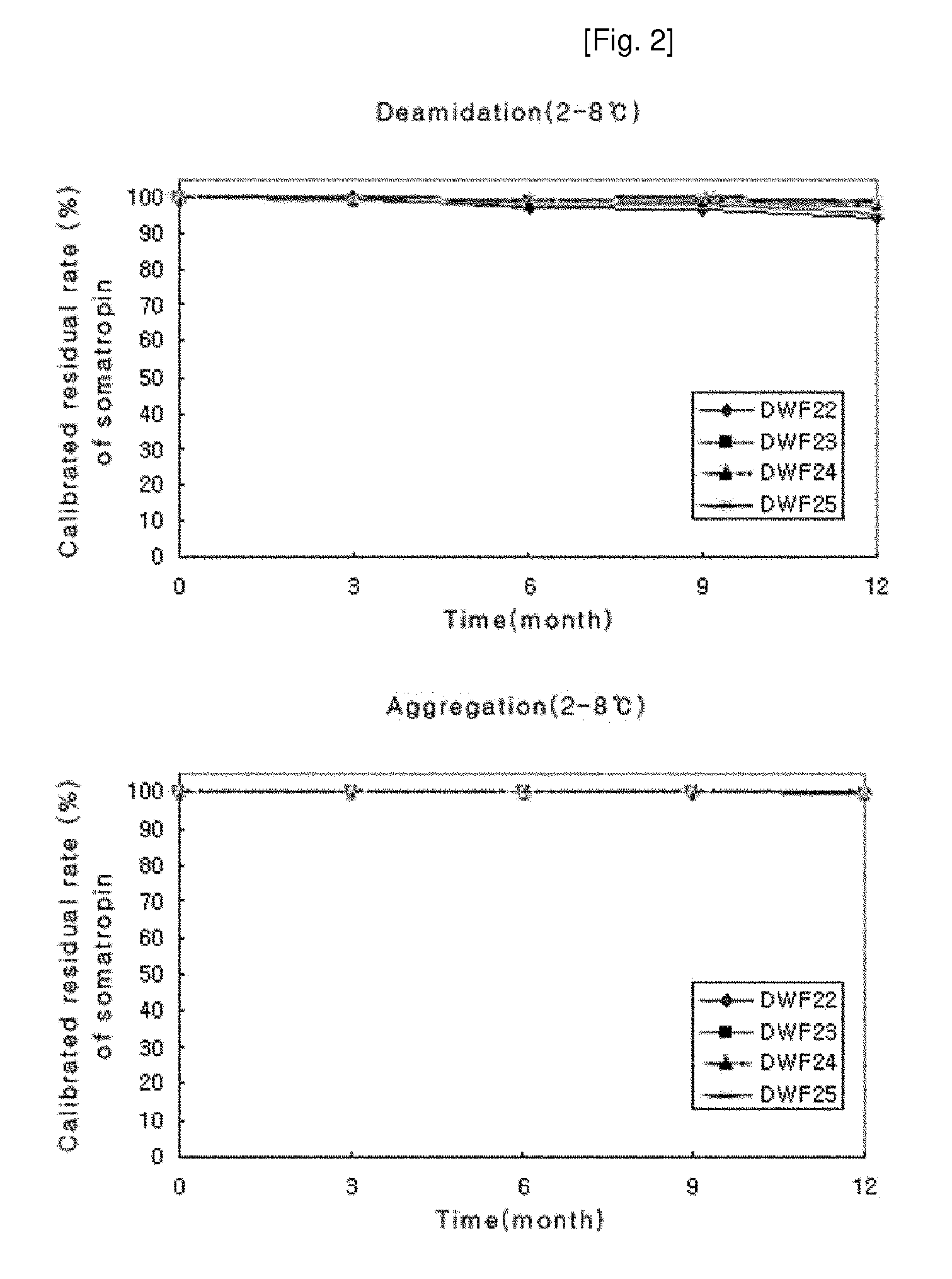
ActiveUS8409586B2Minimize formationImprove conveniencePeptide/protein ingredientsPeptide preparation methodsHuman growth hormoneArginine
Disclosed herein is a stable liquid formulation comprising human growth hormone; L-lysine, L-arginine or polyethylene glycol 300; and poly(oxyethylene) poly(oxypropylene) copolymer, polyethylene glycol-15 polyoxystearate or polyethylene glycol-35 castor oil.
Owner:DAEWOONG CO LTD
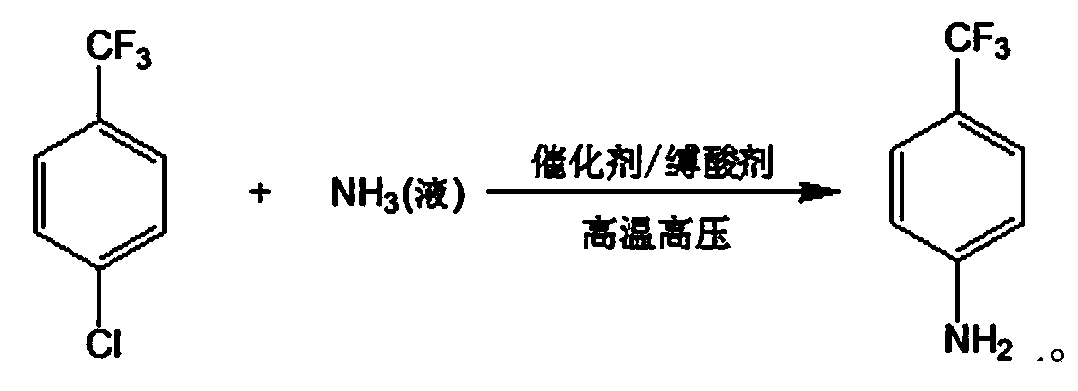
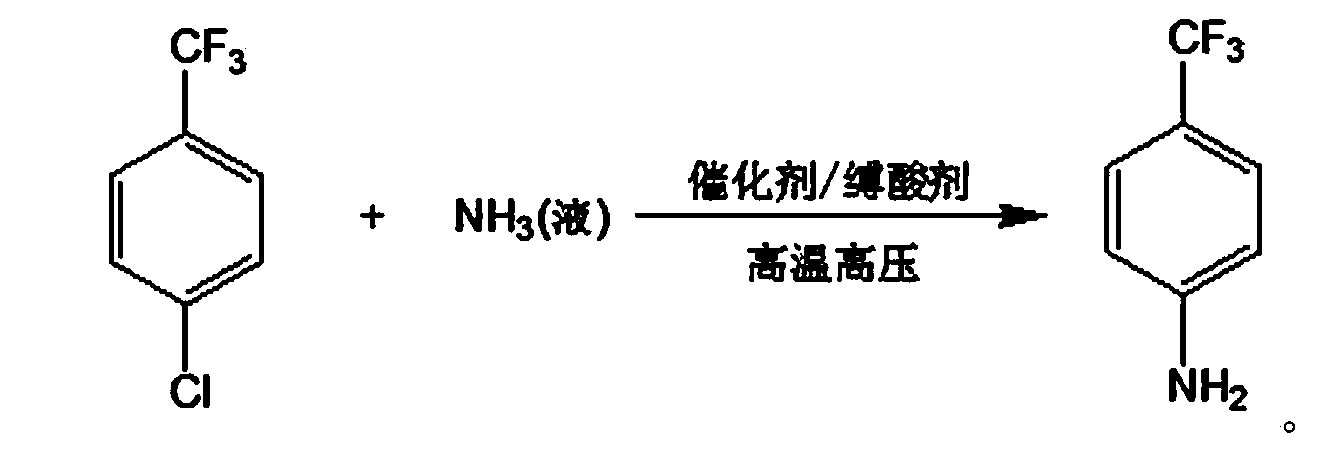
Method for preparing p-trifluoromethylaniline by performing high pressure ammonolysis
ActiveCN103408436AReduce lossHarm reductionOrganic compound preparationAmino compound preparationN dimethylformamideChlorobenzene
The invention relates to a new method for preparing p-trifluoromethylaniline by performing high pressure ammonolysis. P-trifluoromethyl chlorobenzene serving as a raw material is subjected to high-temperature high pressure ammonolysis reaction in a solvent under the action of a catalyst, liquid ammonia and an acid-binding agent to generate p-trifluoromethyl phenylamine; the catalyst is a mixture of cuprous chloride and copper powder; the acid-binding agent is one or two of inorganic base mixtures of sodium hydroxide and the like, or one or two of organic base mixtures of pyridine and triethylamine; and the solvent is one or two of mixing solvents of methanol, ethanol, polyethylene glycol 300-3,000 and N,N-dimethylformamide. The method has the advantages that the inorganic base, serving as the acid-binding agent, with low cost and easy availability is adopted, the loss of liquid ammonia in the reaction process is greatly reduced, and the production efficiency is improved; and the catalyst can be prepared from organic solvents with the cost as low as that of ethanol and less harm, so that the generation of hydrolysis side reaction is avoided, the purity of the product is high and the quality is high; and raw materials without being totally reacted can be recycled.
Owner:JIANGSU FENGHUA CHEM IND
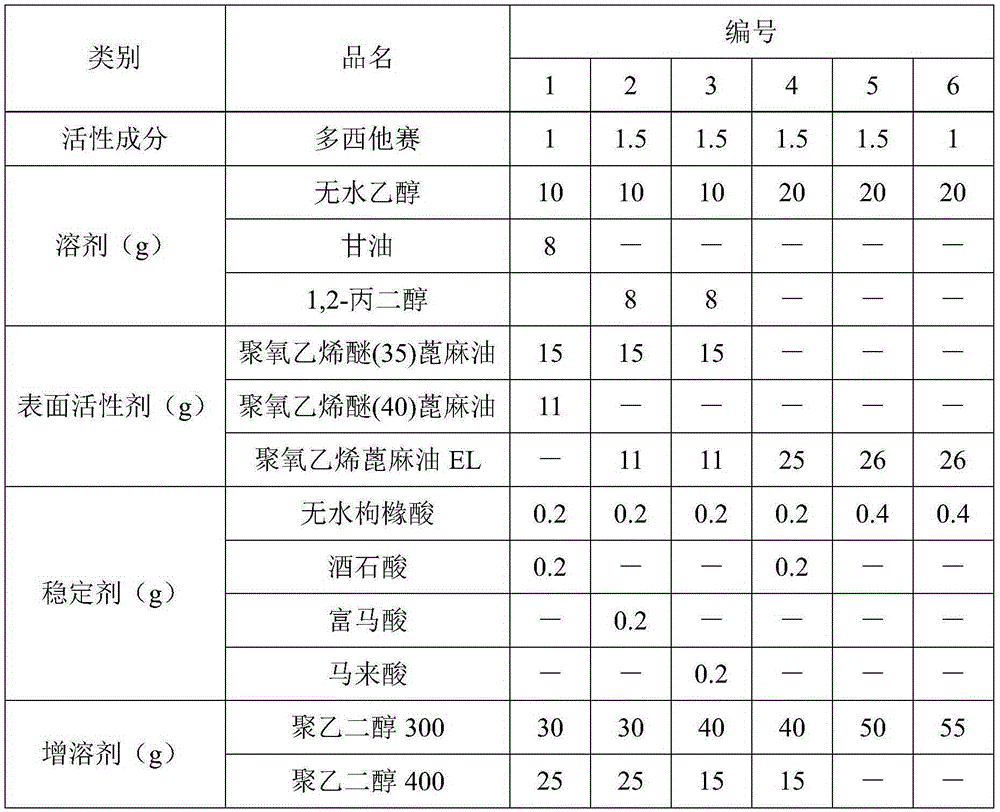
Docetaxel injection and preparation method thereof
InactiveCN105395540ANo hemolysisImprove securityOrganic active ingredientsPharmaceutical delivery mechanismHemolysisGlycerol
The invention discloses docetaxel injection. The docetaxel injection is prepared from the following components in parts by weight: 1-2 parts of docetaxel, 18-25 parts of a solvent, 20-30 parts of a surfactant, 0.3-0.6 part of a stabilizer, and 40-60 parts of a solubilizer, wherein the solvent is one or more of absolute ethyl alcohol, glycerol and propylene glycol, the surfactant is polyoxyethylenated castor oil, the stabilizer is one or more of citric acid, tartaric acid, fumaric acid and maleic acid, and the solubilizer is polyethylene glycol 300 and / or polyethylene glycol 400. The docetaxel injection provided by the invention is free of hemolysis, good in safety, good in stability, stable in content of active ingredients, and low in contents of related substances and impurities, and can be stored under the condition with the temperature being 20-40 DEG C.
Owner:HAINAN GENERAL & KANGLI PHARMA
Stable liquid formulation of human growth hormone
ActiveUS20090298768A1Minimize formationImprove conveniencePeptide/protein ingredientsPeptide preparation methodsHuman growth hormoneArginine
Disclosed herein is a stable liquid formulation comprising human growth hormone; L-lysine, L-arginine or polyethylene glycol 300; and poly(oxyethylene) poly(oxypropylene) copolymer, polyethylene glycol-15 polyoxystearate or polyethylene glycol-35 castor oil.
Owner:DAEWOONG CO LTD
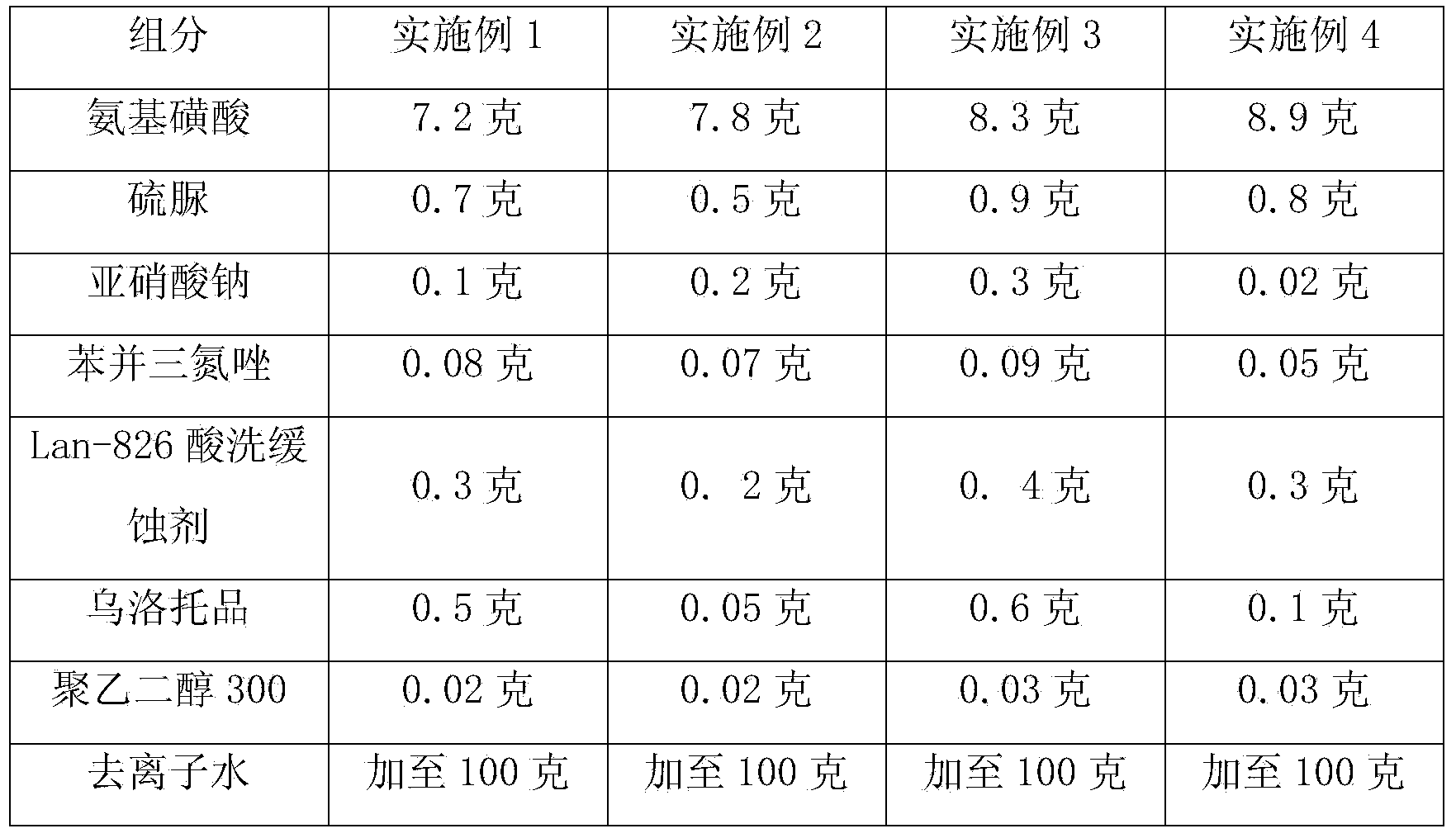
Cleaning solution for indissolvable water scale in mining automobile engine cooling system
The invention provides a cleaning solution for indissolvable water scale in a mining automobile engine cooling system. The cleaning solution is characterized by comprising following components by weight: 7.0-9.0% of sulphamic acid, 0.4-1.0% of thiourea, 0.02-0.35% of sodium nitrite, 0.05-0.1% of benzotriazole, 0.15-0.4% of a Lan-826 acid-washing corrosion inhibitor, 0.05-0.70% of urotropine, and 0.02-0.03% of polyethylene glycol 300, with the balance being deionized water. After cleaning with the cleaning solution, the fuel consumption amount can be largely reduced, the power property can be significantly improved, and the rate of fuel saving reaches 1-3%. The cleaning solution is re-used in mining automobile engines. The cleaning solution can clean indissolvable water scale in one time, is free of preparation on site, simple in operation, short in cleaning time, time saving, labor saving, green, and free of corrosion to metal and rubber in the cooling system, and protects systems. The cleaning solution and cooling liquids at present are compatible. The cleaning solution is low in cost and high in performance cost ratio.
Owner:ANSTEEL GRP MINING CO LTD
Sample pad treatment fluid improving analysis sensitivity of aflatoxin B1 colloidal gold detecting test strip
InactiveCN110308276AEasy to operateLow costBiological material analysisBiological testingPolyethylene glycolSodium azide
The invention relates to a sample pad treatment fluid improving analysis sensitivity of an aflatoxin B1 colloidal gold detecting test strip. Every 1000mL of the sample pad treatment fluid is preparedby fully mixing 11-13g of a buffer solution, 20-70g of Trehalose, 5-10g of Tween-80, 5-10g of Triton X-405, 1-5g of polyethylene glycol 300, 10-40g of bovine serum albumin, 0.05-0.15g of sodium azide,0.1-1g of sodium alginate, 0.01-0.5g of dextran sulfate, 0.01-0.5g of polyacrylamide and the residues of water or deionized water, and using 4M of hydrochloric acid to adjust the pH value to 8.0. Operation is simple, the cost is low, materials are easy to obtain, the detecting sensitivity is doubled or more, and the social and economical values are high.
Owner:ZHENGZHOU UNIVERSITY OF LIGHT INDUSTRY +1
SiO2 acid-base composite nanometer coating with porous structure and preparation method thereof
The invention discloses a SiO2 acid-base composite nanometer coating with a porous structure and a preparation method thereof. The coating consists of a multi-slit film with the thickness of 150-200 nm, and a substrate is covered with the multi-slit film, wherein the multi-slit film is formed by placing SiO2 nanometer chains containing mesopores between SiO2 nanoparticles which are distributed in a three-dimensional net shape. The method includes the steps that separately prepared tetraethyl silicate ethanol solutions are dropwise added into a mixed aqueous solution and stirred for aging first, water and a mixed solution of ethanol and ethyl tetrasilicate are sequentially added into obtained SiO2 alkaline sol after an aqueous solution of nitric acid is added dropwise into the SiO2 alkaline sol, stirring is carried out to obtain acid-base composite sol, and then polyethylene glycol 300 is added into the acid-base composite sol and stirred for aging; the substrate is placed in the obtained sol for film coating and then subjected to immersing, pulling, film coating and air-drying, and then an obtained film-coated substrate is subjected to annealing to obtain a target product. The nanometer coating has good hydrophilicity and wear resistance and high light transmission performance and is extremely likely to be commercially used in the fields of fog resistance, automatic cleaning, wide-spectrum reflection enhancement and the like in a wide range.
Owner:HEFEI INSTITUTES OF PHYSICAL SCIENCE - CHINESE ACAD OF SCI +1
Isopycnic cutting slurry for linear cutting of solar wafer and manufacturing method thereof
InactiveCN102206536AControl environmentReduce cutting costsLubricant compositionPolyethylene glycolSlurry
Disclosed are an isopycnic cutting slurry for linear cutting of a solar wafer and a manufacturing method thereof. The cutting slurry comprises, by weight, 100 parts of acrylic monomers, 4 parts of an initiator, 300 parts of polyethylene glycol and 280 parts of silicon carbide particles. The manufacturing method for the isopycnic cutting slurry comprises the following steps: a, weighing 3 / 4 of thetotal polyethylene glycol to dissolve all the acrylic monomers except acrylic acid; b, weighing acrylic acid to dissolve 4 / 5 of the total initiator; c. evenly mixing the two solutions, weighing a half of the mixed solution, adding the half of the mixed solution into a synthesis device, and adding another half of the mixed solution through a feeding device under the condition of a backflow at a temperature of 75 to 80 DEG C; e, after 2.0 hours, adding a mixed liquor consisting of the rest 1 / 4 of the polyethylene glycol and 1 / 5 of the initiator to the synthesis device and controlling the addingto be finished within 30 min; f. after 2.0 hours of further reaction, stopping heating and stirring, cooling the solution to a temperature of 25 DEG C for discharging. With utilization of the method in the invention, accessory environment can be controlled and cutting cost can be reduced.
Owner:浙江德圣龙窗帘有限公司
Five-kernel soft capsule and preparation method thereof
The invention discloses a five-kernel soft capsule which comprises main materials and auxiliary materials, wherein the main materials consist of the following raw material extracts in parts by mass: 9-15 parts of fructus cannabis kernels, 10-15 parts of bunge cherry kernels, 5-10 parts of platycladi kernels, 10-15 parts of trichosanthes kirilowii kernels and 5-10 parts of sweet almond kernels; the auxiliary materials comprise one or more of soybean oil, polyethylene glycol, glycerinum and dimethicone; the polyethylene glycol is one or more of polyethylene glycol 200, polyethylene glycol 300, polyethylene glycol 400 and polyethylene glycol 600; the mass percentages of the main materials and the auxiliary materials are that the main materials account for 5-80% and the auxiliary materials account for 95-20%. A preparation method comprises the following steps: (1) extracting effective components; (2) adding the auxiliary materials, uniformly, and treating in a machine so as to obtain the soft capsule; (3) filling the capsule into a bottle, or pressing into an aluminum foil bubble-cap plate, packaging, and inspecting, thereby obtaining the qualified five-kernel soft capsule. The five-kernel soft capsule disclosed by the invention is rapid in absorption, good in taste, gentle in action, free of adverse reaction and applicable to patients suffering from intestinal dryness with constipation, and has the functions of moistening intestines and relaxing bowel and nourishing bodies and tonifying deficiency.
Owner:贵阳护理职业学院
Aspirin injection used for relieving pain
InactiveCN107550856AQuick resultsImprove liquidityOrganic active ingredientsNervous disorderSide effectSolvent
The invention discloses an aspirin injection used for relieving pain. The aspirin injection used for relieving pain contains aspirin, a solvent, and an anti-oxidant at a ratio (w / v / v) of 1: (7-45) : (2-5), and preferably at 1: (30-50) : (2-3); the solvent is one randomly selected from methoxy polyethylene glycol, polypropylene glycol, and polyethylene glycol; the anti-oxidant is phenthiazine witha mass concentration of 0.25% to 0.5%; the concentration of polyethylene glycol 300 ranges from 10 to 30%; the concentration of methoxy polyethylene glycol ranges from 10 to 25%; the concentration ofpolypropylene glycol ranges from 10 to 25%. The aspirin injection is used for parenteral administration instead of oral administration, so that stomach and cardiovascular side effects usually caused by oral pain relievers in clinical treatment are avoided; sustained slow release is realized; and long lasting drug release is realized.
Owner:宫丽丽
Isopycnic cutting slurry for linear cutting of solar wafer and manufacturing method thereof
InactiveCN102206536BControl environmentReduce cutting costsLubricant compositionPolyethylene glycolMicroparticle
Disclosed are an isopycnic cutting slurry for linear cutting of a solar wafer and a manufacturing method thereof. The cutting slurry comprises, by weight, 100 parts of acrylic monomers, 4 parts of an initiator, 300 parts of polyethylene glycol and 280 parts of silicon carbide particles. The manufacturing method for the isopycnic cutting slurry comprises the following steps: a, weighing 3 / 4 of thetotal polyethylene glycol to dissolve all the acrylic monomers except acrylic acid; b, weighing acrylic acid to dissolve 4 / 5 of the total initiator; c. evenly mixing the two solutions, weighing a half of the mixed solution, adding the half of the mixed solution into a synthesis device, and adding another half of the mixed solution through a feeding device under the condition of a backflow at a temperature of 75 to 80 DEG C; e, after 2.0 hours, adding a mixed liquor consisting of the rest 1 / 4 of the polyethylene glycol and 1 / 5 of the initiator to the synthesis device and controlling the addingto be finished within 30 min; f. after 2.0 hours of further reaction, stopping heating and stirring, cooling the solution to a temperature of 25 DEG C for discharging. With utilization of the method in the invention, accessory environment can be controlled and cutting cost can be reduced.
Owner:浙江德圣龙窗帘有限公司
A reduction cleaning agent for synthetic fibers, its preparation method and application
ActiveCN105714585BBreakthrough progressThe cleaning effect is more thanDyeing processPolyethylene glycolCleansing Agents
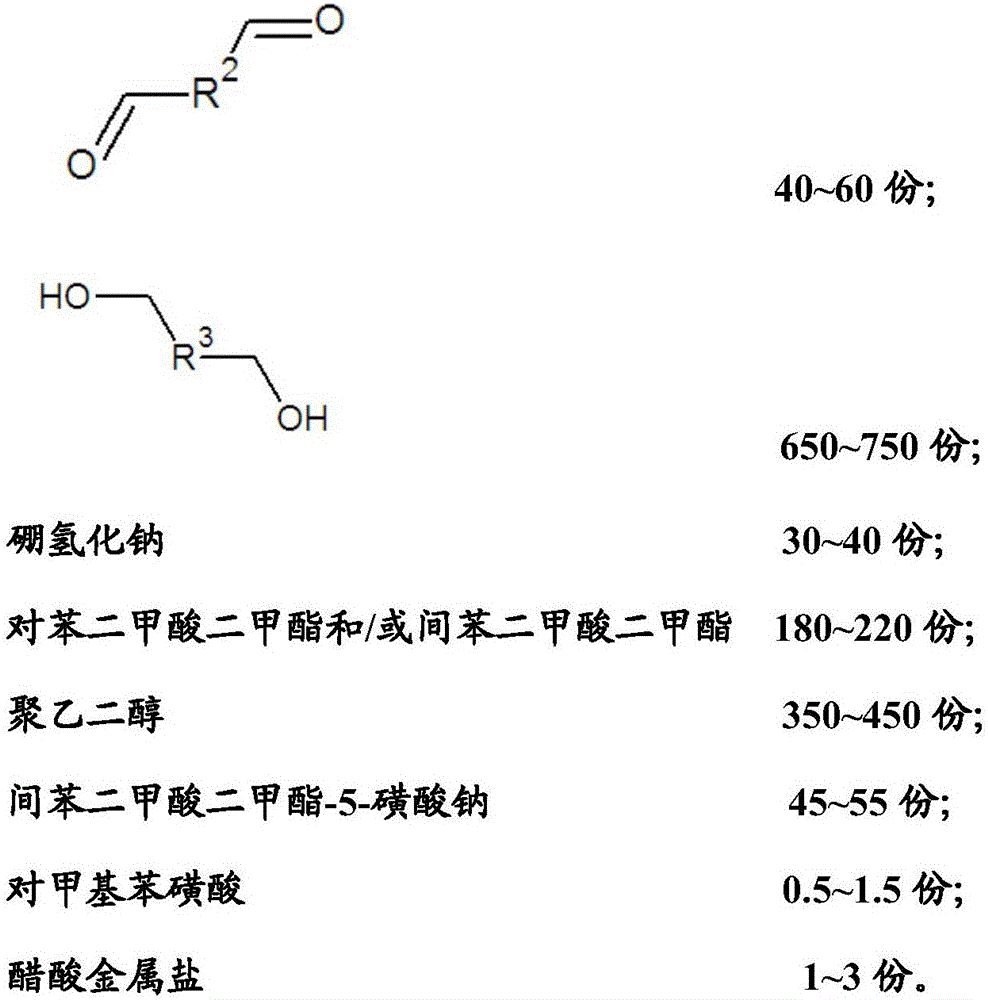
The invention relates to a reductive cleaning agent for synthetic fibers as well as a preparation method and an application of the reductive cleaning agent. The reductive cleaning agent is prepared from raw materials in parts by weight as follows: 250-350 parts of a substance A shown in the specification, 30-80 parts of a substance B shown in the specification, 600-800 parts of a substance C shown in the specification, 20-40 parts of sodium borohydride, 150-250 parts of dimethyl terephthalate / dimethyl isophthalate, 300-500 parts of polyethylene glycol, 35-65 parts of sodium dimethyl isophthalate-5-sulfonate, 0.5-1.5 parts of p-methylbenzene sulfonic acid and 1-3 parts of acetic metal salt. The reductive cleaning agent has a good cleaning effect, a durable effect and a sufficient cleaning effect when used, cannot lose efficacy at high temperature, is stable after meeting with water or absorbing moisture, cannot decompose or release combustible gas or corrosive gas, does not contain carcinogenic aromatic amine or environmental hormone, cannot be influenced by poor storage environment and transportation environment, requires lower transportation cost, has few transportation risks and is a real environment-friendly and efficient printing and dyeing auxiliary.
Owner:DUPLUS CHEM OF ZHANGJIAGANG CITY
Bepotastine besilate nasal spray and preparation method thereof
ActiveCN103816121BOrganic active ingredientsAerosol deliveryMucous membrane inflammationPolyethylene glycol
The invention discloses a bepotastine besilate nasal spray and a preparation method thereof. For the nasal spray, 1-10 g of bepotastine besilate and 3-15 g of solubilizing composition are added in 100 mL of water; the solubilizing composition comprises components in percentage by weight as follows: 80%-95% of propylene glycol, polyethylene glycol 300 or polyethylene glycol 400 and the balance of caprylocaproyl macrogolglycerides; and pH of a nasal spray solution ranges from 6 to 8. In the composition, bepotastine besilate does not exist in a solid particle mode and cannot be crystallized during a long-term storage process, so that not only is rapid medicine absorption facilitated, but also a spray pump port is not blocked easily, and anaphylactic rhinitis and mucous membrane inflammation related to rhinitis can be cured effectively.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
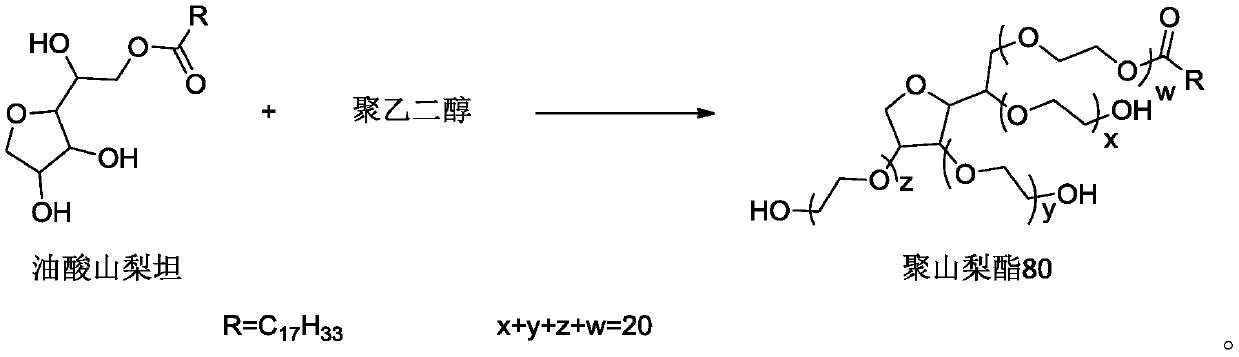
Polysorbate 80 for injection, and preparation method thereof
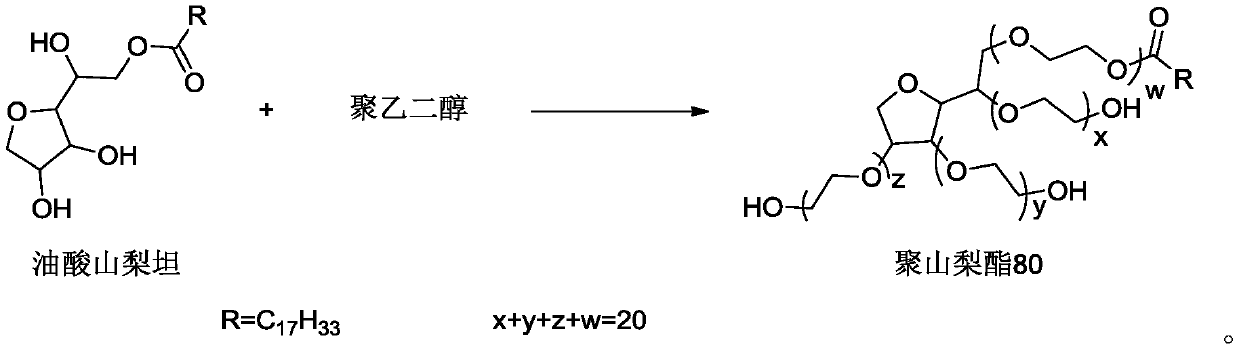
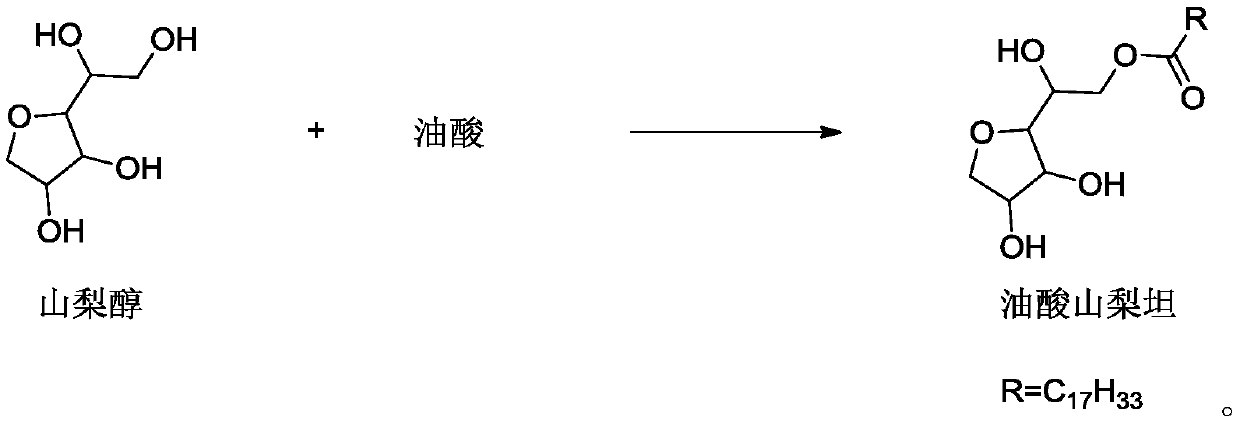
InactiveCN110591074AReduce allergiesReduce riskPharmaceutical delivery mechanismPharmaceutical non-active ingredientsPEG 400Polymer science
The invention discloses polysorbate 80 for injection, and a preparation method thereof. The preparation method comprises: in the presence of a catalyst, carrying out an etherification reaction on sorbitan oleate and polyethylene glycol to obtain polysorbate 80, wherein the polyethylene glycol is one or a plurality of materials selected from polyethylene glycol 300, polyethylene glycol 400 and polyethylene glycol 600, a mass ratio of the polyethylene glycol 300 to the sorbitan oleate is a, a mass ratio of the polyethylene glycol 400 to the sorbitan oleate is b, a mass ratio of the polyethyleneglycol 600 to the sorbitan oleate is c, a is more than or equal to 0 and less than or equal to 2.93, b is more than or equal to 0 and less than or equal to 2.20, c is more than or equal to 0 and lessthan or equal to 1.47, and a, b and c are not 0 at the same time. According to the present invention, the product has advantages of low allergy and hemolysis risks and high safety.
Owner:HUBEI GEDIAN HUMANWELL PHARMA EXCIPENTS
Stable human growth hormone liquid preparation
PendingCN105412910APeptide/protein ingredientsPharmaceutical delivery mechanismArginineHuman growth hormone
The invention discloses a stable liquid preparation, which consists of human growth hormone, and L-lysine, L-arginine or polyethylene glycol 300, as well as a poly (ethylene oxide)-poly (propylene oxide) copolymer, polyethylene glycol-15 poly-hydroxy stearate or polyethylene glycol-35 castor oil.
Owner:DAEWOONG CO LTD
Stable human growth hormone liquid preparation
PendingCN105363022APeptide/protein ingredientsPharmaceutical delivery mechanismArginineHuman growth hormone
A stable liquid preparation is disclosed, and comprises the following parts: human growth hormone; L-lysine, L-arginine or polyethylene glycol 300; and poly (ethylene oxide) poly (propylene oxide) copolymer, polyethylene glycol-15 poly hydroxy stearate or polyethylene glycol-35 castor oil.
Owner:DAEWOONG CO LTD
Long-acting kanamycin sulfate injection for veterinary use and preparation method of injection
InactiveCN107412154AProlong the action timeIncrease viscosityAntibacterial agentsOrganic active ingredientsRetention timePolyethylene glycol
The invention discloses a long-acting kanamycin sulfate injection for veterinary use and a preparation method of the injection, and belongs to the technical field of preparations for veterinary use. Each 1000L of the injection consists of 50-250kg of kanamycin sulfate, 40-60g of edetate disodium, 1-5kg of sodium hydrogen sulfite, 50-150kg of polyvinylpyrrolidone, 50-150kg of polyethylene glycol 300 and the balance of injection water. The long-acting kanamycin sulfate injection for veterinary use prepared by the invention, in comparison a national-standard kanamycin sulfate injection, can double an effective drug peak concentration retention time, so that efficacy of antipyretics is prolonged and clinical operations are reduced; and the injection is more conducive to rapid recovery of diseased pigs.
Owner:浙江大飞龙动物保健品股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com