Stable preparation formula of erythopoietin
A technology of erythropoietin and stabilizer, which is applied in the fields of pharmaceutical formulations, extracellular fluid diseases, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
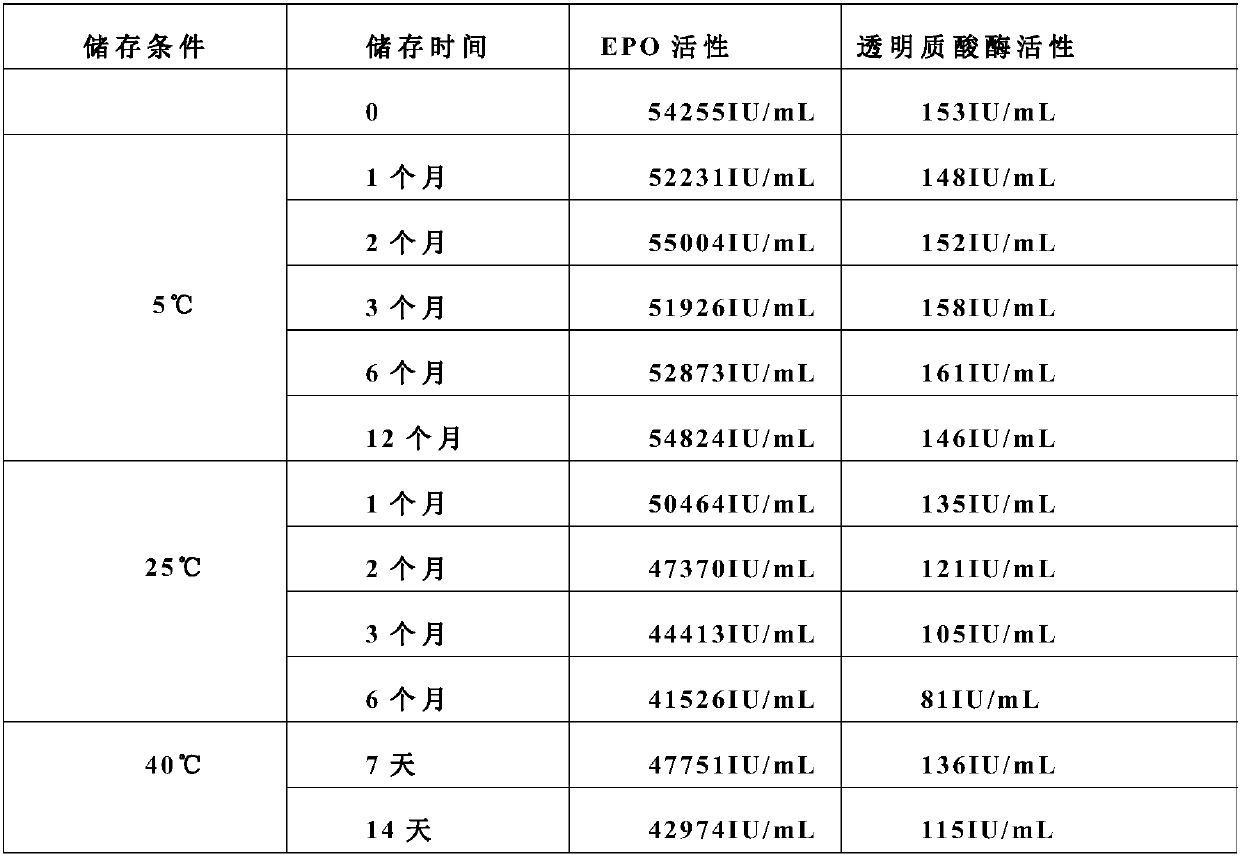
[0039] A stable erythropoietin formulation consists of the following components: 500ug / mL EPO, 10mM citrate buffer, pH7.0, 2.5mg / mL human albumin, 150IU / mL hyaluronidase, 0.01% polysorbate Ester 20.
[0040]
Embodiment 2
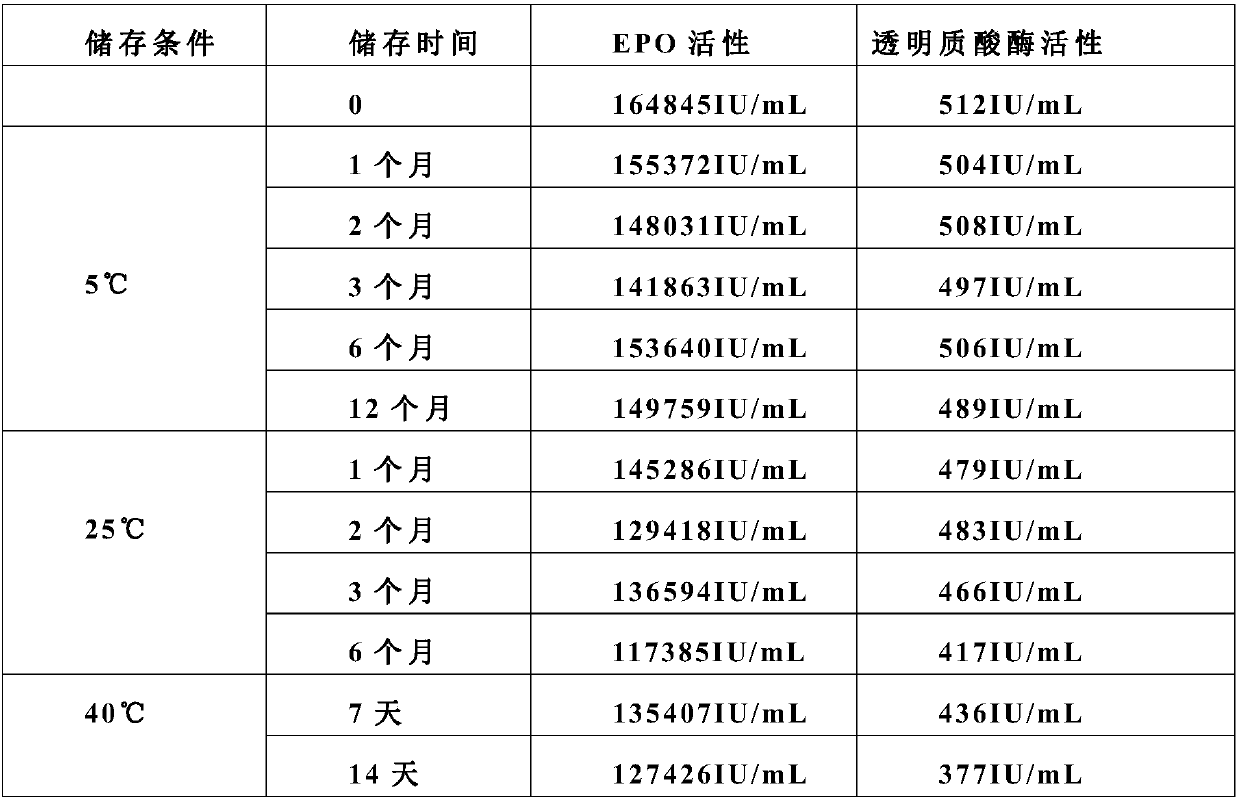
[0042] A stable erythropoietin formulation consists of the following components: 1.5mg / mL LEPO, 10mM citrate buffer, pH 7.0, 100mM trehalose dihydrate, 500IU / mL hyaluronidase, 0.04% polysorbate 20.
[0043]
Embodiment 3
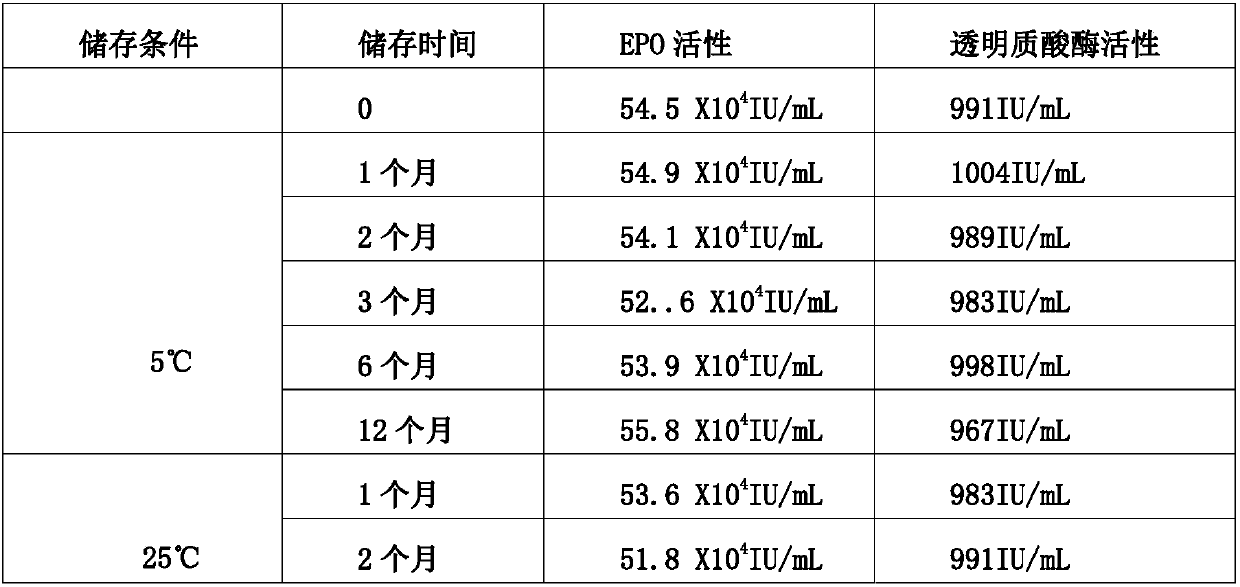
[0045] A stable erythropoietin formulation consists of the following components: 5 mg / mL EPO, 10 mM phosphate buffer, pH 6.8, 150 mM trehalose dihydrate, 1000 IU / mL hyaluronidase, 0.04% polysorbate 20 .
[0046]
[0047]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com