Preparation method of ivermectin
A technology for ivermectin and avermectin, which is applied in the preparation of sugar derivatives, chemical instruments and methods, and bulk chemical production, can solve the problems of not being suitable for industrial production, and achieves ease of preparation conditions and high yield , the effect of a wide range of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
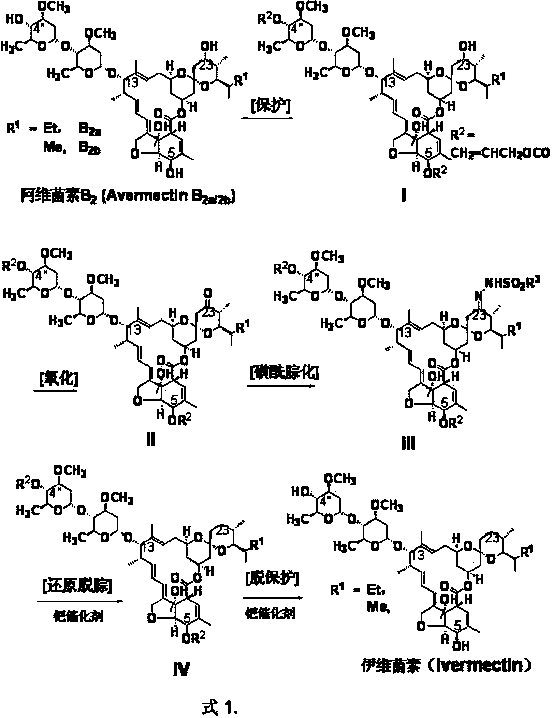
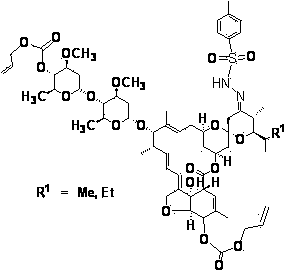
[0023]Example 1 4", 5-bis(allyloxycarbonyloxy)-23-deoxy-(4-methylbenzenesulfonylhydrazone)-abamectin B 2 preparation of
[0024]
[0025] In an inert gas (argon) atmosphere, 10 g of abamectin B 2 (content B 2a 93.3%, B 2b 3.2%) was dissolved in 100m1 dichloromethane, cooled to -10°C, added 4.2g allyl chloroformate and 3.0g tetramethylethylenediamine, and stirred for 2 hours. Heat up to 20°C and add 8.0g of tetramethylethylenediamine, 8.0g of dimethyl sulfoxide and 8.0g of phenoxyphosphoryl chloride, and stir for 10 hours. Add phosphoric acid solution and sodium hydroxide solution, first adjust the pH value to 2, then adjust to 7-8, separate layers, dry the organic phase with sodium sulfate, and remove the solvent under reduced pressure.
[0026] In an inert gas (argon) atmosphere, the residue was dissolved in 80ml of 95% ethanol, and 2.5g of tosylhydrazide (TsNHNH 2 ), heated to 50~60 o C, and stirred the reaction for 8 hours, then cooled to 25 o C, filtered, and d...
Embodiment 2
[0027] Example 2 Preparation of ivermectin.
[0028] In an inert gas (argon) atmosphere, the 4", 5-di(allyloxycarbonyl oxygen)-23-deoxy-(4-methylbenzenesulfonylhydrazone)-abamectin B obtained in Example 1 2 (10.2g) into 100ml sec-butyl acetate, add 0.2g triphenylphosphine and 0.1g Pd(OAc) 2 , heated to 25 o C add 0.5g NaBH in 3 times 4 , add 10 ml methanol after 1 hour, 25 o C was stirred for 5 hours. heat up to 50 o C continued to stir at constant temperature for 8 hours. Add 20ml of 5% acetic acid aqueous solution to wash the reaction solution, and then wash with NaHCO 3 Wash the toluene with aqueous solution, and crystallize in ethanol after removing the solvent under reduced pressure to obtain 6.5g white ivermectin crystals. Analysis of 22,23-Dihydroabamectin B by HPLC External Standard Method 1 Content 98.3% (of which 22,23-dihydro B 1a 96.1%, 22,23-Dihydro B 1b 2.1%). 1 HNMR and 13 CNMR further confirmed its main component structure 22,23-dihydro-B 1a...
Embodiment 3
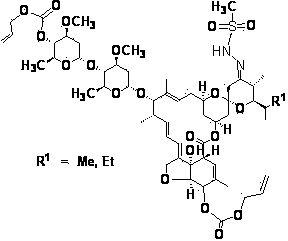
[0029] Example 3 4”, 5-bis(allyloxycarbonyloxy)-23-deoxy-methylsulfonylhydrazone-abamectin B 2 preparation of
[0030]
[0031] 4 ", 5-bis(allyloxycarbonyloxy)-23-deoxy-(4-methylbenzenesulfonylhydrazone)-abamectin B of synthetic method steps and example 1 2 Synthetic method is the same, p-toluenesulfonyl hydrazide (TsNHNH 2 ) replaced by methylsulfonylhydrazide (MsNHNH 2 ), to obtain 4”, 5-di(allyloxycarbonyloxy)-23-deoxy-methylsulfonylhydrazone-abamectin B 2 . Liquid mass analysis LC-MS [M+H] + 1149, l H NMR (CDCl 3 , 200Hz) 5.68 (m, 1H ), 5.45 (m, lH),5.23(m, 4H), 5.02 (m, lH), 4.83 (m, 5H), 4.67 (m, 2H), 4.36 (br d, J = 6.1, lH), 4.00 (m, lH), 3.97 (m, lH), 3.96 (br s, lH), 3.89 (m, 2H), 3.86 (m, lH), 3.75 (m, lH), 3.56 (m, lH), 3.51 (m, lH), 3.43 (s, 3H), 3.45 (s, 3H), 3.22 (m, 2H), 3.25 (m, lH), 2.90 (m, lH), 2.50 ( s, 3H), 2.53 (m, lH), 2.30-2.26 (m, 3H), 2.22 (dd, J = 12.7, 5.0, lH), 2.00-1.90 (m, 2H), 1.88 (br s, 4H), 1.80 (m, 2H), 1.76 (m, lH), 1.60-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com