Methoxy polyethylene glycol-modified erythropoietin mimic peptide derivative
A technology of methoxypolyethylene glycol and erythropoietin, which is applied in the field of cytopoietin receptors or erythropoietin hormones, can solve the problems of short half-life and low EC50, and achieve high bioavailability and biological active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example
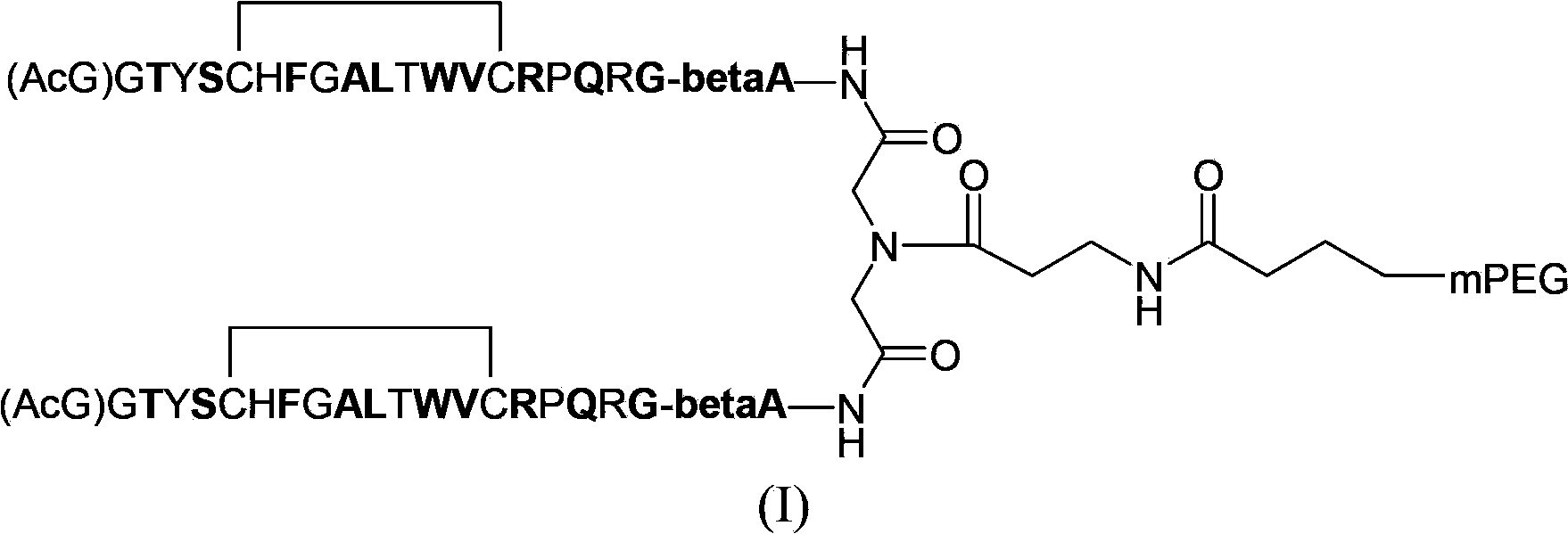
[0035] Reference Example: Preparation of HH-EPO-018(2)
[0036] 1. Synthesis of erythropoietin mimic peptide derivative monomer peptide SEQ ID NO:8
[0037] The synthesis of monomeric peptides of erythropoietin-mimetic peptide derivatives adopts the method of solid-phase peptide synthesis. This type of peptide synthesis method has been reported in many literatures. See stewart, J.M., and Young, J.D., solid phase peptide synthesis 2deditioN, Novabiochem peptide synthesis notes. The erythropoietin mimetic peptide derivative monomer peptide provided by the present invention is manually synthesized, the resin is rink amind resin, the α-amino group of the amino acid derivative used is protected by Fmoc (fluorenylcarbonyl), and the cysteine side Chain sulfhydryl group, glutamine side chain amino group, histidine side chain imidazole group are protected by Trt (trityl), arginine side chain guanidine group is protected by Pbf (2,2,4,6,7-pentamethyl) Dihydrobenzofuran-5-sulfonyl) p...
Embodiment 1
[0052] The preparation of embodiment one HH-EPO-018 (22)
[0053] 1. Synthesis of erythropoietin-mimicking peptide derivative monomer peptide SEQ ID NO: 8: refer to the reference example.
[0054] 2. Preparation of HH-EPO-008: refer to the reference example.
[0055] 3. Preparation of mPEG2-OSU (20kD+20kD)
[0056]
[0057] (1) Preparation of intermediate three
[0058] Add glycerol (15.3mg) into the reactor, dissolve it with boric acid buffer (pH=8), add mPEG-OSU (20KD) (10.0g), stir at room temperature overnight, then add 50ml of deionized water to dilute and adjust with oxalic acid PH≈3, extract completely with dichloromethane (150ml×3), dry over anhydrous magnesium sulfate, concentrate under reduced pressure, settle the residue with ether, filter out the solid, and dry the solid under vacuum to obtain 9.71g of white solid (crude intermediate 3) .
[0059] Dissolve 9.61g of the crude intermediate 3 in 150ml of deionized water and purify it by column chromatography on...
Embodiment 2
[0065] The preparation of embodiment two HH-EPO-018 (31)
[0066]1. Synthesis of erythropoietin-mimicking peptide derivative monomer peptide SEQ ID NO: 8: refer to the reference example.
[0067] 2. Preparation of HH-EPO-008: refer to the reference example.
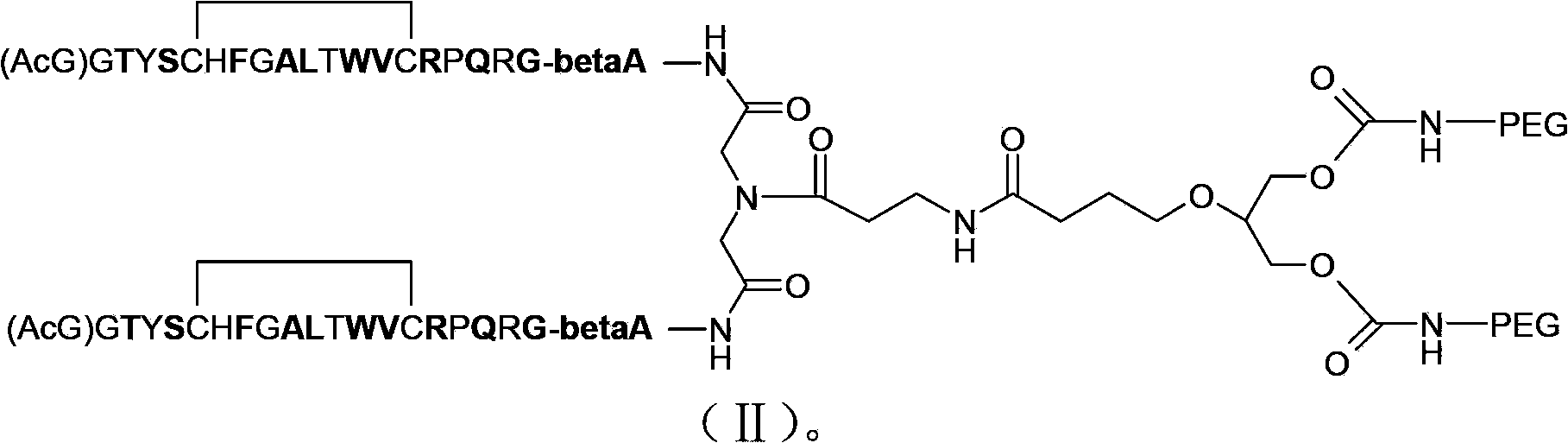
[0068] 3. Preparation of mPEG2-OSU (30kD+10kD)
[0069]
[0070] (1) Preparation of intermediate four
[0071] Add glycerol (460mg) into the reactor, dissolve it with boric acid buffer (pH=8), add mPEG-OSU (10KD) (2.5g), stir at room temperature overnight, then add 50ml of deionized water to dilute and adjust the pH with oxalic acid ≈3, extracted completely with dichloromethane (150ml×3), dried over anhydrous magnesium sulfate, concentrated under reduced pressure, the residue was settled with ether, and the solid was filtered out, and the solid was dried in vacuo to obtain 2.48g of white solid. Dissolve 2.48g of white solid in 5ml of TFA / DCM (1 / 4), stir for 30min, settle into diethyl ether and filter out the solid. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com