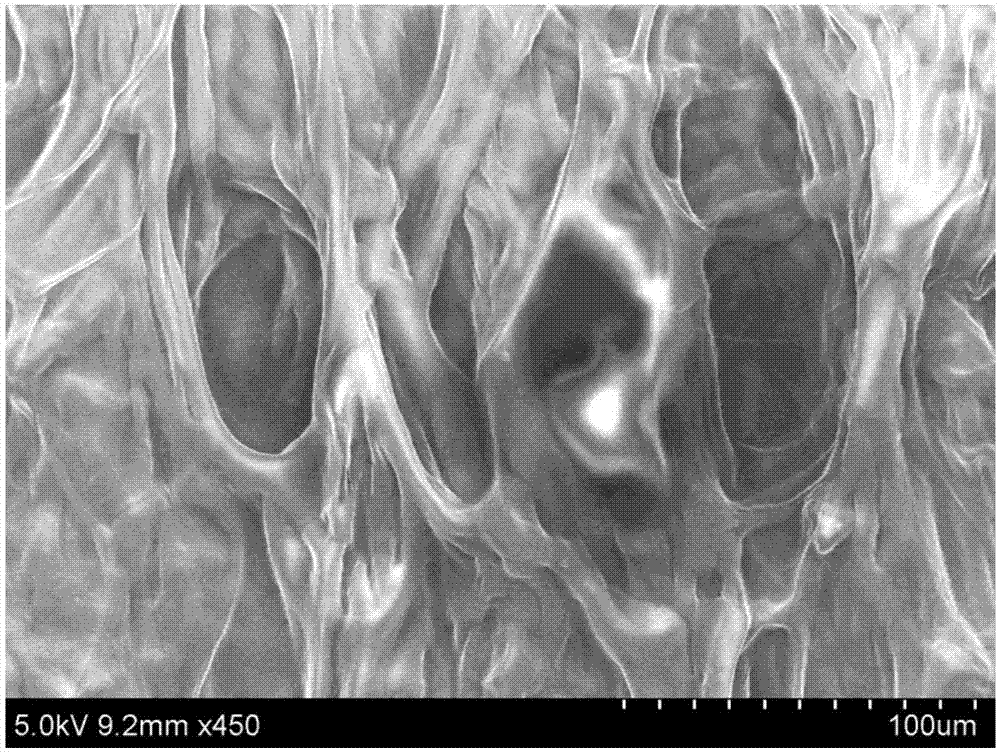
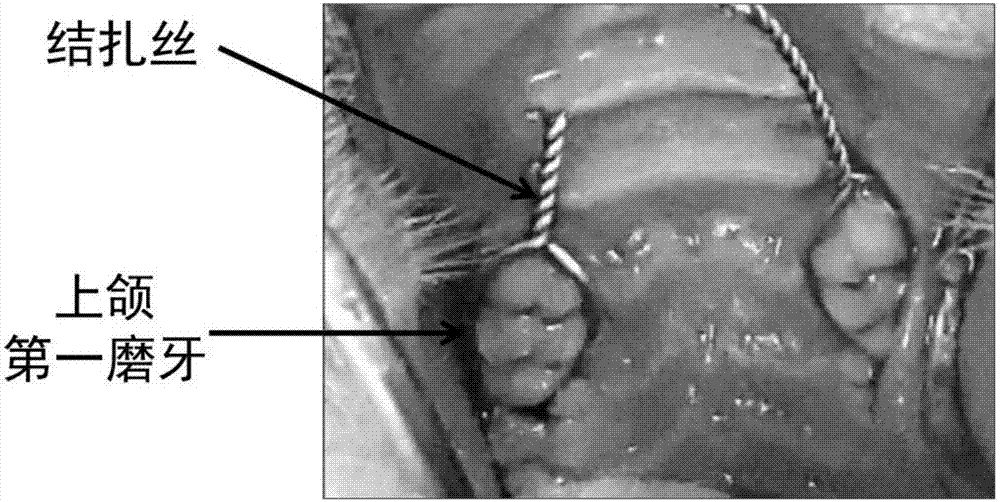
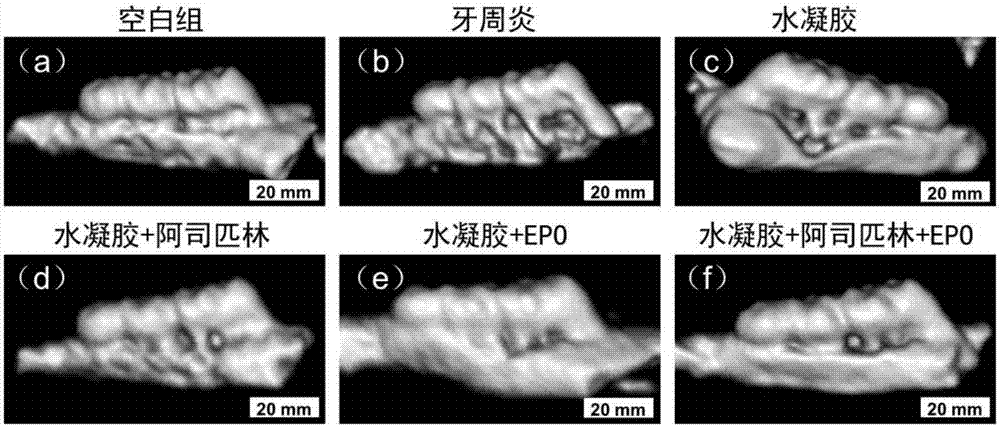
Chitosan-based periodontal local drug slow-release hydrogel and application thereof
A topical drug and hydrogel technology, which is applied in drug combinations, medical preparations containing active ingredients, and pharmaceutical formulas, to achieve the effects of low risk, simple experimental operation, and good experimental repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] (1) Preparation of each component solution of the hydrogel: 20 mg of aspirin (US Sigma Company) was added to 20 mL of 0.1 mol / L hydrochloric acid (Beijing Chemical Plant) solution, and 0.4 g of chitosan (US Sigma Company) was added. ), stir to obtain aspirin-containing chitosan solution. 1 g of sodium β-glycerophosphate (Sigma, USA) was added to 2 mL of deionized water, and 6000 U of EPO (China Sansheng Pharmaceutical Co., Ltd.) was added, and stirred evenly to obtain a sodium β-glycerophosphate solution containing EPO. Add 20 mg of gelatin (Sigma, USA) into 10 mL of deionized water, and stir evenly to obtain a gelatin solution.
[0018] (2) Gelation of chitosan thermosensitive hydrogel loaded with aspirin and EPO: take 2mL of the above-mentioned chitosan solution containing aspirin, add 0.25mL of the above-mentioned β-glycerol dropwise at a volume ratio of 8:1:0.25 Sodium phosphate solution and 62.5 μL of the above-mentioned gelatin solution were stirred evenly, place...
Embodiment 2
[0021] (1) Preparation of the solutions of the various components of the hydrogel: 40 mg of aspirin was added to 40 mL of 0.1 mol / L hydrochloric acid solution, and 0.8 g of chitosan was added, and stirred evenly to obtain a chitosan solution containing aspirin. Add 2 g of sodium β-glycerophosphate to 4 mL of deionized water, and add 10000 U of EPO, and stir evenly to obtain a solution of sodium β-glycerophosphate containing EPO. Add 100 mg of gelatin into 20 mL of deionized water, and stir evenly to obtain a gelatin solution.
[0022] (2) Gelation of chitosan thermosensitive hydrogel loaded with aspirin and EPO: Take 4 mL of the above chitosan solution, add 1 mL of the above β-glycerophosphate dropwise at a volume ratio of 12:3:1 Solution and 333 μL of the above-mentioned gelatin solution were stirred evenly, and placed in constant temperature water baths at 33°C, 34°C, 35°C, 36°C, 37°C, 38°C and 40°C respectively, and their gelatinization at different temperatures were detect...
Embodiment 3
[0024] (1) Preparation of the solution of each component of the hydrogel: 30 mg of aspirin was added to 30 mL of 0.1 mol / L hydrochloric acid solution, and 0.5 g of chitosan was added, and stirred evenly to obtain a chitosan solution containing aspirin. Add 1.5 g of β-sodium glycerophosphate to 3 mL of deionized water, and add 8000 U of EPO, and stir evenly to obtain a solution of β-sodium glycerophosphate containing EPO. Add 50 mg of gelatin into 15 mL of deionized water, and stir evenly to obtain a gelatin solution.
[0025](2) Gelation of chitosan temperature-sensitive hydrogel loaded with aspirin and EPO: Take 3mL of the above-mentioned chitosan solution, add 0.6mL of the above-mentioned β-glycerophosphate dropwise at a volume ratio of 10:2:0.5 Sodium solution and 150 μL of the above gelatin solution, stir well.
[0026] (3) In order to investigate the control effect of the aspirin and EPO-loaded chitosan thermosensitive hydrogel on periodontal inflammation and the effect ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com