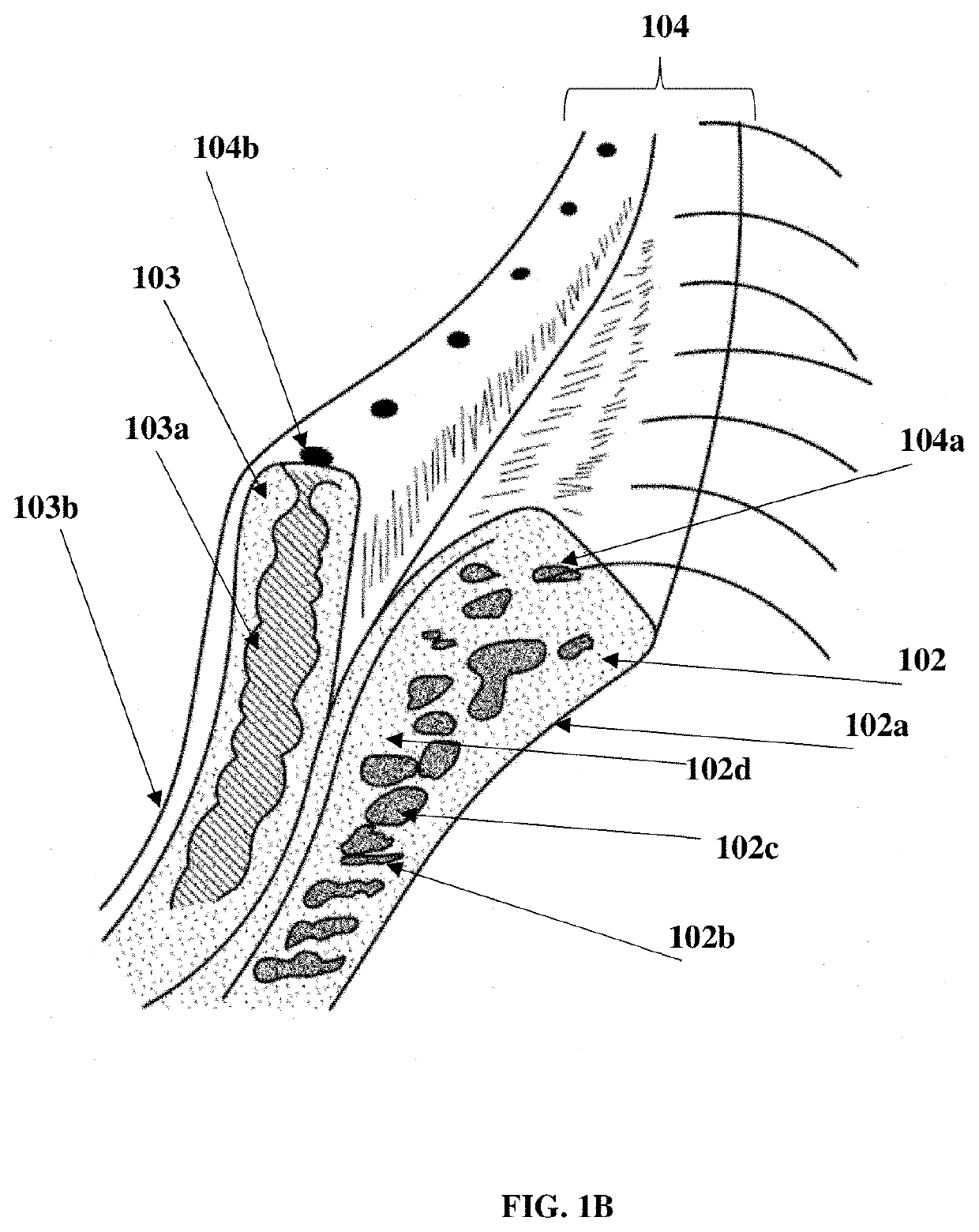
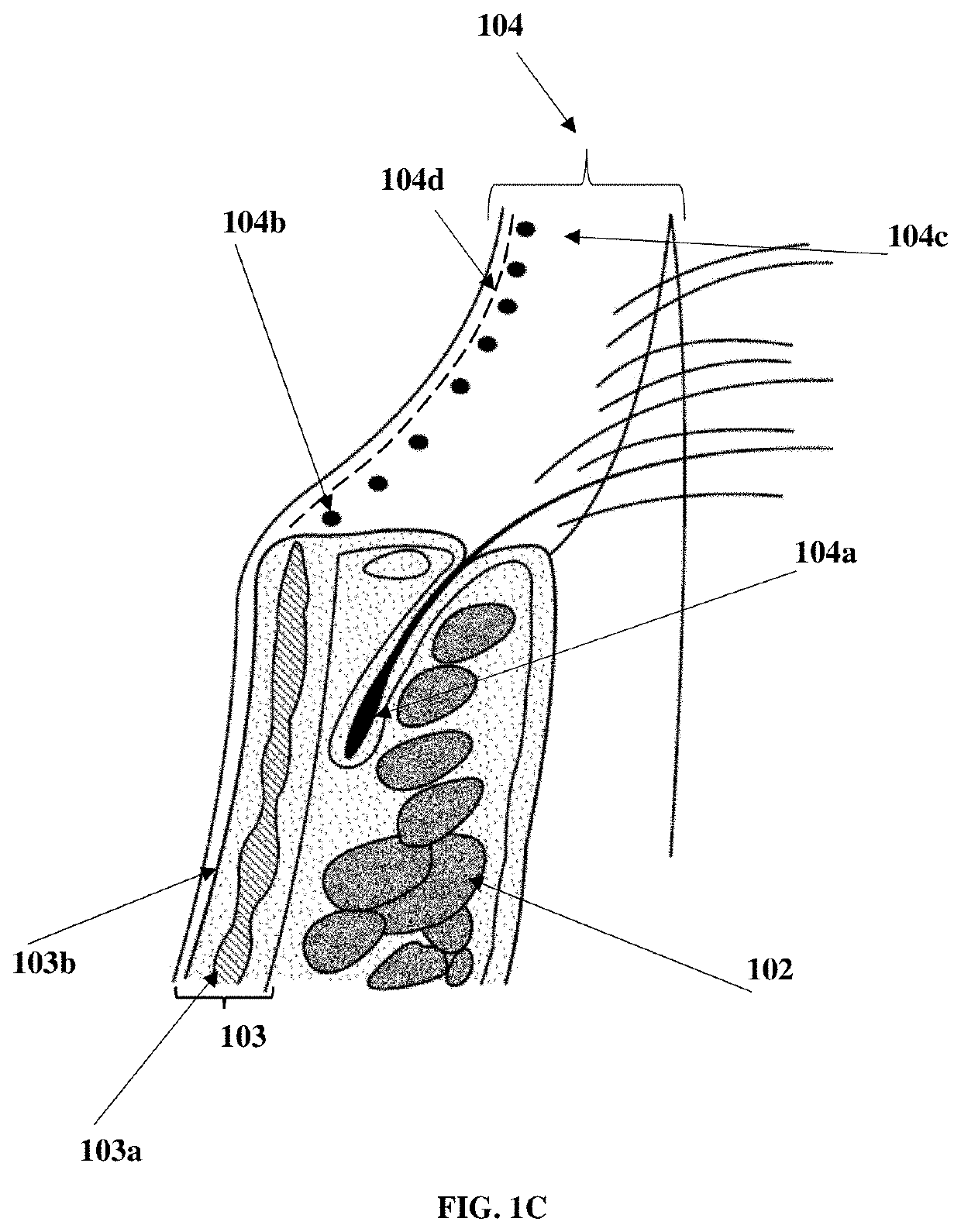
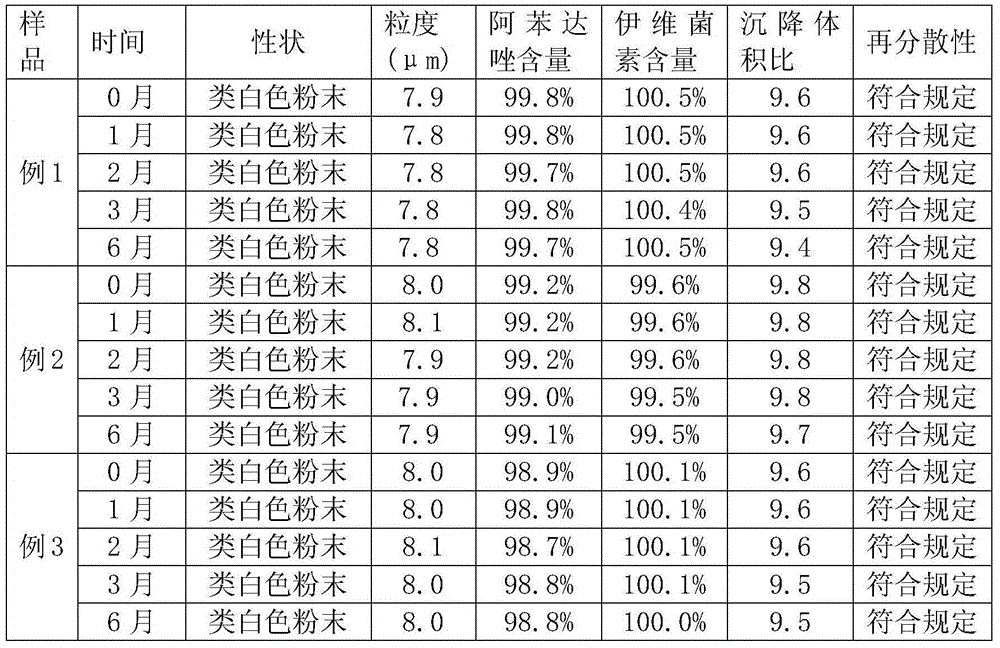
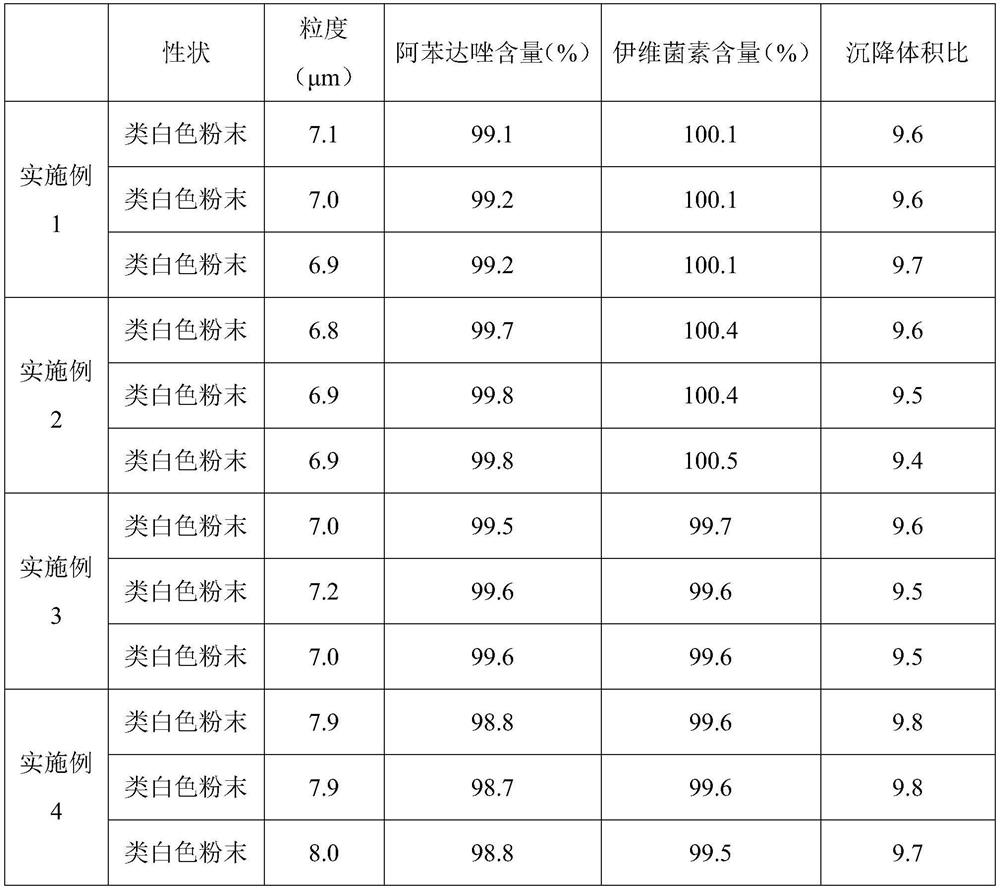
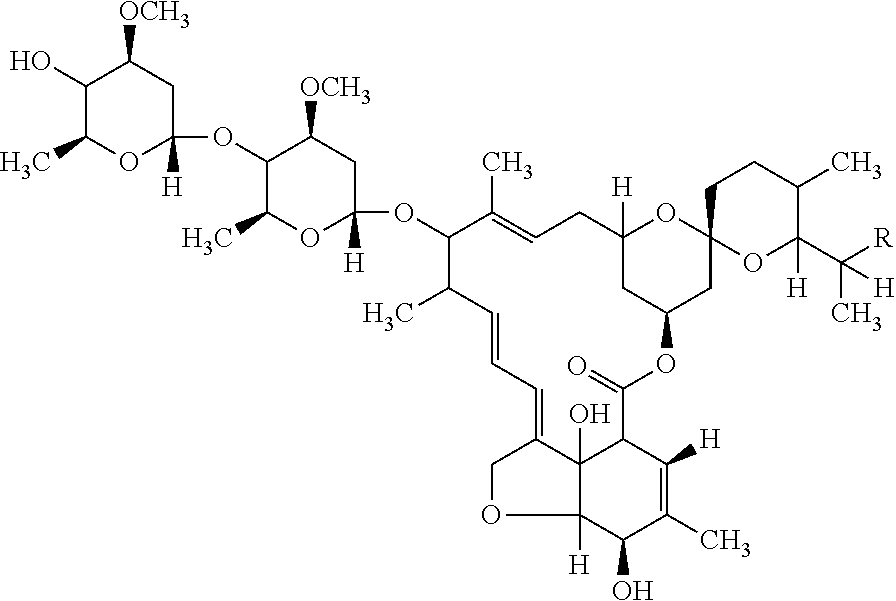
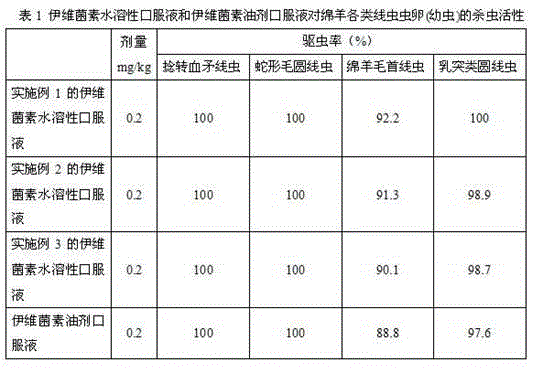
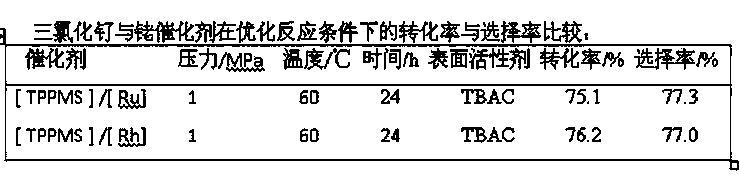
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
68 results about "Ivermectinum" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
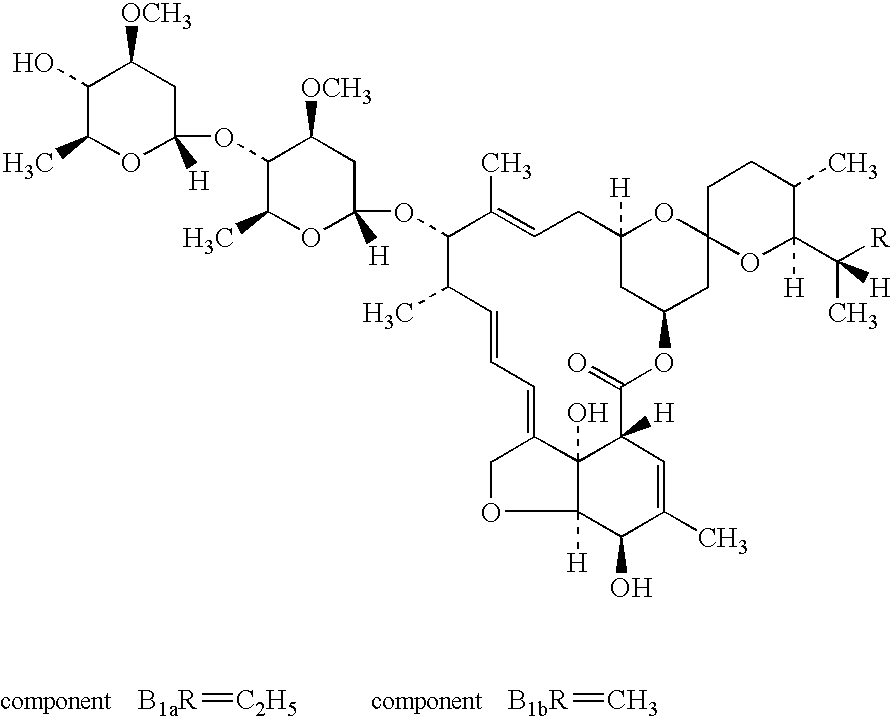
Topical ivermectin composition
Owner:MERCK SHARP & DOHME CORP +1
Treatment of inflammatory lesions of rosacea with ivermectin
ActiveUS20150011489A1Quick reliefLong maintenance periodBiocideInorganic non-active ingredientsTopical treatmentPharmaceutical medicine
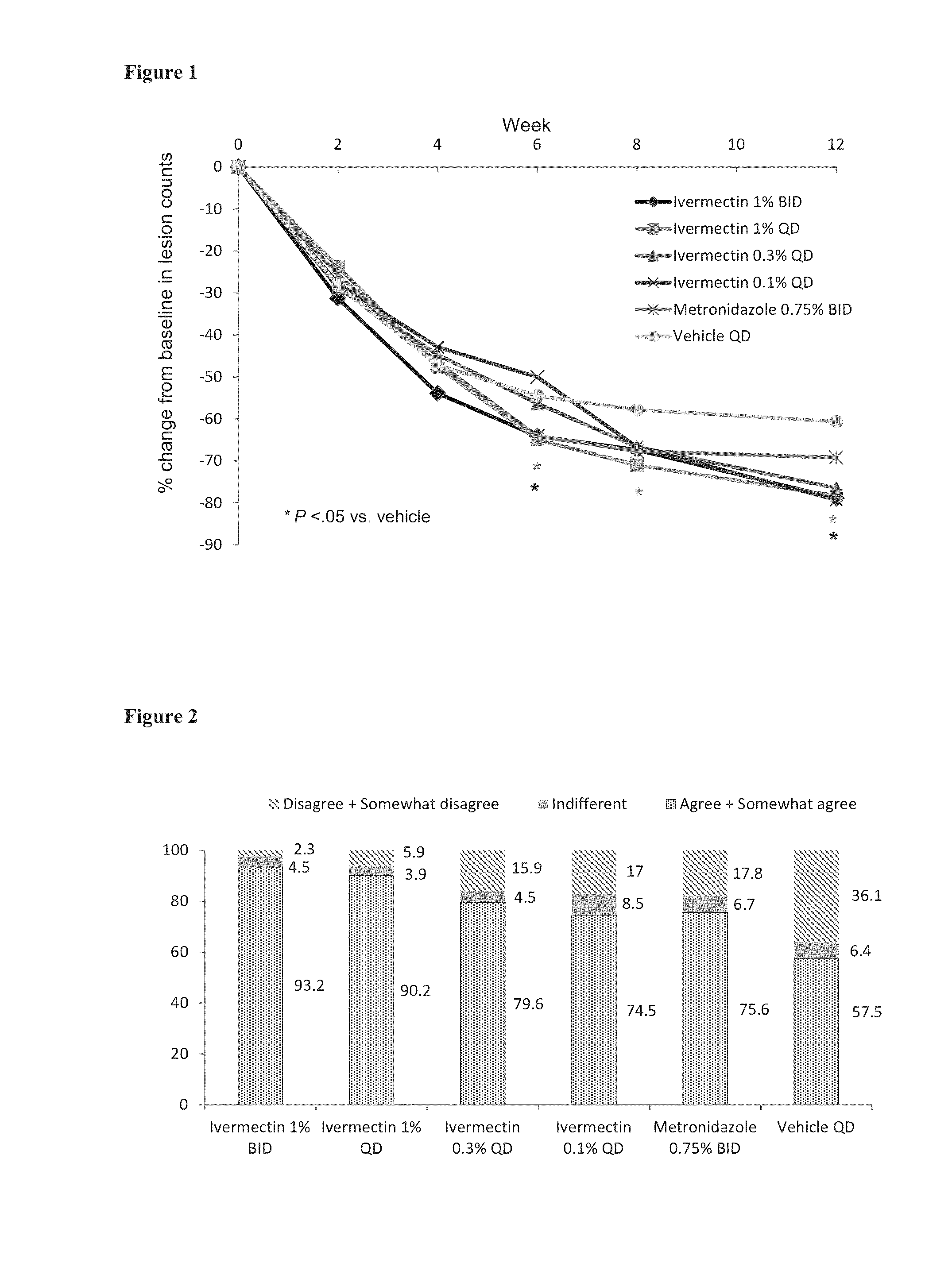
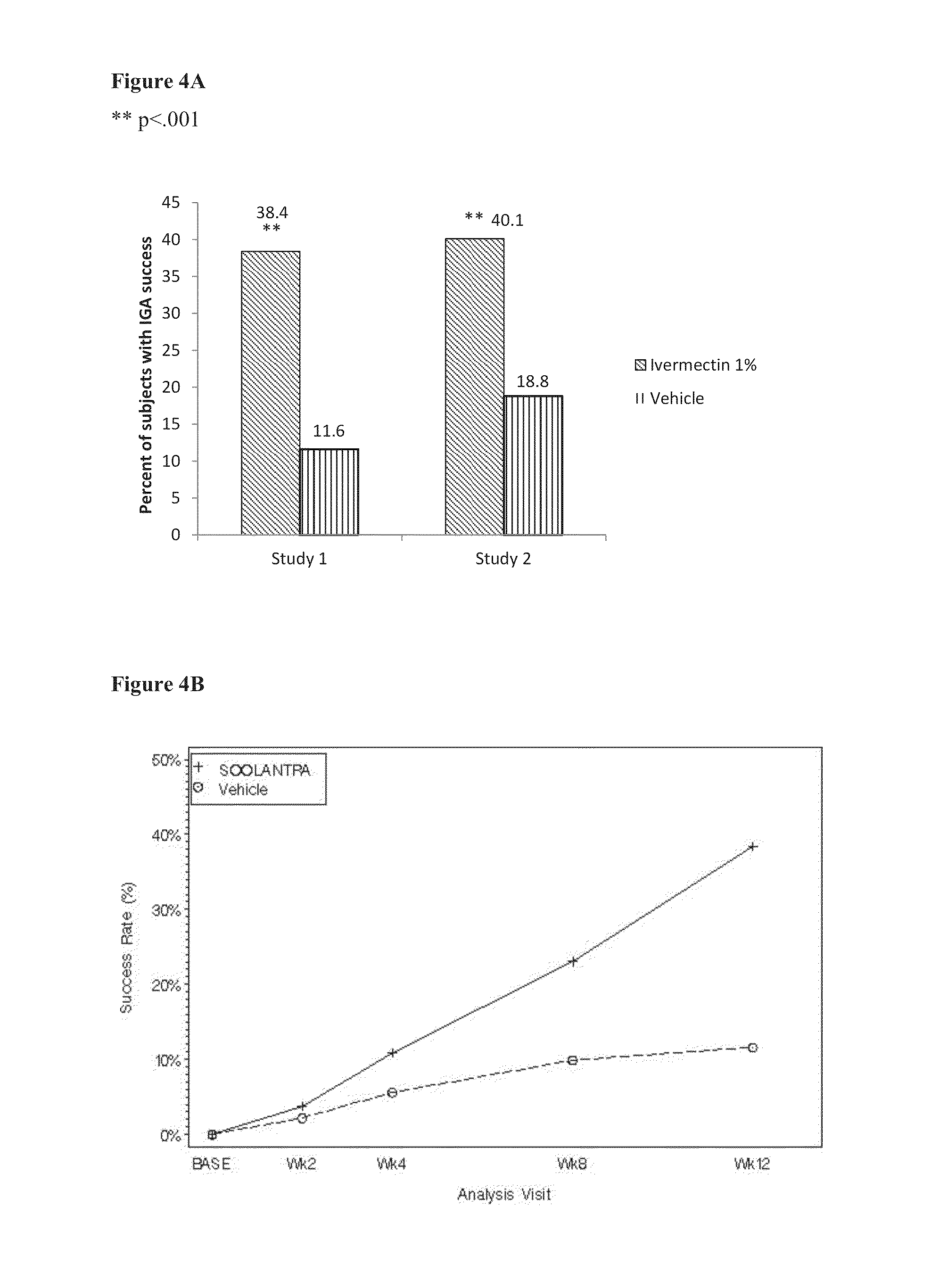
Methods for safe and effective treatment of inflammatory lesions of rosacea in a subject are described. The methods involve once daily topically applying to an affected skin area a topical composition containing ivermectin and a pharmaceutically acceptable carrier. It has been demonstrated that once daily topical treatment with ivermectin is significantly superior than twice-daily topical treatment with metronidazole in reducing inflammatory lesion counts.
Owner:GALDERMA HLDG SA
Anti-parasitic ivermectin transdermal solution used for livestock and preparation method thereof
InactiveCN101579309AFully reflect the advantages of preparationsPromote percutaneous absorptionOrganic active ingredientsPharmaceutical delivery mechanismSide effectThird generation
The invention relates to an anti-parasitic ivermectin transdermal solution used for livestock and a preparation method thereof. The anti-parasitic ivermectin transdermal solution comprises the following components respectively according to parts by volume: 5 to 15 parts of ethyl acetate, 20 to 30 parts of dimethyl sulfoxide, 2 to 6 parts of azone, 1 to 3 parts of isopropanol and 50 to 70 parts of propanediol; and the content of ivermectin is 0.3g to 1g in a solution of 100ml, and the finished product is prepared by the processes of dissolving, mixing, filtering and filling. The azone related in the prescription of the invention is a novel skin penetration enhancer, can increase the transdermal absorption of the skin to various medicines of different types, effectively avoids the pass effect of hepar, avoids peak and valley phenomena in the absorption process of the medicine, reduces the toxic or side effect caused by the correlative dosage in the medicine, prolongs the effective effect time, changes the administration area, effectively regulates the administration dosage and reduces differences among individuals. The invention has the characteristic of simple, convenient and fast use, and is more suitable for the anti-parasitic prevention and treatment of a pasturing area compared with other preparation forms.
Owner:TIANJIN BIJIA PHARMA CO LTD
Compositions comprising C-13 alkoxyether macrolide compounds and phenylpyrazole compounds
ActiveUS20070293446A1High instantaneous effectivenessLong effectivenessBiocideSugar derivativesMedicinal chemistryParasite infestation
Provided is a novel ivermectin derivative and compositions for the treatment or prophylaxis of parasite infestations in mammals or birds which comprise: (A) a pharmaceutically effective amount of a 1-N-phenylpyrazole compound; (B) a pharmaceutically effective amount of an ivermectin derivative of formula (II) wherein: R14 represents —(CH2)sO—Z wherein, s is 1 or 2; Y represents —CH(OR15)—, —C(═O)— or —C(═NOR15); R15 represents hydrogen, alkyl or phenyl; and R16 represents —CH3 or —CH2CH3; Z is alkyl, alkenyl, alkynyl, acyl, alkylalkoxy, aryl, alkanoyloxy, alkoxycarbonyl, alkenoyl, alkynoyl, or aroyl.
Owner:MERIAL INC
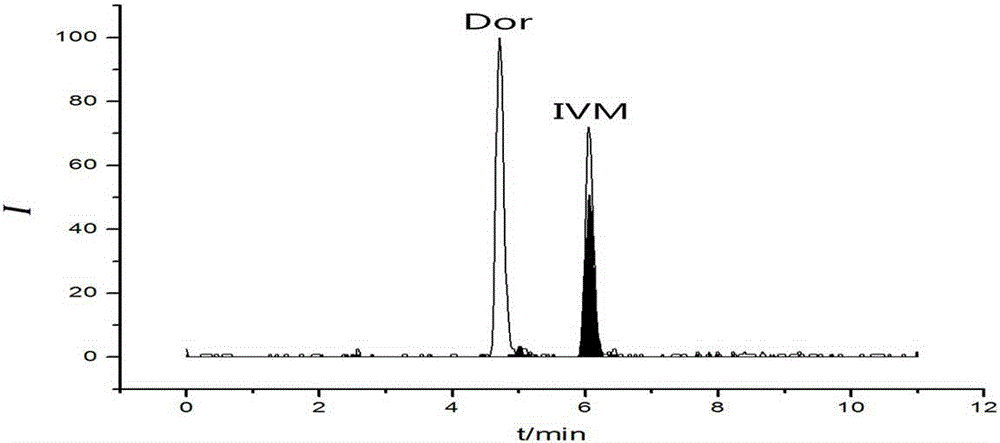
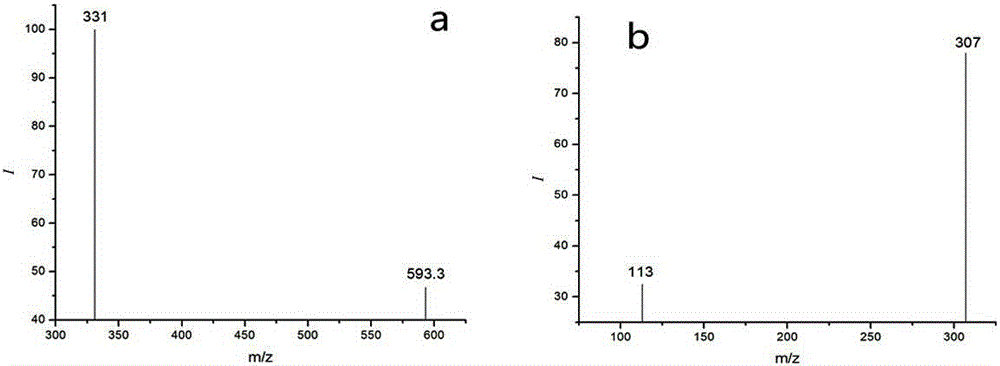
Method for detecting residual quantity of ivermectin in sheep muscle tissues by using liquid chromatograph/mass spectrometer with doramectin as internal standard substance
InactiveCN105181839AEliminate distractionsHigh enrichment efficiencyComponent separationMuscle tissueInternal standard
The invention discloses a method for detecting the residual quantity of ivermectin in sheep muscle tissues by using a liquid chromatograph / mass spectrometer with doramectin as an internal standard substance. The method comprises the following steps: extracting ivermectin residual in the sheep muscle tissues with acetonitrile as an extraction solvent, adopting an impurity adsorption and solid phase extraction mode, adopting an alkaline alumina column as a solid phase extraction column, introducing acetonitrile, eluting, and collecting all obtained eluate. The method solves the problems of complex extraction and purification processes and high detection limit of traditional detection methods, realizes enrichment and purification of ivermectin residual in the sheep muscle tissues, improves the detection sensitivity, repeatability and accuracy, and is suitable for batch sample detection.
Owner:LANZHOU INST OF ANIMAL SCI & VETERINARY PHARMA OF CAAS
Compound ivermectin albendazole transdermal agent for livestock and application method thereof
ActiveCN103494832AEasy to prepareSimple MedicationOrganic active ingredientsSolution deliveryGastric emptyingTherapeutic effect
The invention belongs to the technical field of preparation of medicament for livestock, and particularly relates to a compound ivermectin albendazole transdermal agent for livestock and an application method thereof. The transdermal agent is a mixture of two or three components below at any proportion: ivermectin, albendazole, solubilizer tween-80, a laurocapram transdermal accelerant, a 95% ethanol diluent, an N-methyl-pyrrolidone and propylene glycol. When the transdermal agent is used for treating parasitic diseases of animals such as pigs and sheep, the transdermal agent is flow-coated from the head part to the tail root part along a dorsal line after hairs at the back part are uniformly pushed aside towards two sides, and good treatment effect can be obtained after the transdermal agent is used continuously for three days. The preparation method of the compound ivermectin albendazole transdermal agent for livestock is simple; in comparison with other dosage form in the prior art, the medicine use way is simpler and more convenient, and more dosage is saved; as no liver first-pass effect exists, influence from gastric emptying rate and the like is avoided, the irritability reaction degree of the animals is low, the treatment effect is better, the treatment way is convenient and fast, and the operation is easy.
Owner:河南亚卫动物药业有限公司
Medium for producing ivermectin by fermenting streptomyces avermitilis and fermenting method
ActiveCN103642865AStable productionEfficient productionMicroorganism based processesFermentationBiotechnologyMicrobiology
The invention relates to a medium for producing ivermectin by fermenting streptomyces avermitilis and a fermenting method. By adding substances such as formula molasses, formula earthworm powder and the like into the medium for optimizing the medium formula, and corresponding optimizing the fermenting technology, the medium and the cultivation method for enabling ivermectin fermentation unit to be stable are provided, the average fermentation unit of ivermectin is 6000 mu g / mL or more and is improved by 20% or more compared with domestic conventional fermentation level. The fermentation period of ivermectin is averagely shortened by 10 h or more, so that the problem is effectively solved that conventional fermentation technology is high in cost; and the environmental influence on sources of raw materials and auxiliary materials is furthest reduced, and the raw materials and the auxiliary materials are guaranteed to be sufficiently supplied, so that the stable efficient production of ivermectin is realized.
Owner:宁夏泰瑞制药股份有限公司
Topical Composition of Ivermectin
A topical water-in-oil composition of ivermectin is provided. The topical water-in-oil composition contains an oily phase, an aqueous phase and a surfactant emulsifier. The emulsion is essentially devoid of any glycols.
Owner:OAKDENE HLDG LLC
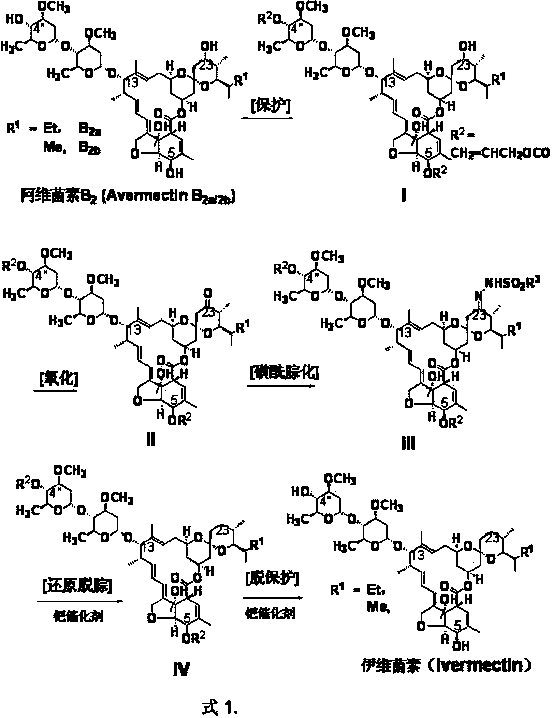
Preparation method of ivermectin
ActiveCN103396464AHigh yieldReduce stepsSugar derivativesSugar derivatives preparationSulfohydrazideHydrazone
The invention relates to a preparation method of ivermectin. The ivermectin is prepared by taking an abamectin B2 component as a raw material and removing 23-position sulfonyl hydrazone of the abamectin B2 through reduction, and the process comprises the following steps: (1) protecting 4''-position hydroxyl and 5-position hydroxyl of the abamectin B2 with allyl carbonate; (2) oxidizing 23-position hydroxyl of the abamectin B2 into keto carbonyl; (3) converting the 23-position keto carbonyl of the abamectin B2 with organic sulfonyl hydrazine into hydrazone, thus generating the 23-position sulfonyl hydrazone of the abamectin B2; and (4) reducing with hydroborate to remove the 23-position sulfonyl hydrazone of the abamectin B2, and simultaneously removing the 4''-position protecting group and the 5-position protecting group to obtain the ivermectin. The total yield of the reaction is up to 67-72%. According to the invention, the ivermectin is prepared by taking the abamectin B2 component as the raw material, the utilization range of the abamectin B2 is widened, the cost of the ivermectin is reduced, the preparation conditions are mild, the yield is high, and the use of virulent tributyltin hydride Bu3SnH is avoided. Thus, the invention is suitable for industrial production.
Owner:河北威远药业有限公司
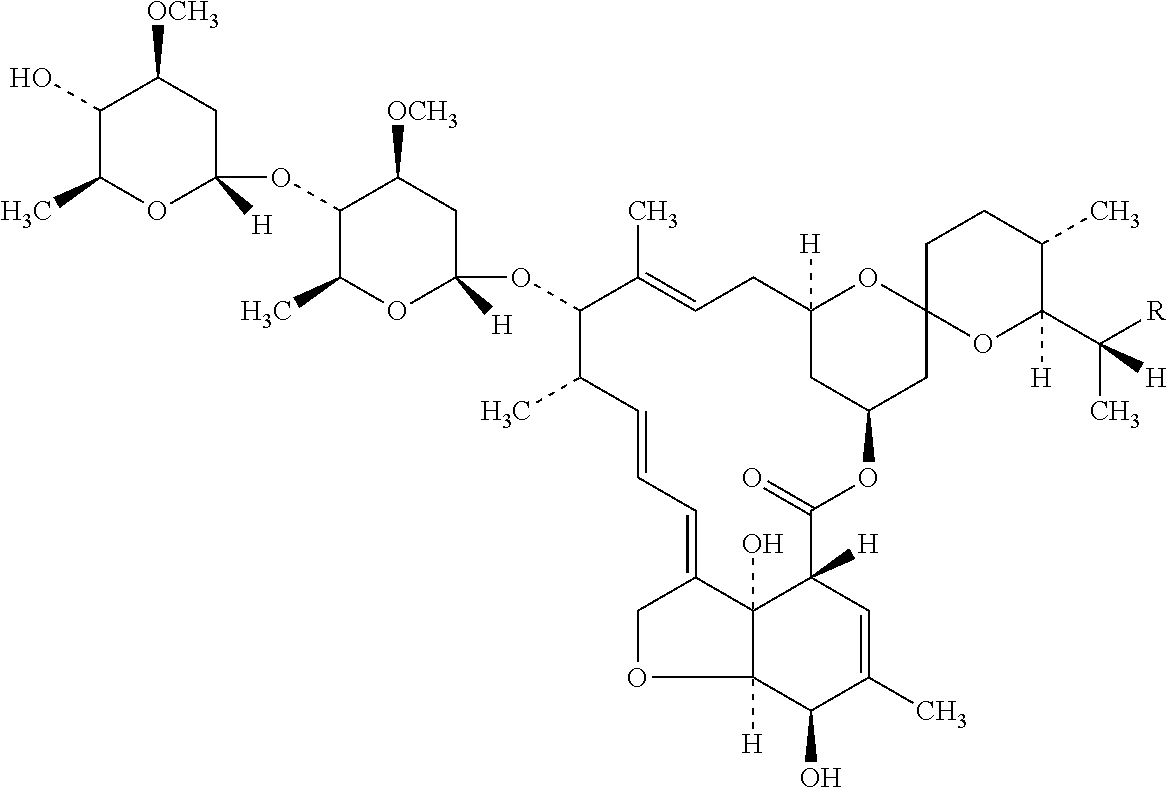
Ivermectin and application of derivative thereof
ActiveCN102872066AOrganic active ingredientsMetabolism disorderAcute hyperglycaemiaInsulin resistance
The invention discloses ivermectin and application of a derivative thereof, and relates to ivermectin. The ivermectin is a derivative of macrolide abamectin generated by streptomyces, and the ivermectin and the derivative thereof are used for preparing medicines for treating metabolism-related diseases such as mammalian hyperglycemia, insulin resistance, hypertriglyceridemia, hypercholesterolemia, diabetes and obesity, and can be applied to medicines for treating diseases such as farnesol receptor-mediated bile obstruction, gallstone, non-alcoholic fatty liver diseases, atherosclerosis, inflammation and cancer.
Owner:丁佳隆
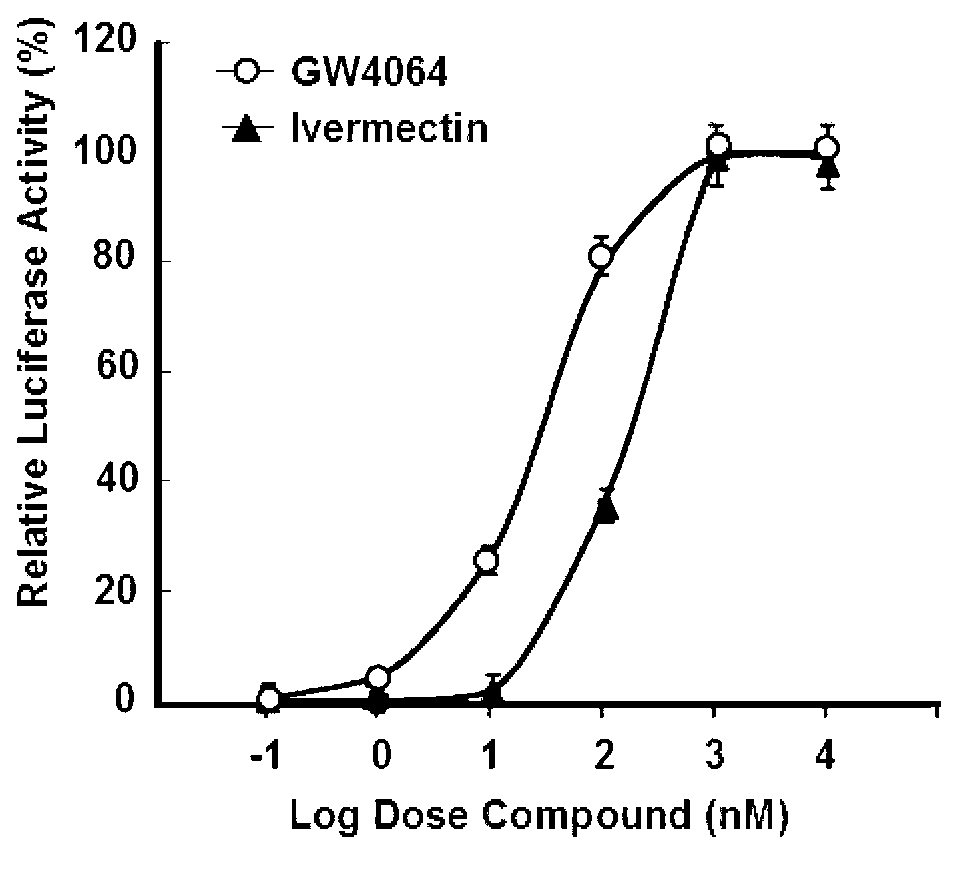
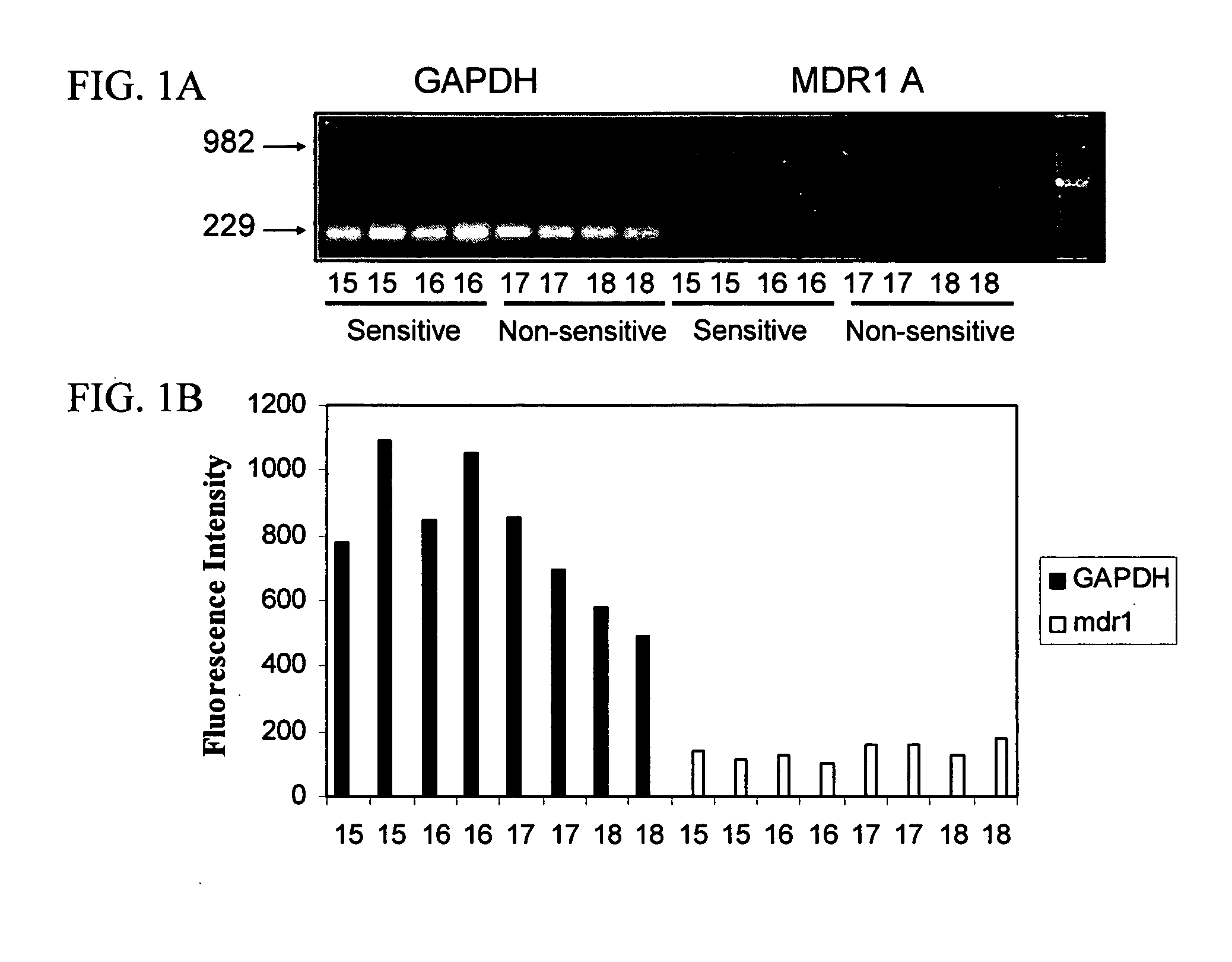
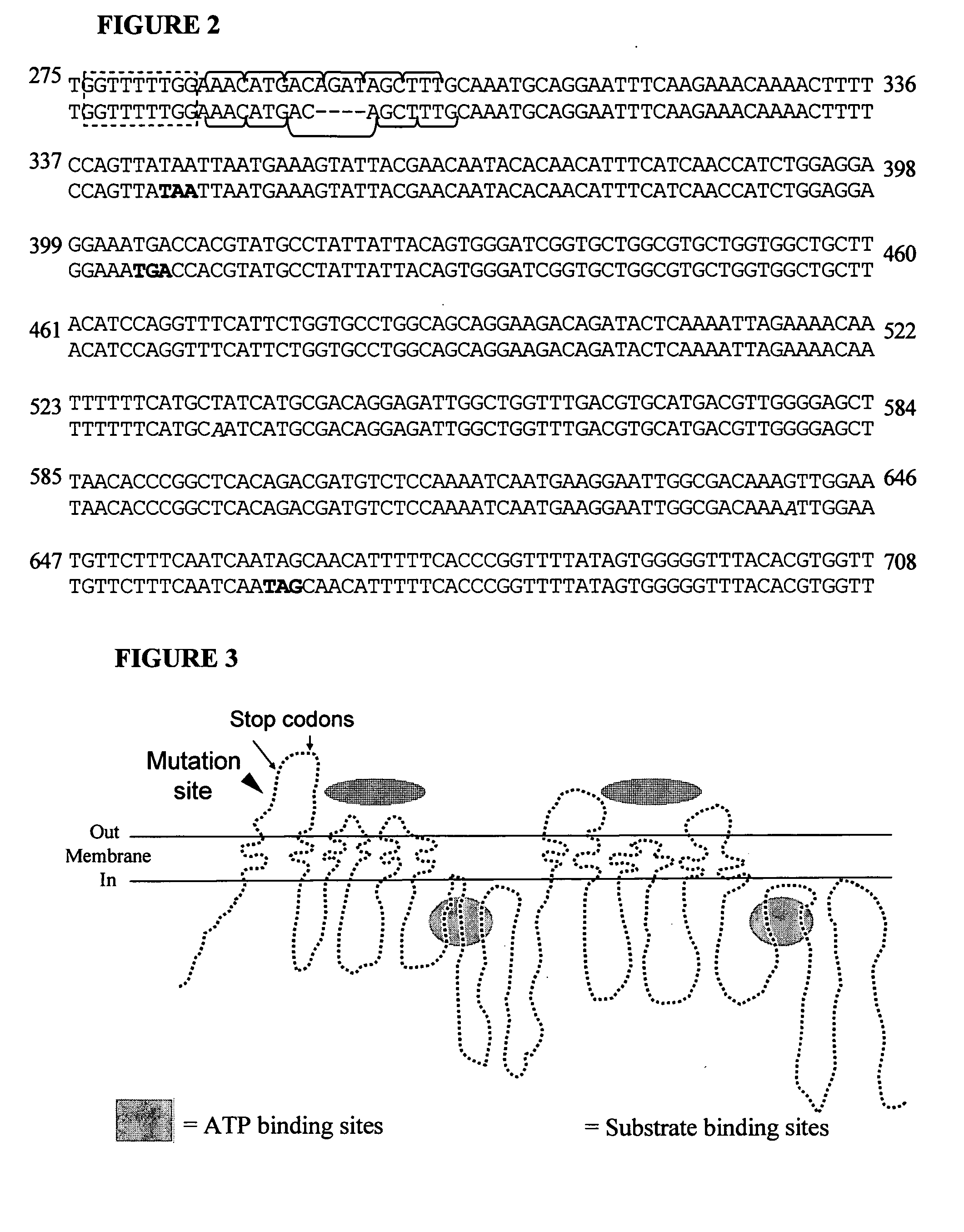
Mdr1 variants and methods for their use
InactiveUS20040265896A1Simple methodCompounds screening/testingMicrobiological testing/measurementMdr1 genePharmaceutical drug
This invention provides the identification of a truncation polymorphism of the mdr1 gene that is linked to ivermectin sensitivity in subjects, such as collies. Also provided are methods for detecting drug transport sensitivity in a subject, and animal models and in vitro cell systems using cells from animals having an mdr1 truncation.
Owner:WASHINGTON STATE UNIVERSITY
Compositions comprising at least one aqueous phase and at least one fatty phase which comprises avermectin compounds
ActiveUS20130108563A1Increase percentageImprove toleranceCosmetic preparationsBiocideRosacea flaccidaPharmaceutical Substances
Pharmaceutical / dermatological emulsions containing at least one avermectin compound, notably ivermectin, include at least one fatty phase and at least one aqueous phase, the at least one avermectin compound being solubilized in the fatty phase, which emulsions are useful for the treatment of a variety of dermatological conditions / afflictions, in particular rosacea.
Owner:GALDERMA HLDG SA
Water based ivermectin O/W injection and preparation method thereof
ActiveCN103417478AHarm reductionSolve pollutionOrganic active ingredientsPharmaceutical non-active ingredientsHydroxystearic AcidPolyethylene glycol
The invention discloses a water based ivermectin O / W (oil-in-water) injection and a preparation method thereof. The O / W injection consists of medium-chain triglycerides, soybean lecithin, 1, 2-propylene glycol, polyoxyethylated 12-hydroxystearic acid, ivermectin and injection water. The key of the ivermectin O / W injection comprises the formula compositions of the injection and the content of each component. The injection has water as the matrix and contains a small amount of an organic solvent, so that the influences of the organic solvent on livestock and poultry and environment are reduced, and the injection has little harm to producers and users, and is safer to store and transport; and also the injection has little toxic and side effects on livestock and poultry, and helps to solve the problems of harm to livestock and poultry and pollution on environment caused by conventional dosage forms during production and use.
Owner:LANZHOU INST OF ANIMAL SCI & VETERINARY PHARMA OF CAAS
Preparation and application of macrolide compound
ActiveCN104231022AExcellent agricultural insecticidal and acaricidal activityOrganic active ingredientsBiocideMacrolide resistanceDomestic animal
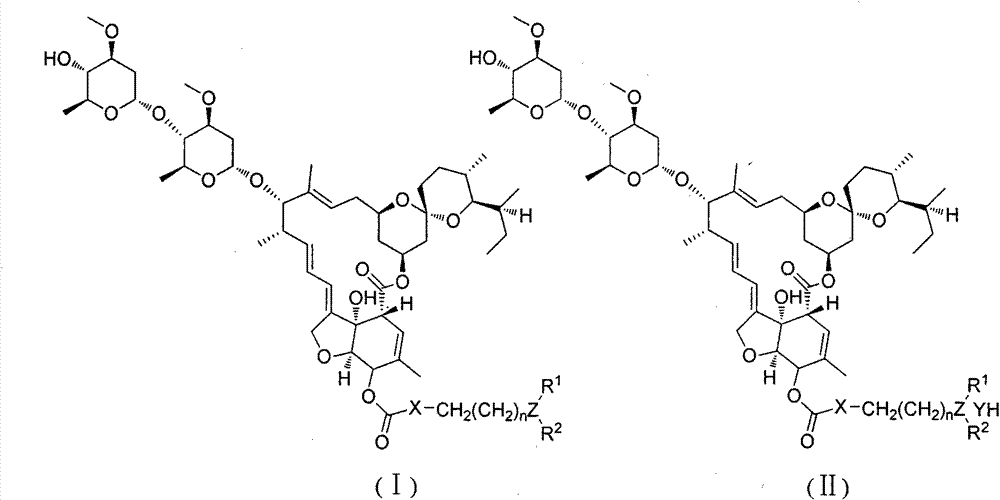
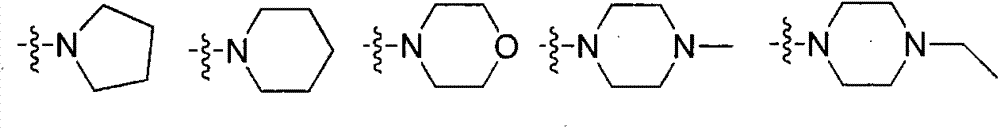
The invention relates to a macrolide compound as well as a preparation method and application thereof, in particular a modified ivermectin derivative as well as a preparation method and application thereof. General formulas (I) (II) of the compound of the macrolide derivative and salt thereof are as shown in the specification. The compound disclosed by the invention is efficient, safe, stable, wide in spectrum and low in toxicity, and has an excellent agricultural insecticidal and acaricidal activity as well as an activity of killing parasites of domestic animals and medical uronema, wherein n represents 0, 1, 2, 3, 4, 5, 6 or 7; X is either N or O; Y represents Cl<->; Z is either N or O; R1 and R2 are selected from H and C1-C4 alkyl; or ZR1R2 is selected from the following structures as shown in the specification.
Owner:NANKAI UNIV +1
Veterinary compound pharmaceutical composition containing albendazole and ivermectin
ActiveCN103356714AReduce the risk of diseaseLittle side effectsOrganic active ingredientsBacteria material medical ingredientsSulfamonomethoxineSulfanilamide
The invention discloses a veterinary compound pharmaceutical composition containing albendazole and ivermectin, and relates to the field of veterinary drug technical preparation. The veterinary compound pharmaceutical composition comprises the following components: albendazole, ivermectin, sulfamonomethoxine, lactobacillus and soluble starch. The veterinary antiparasitic pharmaceutical composition is convenient to use, is mutually synergistic, has broad spectrum of insect resistance and good insect-resistant effect, can increase gastric motility, increases feed intake, and is in favor of the growth of livestock. The pharmaceutical compound of the invention can be a dry suspension, can be directly added in an automatic drinking device in proportion, is convenient to use, and has wide application prospects.
Owner:莒南县华源动物无害化处理有限公司
Pharmaceutical composition for treating chicken coccidiosis and preparation method thereof
InactiveCN105381024AExpand new areas of applicationNot prone to residueOrganic active ingredientsAntiparasitic agentsBiotechnologyFormulary
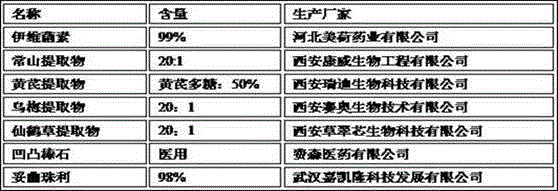
The invention discloses a pharmaceutical composition for treating chicken coccidiosis. The pharmaceutical composition is prepared from the following components by weight: 0.25 to 2.5 parts of ivermectin, 2.5 to 25 parts of antifeverile dichroa root extract, 15 to 25 parts of astragalus extract, 15 to 25 parts of dark plum extract, 10 to 15 parts of hairyvein agrimony extract and 500 parts of purified attapulgite. The invention also provides a preparation method for the pharmaceutical composition. The invention has the following advantages: the pharmaceutical composition provided by the invention has an anticoccidial index of more than 195 and is composed of both traditional Chinese medicines and western medicines; and an adjuvant material in an oral preparation is attapulgite, so slow release effect is exerted, residues are hardly left, so the pharmaceutical composition has obvious advantages. According to results of in-vitro anticoccidial sporlating test and artificial treatment effect test of chicks infected with coccidia, the traditional Chinese medicine composition provided by the invention has anticoccidial effect; so the composition facilitates further confirmation of the application scope of a compound medicine, provides related enterprises with a novel approach to develop high-quality products, broadens novel application fields of medicine resources, lays a good foundation for research and development of a novel anticoccidial medicine and is of obvious significance.
Owner:河北美荷药业有限公司
Ivermectin controlled-release capsule and preparation method and application thereof
InactiveCN107773554ARelease fullyHigh protection rateOrganic active ingredientsAntiparasitic agentsTherapeutic effectPhospholipid
The invention discloses an ivermectin controlled-release capsule and a preparation method and application thereof. The ivermectin controlled-release capsule comprises, by mass, 0.1-1% of ivermectin raw material, 20-60% of water-soluble carrier and 30-70% of enteric-soluble wrapping material, the water-soluble carrier is one or multiple of hydroxypropyl-beta-cyclodextrin, methyl-beta-cyclodextrin,HPMC, PVP, PEG, poloxamer 188, mannitol, D-alpha-tocopherol PEG 1000 succinate, cholate / phospholipid mixed micelle, polyethylenediamine dendritic polymer, phospholipid or cholesterol, and the enteric-soluble wrapping material is one or multiple of hydroxypropyl methyl cellulose phthalic acid, acrylic resin II, acrylic III, cellulose acetate phthalate, polyethylene diacetate phthalate or hydroxypropyl methyl cellulose acetate succinate. The ivermectin controlled-release capsule has the advantage of outstanding acid resistance, enables drug to reach intestinal tracts to be disintegrated and released, remarkably improves bioavailability and has remarkable treatment effect.
Owner:SOUTH CHINA AGRI UNIV
Albendazole and ivermectin premix as well as preparation method and application thereof
PendingCN114099529AWell mixedGood dispersionOrganic active ingredientsPharmaceutical non-active ingredientsBiotechnologyAnimal science
The invention discloses an albendazole and ivermectin premix as well as a preparation method and application thereof, and relates to the technical field of veterinary drugs. The albendazole and ivermectin premixing agent is prepared from the following components in percentage by mass: 5.5 to 6.5 percent of albendazole, 0.2 to 0.3 percent of ivermectin, 4 to 6 percent of activated carbon, 55 to 65 percent of polyethylene glycol and 25 to 35 percent of glyceryl monostearate. The preparation method comprises the following steps: heating, melting, emulsifying and stirring the components to form a medicine suspension, and carrying out spray drying and condensation on the medicine suspension to form spherical particles. According to the albendazole and ivermectin premixing agent provided by the invention, the dosage of albendazole and ivermectin is controlled, specific auxiliary materials are selected, albendazole and ivermectin are dispersed in molten liquid of the auxiliary materials, and spherical fine particles are formed through spray drying and condensation, so that the albendazole and ivermectin premixing agent is prepared. The albendazole and ivermectin premixing agent prepared by the invention can be uniformly mixed and is good in dispersity.
Owner:江西博莱兽药科技协同创新有限公司
Ivermectin derivative, monoclonal antibody for resisting abamectin type drug and application thereof
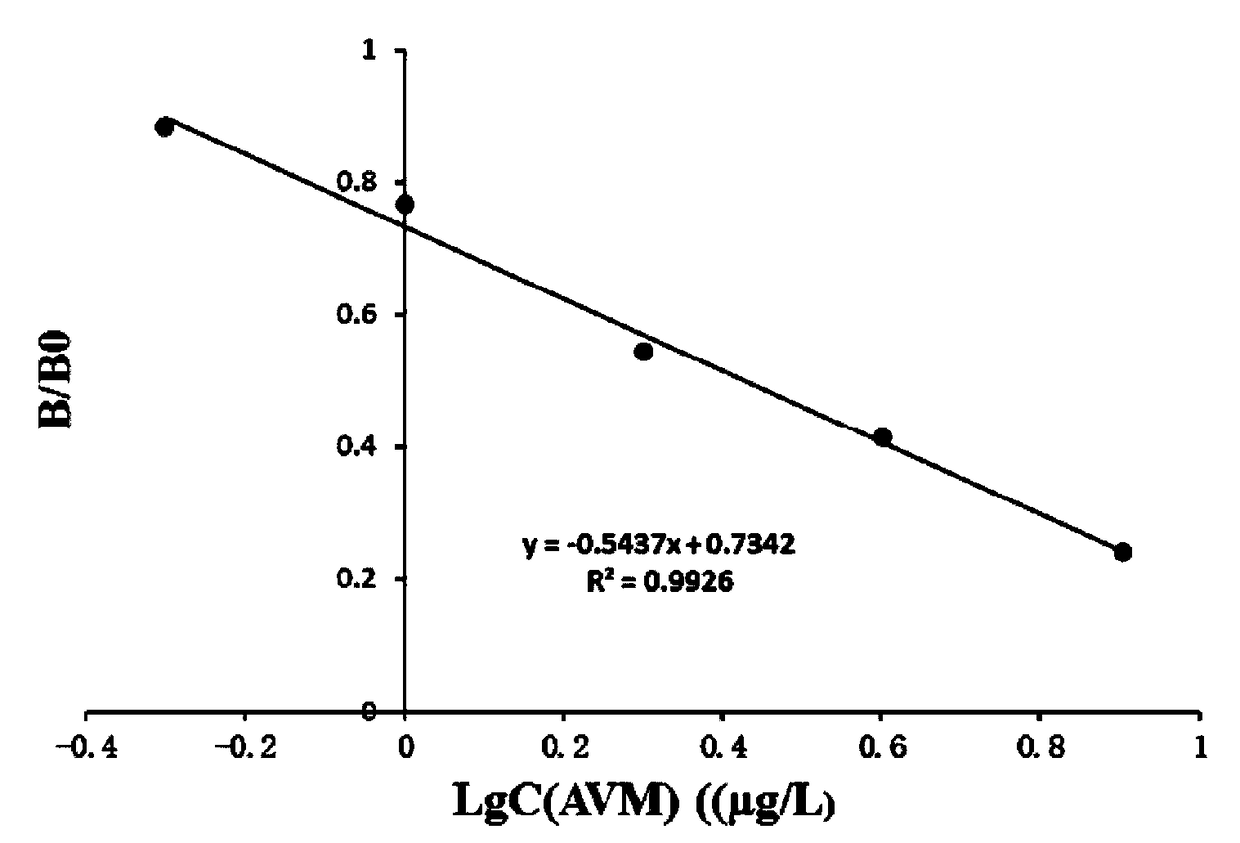
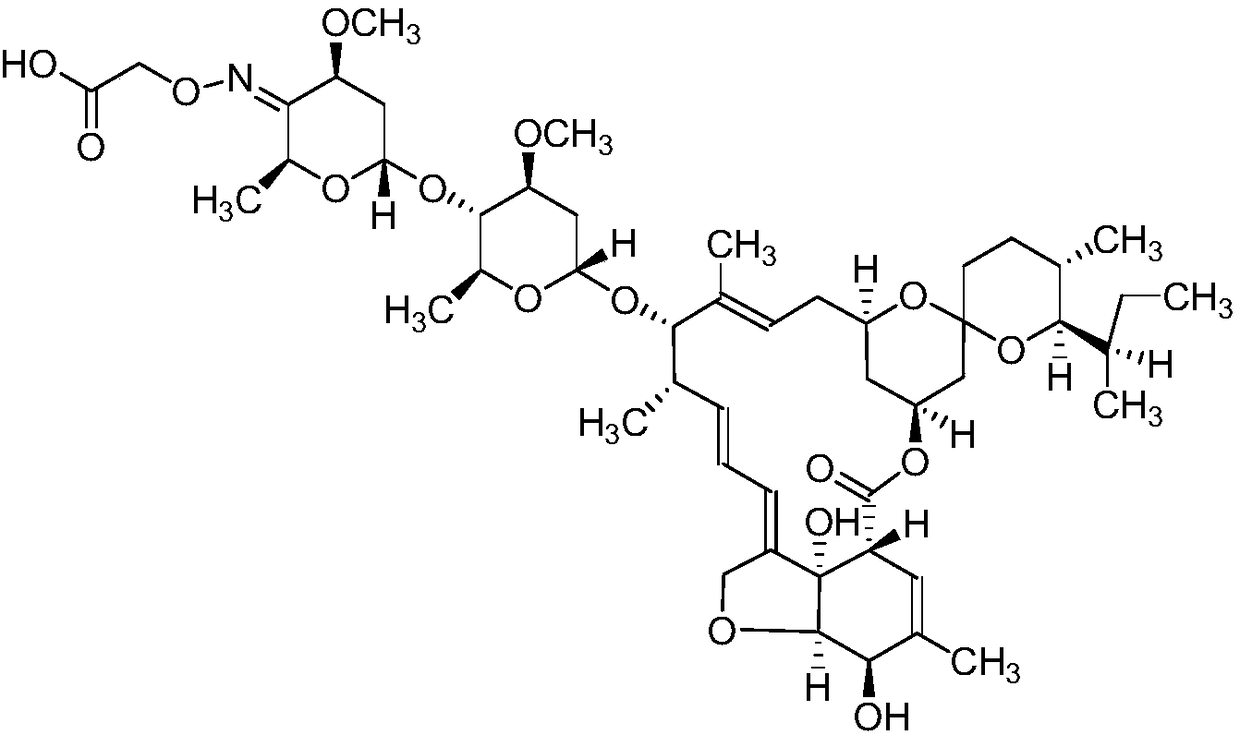
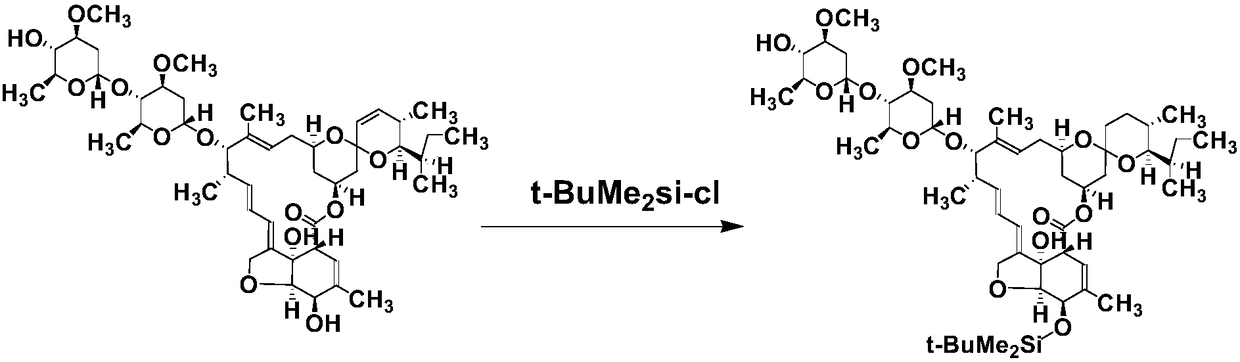
ActiveCN109180760ACross-reactivity rate noSensitive detectionSugar derivativesImmunoglobulinsAvermectinMonoclonal antibody
The invention discloses a novel ivermectin hapten. The novel ivermectin hapten is obtained through reacting with carboxymethoxylamine hemihydrochloride at the C4-OH position of ivermectin and substituting hydroxyl on C4 with carboxymethyl hydroxyamino; the derivative is coupled with carrier protein to be used as an immunogen, and can be used for preparing a monoclonal antibody for resisting an abamectin type drug. The invention further discloses one broad-spectrum monoclonal antibody for resisting the abamectin type drug; the monoclonal antibody can be used for simultaneously and specificallyidentifying abamectin, ivermectin, eprinomectin and emamectin; and an enzyme immunoassay method and a kit, which are established by utilizing the monoclonal antibody are suitable for detecting residues of the abamectin type drug in animal tissues and have the advantages of sensitivity in detection, high accuracy, good precision and the like.
Owner:HUAZHONG AGRI UNIV
Compound ivermectin injection and preparation method thereof
InactiveCN104306388AGood insect resistanceBroad spectrum insect resistanceOrganic active ingredientsPharmaceutical delivery mechanismSulfite saltPyrrolidinones
The present invention discloses a composing prescription of a compound ivermectin injection and a preparation method of the compound ivermectin injection, and belongs to the veterinary medicine technology. The compound ivermectin injection comprises main drugs, a solvent and an antioxidant so as to prepare the stable high-concentration compound ivermectin injection. The injection has characteristics of advanced technology, good stability, broad spectrum, high efficiency, low irritation on the injected site, easy use, and clinical efficiency superior to the single-formula injection. According to the present invention, the raw materials comprise 0.5-5.0% of ivermectin, 2-20.0% of mebendazole, 0.5-5.0% of levamisole hydrochloride, 0.1-1% of the antioxidant, and the balance of the solvent, wherein the antioxidant is one or a mixture selected from thiourea, L-cysteamine hydrochloride, vitamin C, ethylenediamine, sodium hydrogen sulfite, anhydrous sodium sulfite, sodium formaldehydesulfoxylate dihydrate, sodium metabisulfite and the like, and the solvent is one or a mixture selected from propylene glycol, ethanol, glycerol, glycerol formal, dimethylformamide, dimethylacetamide, 2-pyrrolidone and the like.
Owner:JIANGXI NUCLEAR IND TIANDIHE PHARMA
Application of composition to preparation of veterinary anti-parasitic drug, transdermal solution of veterinary anti-parasitic drug and preparation method of transdermal solution
InactiveCN111514157ASolve the problem of small range of deworming by single applicationReduce labor costsOrganic active ingredientsPharmaceutical delivery mechanismAntiparasiticGlycerol
The invention belongs to the technical field of an anti-parasitic drug, and particularly relates to an application of a composition containing ivermectin and levamisole hydrochloride to preparation ofa veterinary anti-parasitic drug, a transdermal solution of the veterinary anti-parasitic drug and a preparation method of the transdermal solution. The transdermal solution comprises components in parts by volume as follows: 20-50 parts of isopropanol, 10-40 parts of glycerol, 10-30 parts of absolute ethyl alcohol, 5-20 parts of dimethyl sulfoxide and 2-6 parts of azone; the content of ivermectin is 0.3-1 g per 100 mL of solution, and the content of levamisole hydrochloride is 5-15 g per 100 mL of solution. According to the invention, ivermectin and levamisole hydrochloride are combined, a synergistic effect is realized by simultaneous use of ivermectin and levamisole hydrochloride, and an anti-parasitic spectrum is improved; and meanwhile, with adoption of a transdermal absorption dosage form, toxic and side effects caused by correlation of the drug and the dosage are reduced, and a simultaneous internal and external anti-parasitic effect of the preparation is realized.
Owner:吉林吉力生物技术研究有限公司 +2
Inclusion complex formed by ivermectin and cyclodextrin and preparation method of inclusion complex
InactiveCN105288642APromote absorptionSmall toxicityOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityOrganic solvent
The invention discloses an inclusion complex formed by ivermectin and cyclodextrin and a preparation method of the inclusion complex. The inclusion complex is prepared by raw ivermectin and beta-cyclodextrin according to the mass ratio of 1:10-1:25. The preparation method includes: dissolving the raw ivermectin into an organic solvent, evenly spraying onto the pasty cyclodextrin, well mixing through colloid grinding, and feeding nitrogen and drying to obtain the inclusion complex. The inclusion complex and the preparation method of the inclusion complex have the advantages that the cavity structure of the cyclodextrin is used to wrap the ivermectin so as to increase the stability and water solubility of the ivermectin, the ivermectin can be prepared into a water solution, and consumption of the organic solvent is reduced.
Owner:镇江泰飞尔医药有限公司
Compositions and methods for the treatment of anterior blepharitis and posterior blepharitis
PendingUS20210052490A1Reduced bioavailabilityDilution effectOrganic active ingredientsSenses disorderPosterior blepharitisPharmaceutical drug
Disclosed herein are pharmaceutical compositions and methods for the treatment of anterior blepharitis and posterior blepharitis which may be of primary origin and not secondary to other factors such as infections, infestations or rosacea. The composition comprises Ivermectin in the range of about 0.001% to 20% by weight of the total composition. Topical administration of said compositions precisely to the eyelid margin provides therapeutic benefit to patients suffering from anterior blepharitis and posterior blepharitis.
Owner:PADMANABHAN VISHWANATH
Veterinary albendazole/ivermectin dry suspension
InactiveCN103599118AConvenience of drug deliveryAvoid side effectsPowder deliveryOrganic active ingredientsBiotechnologyAnimal science
The invention relates to a veterinary anthelmintic, and particularly relates to a veterinary albendazole / ivermectin dry suspension with using convenience and significant drug effects. The dry suspension is prepared by steps of stirring 0.1-0.2 part of ivermectin and 2-4 parts of sodium carboxymethylcellulose and mixing, adding 2-4 parts of a flavoring agent after the powder is mixed uniformly, and mixing uniformly again; stirring 5-15 parts of albendazole and 10-20 parts of sodium chloride and mixing, adding 5-10 parts of sodium citrate after the powder is mixed uniformly, and mixing uniformly again; adding the former into the latter through an equivalent increment method, and stirring uniformly; adding the balance of other auxiliary material, and further uniformly stirring and mixing; and subpackaging to obtain the finished product. The other auxiliary material is soluble starch or microcrystalline cellulose. The dry suspension can be proportionally added in an automatic drinker directly for use, thus facilitating all-round dosing in a large-size livestock farm. The dry suspension avoids the toxic and side effect caused by non-uniform feed mixing, and is more convenient to use. The drug effect is remarkably enhanced, and the cost is obviously lowered in comparison with the prior art. The dry suspension has a wide application prospect.
Owner:SICHUAN DERUNTONG BIOTECH
Albendazole ivermectin powder and preparation method thereof
PendingCN112618492AImprove palatabilityImprove stabilityPowder deliveryOrganic active ingredientsBiotechnologyAlbendazole
The invention discloses albendazole ivermectin powder and a preparation method thereof. The problems that existing albendazole ivermectin is poor in stability and dispersity and poor in palatability and influences the insecticidal effect can be solved. The albendazole ivermectin powder is prepared from the following raw materials of, in parts by weight, 6.0-18.0 parts of albendazole, 0.2-1.3 parts of ivermectin, 25.0-40.0 parts of a buffering agent, 45.0-55.0 parts of starch and 3.5-6.0 parts of a flavoring agent; the flavoring agent is maltol; and the starch is soluble starch. The albendazole ivermectin powder has the advantages of improved stability, improved dispersibility, good palatability and the like.
Owner:四川乾通动物药业有限公司
Pharmaceutical Composition of Ivermectin and Process for Preparation thereof
ActiveUS20190282538A1Good storage stabilityPharmaceutical non-active ingredientsEmulsion deliverySerum rashActive agent
The invention relates to a topical pharmaceutical composition comprising effective amount of ivermectin as an active agent, process of preparation thereof and method of treating dermatological conditions such as inflammatory lesions of rosacea, common acne, seborrheic dermatitis, perioral dermatitis, acne form rashes, and the like.
Owner:AUROBINDO PHARMA LTD
A water-soluble ivermectin oral liquid for veterinary use and a preparing method thereof
InactiveCN105748403AReduce solubilityLong shelf lifeOrganic active ingredientsPharmaceutical delivery mechanismMouth mucosaDisodium phosphate
A water-soluble ivermectin oral liquid for veterinary use and a preparing method thereof are disclosed. Each 1000 mL of the oral liquid comprises 0.1-2 g of ivermectin, 1-20 mL of ethyl acetate, 50-150 mL of Tween-80, 1-10 g of sodium hydrogen phosphate, 1-10 g of sodium dihydrogen phosphate and 0.001-1 g of thiomersal, with the balance being water. The oral liquid adopts the water as a solvent, the ivermectin is low in solubility in water, but a solution can be uniform through adding the water after the ethyl acetate and the tween-80 which are high in dose are utilized to promote solution of the ivermectin, and therefore the ivermectin oral liquid is uniform, stable and long in shelf life, and is nonirritant to mouth mucosa, lower in production cost and good in absorption when being compared to oil oral liquids.
Owner:CHONGQING BULL ANIMAL PHARMA +1
Method for synthesizing ivermectin
ActiveCN103387594BFix expensive bugsPhysical/chemical process catalystsSugar derivativesPtru catalystOrganosolv
The invention belongs to the field of veterinary drug synthesis and discloses a method for synthesizing ivermectin. In the method, under the catalysis of ruthenium trichloride and monosulfonated triphenylphosphine sodium salt, hydrogenation reaction occurs between abamectin and hydrogen to obtain ivermectin. Compared with the rhodium catalyst, the ruthenium trichloride used in the present invention is cheap, and can effectively reduce the production cost of abamectin hydrogenation to prepare ivermectin. The hydrogenation reaction is carried out in a water-organic solvent two-phase system, the separation of the catalyst, the reactant and the product is convenient, the catalyst is easy to recover, and at the same time, the residual amount of heavy metals in the product is reduced, and the cost of product purification is saved. The conversion rate of abamectin hydrogenation to synthesize ivermectin by adopting the method of the invention reaches 75%, and the selectivity reaches 77%, which is suitable for industrial application.
Owner:河北美荷药业有限公司
Anthelmintic composition
InactiveCN101107002AOrganic active ingredientsBacteria material medical ingredientsAnthelmintic drugMacrocyclic lactone
A topical formulation comprising as active ingredients, at least one effective agent derived from Streptomyces avermitilis, i.e. a macrocylic lactone e.g. an avermectin or chemically modified or synthetic derivative thereof, e.g. ivermectin, together with another anthelmintic of the sulphonamide type, e.g. clorsulon, in a carrier that facilitates topical administration and delivery of the active ingredients transdermal Iy, e.g. a carrier that is useful for this purpose comprises alcoholic solvents, such as ethanol, and isopropanol, with optional excipients and formulation aids, which may comprise a polymeric species such as PVP or a poloxamer.
Owner:NORBROOK LABORATORIES LIMITED
Topical application of ivermectin for the treatment of dermatological conditions/afflictions
Dermatological conditions / afflictions such as rosacea, common acne, seborrheic dermatitis, perioral dermatitis, acneform rashes, transient acantholytic dermatosis, and acne necrotica miliaris, most notably rosacea, are treated by topically applying onto the affected skin area of an individual in need of such treatment, a topical pharmaceutical composition which comprises a thus effective amount of ivermectin.
Owner:GALDERMA HLDG SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com