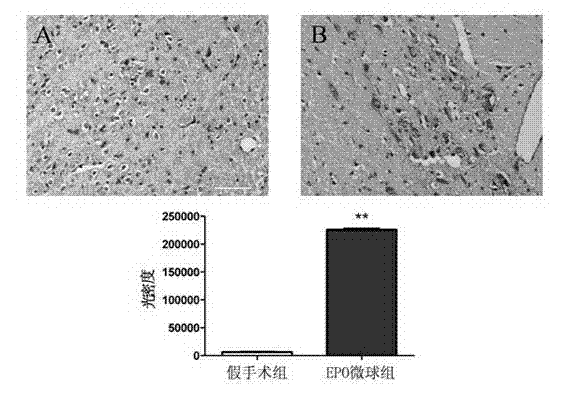
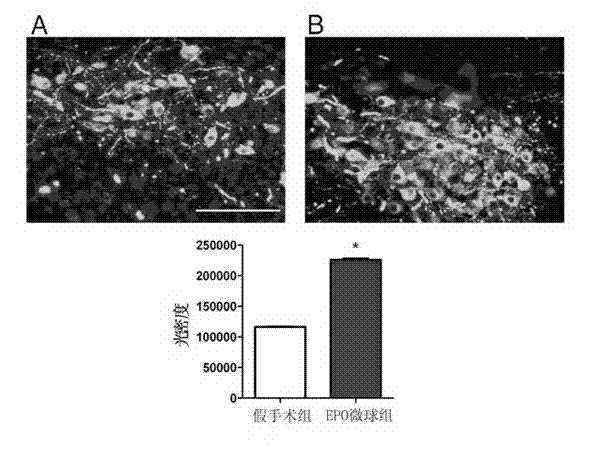
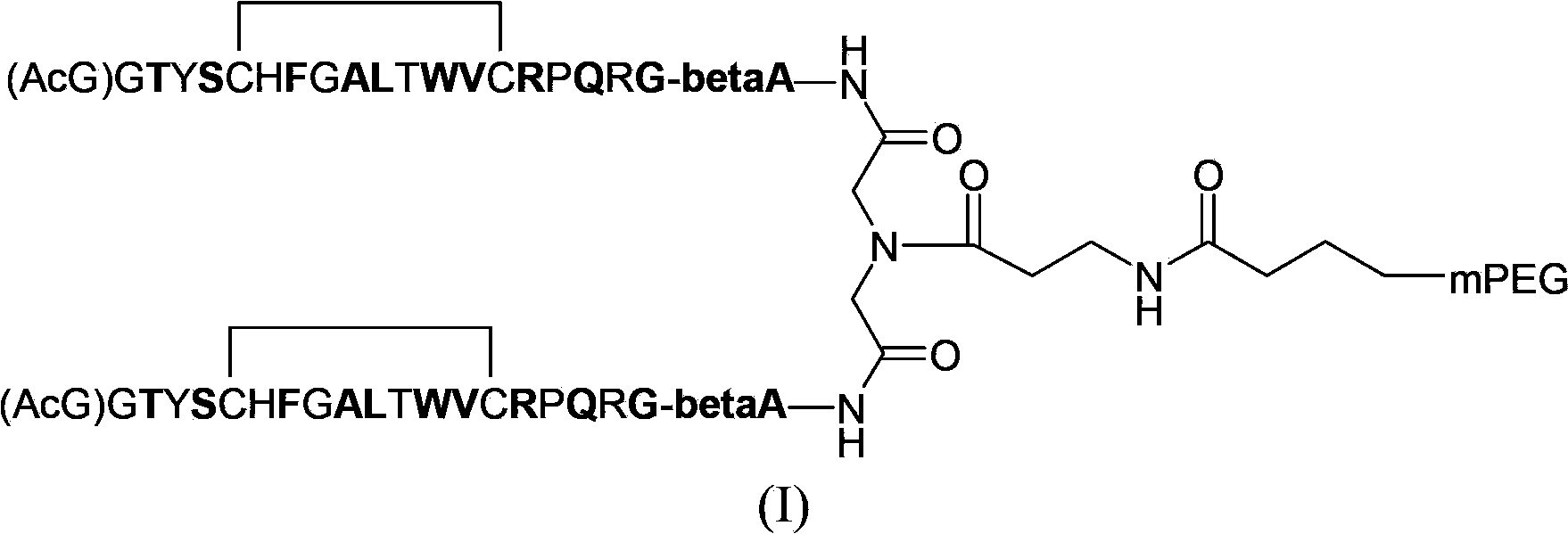
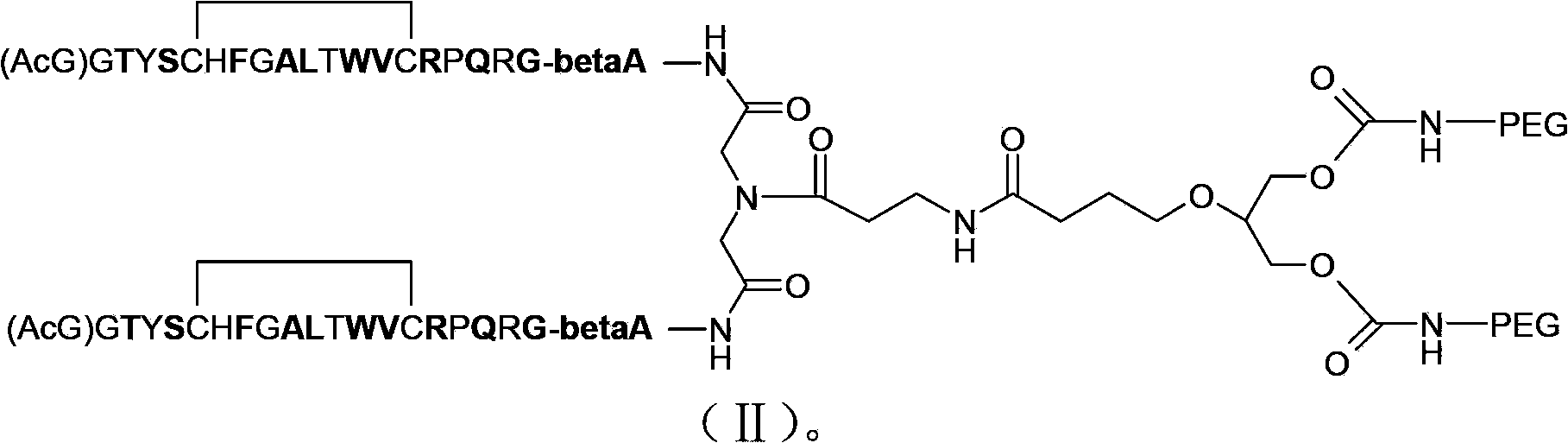
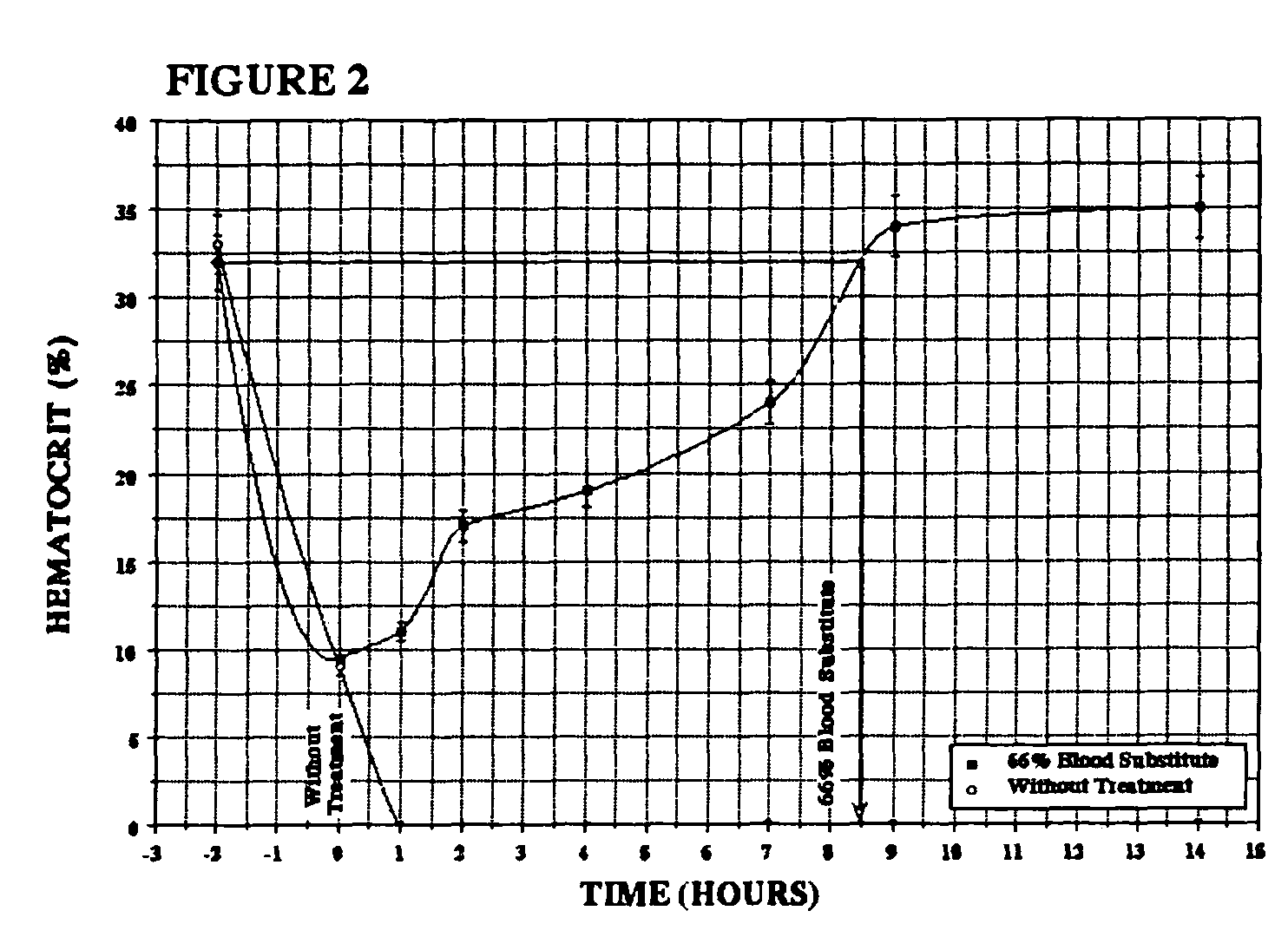
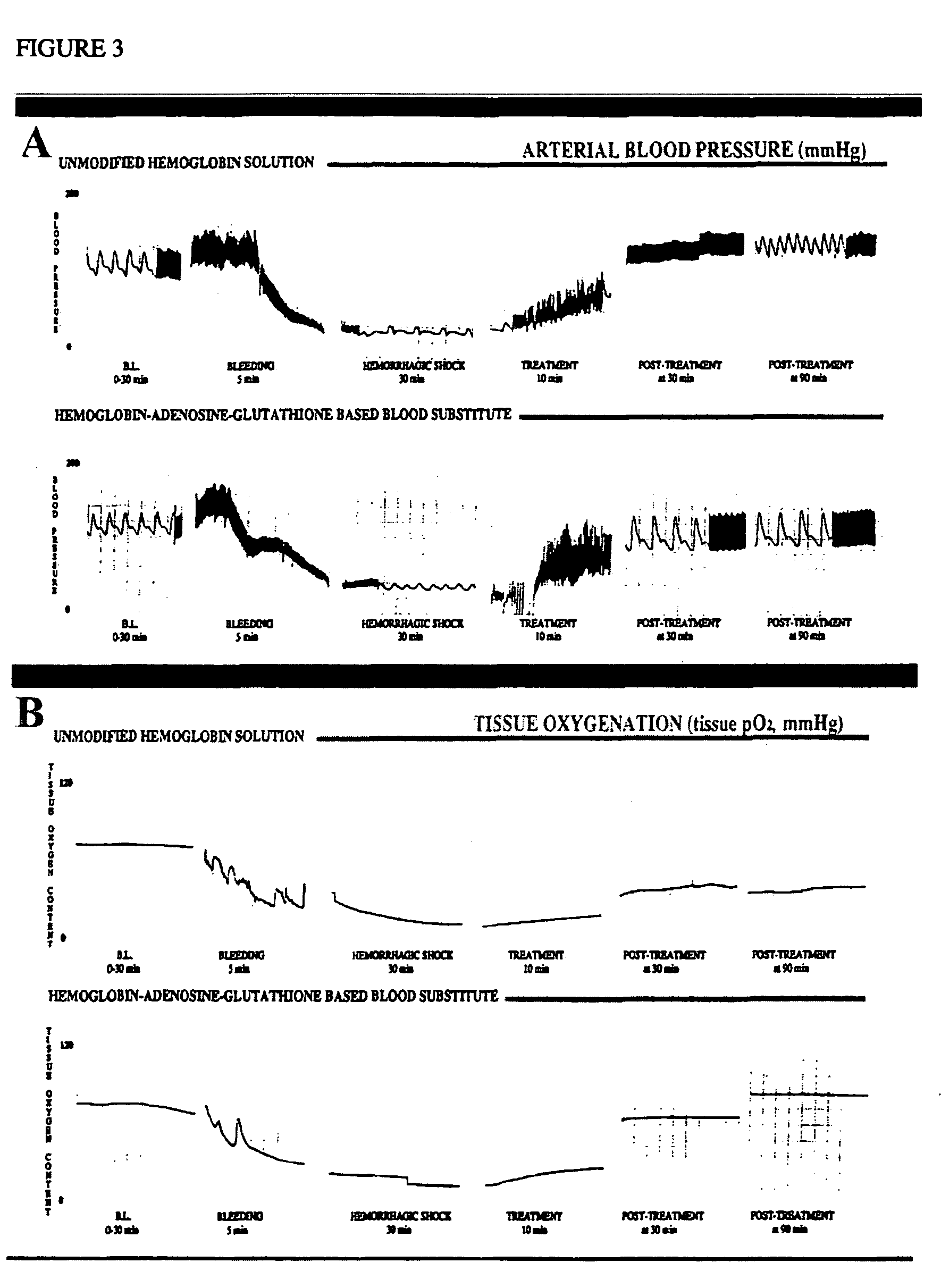
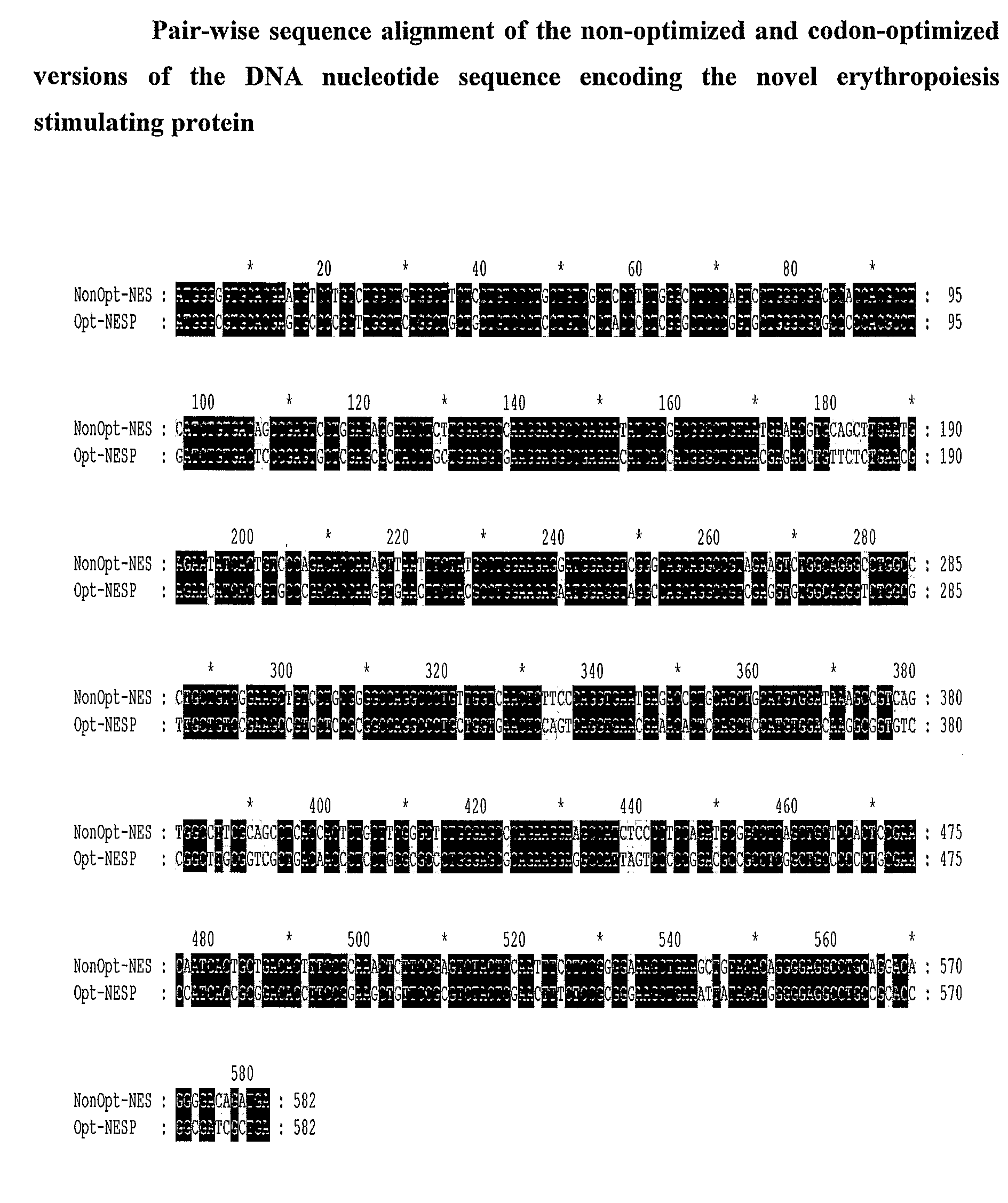
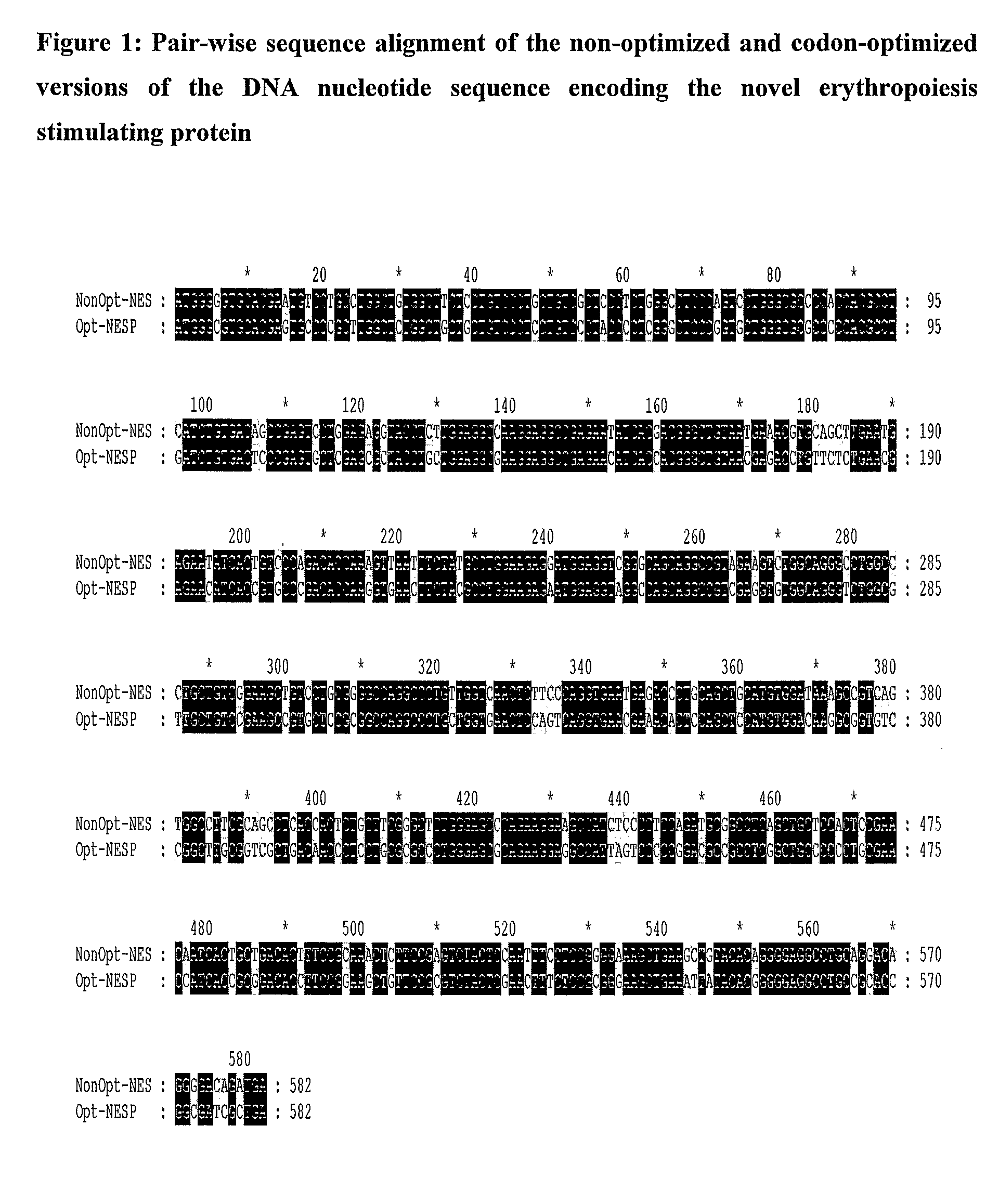
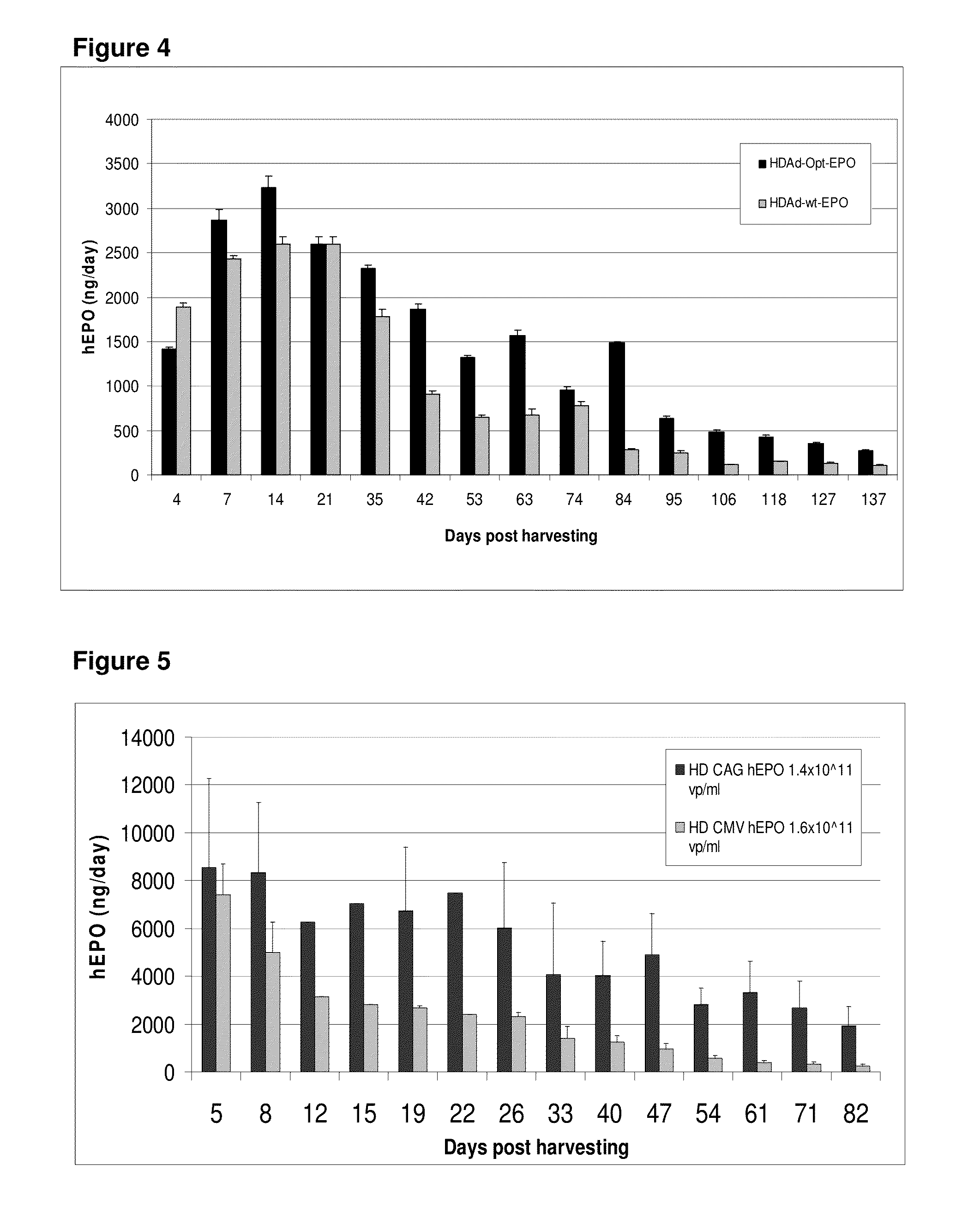
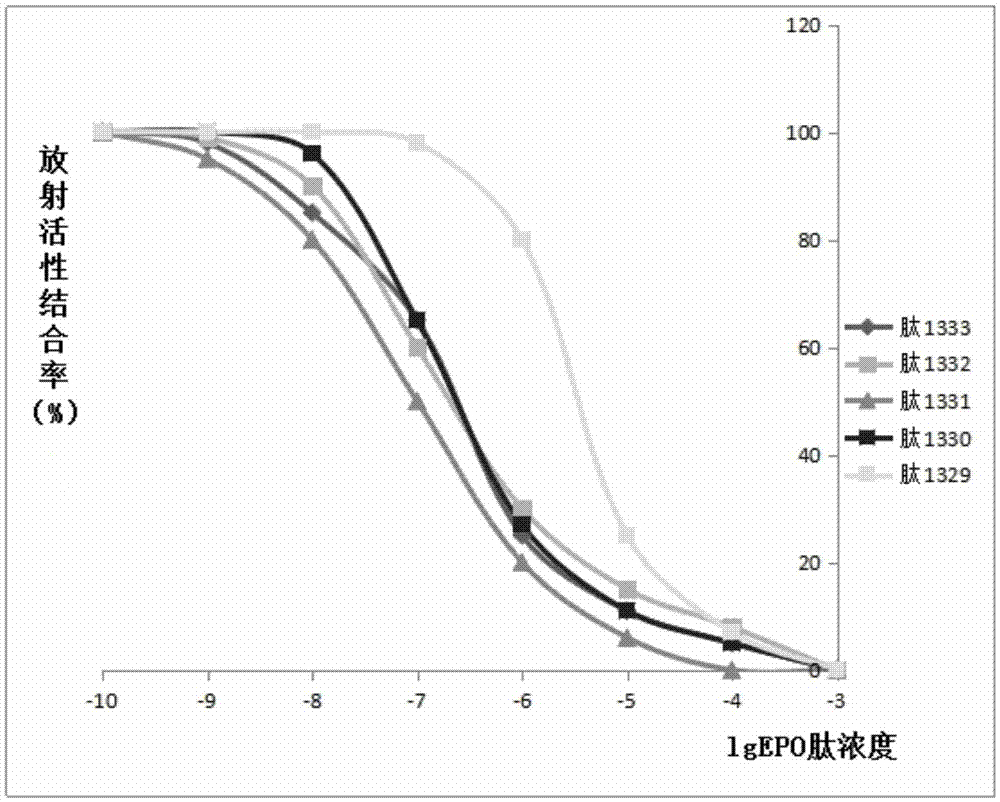
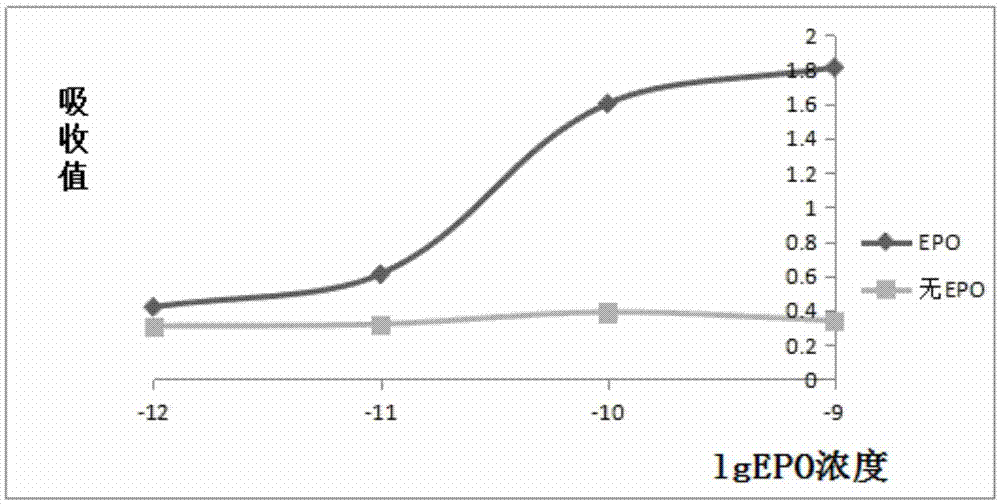
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
88 results about "Erythrogenin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Glycosylation analogs of erythropoietin
ActiveUS7217689B1Increase the number ofHigh sialic acid contentPeptide/protein ingredientsTissue culturePlasmidDNA
Erythropoietin analogs having at least one additional site for glycosylation, or a rearrangement of at least one site for glycosylation are disclosed. The invention also relates to DNA sequences encoding said erythropoietin analogs, and recombinant plasmids and host cells for analog expression.
Owner:AMGEN INC
Treatment of mitochondrial diseases with an erythropoietin mimetic
InactiveUS20090291092A1Stimulating erythropoiesisNervous disorderPeptide/protein ingredientsDiseaseRed blood cell
Methods of treating mitochondrial disorders that are not respiratory chain disorders using compositions comprising EPO mimetic compounds or compounds capable of increasing endogenous EPO levels or stimulating erythropoiesis are disclosed. Methods of treating Friedreich's ataxia, Leigh's syndrome, or other disorders by increasing the expression of frataxin with an EPO mimetic compound or a compound capable of increasing endogenous EPO levels or stimulating erythropoiesis are also disclosed.
Owner:EDISON PHARMA
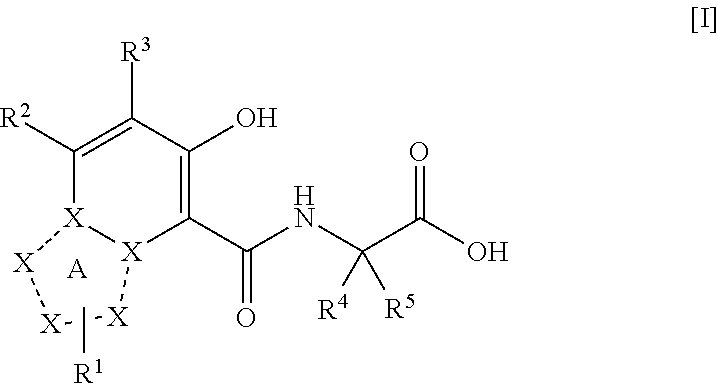
Triazolopyridine compound, and action thereof as prolyl hydroxylase inhibitor or erythropoietin production-inducing agent
ActiveUS20110077267A1Easy to produceReduce productionBiocideGroup 5/15 element organic compoundsDiseaseMedicine
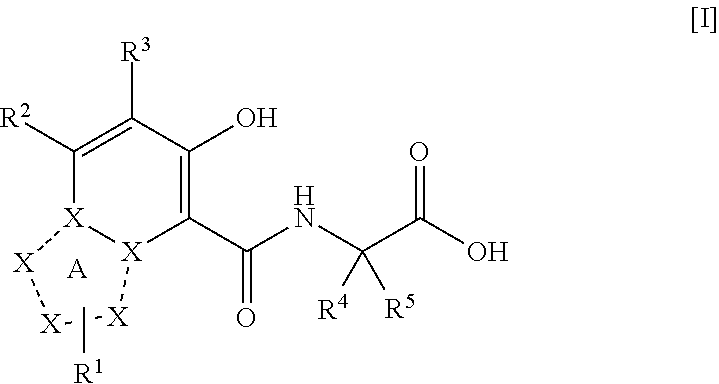
The present invention provides a triazolopyridine compound having a prolyl hydroxylase inhibitory action and an erythropoietin production-inducing ability. The present invention relates to a compound represented by the following formula [I]:wherein each symbol is as defined in the specification, or a pharmaceutically acceptable salt thereof, or a solvate thereof, as well as a prolyl hydroxylase inhibitor or erythropoietin production-inducing agent containing the compound. The compound of the present invention shows a prolyl hydroxylase inhibitory action and an erythropoietin production-inducing ability and is useful as a prophylactic or therapeutic agent for various diseases and pathologies (disorders) caused by decreased production of erythropoietin.
Owner:JAPAN TOBACCO INC
Fusion protein having enhanced in vivo erythropoietin activity
ActiveUS7091326B2Enhanced human EPO activityProlong half-life in vivoPeptide/protein ingredientsAntibody mimetics/scaffoldsRed blood cellHalf-life
Provided is a fusion protein comprising, at its carboxy terminal of human erythropoietin (EPO), a mutant having one to four amino acid substitutions in the carboxy terminal peptide (CTP) fragment of a human chorionic gonadotropin (HCG) β subunit, for increasing an in vivo half-life activity of EPO. The in vivo half-life can be greatly elongated while retaining the intrinsic activity of the EPO, without increasing the sugar chain content.
Owner:CJ HEALTHCARE CORP
Erythrocyte-stimulating factor mimic peptide derivative, medical salts thereof, preparation method and use thereof
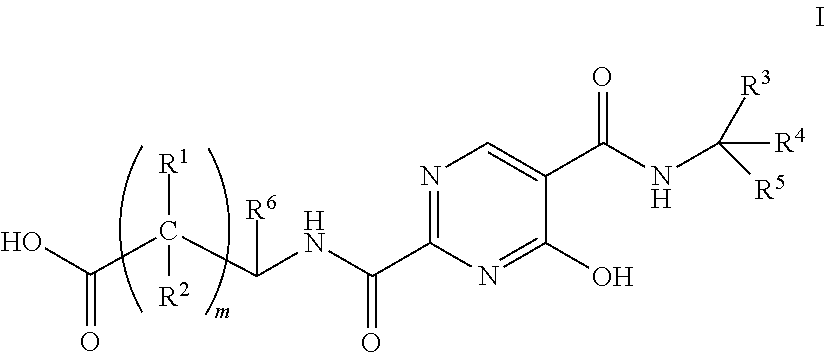
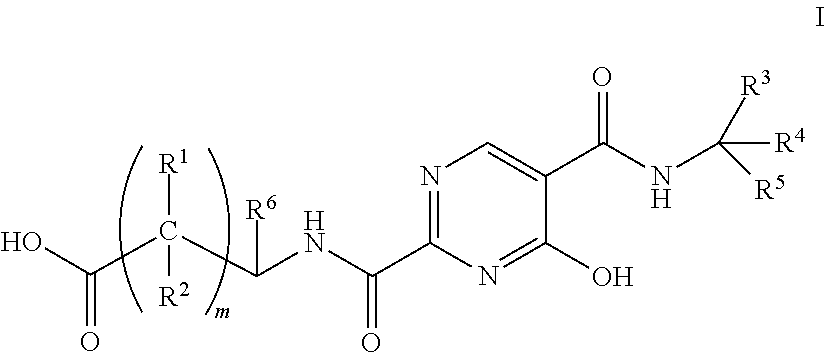
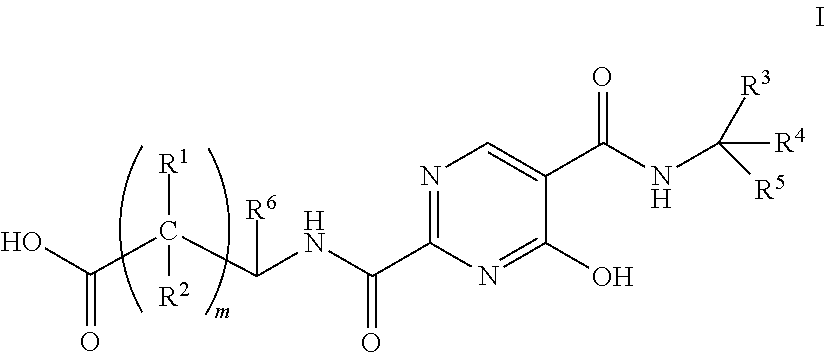
The invention relates to erythropoietin simulated peptide derivatives with a general formula (I) and medicinal use salts and a preparation method thereof, wherein R1, R2, R3, R4, R5, n1 and n2 are defined in an instruction book. The invention also relates to medicine compositions containing the erythropoietin simulated peptide derivatives with the general formula (I) and the medicinal use salts thereof. The erythropoietin simulated peptide derivatives with the general formula (I) and the medicinal use salts thereof, or the medicine compositions containing the erythropoietin simulated peptide derivatives with the general formula (I) and the medicinal use salts thereof can be widely applied to treating the diseases characterized by the deficiency of erythropoietin or the shortage or defect of erythrocyte groups.
Owner:JIANGSU HANSOH PHARMA CO LTD
Application of erythropoietin microspheres to preparation of drugs for treating motor complications in Parkinson's disease
InactiveCN102871969ANo side effectsImprove compliancePowder deliveryNervous disorderSide effectMicrosphere
The invention discloses application of erythropoietin microspheres to preparation of drugs for treating motor complications in the Parkinson's disease. The erythropoietin microspheres are obtained by means of preparing one of naturally extracted erythropoietin, genetic recombination expression erythropoietin, polyethylene glycol modified erythropoietin, glycosylation modified erythropoietin or human serum fused erythropoietin with polysaccharides into particles, and preparing the particles with sustained-release materials into the microspheres. The application has the advantages that a novel medicinal use of the erythropoietin microspheres is cultivated, a novel application field is developed, treating and preventing the motor complications in the Parkinson's disease by the erythropoietin microspheres have the advantages of long-acting sustained release, no toxic and side effects, fine compliance, patient pain relieving, low cost and the like and are easily accepted by patients, and the erythropoietin microspheres are simple in preparation process and environment-friendly and have excellent application prospects in treating and preventing the motor complications in the Parkinson's disease.
Owner:XIN HUA HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Methoxy polyethylene glycol-modified erythropoietin mimic peptide derivative
InactiveCN103570834AImprove biological activityImprove bioavailabilityNervous disorderPeptide/protein ingredientsDiseaseRed Cell
The present invention relates to a methoxy polyethylene glycol-modified erythropoietin mimic peptide derivative represented by a general formula (I), a pharmaceutically acceptable salt of the derivative, a preparation method of the derivative and the pharmaceutically acceptable salt, and uses of the derivative and the pharmaceutically acceptable salt in preparation of drugs for treatment of diseases adopting erythropoietin deficiency or red cell mass shortage or defect as characteristics.
Owner:JIANGSU HANSOH PHARMA CO LTD
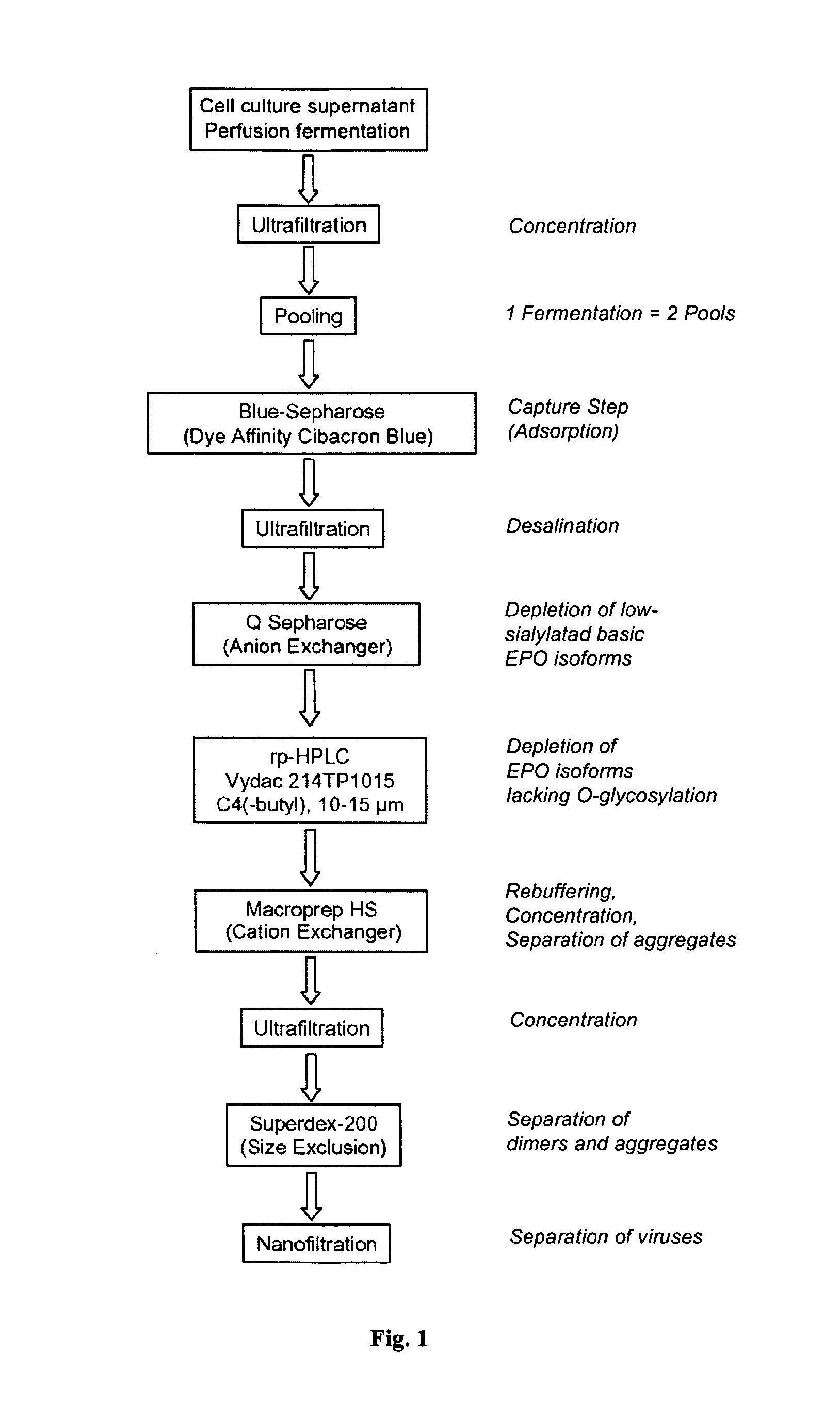
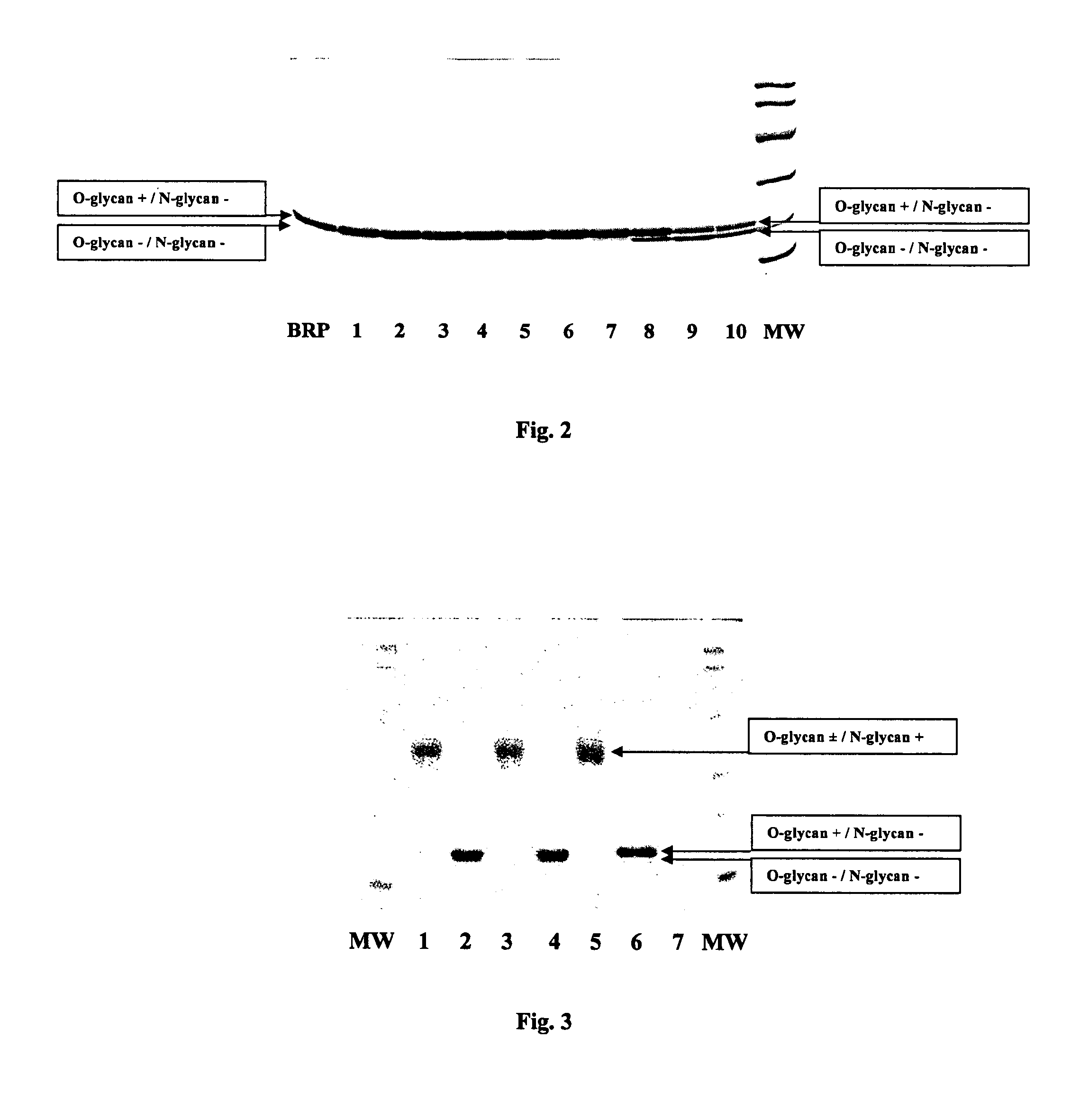
Process for the purification of recombinant human erythropoietin (EPO), epo thus purified and pharmaceutical compositions comprising same
InactiveUS20120264688A1Increase virus safetyEffective stepPeptide/protein ingredientsPeptide preparation methodsMedicineRed blood cell
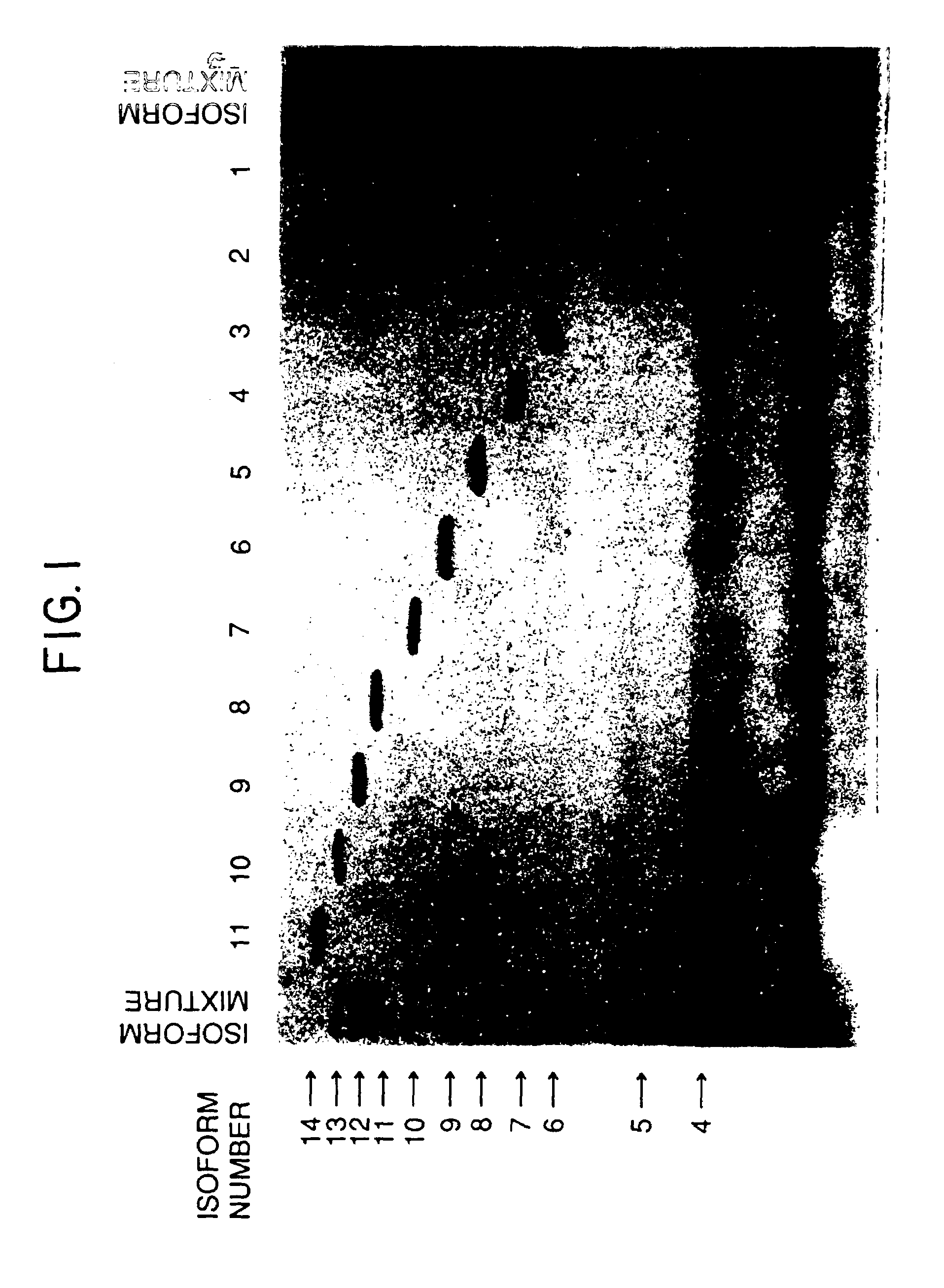
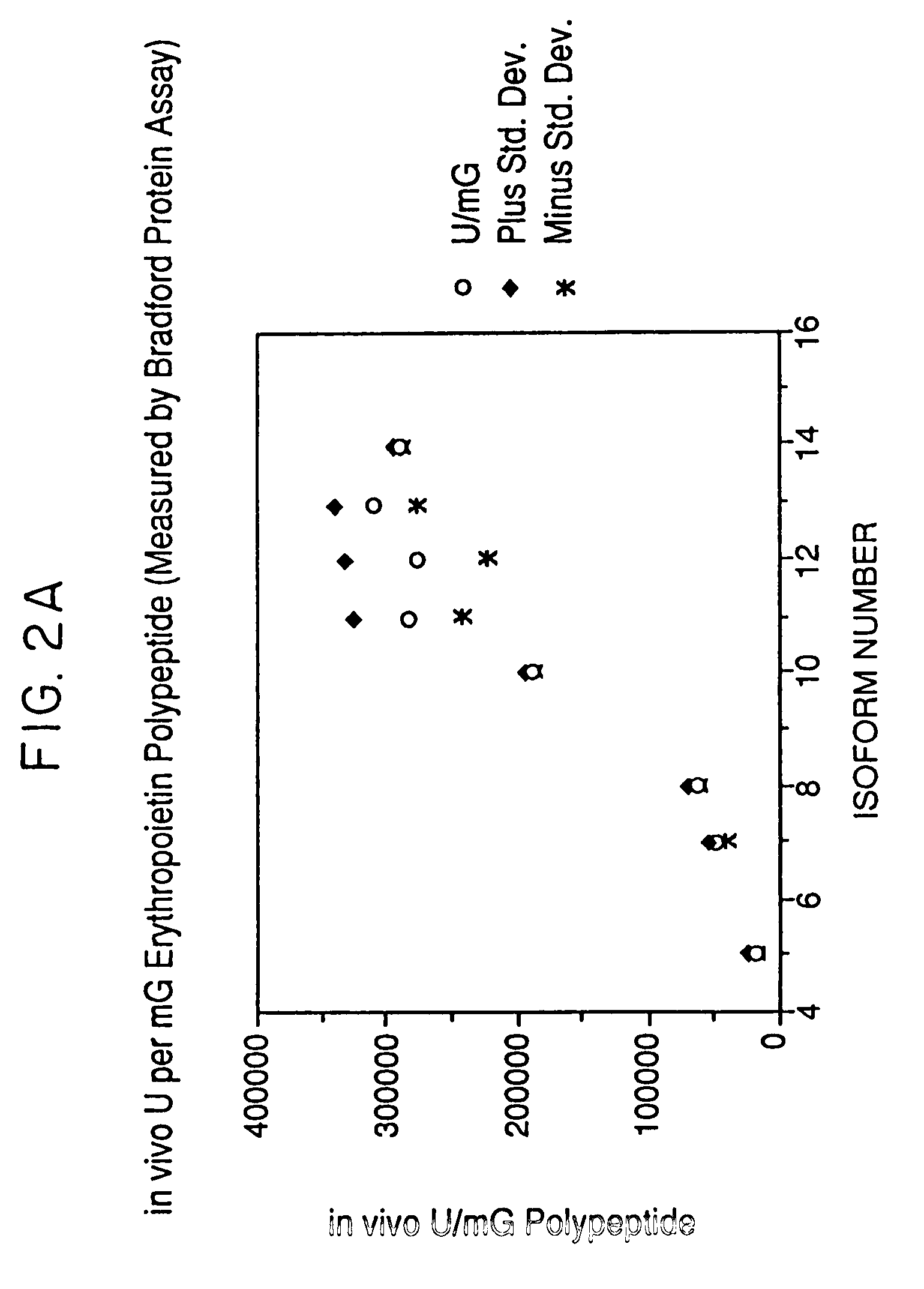
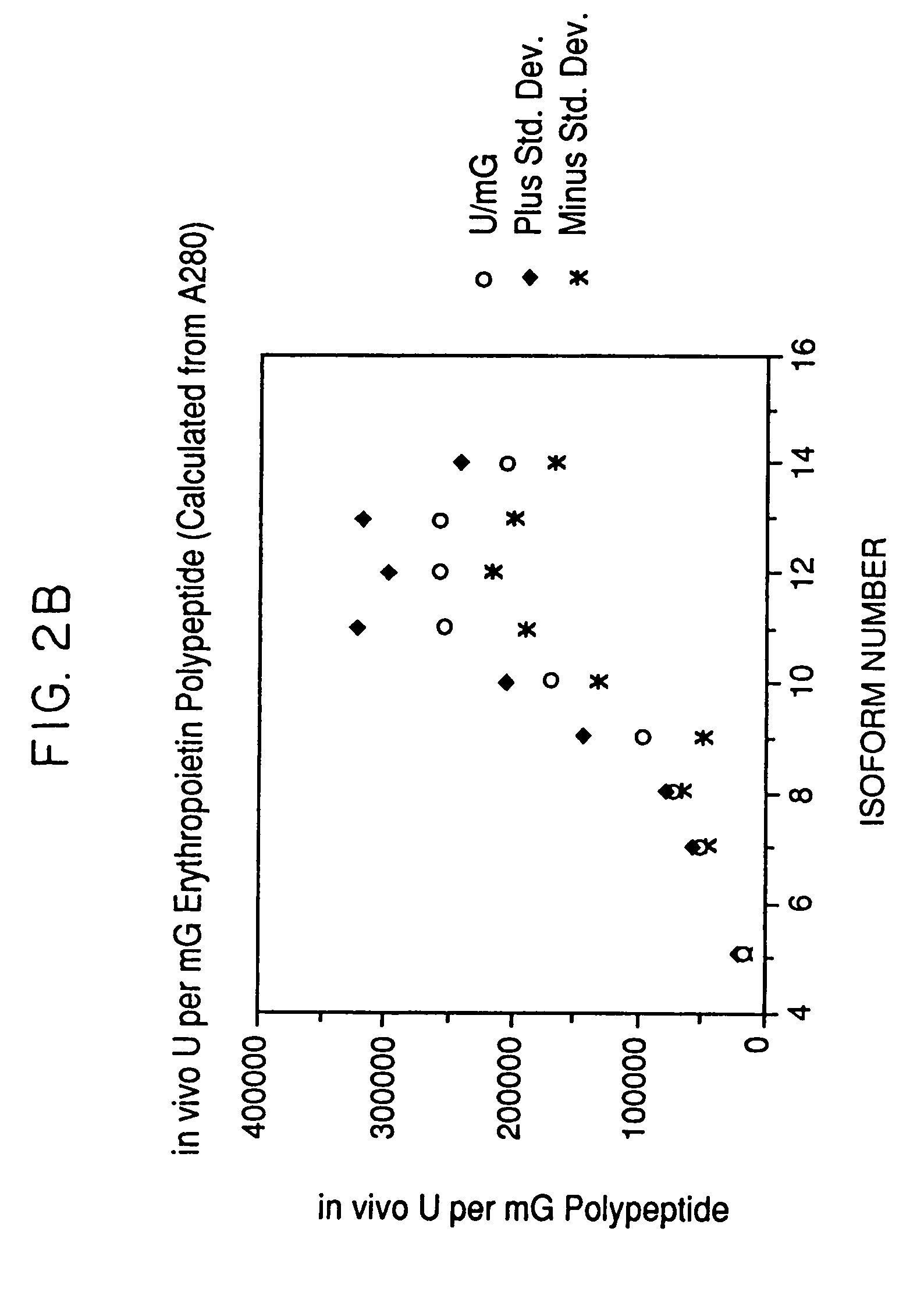
A procedure for the production of erythropoietin (EPO), in particular recombinant human EPO (rhEPO) with a defined composition of glycoforms in a highly pure form, i.e., with a high amount of O-glycosylated EPO isoforms is provided.
Owner:RATIOPHARM GMBH
Erythropoietin production accelerator
The present invention relates to a preventive or therapeutic agent for pathological conditions caused by reduced production of erythropoietin, or for anemia, or for chronic anemia, renal anemia, aplastic anemia, or pure red cell aplasia, the agent comprising, as an active ingredient, a cyclic amine compound represented by the following formula (1): wherein, R1, R2 and R3 each independently represent a hydrogen atom, a halogen atom, or hydroxy, alkyl, halogen-substituted alkyl, alkoxy, alkylthio, carboxyl, alkoxycarbonyl or alkanoyl group; W1 and W2 each independently represent N or CH; X represents O, NR4, CONR4 or NR4CO; R4 each represents a hydrogen atom, or an alkyl, alkenyl, alkynyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted aralkyl, or substituted or unsubstituted heteroaralkyl group; and l, m and n each represents a number of 0 or 1, or a salt thereof or a solvate thereof.
Owner:KOWA CO LTD
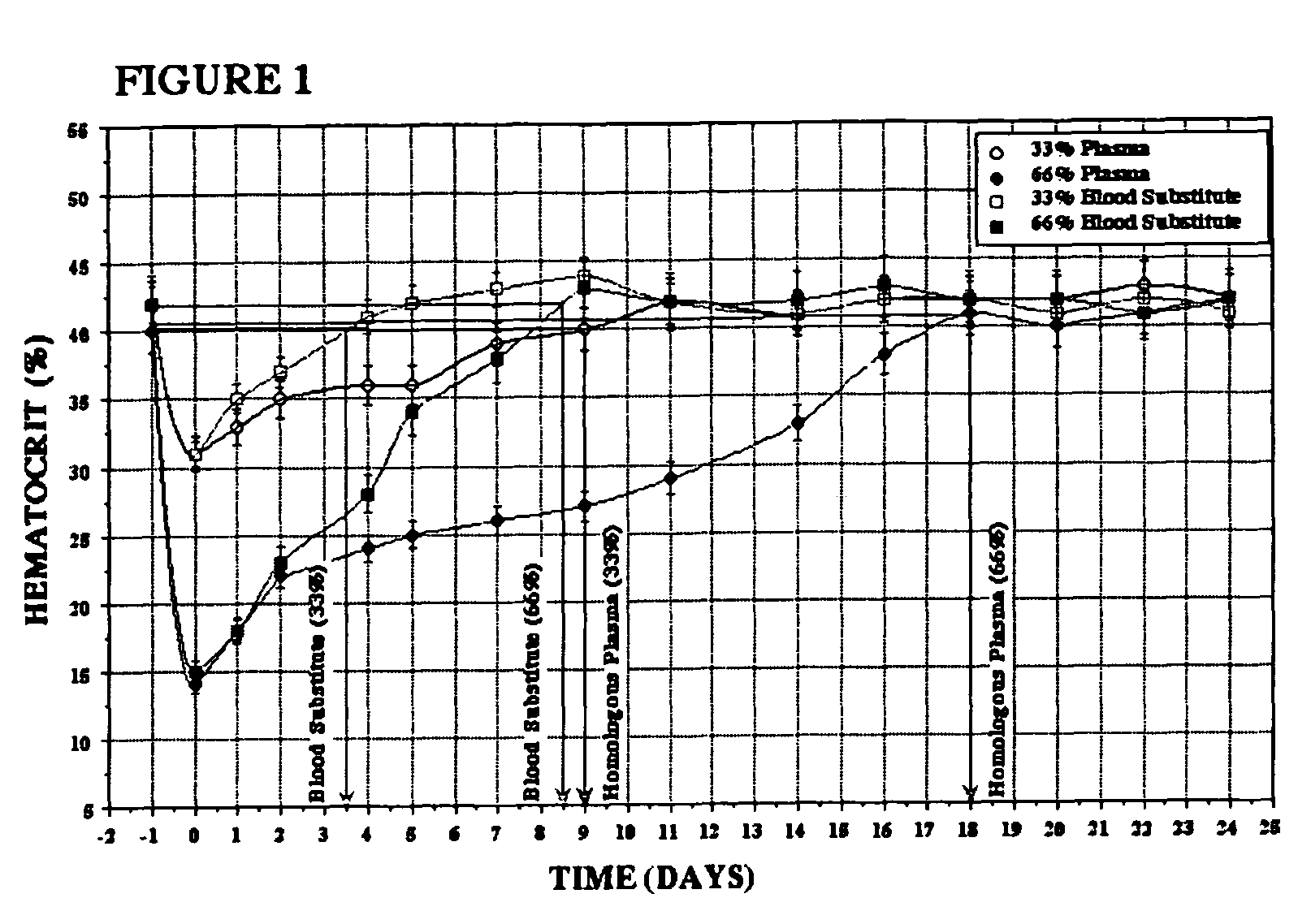
Methods of treating acute blood loss
InactiveUS7759306B2Blood lossRestore blood flowPeptide/protein ingredientsAntinoxious agentsCross-linkDouble-time
Owner:TEXAS TECH UNIVERSITY HEALTH SCIENCES CENTER
Erythropoietin and fibronectin compositions for therapeutic and cosmetic applications
InactiveUS20110123481A1Promoting wound healing and connective tissue reconstructionPromote wound healingPeptide/protein ingredientsOintment deliveryDrugInjury mouth
A method of promoting wound healing or connective tissue reconstruction and a method of treating ischemia in a subject in need thereof are disclosed. The methods comprising topically administering to the subject about 10-30 mg per cm2 wound tissue of Erythropoietin and about 100-300 mg per cm2 wound tissue of Fibronectin, thereby promoting wound healing or connective tissue reconstruction or treating ischemia in the subject. Unit dosage forms, pharmaceutical compositions, cosmetic compositions and formulations comprising Erythropoietin and / or Fibronectin are also disclosed.
Owner:REMEDOR BIOMED
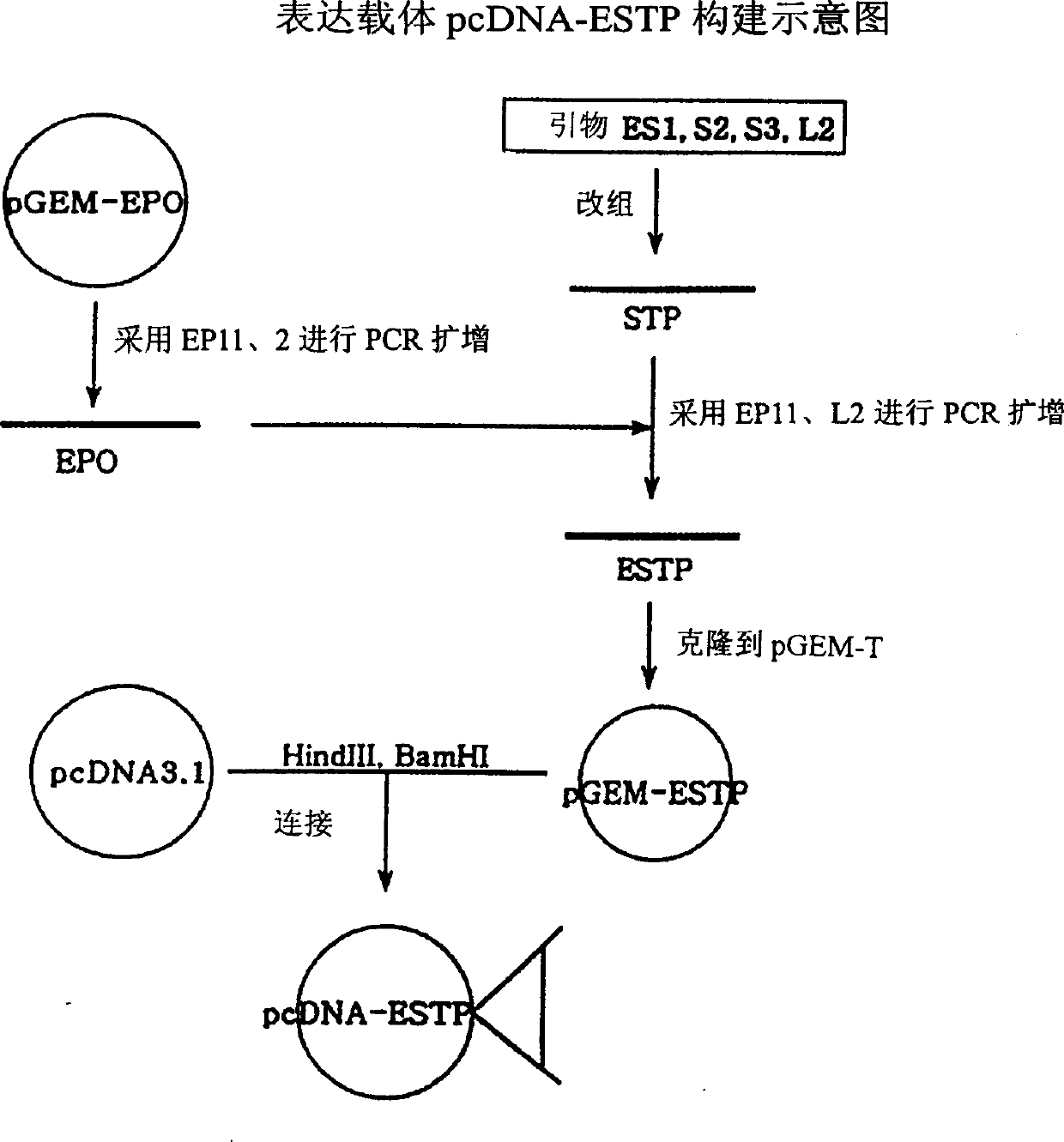
Recombinant Method for Production of an Erythropoiesis Stimulating Protein
InactiveUS20090029907A1Increase the number ofHigh sialic acid contentPeptide/protein ingredientsRecombinant DNA-technologyErythroid cellBULK ACTIVE INGREDIENT
The present invention relates to the recombinant method used for the production of a highly glycosylated form (in total five N linked glycosylations as opposed to three N linked glyosylations in the natural EPO) of erythropoietin. The added sites for glycosylation will result in greater number of carbohydrate chains, and higher sialic acid content than human EPO, which in turn would impart to the recombinant molecule a longer half-life. The invention further relates to the construction of expression cassettes comprising nucleic acid sequences encoding for the highly glycosylated form of Erythropoietin and stable expression in the host cells. The invention further relates to the optimized method for purification of the erythropoiesis stimulating protein. The recombinant EPO according to the invention, and the salts and functional derivatives thereof, may comprise the active ingredient of pharmaceutical compositions for an increase in the hematocrit for treatment of anemia and for restoration of patient well being and quality of life.
Owner:AVESTHAGEN
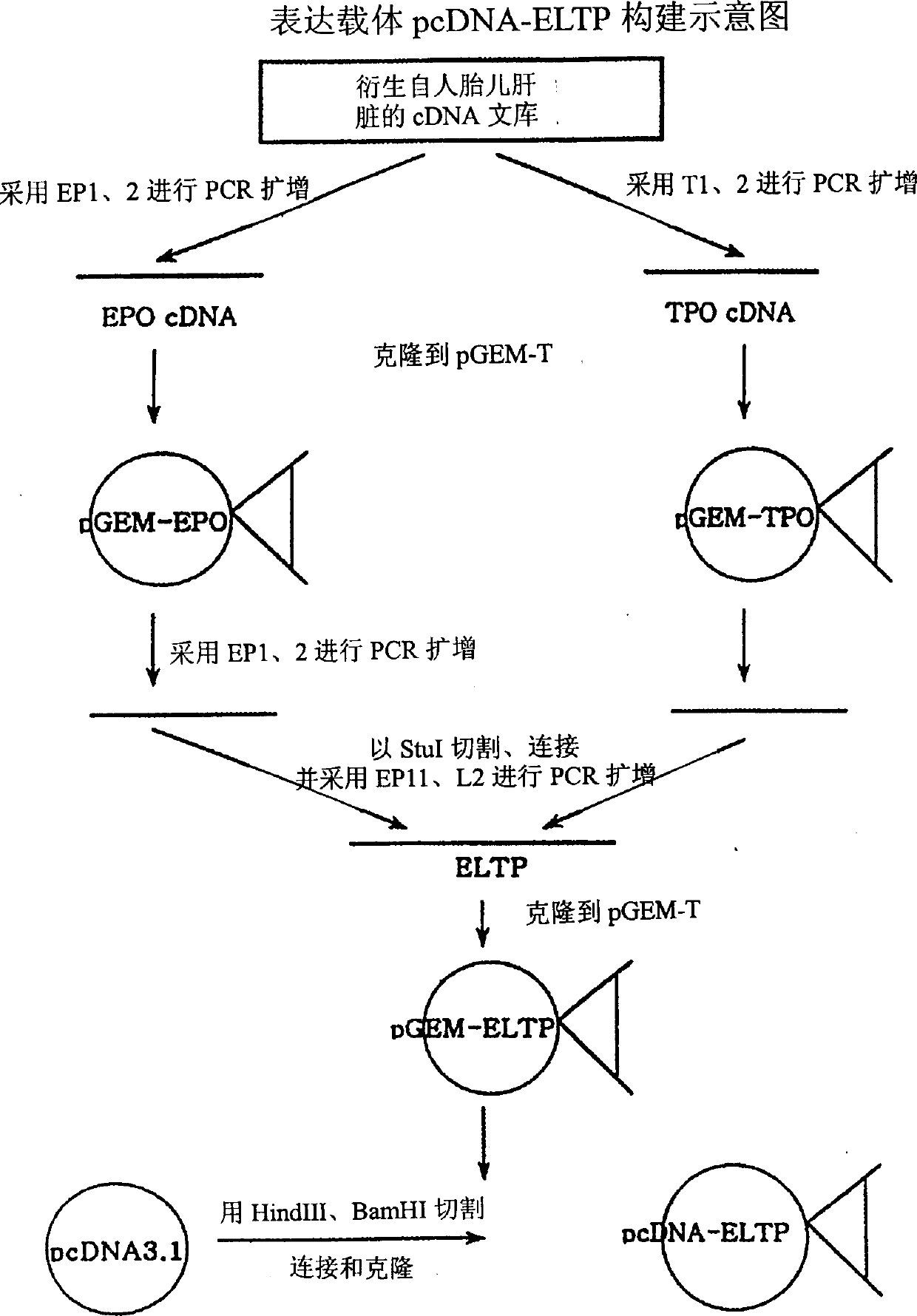
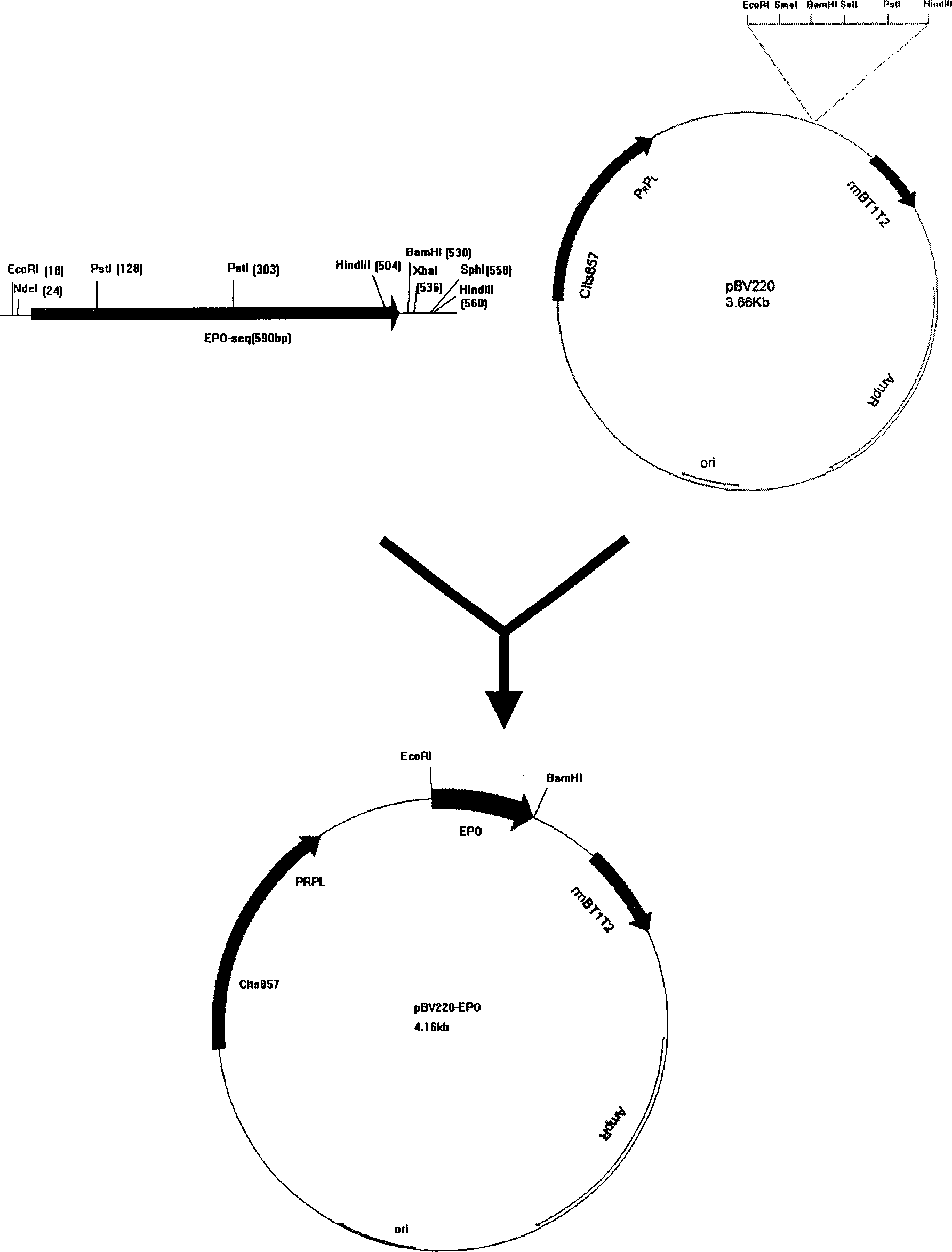
Method for activating expression of erythrogenin genes
ActiveCN103667344ANo adverse reactions such as regenerative disordersGenetic material ingredientsGene therapyEnzyme digestionMesenchymal stem cell
The invention relates to the field of biotechnology, and particularly relates to a method for activating the expression of erythrogenin genes, namely, activating the expression of erythrogenin genes in target cells by using a TALE technology. The method comprises the following steps: 1) searching a TALE target spot sequence at the upstream of an erythrogenin gene promoter, and designing a target spot identification module which is used for specifically identifying the TALE target spot sequence and composed of TAL nucleic acid identification units; 2) building a coding sequence of the target spot identification module; 3) connecting the coding sequence of the target spot identification module prepared in the step 2) with a skeleton carrier by using an enzyme digestion and connection method so as to obtain a recombinant plasmid; 4) transforming the recombinant plasmid to a target cell and culturing the cell. According to the invention, the expression of erythrogenin genes is activated in autologous mesenchymal stem cells by using a TAL technology, so that the method provides a new source of cells for gene therapy, and can play an important role in tissue engineering and cell therapy.
Owner:SHANGHAI GENECHEM
Fusion protein with reinforced erythrocytin activity in vivo
InactiveCN1421461AImprove in vivo activityThrombopoietinPeptide/protein ingredientsIn vivoC-terminus
Owner:CJ HEALTHCARE CORP
Recombinant erythropoietin with intracorporeal physical activity and modified by macrogol
InactiveCN1680449AReduce the cost of medicationReduce manufacturing costPeptide/protein ingredientsPharmaceutical non-active ingredientsDiseaseRed blood cell
The invention relates to a polyethylene glycol conjugate (PEG-EPOP) of the hematopoietin protein, which generates a recombinant erythropoietin (rhEPOP) with intracorporeal physical activity and modified by macrogol. The invention discloses the method of obtaining EPOP with low cost by the prokaryotic expression system so as to obtain polyethylene glycol conjugate (PEG-EPOP) with low cost for producing the medicine for treating anemic diseases and increasing erythrocyte.
Owner:CHENGDU INST OF BIOLOGICAL PROD
Recombinant human erythropoietin preparation without human albumin
ActiveCN104189891AImprove stabilityImprove high temperature stabilityPeptide/protein ingredientsPharmaceutical non-active ingredientsActive agentHuman albumin
The invention discloses a recombinant human erythropoietin preparation without human albumin. The recombinant human erythropoietin preparation without human albumin is characterized by comprising the following components: dosages of human erythropoietin, 0.1% to 2% (W / V) of amino acid and salt derivatives thereof, 0.001% to 0.01% (V / V) of non-ionic surface active agent, a physiological soluble buffer solution which can control the pH of the preparation to be 5.0 to 8.0, an iso-osmotic agent consisting of 1% to 5% (W / V) of mannitol or 0.1% to 0.9% (W / V) of sodium chloride, and the balance of water. With the adoption of the preparation, the risks of potential virus threats and protein allergy caused by human albumin in use can be avoided, the content accuracy of recombinant human erythropoietin injection can be ensured, and the stability of high-temperature storage is improved.
Owner:BEIJING FOUR RINGS BIOPHARM
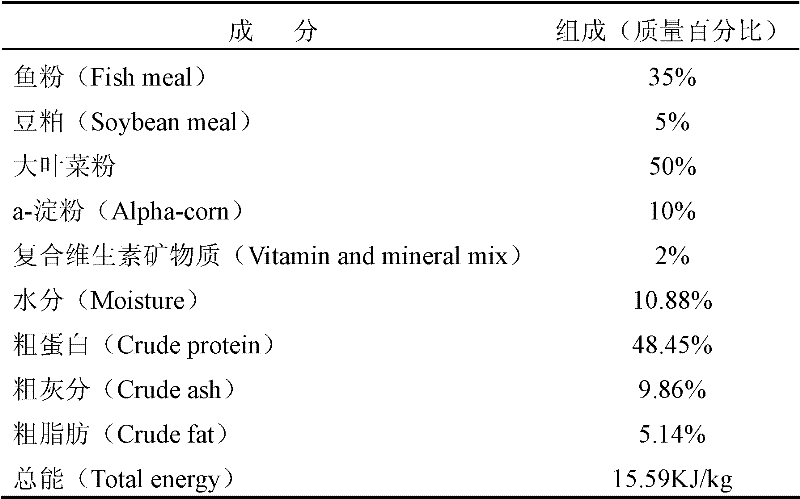
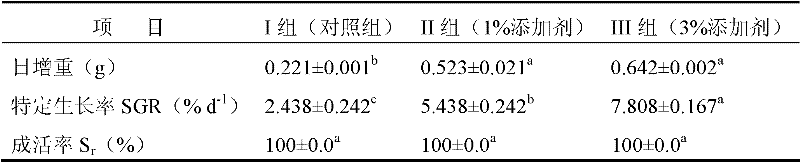
A kind of sea cucumber species ginseng ripening-promoting feed additive
InactiveCN102265985APromote gonad developmentIncrease the maturity ratioClimate change adaptationAnimal feeding stuffAstaxanthinPhospholipid
The invention discloses a feed additive for promoting ripening of sea cucumber seeds. The additive consists of the following components in percentage by mass: 5 to 10 percent of docosahexaenoic acid (DHA), 20 to 35 percent of eicosapentaenoic acid (EPA), 0.5 to 2 percent of astaxanthin, 2 to 4 percent of cholesterol, 0 to 10 percent of phospholipid, 5 to 15 percent of sand hopper powder, 0.5 to 1percent of vitamin A, 0.5 to 1 percent of vitamin C, 0.5 to 1 percent of vitamin E, 10 to 30 percent of pine needle powder, 5 to 15 percent of malt, 10 to 20 percent of egg yolk powder, 0.5 to 2 percent of globulin, 0.5 to 1 percent of erythrogenin and 0.5 to 1 percent of saccharicterpenin. The additive is suitable for promoting ripening of the sea cucumber seeds, and can obviously promote the gonad development of the sea cucumber seeds, quicken sexual maturity and improve the egg laying amount and the ripening ratio, fertility rate, hatching rate and sea cucumber seedling survival rate of the colony; and the additive does not contain hormone or antibiotics, has no pollution or no toxic or side effect, has low consumption, and is stable in raw material source, low in price and simple in production process.
Owner:DALIAN UNIV OF TECH
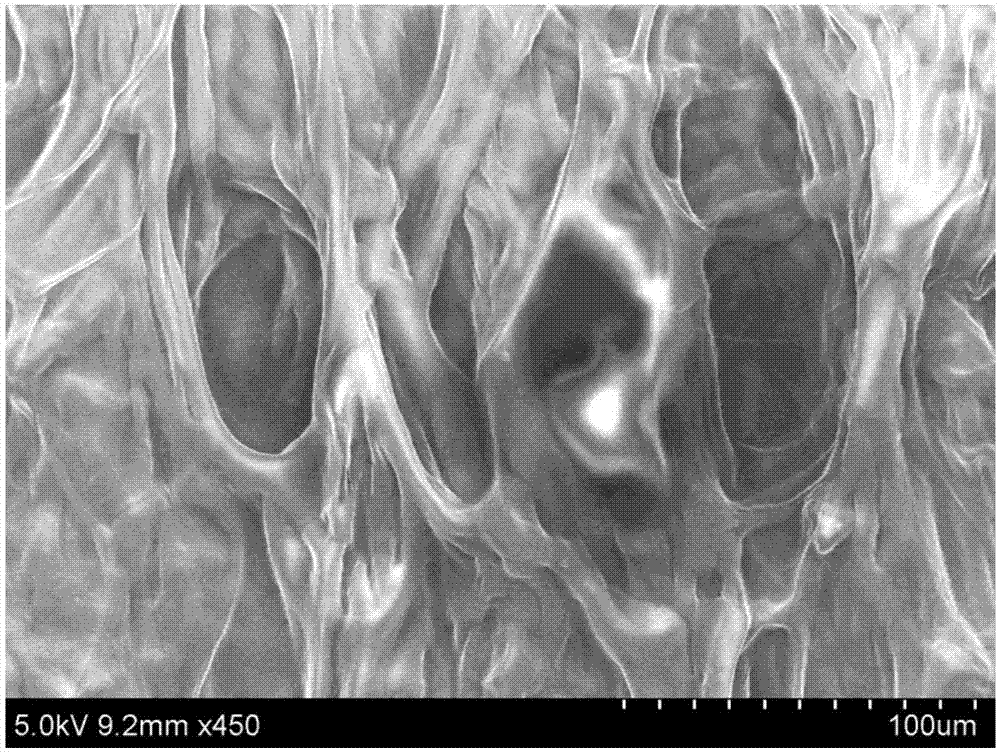
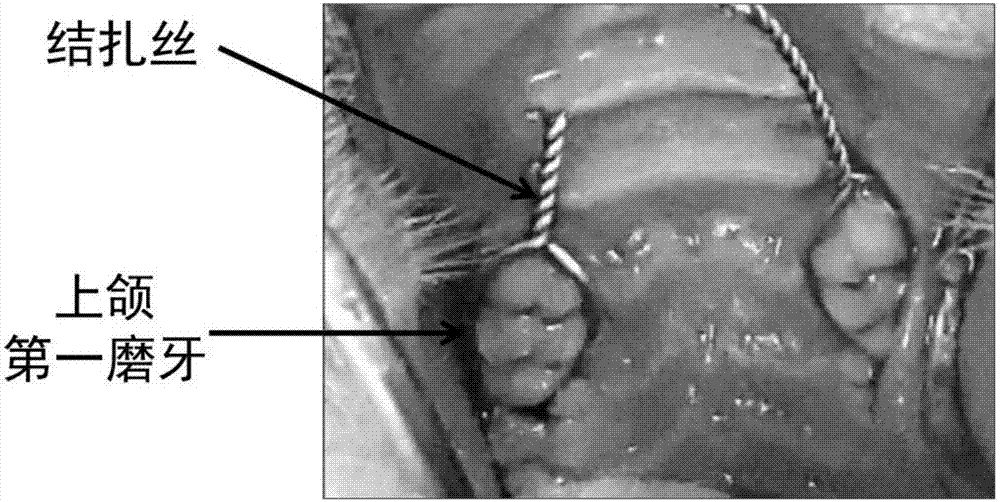
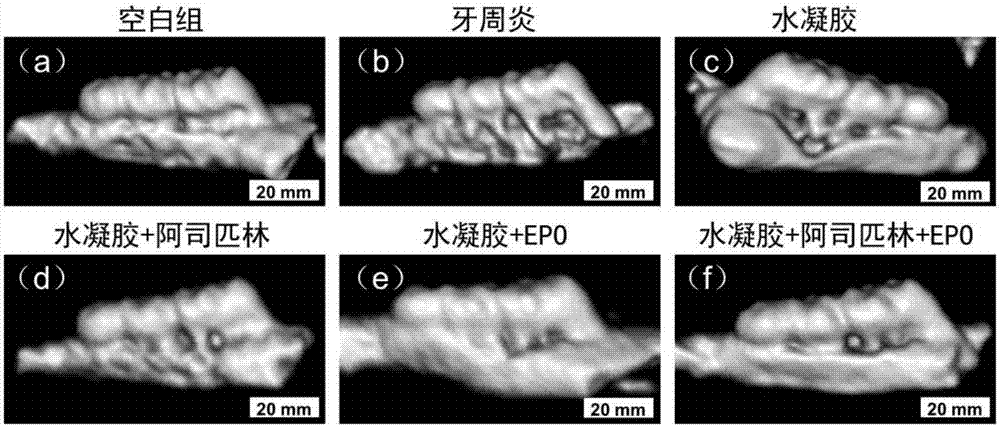
Chitosan-based periodontal local drug slow-release hydrogel and application thereof
InactiveCN107375195APromote regenerationEasy to operateOrganic active ingredientsPeptide/protein ingredientsPhosphateSodium glycerophosphate
The invention relates to chitosan-based periodontal local drug slow-release hydrogel and application thereof, belonging to the technical field of preparation of local drug slow-release carriers. The invention in particular relates to chitosan temperature-sensitive hydrogel loading aspirin and erythrogenin (EPO), and the chitosan temperature-sensitive hydrogel is obtained by mixing an aspirin-containing chitosan solution, an EPO-containing beta-sodium glycero-phosphate solution and a gelatin solution according to a proportion at the temperature of 34-40 DEG C. The surface pore size of the chitosan temperature-sensitive hydrogel loading the aspirin and the EPO prepared by the method is about 40-70mu m and can be gelatinized at the temperature of 34-40 DEG C; in a rat maxillary first molar periodontitis model constructed by using ligature wires, the effects of controlling periodontal inflammation and promoting periodontal tissue regeneration can be achieved by locally injecting the hydrogel. Therefore, the chitosan-sodium glycerophosphate-gelatin injectable temperature-sensitive hydrogel loading the aspirin and the EPO has better application value and prospects when being used as a local drug slow-release carrier for periodontitis treatment.
Owner:JILIN UNIV
Erythropoietin Expression Promoter
ActiveUS20150353489A1Increase volumeSuppression amountOrganic active ingredientsOrganic chemistryMedicineLiver function
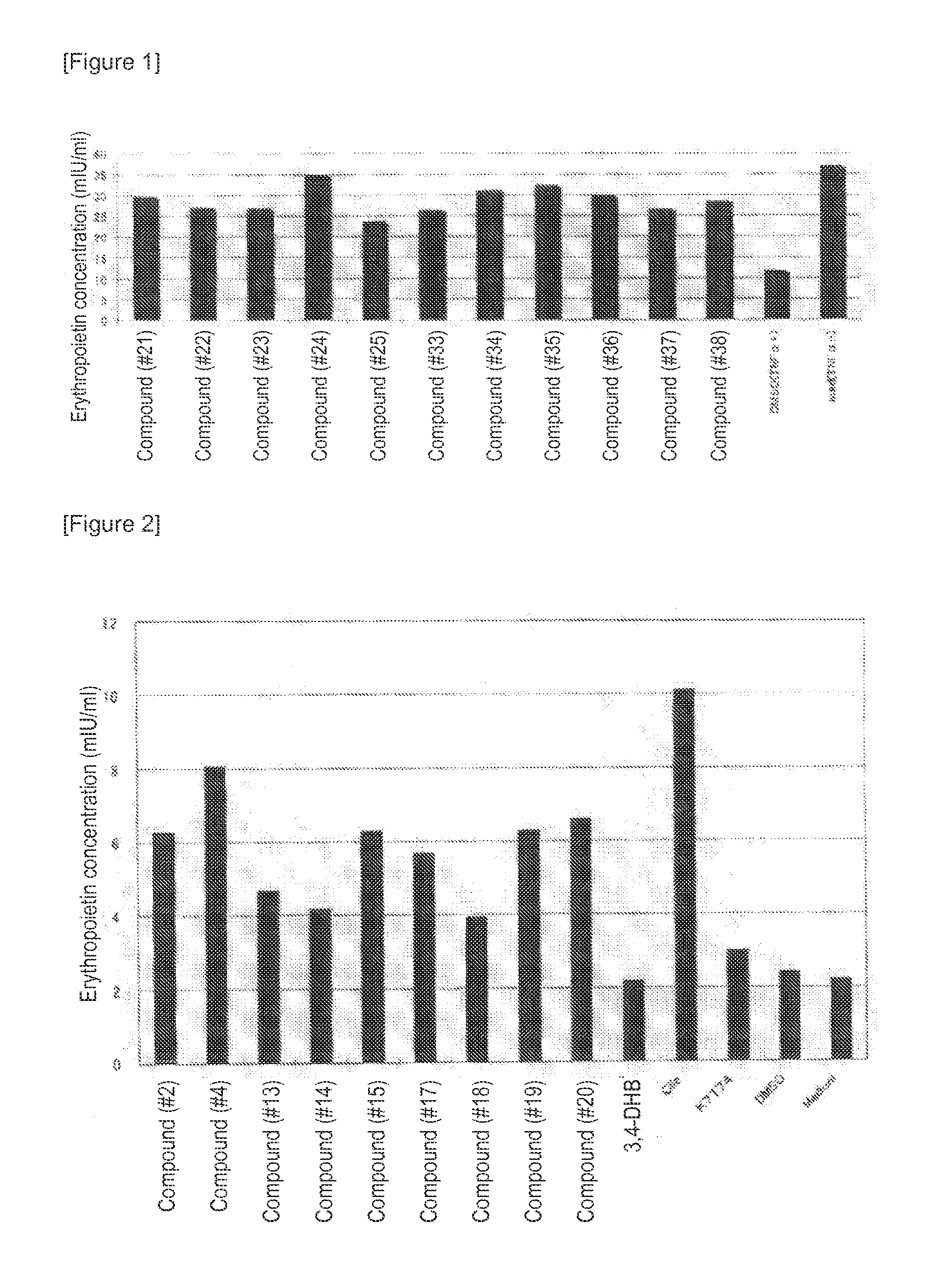
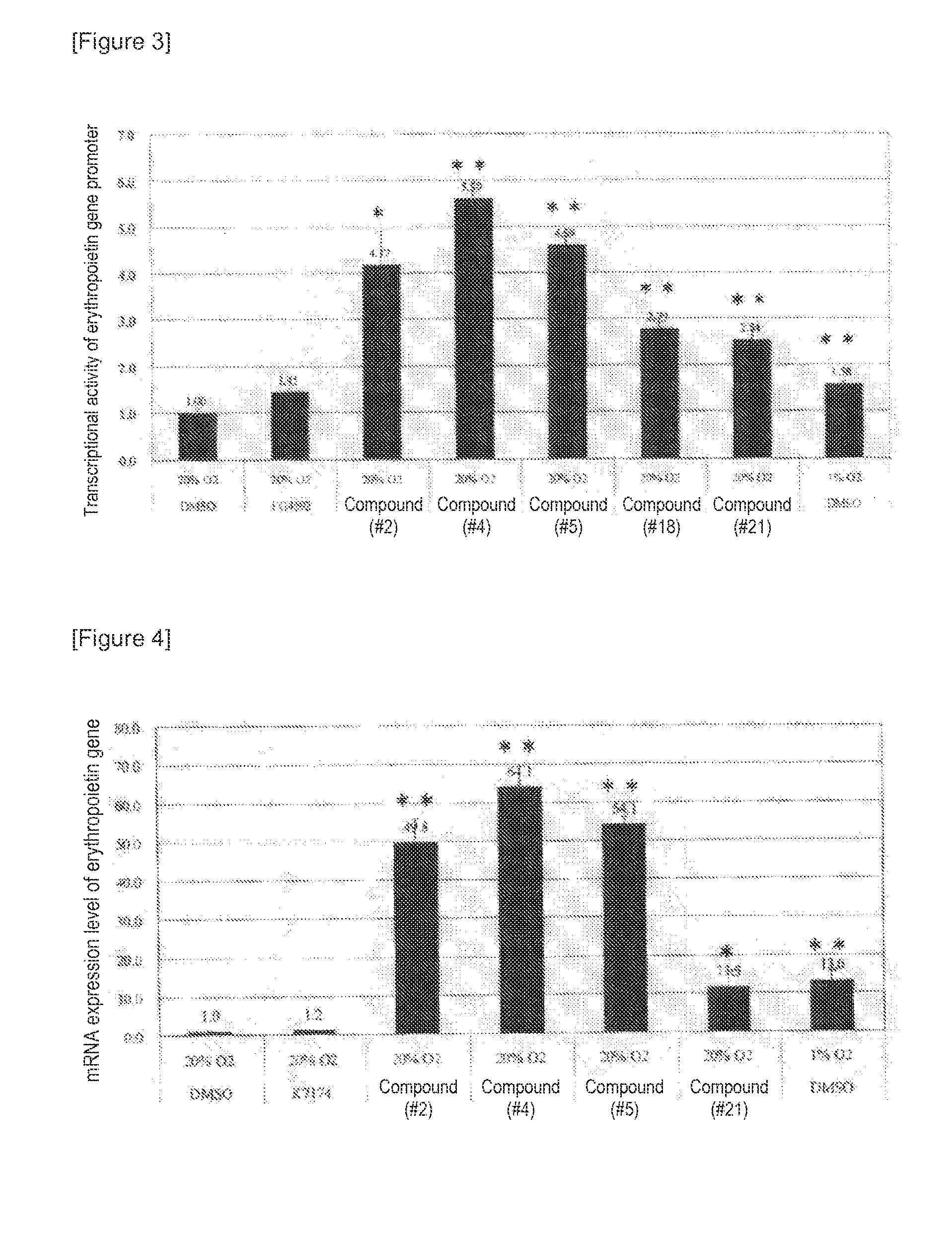
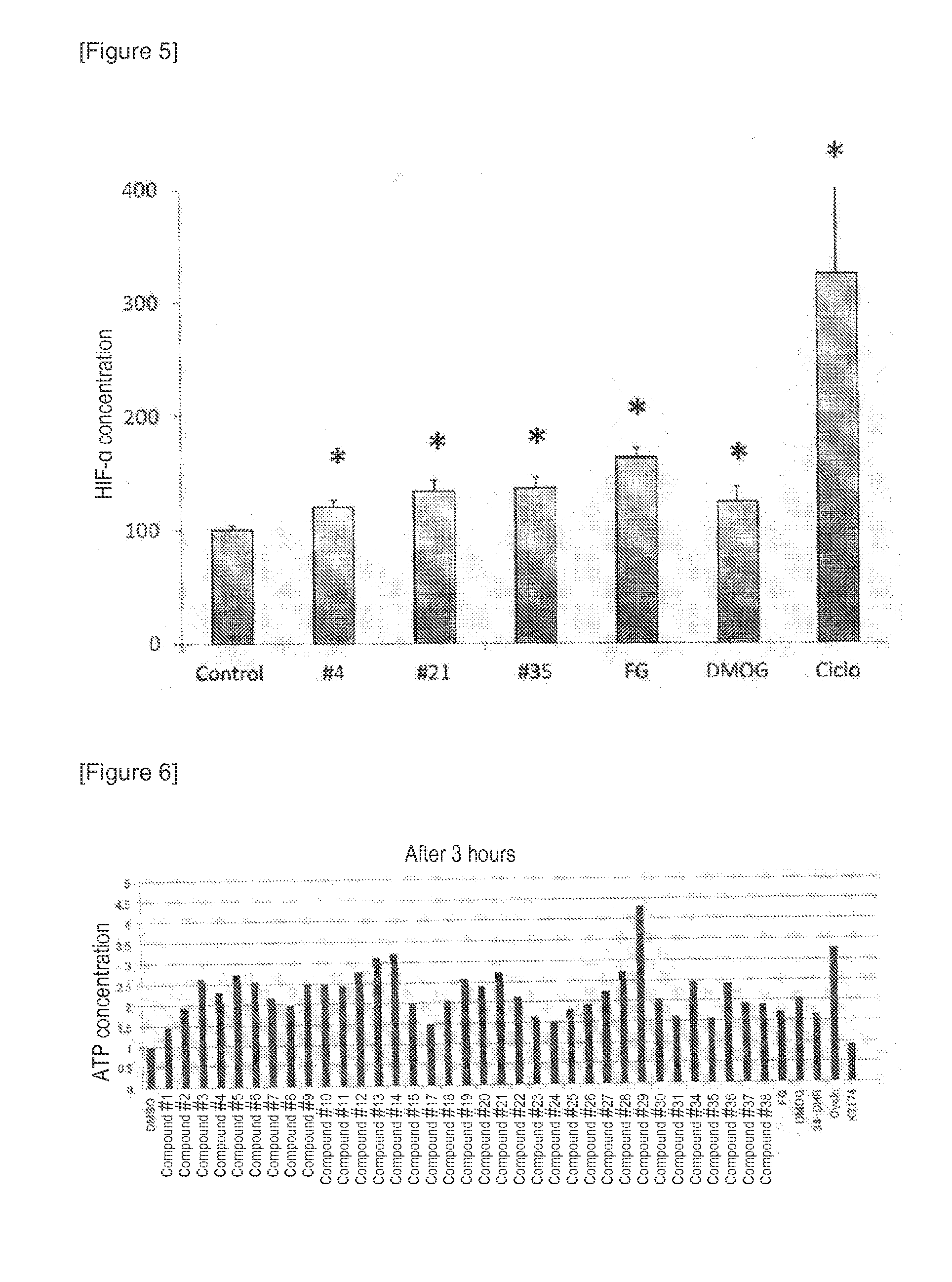
The present invention provides an erythropoietin expression-enhancing agent that can cancel the suppression of erythropoietin production or promote erythropoietin production, and a therapeutic or preventive drug for anemia, a liver function-improving agent, an ischemic injury-improving agent, a renal protective agent, and an insulin secretagogue comprising the erythropoietin expression-enhancing agent. The erythropoietin expression-enhancing agent of the present invention comprises one or more compounds selected from the group consisting of compounds represented by the following general formulas (I), (II), and (III) and pharmaceutically acceptable salts thereof when R3 is OH.
Owner:TOHOKU UNIV +2
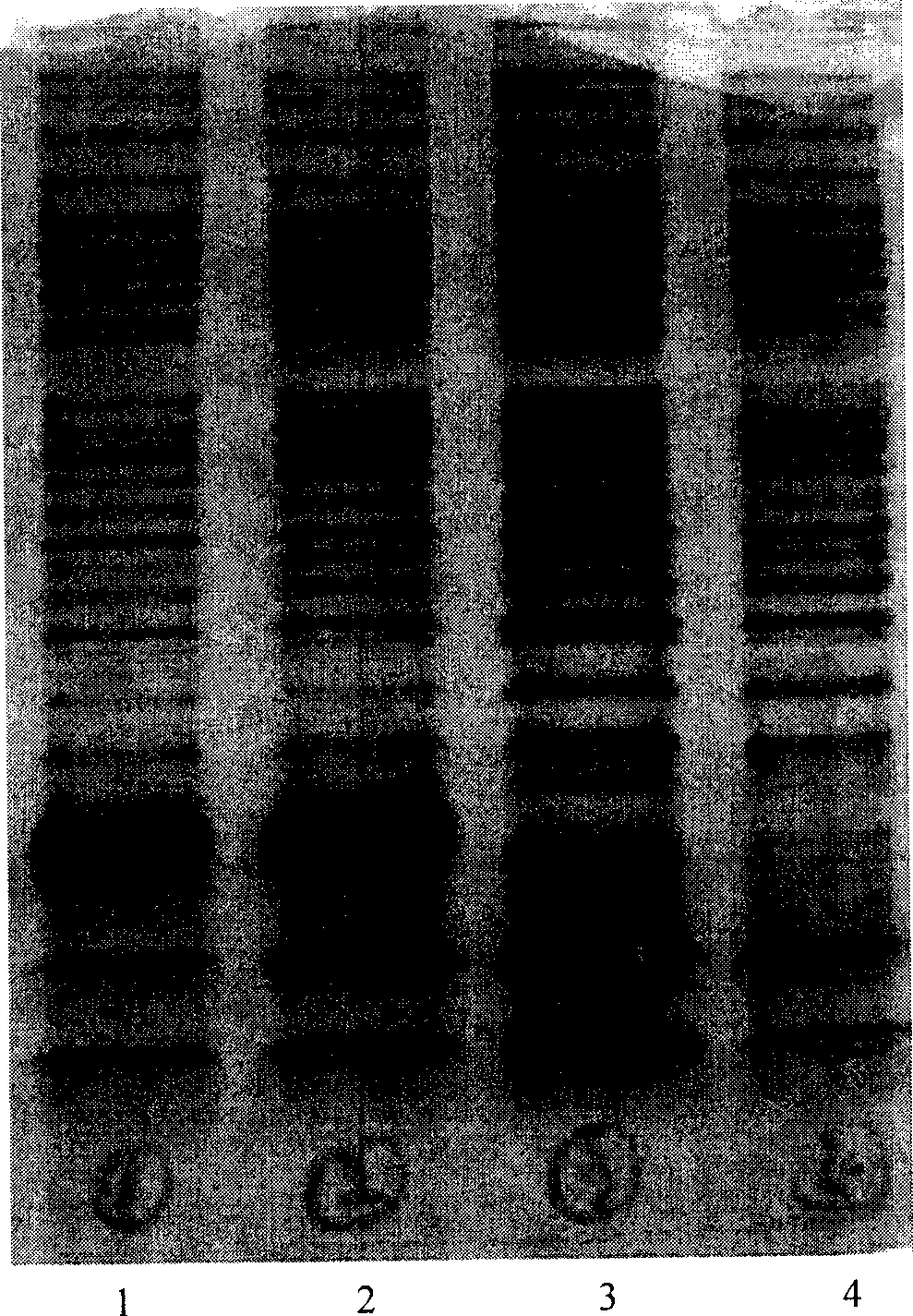
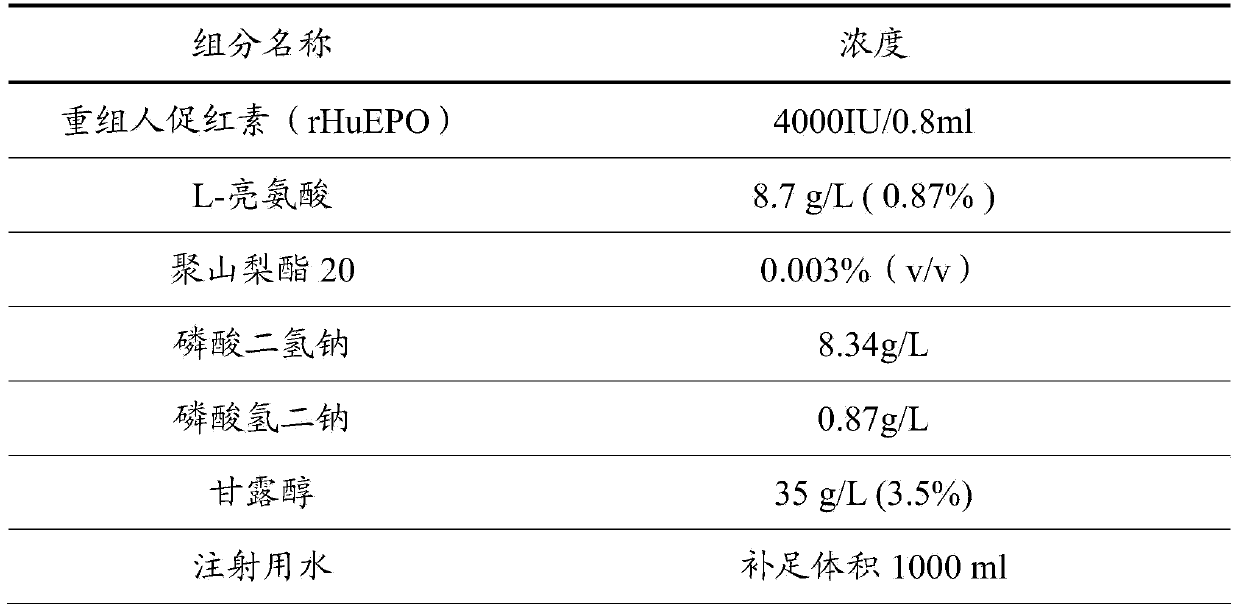
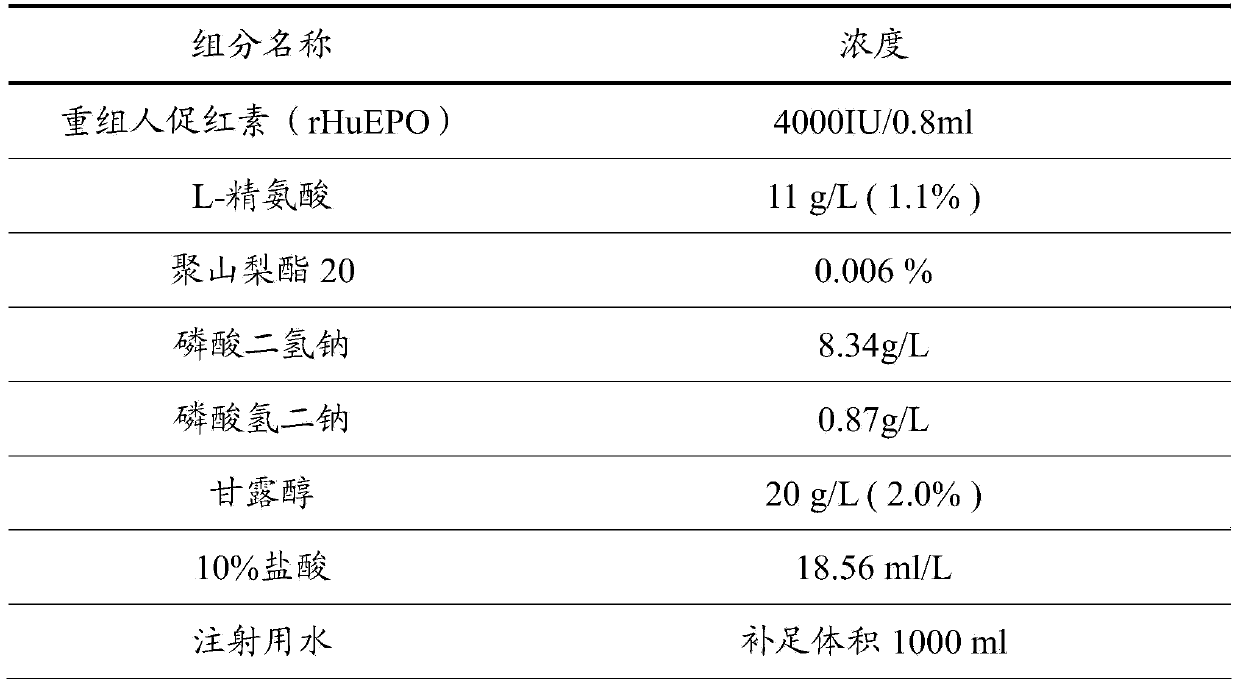
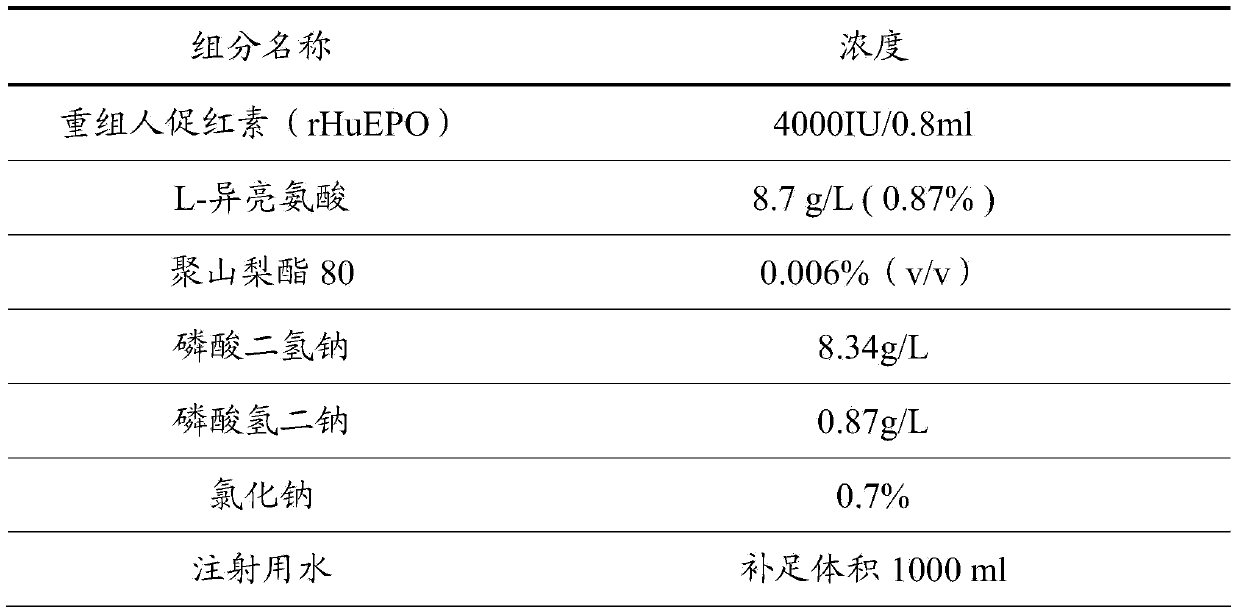
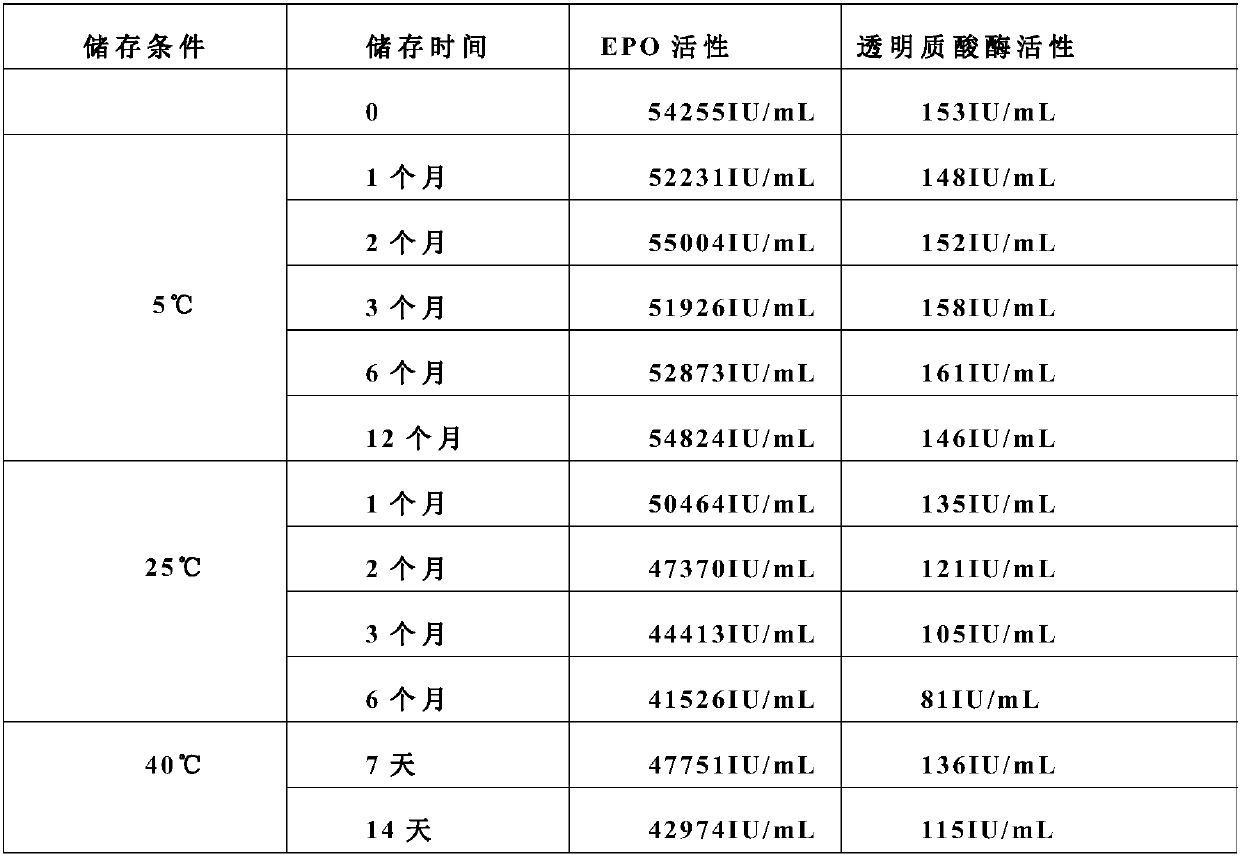
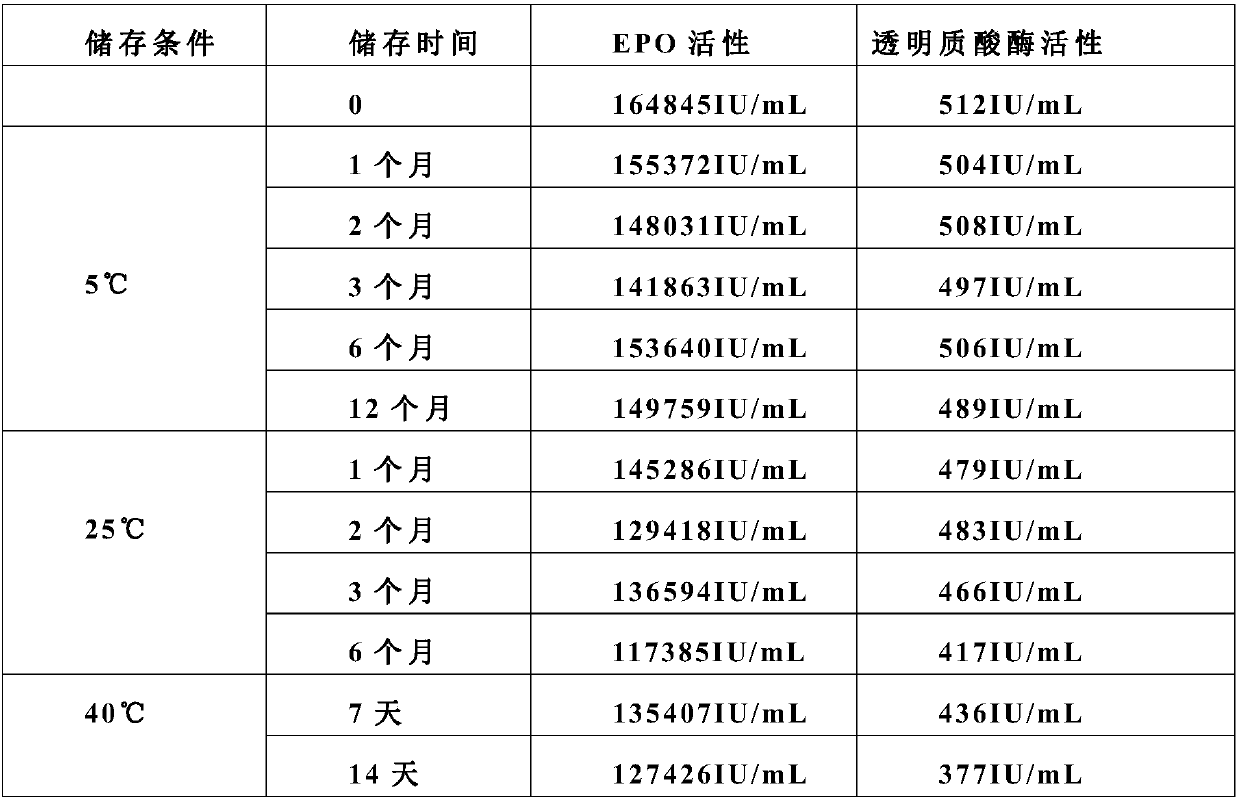
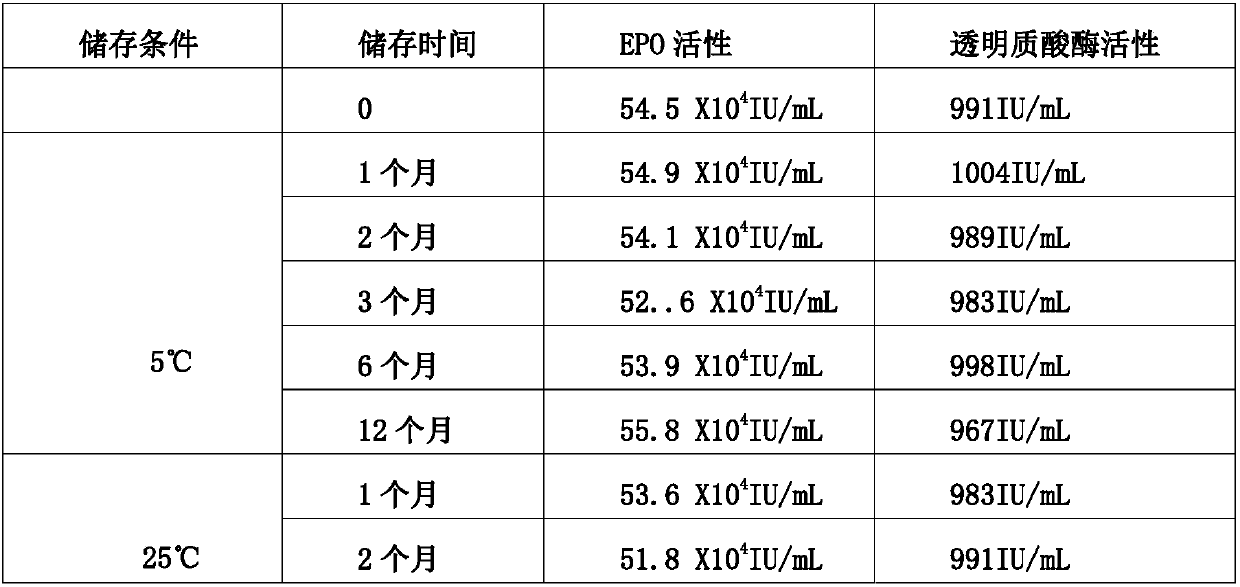
Stable preparation formula of erythopoietin
InactiveCN107596349APeptide/protein ingredientsDermatological disorderBuffering agentNonionic surfactant
The invention provides a stable preparation formula of erythopoietin (EPO). The stable preparation formula comprises erythopoietin, hyaluronidase, a buffering agent, a stabilizing agent and a nonionicsurfactant. The stable preparation formula of the EPO is used for subcutaneous or intramuscular injection, and the dosage form is an injection or freeze-dried powder injection. The EPO can be used for treating the diseases such as anemia caused by kidney failure, anemia caused by radiotherapy and chemotherapy of patients with cancer, autologous transfusion before operation, neonatal anemia and the like, and also can be used for cosmetics and the like.
Owner:刘冬连 +1
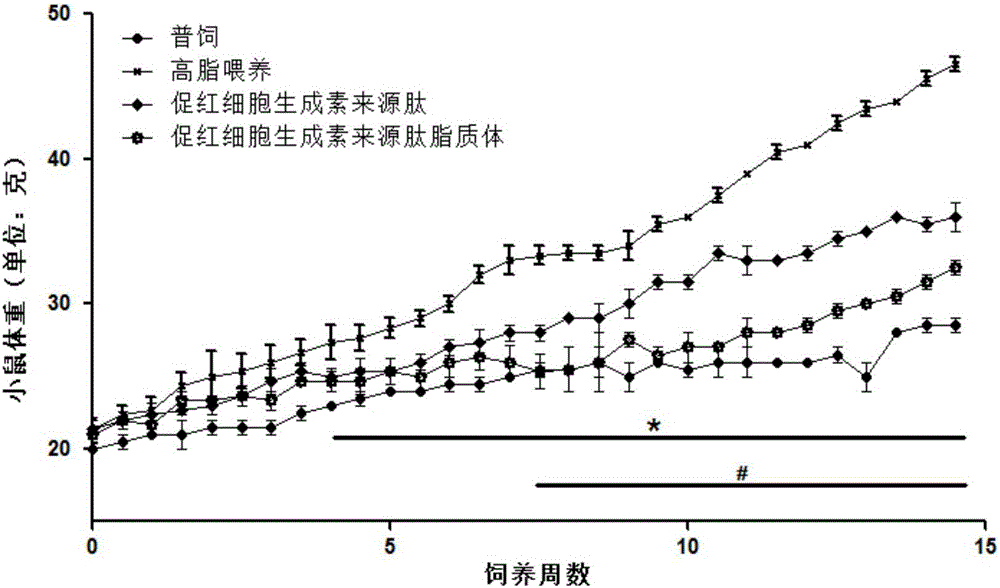
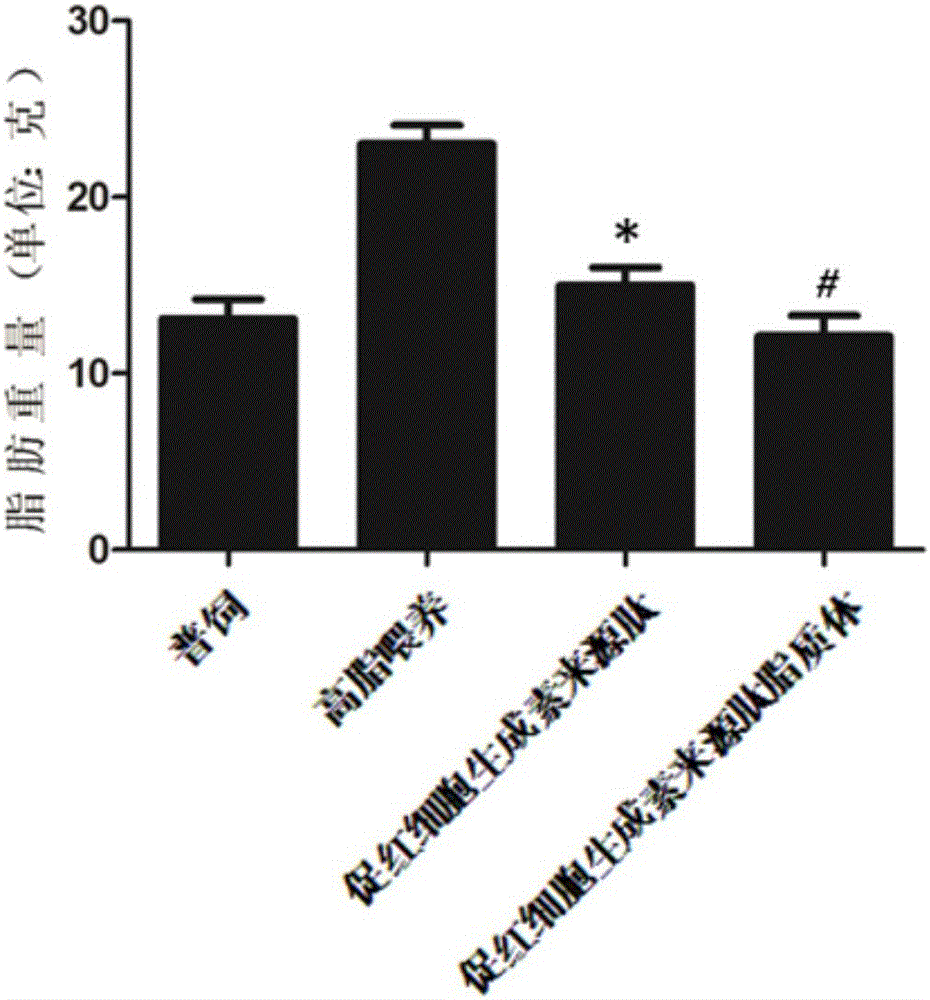
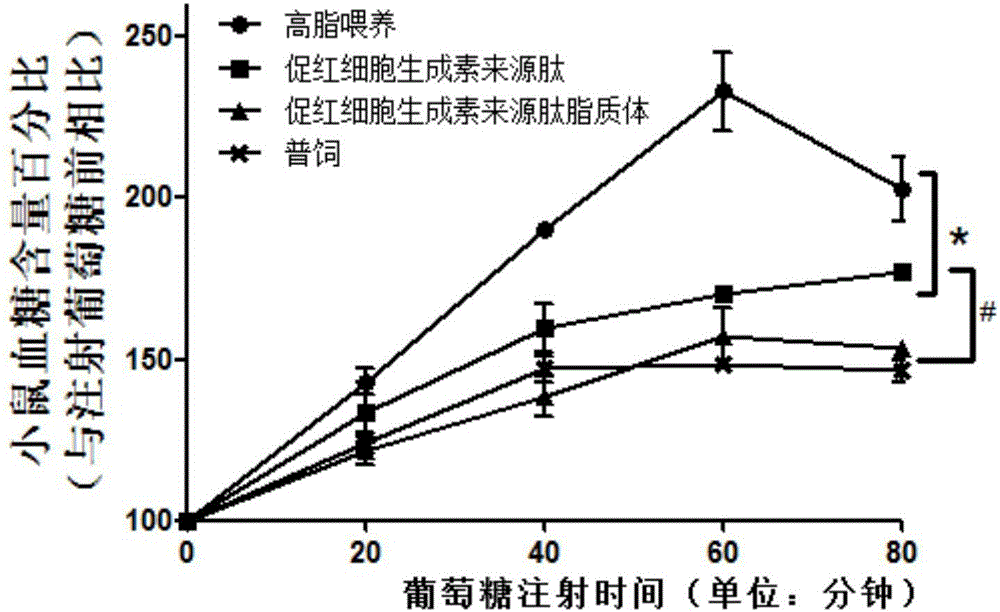
Application of hemopoietin sourced peptide in preparation of medicine for treating metabolic syndrome
ActiveCN105148257AProlong the action timeIncrease fat solubilityPeptide/protein ingredientsMetabolism disorderSolubilitySide effect
The invention belongs to the field of bio-medicines, and particularly relates to an application of a hemopoietin sourced peptide in preparation of a medicine for treating the metabolic syndrome. The application includes an application of preparation of medicines for treating obesity, diabetes mellitus and hyperlipemia, The hemopoietin sourced peptide can be prepared into lipidosome, and the metabolic syndrome can be treated by the hemopoietin sourced peptide and the hemopoietin sourced peptide lipidosome, the syndromes of the hemopoietin sourced peptide are remarkably relieved after treatment, and the effect of the hemopoietin sourced peptide lipidosome is superior to that of the hemopoietin sourced peptide, and the administration frequency can be reduced. The application of the hemopoietin sourced peptide lipidosome to medicines can be used for prolonging the medicine acting period, increasing the lipid solubility and stability of the medicines and reducing toxic and side effects. Therefore, compared with an independent application of micro-molecule polypeptide, lipidosome coated micro-molecule polypeptide has the advantages of prolonging half-life period, improving stability and lipid solubility and increasing administration routes. The hemopoietin sourced peptide has an important clinical application value.
Owner:ARMY MEDICAL UNIV
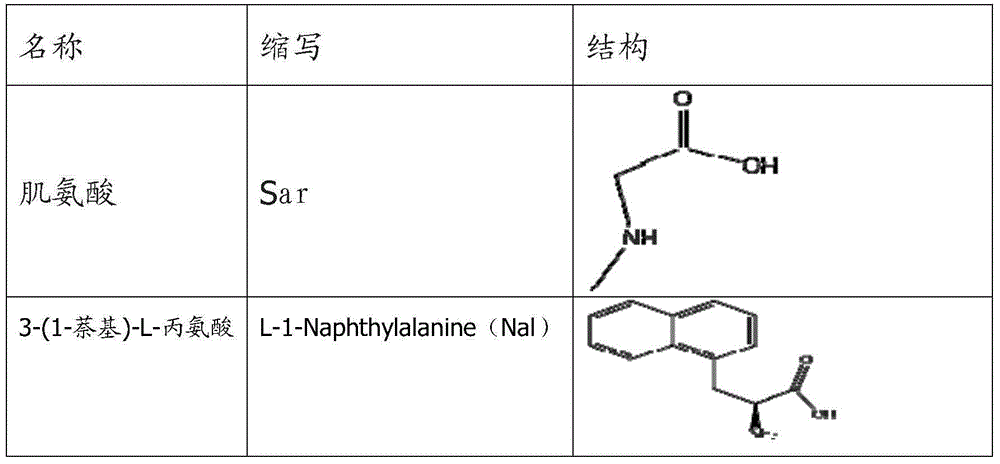
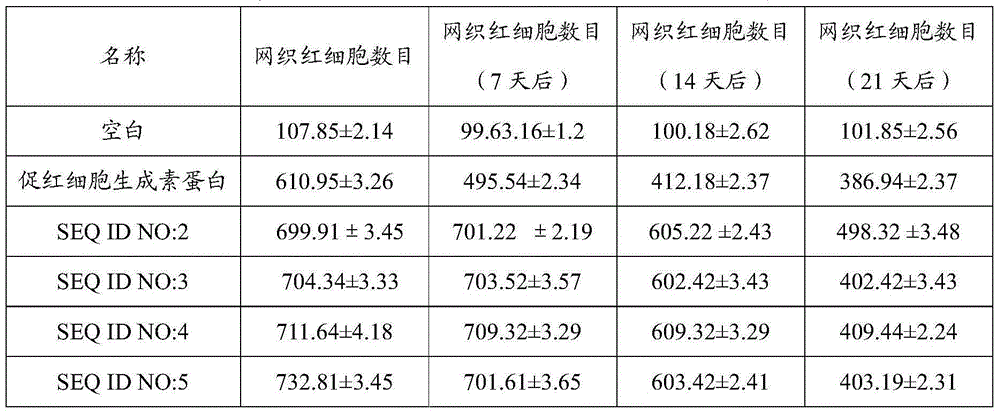
Erythropoietin mimic peptide and preparation method and application thereof
ActiveCN105085653APacking has no appreciable effect onHemoglobin content had no appreciable effectPeptide/protein ingredientsAntipyreticDiseaseAmino acid side chain
The invention relates to erythropoietin mimic peptide capable of lastingly promoting erythropoiesis. Amino acid sequence of the erythropoietin mimic peptide is shown as SEQ ID NO:1GGLYAX1HMGPITX2VX3QPLRX4K, wherein X1 is chosen from lysine or arginine, X2 is 3-(1-naphthyl)-L-alanine, X3 is chosen from aspartic acid or glutamine, X4 is sarcosine, and free amino and carboxyl of an amino acid side chain are cyclically condensed between X1 and X3, and N terminal is acetylized. The invention further provides application of the erythropoietin mimic peptide in drug used for treating diseases featured by erythrocyte deficiency or erythrogenin deficiency or erythrocyte group deficiency or defect. The erythropoietin mimic peptide can promote generation of erythrocytes, and half-life period of the drug in vivo is prolonged remarkably.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
In-vitro culture solution of mesenchymal stem cells and culture and amplification method
InactiveCN106190965AImprove efficiencyHighlight substantive featuresCulture processSkeletal/connective tissue cellsVitamin CFiltration
The invention discloses an in-vitro culture solution of mesenchymal stem cells and a culture and amplification method. According to the in-vitro culture solution, DMEM culture solution is taken as a basic culture medium, compound (I), L-glutamine, vitamin C, insulin, erythrogenin and vascular endothelial growth factors are sequentially added, then sodium hydroxide is adopted for adjusting the pH, finally, fetal calf serum is added, filtration sterilization is carried out, and thus the in-vitro culture solution of the mesenchymal stem cells is obtained, wherein the concentration of the compound (I) is 15-25 mg / L, the concentration of L-glutamine is 200-400 mg / L, the concentration of vitamin C is 60-90 mg / L, the concentration of insulin is 10-20 mU / L, the concentration of the erythrogenin is 20-40 U / L, and the concentration of the vascular endothelial growth factors is 20-120 [mu] g / L. With the adoption of the in-vitro culture solution of mesenchymal stem cells and the culture and amplification method, the amplification efficiency of the human mesenchymal stem cells can be improved, and compared with the prior art, the in-vitro culture solution and the culture and amplification method have the outstanding substantive features and prominent progress.
Owner:袁肇斌
Substituted pyrimidines as inhibitors of hif prolyl hydroxylase
The present invention concerns compounds of Formula I that inhibit HIF prolyl hydroxylase, their use for enhancing endogenous production of erythropoietin, and for treating conditions associated with reduced endogenous production of erythropoietin such as anemia and like conditions, as well as pharmaceutical compositions comprising such a compound and a pharmaceutical carrier.
Owner:MERCK SHARP & DOHME CORP
Chromatographic purification of recombinant human erythropoietin
The invention provides a method for recovering and purifying recombinant human erythropoietin (rhEpo) from a cell culture medium comprising host cells, which method comprises the steps of: (a) removing host cells, cellular constituents and debris from the cell culture medium by centrifugation using a disc stack separator followed by a depeth filtration step to obtain a clarified culture medium supernatant; (b) adjusting the conductivity of the supernatant to 5 mS / cm or less, and a pH of between about 7.0 and 8.0; (c) applying the supernatant from step (b) to a column comprising an anion exchange chromatographic medium, washing the column, eluting the rhEpo from the column, and collecting the peak fraction (s) that contain rhEpo; (d) subjecting the combined peak fractions from step (c) to a reverse phase chromatography step using a polystyrene resin that can be run under medium pressure (< 10 bar) and is resistance to high concentrations of NaOH, such as Source 30RPC, the rhEpo being eluted using a linear gradient of an organic solvent; (e) applying one or more fractions eluted in step (d) which contain rhEpo to a column comprising Q-Seph HP anion exchange chromatographic media, washing the column, and eluting the rhEpo using a linear salt gradient; (f) selecting one or more fractions eluted in step (e) which contain rhEpo based on degree of sialylation of the rhEpo; and (g) subjecting one or more fractions eluted in step (f) which contain rhEpo by one or more size exclusion chromatographic steps using Superdex 75 prep grade to remove potential dimers and higher aggregates; and collecting the eluate containing rhEpo.
Owner:SANDOZ AG
Liquid pharmaceutical composition of conjugated erythropoietin
ActiveUS20170112901A1Stable pharmaceutical compositionPeptide/protein ingredientsInorganic non-active ingredientsSucroseArginine
A liquid pharmaceutical composition having a conjugated erythropoietin, buffer, sugar, tonicity modifier and amino acid as an aggregation inhibitor. The liquid pharmaceutical composition provides a stable pharmaceutical composition which encompasses conjugated erythropoietin, acetate buffer, sucrose, arginine and sodium chloride which is maintained at a pH of about 4.9 to 5.3.
Owner:INTAS PHARM LTD
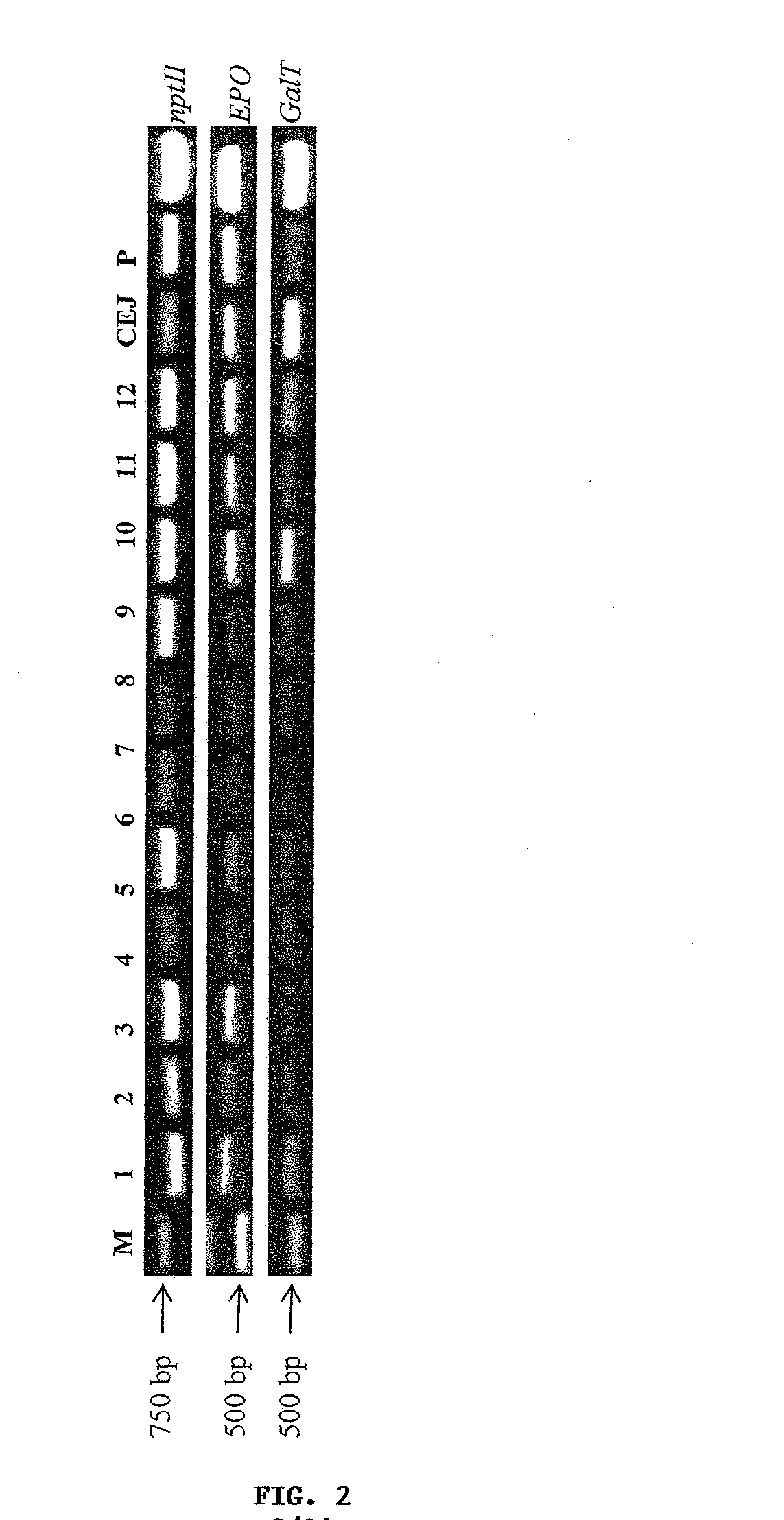
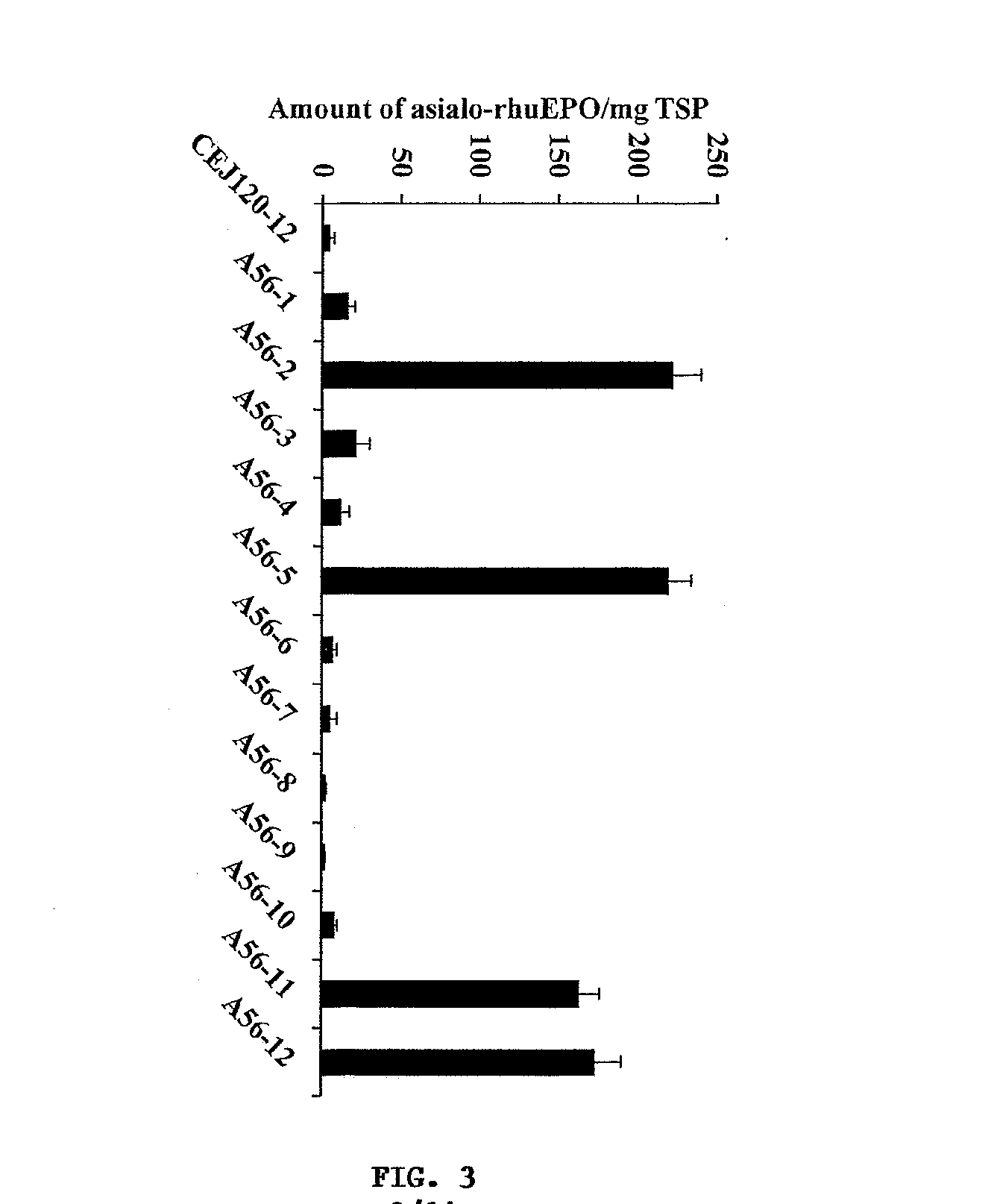
Methods for the production of cytoprotective asialo-erythropoietin in plants and its purification from plant tissues
PendingUS20150119325A1Improve the level ofEasy to producePeptide/protein ingredientsImmunoglobulinsNucleotidePlant cell
The present invention provides methods for the high-level production of recombinant human erythropoietin (rhuEPO) derivative, asialoerythropoietin (asialo-rhuEPO), in plants. The methods for producing asialo-rhuEPO comprise making a plant or at least one plant cell that comprises a promoter that drives expression in a plant cell operably linked to a polynucleotide encoding a human erythropoieting fusion protein and a promoter that drives expression in a plant cell operably linked to a polynucleotide encoding N-glycosylation modification enzyme, particularly a mammalian β1,4-galactosyltransferase. The present invention further provides plants, plant cells, and seeds that have been genetically modified to produce high levels of asialo-rhuEPO. Additionally, provided are methods for purifying asialo-rhuEPO from plant tissues. Such methods comprise removing chlorophyll and / or RuBisCO protein from an aqueous extract of plant tissue comprising asialo-rhuEPO, binding the asialo-rhuEPO in the extract to an immune affinity column, and eluting the bound asialo-rhuEPO from immune affinity column.
Owner:NORTH CAROLINA CENTRAL UNIVERSITY
Long lasting drug formulations
InactiveUS9127084B2Improve the level ofBiocidePeptide/protein ingredientsNucleic acid sequencingRegulatory sequence
The present invention is directed to long-lasting erythropoietin therapeutic formulations and their methods of use wherein the formulation comprises a genetically modified micro-organ that comprises a vector which comprises a nucleic acid sequence operably linked to one or more regulatory sequences, wherein the nucleic acid sequence encodes erythropoietin.
Owner:MEDGENICS MEDICAL ISRAEL
Hemopoietin peptide, hemopoietin peptide derivative, hemopoietin peptide polymer, preparation method of hemopoietin peptide and application of hemopoietin peptide
InactiveCN106928338APreparation curative effect is remarkableLittle side effectsPeptide/protein ingredientsPeptide preparation methodsSide effectTherapeutic effect
The invention relates to the fields of phaseolin polypeptide, and particularly relates to a hemopoietin peptide, a hemopoietin peptide derivative, a hemopoietin peptide polymer, a preparation method of the hemopoietin peptide and an application of the hemopoietin peptide. The sequence of the hemopoietin peptide is SEQ ID No.1:R1YR2CR3R4GPR5TWVCR6R7R8, wherein R1, R2, R5, R6, R7 and R8 are respectively independent L type or D type amino acids; the R3 is Lys, Glu, Asp, Gln, Asn, Met, Ser, Tyr, Pro or Ile, the R3 is an L type or D type amino acid, the R4 is Met, Phe or Ile, and the R4 is an L type amino acid. The hemopoietin peptide can be used to treat anaemia or related diseases caused by anaemia, and to be specific, the hemopoietin peptide is useful for preparing medicines which treats anaemia or related diseases caused by anaemia and are more remarkable in treatment effects and lower in side effects.
Owner:ADLAI NORTYE BIOPHARMA CO LTD
Separation and purification process of recombinant human erythrogenin
InactiveCN1243019CAvoid sex changeAvoid toxicityPeptide preparation methodsErythropoietinSephadexOrganic solvent
The separation and purification process of recombinant human erythrogenin includes the following steps: Blue sepharose F. F. chromatography; metal chelating affinity column chromatography; Sephadex G-25 column chromatography; and Source Q column chromatography, collecting destination protein and 0.22 micron membrane filtering. The process has low cost, and using no reverse chromatography and organic solvent avoids denaturation of protein during chromatography and residue of organic solvent. The combined purification process including Blue sepharose Fast Flow chromatography, metal chelating affinity column chromatography Sephadex G-25 column chromatography and Source Q column chromatography successively can prepare medicine level rHuEPO protein in large scale.
Owner:NANJING NORMAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com