Recombinant human erythropoietin preparation without human albumin
A technology of human serum protein and serum protein, which is applied in the field of recombinant human erythropoietin preparations, can solve problems such as hazards, and achieve good high temperature stability and good room temperature stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
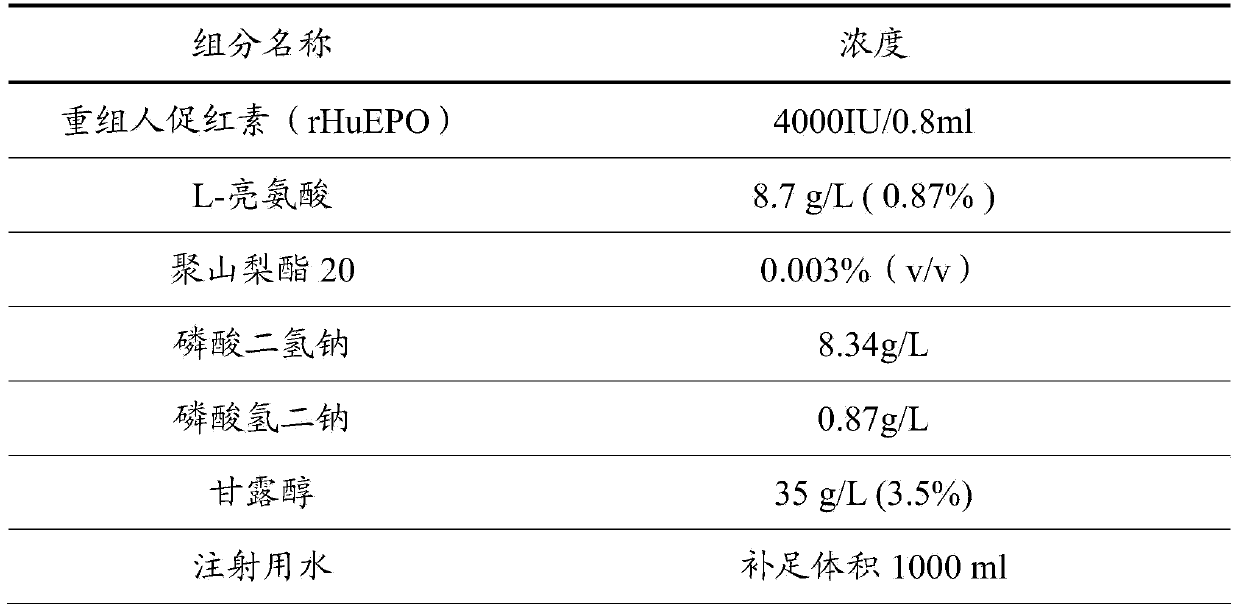
[0025] The components and proportioning ratio of recombinant human erythropoietin preparation in the present embodiment are:
[0026]
[0027] Accurately weigh the above-mentioned components and keep them mixed evenly at room temperature, make up to 1000ml with water for injection to a constant volume, and place them in a sterilized container.
Embodiment 2
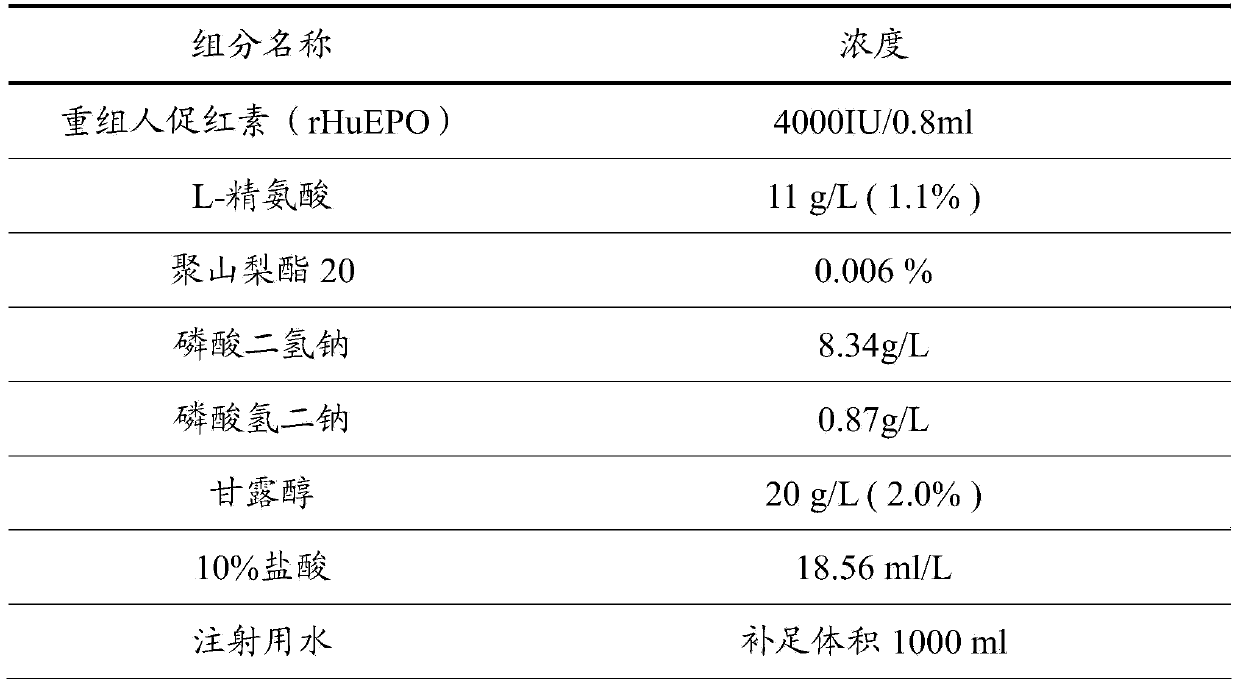
[0029] The difference between this example and Example 1 is that the concentration of recombinant human erythropoietin (rHuEPO) is 2000 IU / ml.
Embodiment 3
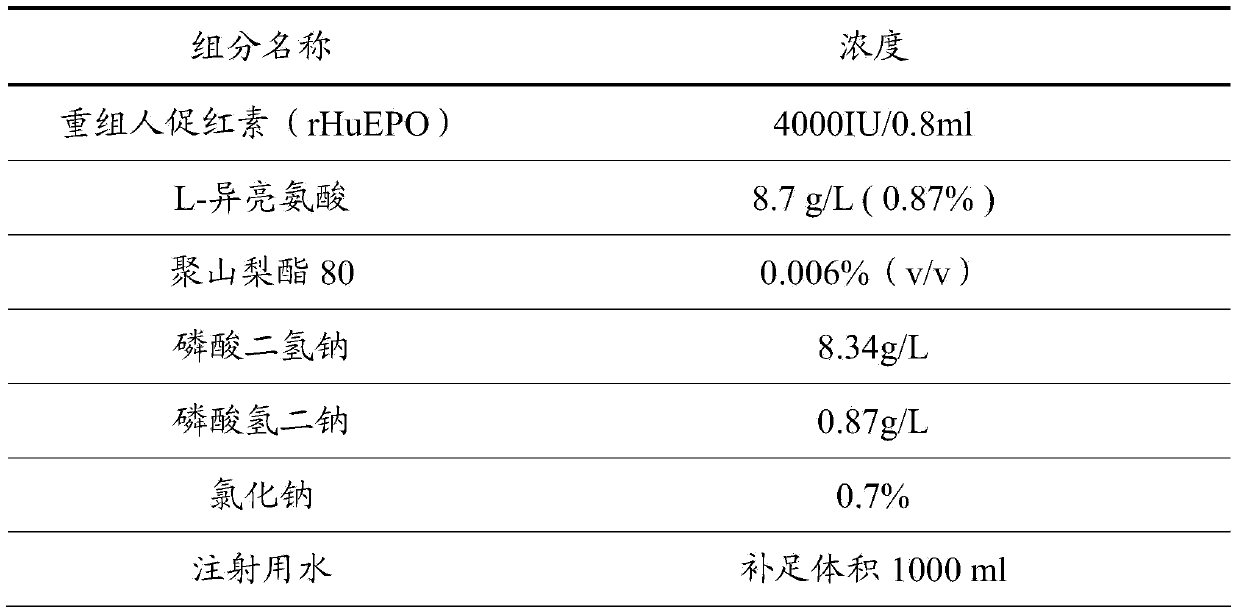
[0031] The difference between this example and Example 1 is that the concentration of recombinant human erythropoietin (rHuEPO) is 20000 IU / ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com