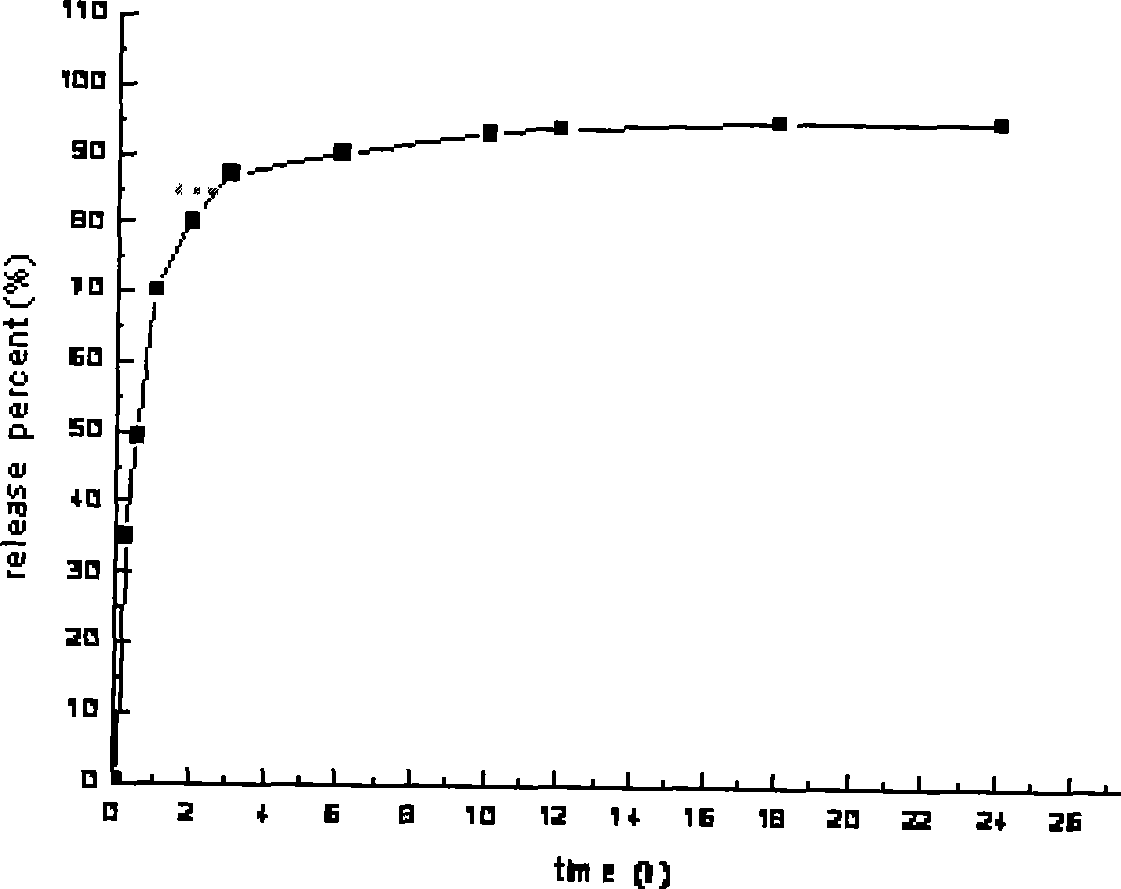
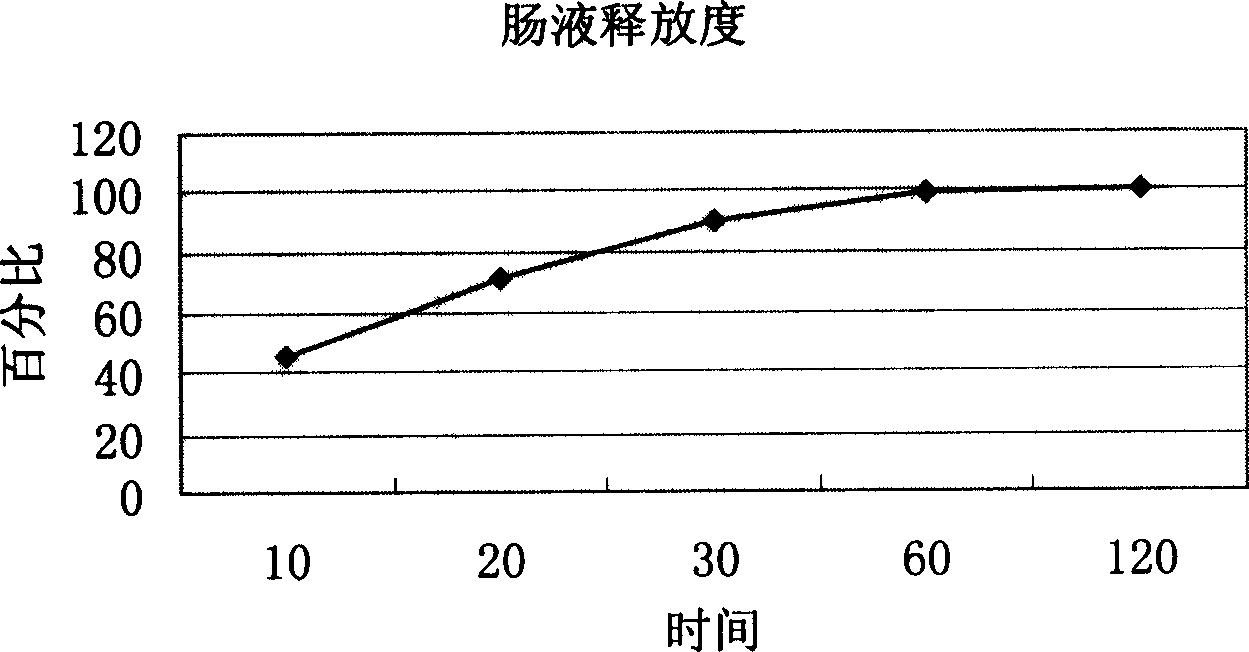
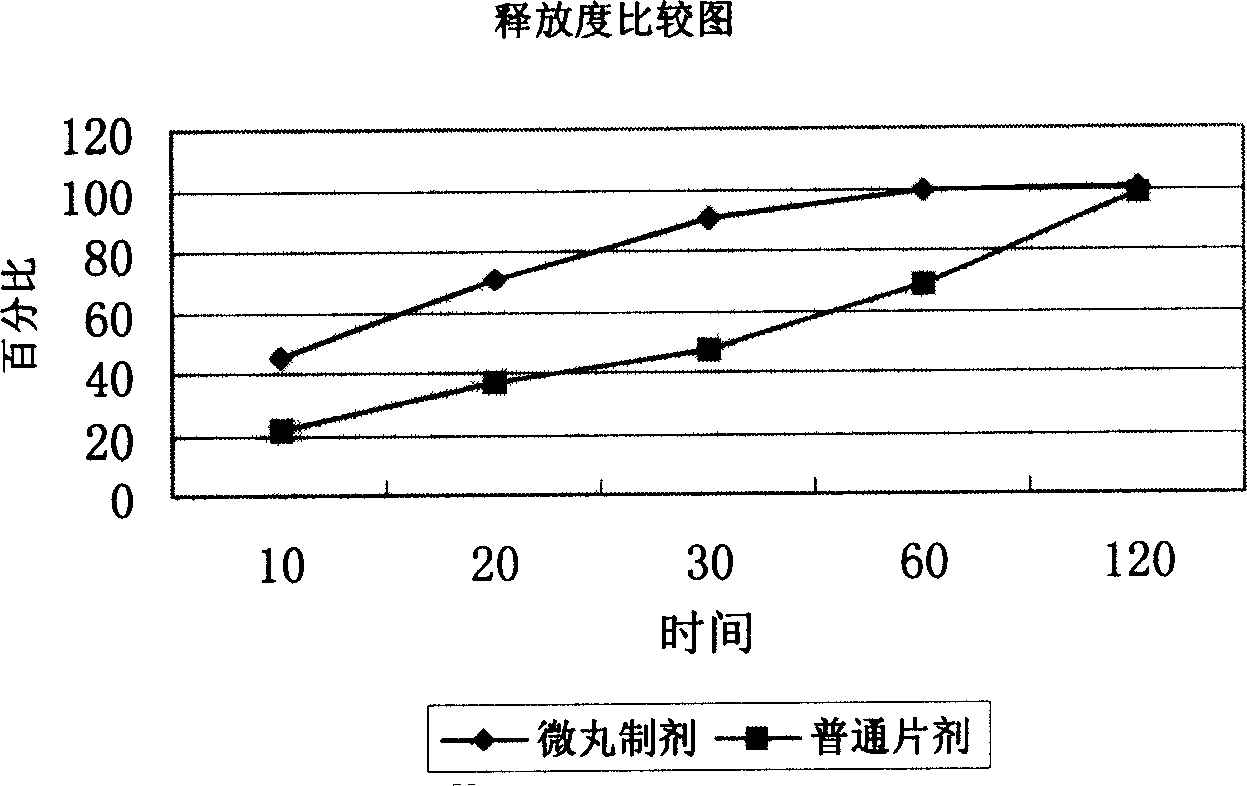
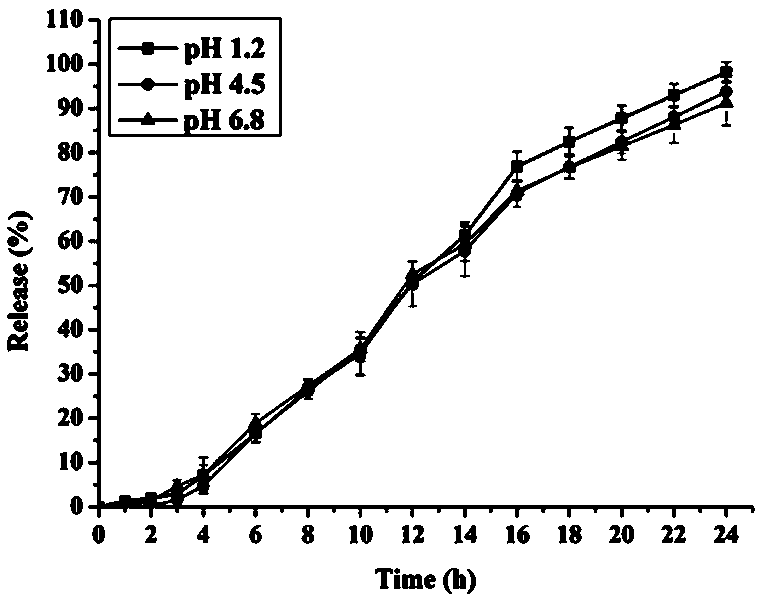
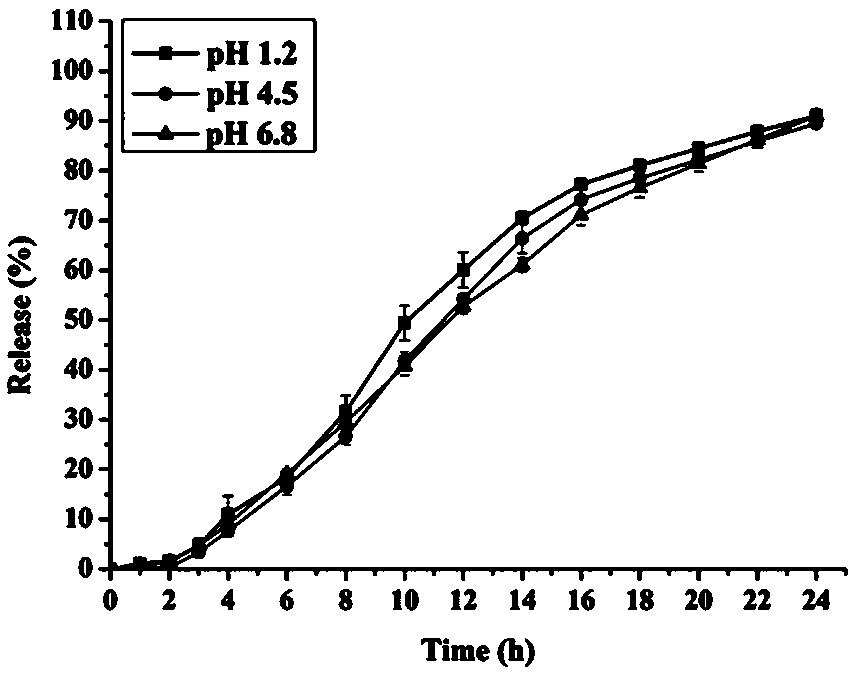
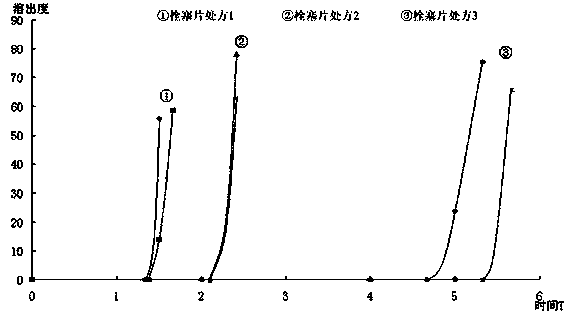
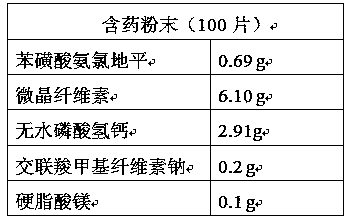
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
80 results about "Controlled Release Capsule" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
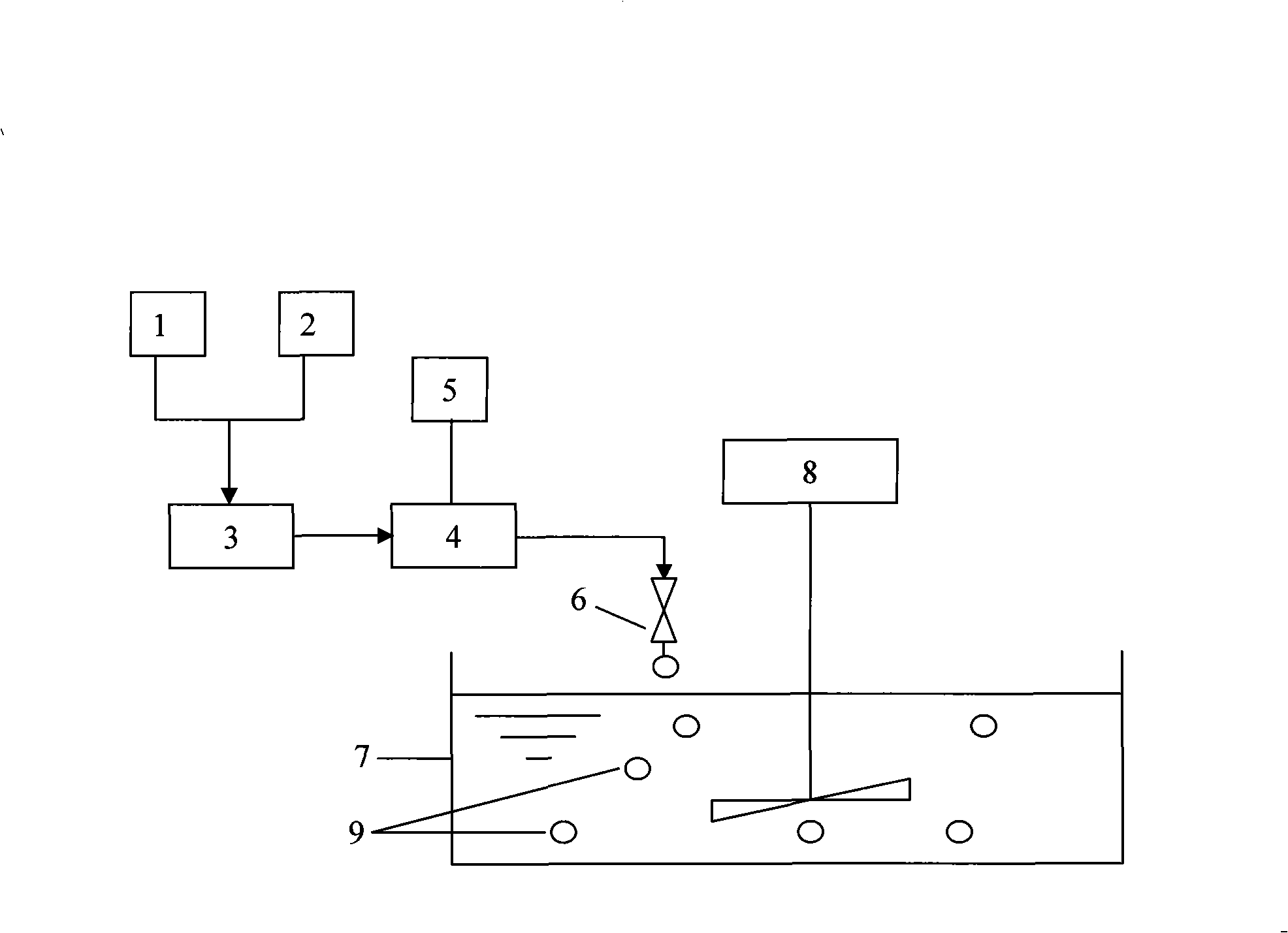
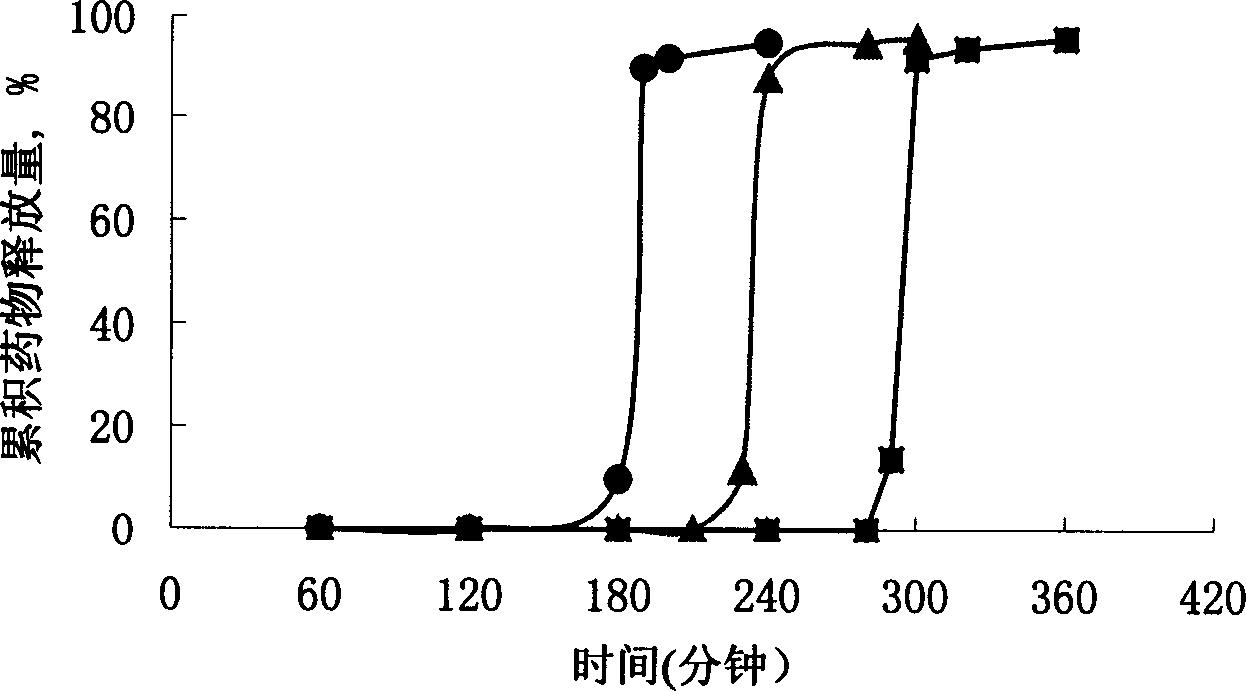
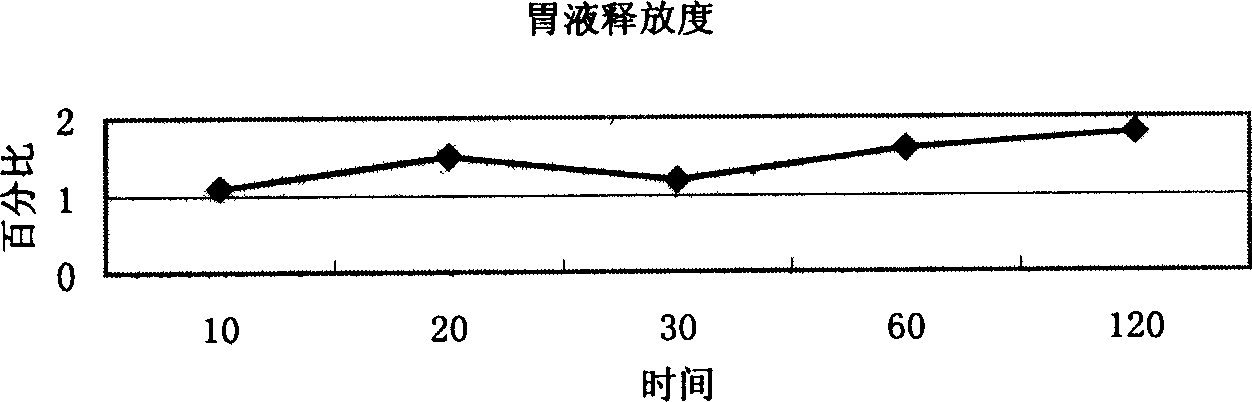
An osmotic, oral, controlled-release capsule is described. This capsule provides drug delivery at fixed delivery rates (T80% = 6 or 14 h) independent of drug properties (e.g., solubility) or drug loading, thereby allowing rapid development of investigational or commercial drugs, especially for proof-of-concept type clinical studies.
Controlled release preparation
Controlled release preparations and capsules are provided. Also provided are emulsions and suspensions, including compositions and methods of manufacturing controlled release capsules, where the fill contains a suspension and / or an emulsion.
Owner:PATHEON SOFTGELS INC
Lithium secondary battery containing capsule for controlled-release of additives
ActiveUS7592095B2Minimizing adverse side reactionDeferred-action cellsCell electrodesLithiumControlled Release Capsule
Provided is a lithium secondary battery comprising a controlled-release capsule which continuously releases a desired amount of additives necessary for electrolytes or electrodes at a constant level and is included in an electrolyte and / or an electrode material, thereby providing inherent effects of additives while simultaneously minimizing adverse side reactions of surplus additives, consequently optimizing the battery performance.
Owner:LG ENERGY SOLUTION LTD
Venlafaxine osmotic device formulation
InactiveUS20070077301A1Reduced food effectReducing food effectBiocideOrganic active ingredientsImmediate releaseNeurological disorder
The present invention provides an osmotic device containing controlled release venlafaxine in the core, wherein the osmotic device exhibits a reduced food effect as compared to a reference controlled release capsule formulation. Some embodiments include venlafaxine in controlled release form in combination with an anti-Alzheimer's or an anti-Parkinson's drug in a rapid release external coat. Memantine is used as an anti-Alzheimer's drug or an anti-Parkinson's drug. Particular embodiments of the invention provide osmotic devices having predetermined release profiles. One embodiment of the osmotic device includes an external coat that has been spray-coated rather than compression-coated onto the device. The device is useful for the treatment of depression in Alzheimer's and / or Parkinson's patients. The device and method can also be used to treat or ameliorate other symptoms associated with Alzheimer's disease, Parkinson's disease or any other neurological disorder. Other dosage forms that provide a controlled, sustained or extended release of venlafaxine in combination with a rapid or immediate release of memantine are useful in the invention.
Owner:OSMOTICA KERESKEDELMI & SZOLGALTATO
Prepn of medicine for treating hepatosis
InactiveCN1403134AImprove bioavailabilityProlong the action timeDigestive systemAntiviralsOrganic solventMedicine
The present invention relates to the preparation of medicine for treating hepatosis. Coarse Ganhuangcao powder is extracted with water or organic solvent to obtain extractive and the extractive is mixed with supplementary material to prepare the medicine in different forms, including tablet, capsule, dropping pill, injection, delayed releasing capsule, etc. The medicine is easy to take, high in biological utilization and long in the time for the active component of Ganhuangcao to act in the body.
Owner:江云 +1
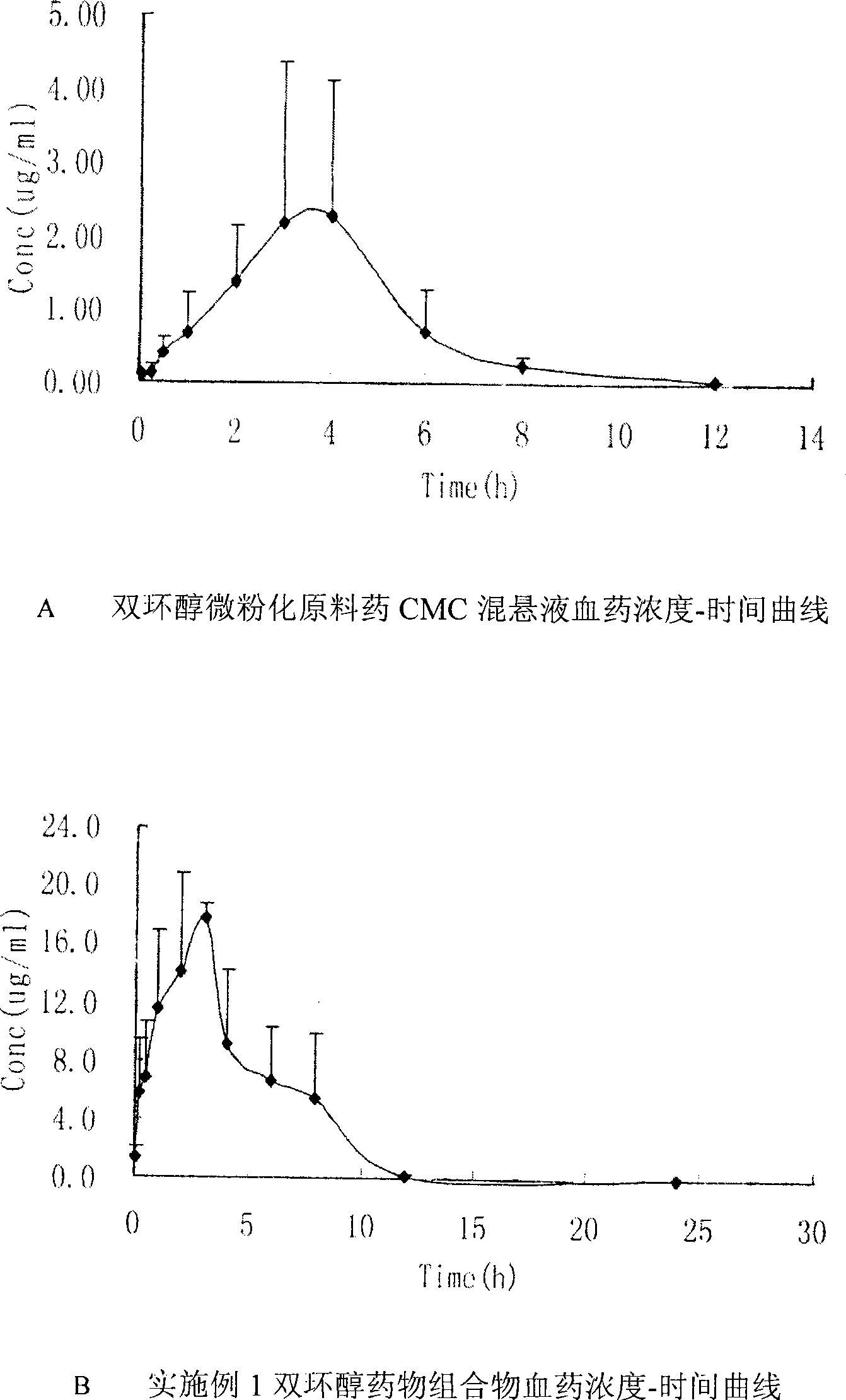
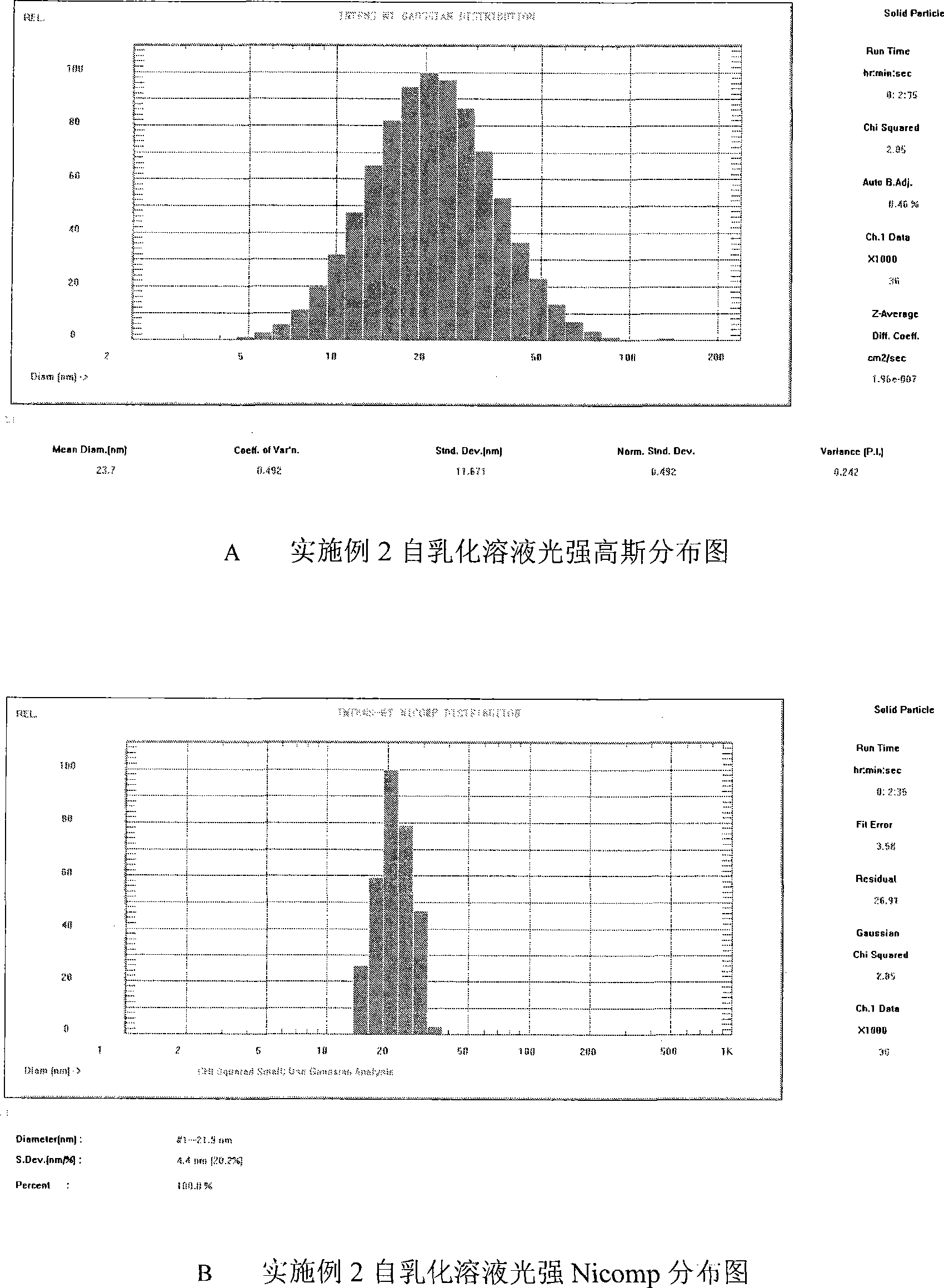
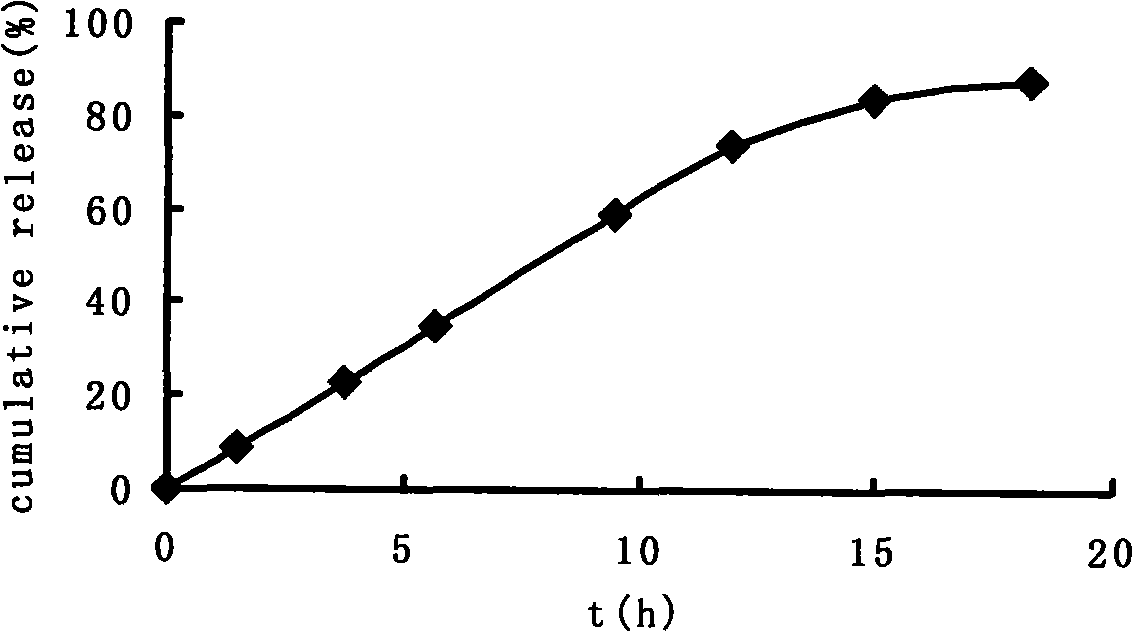
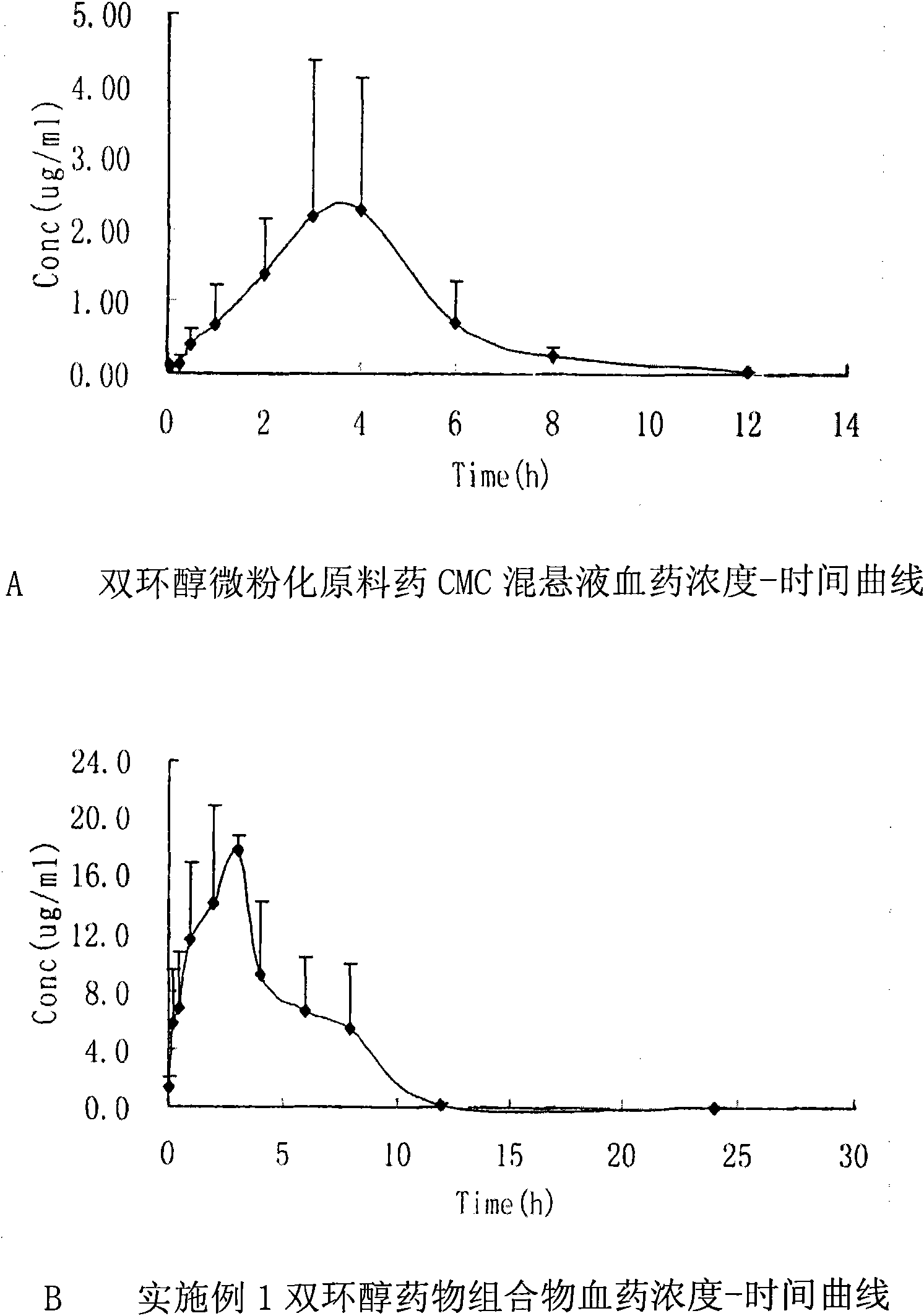
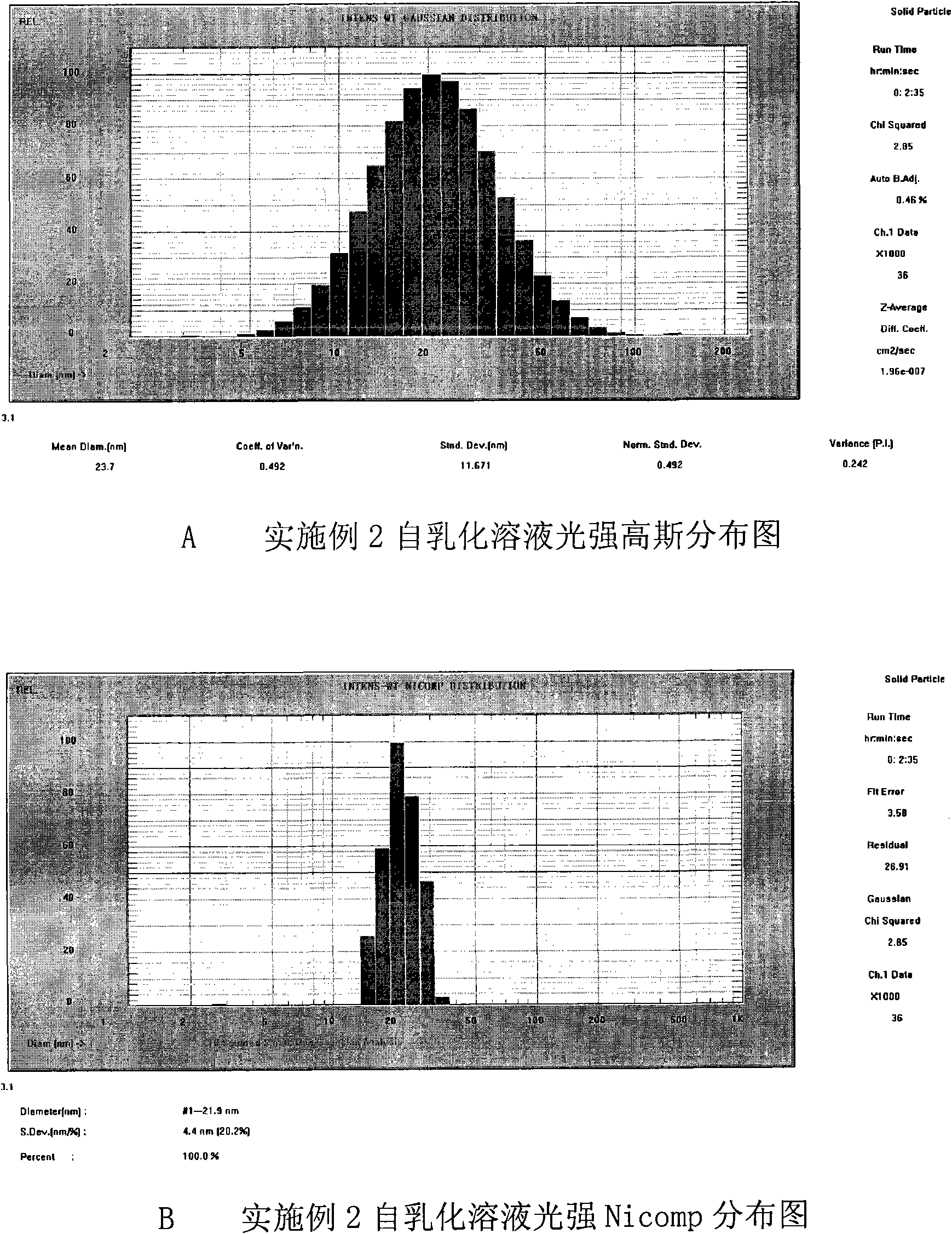
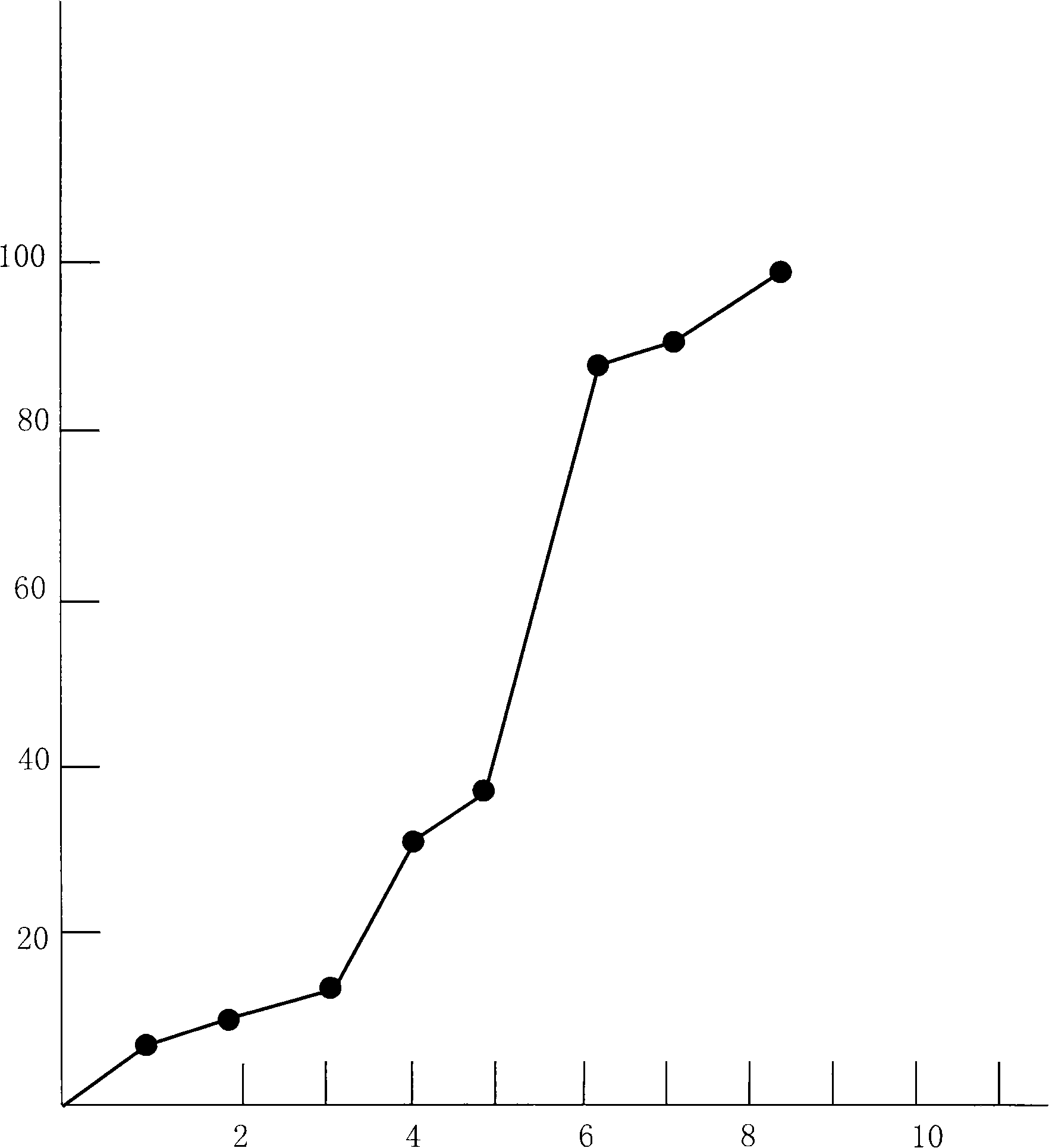
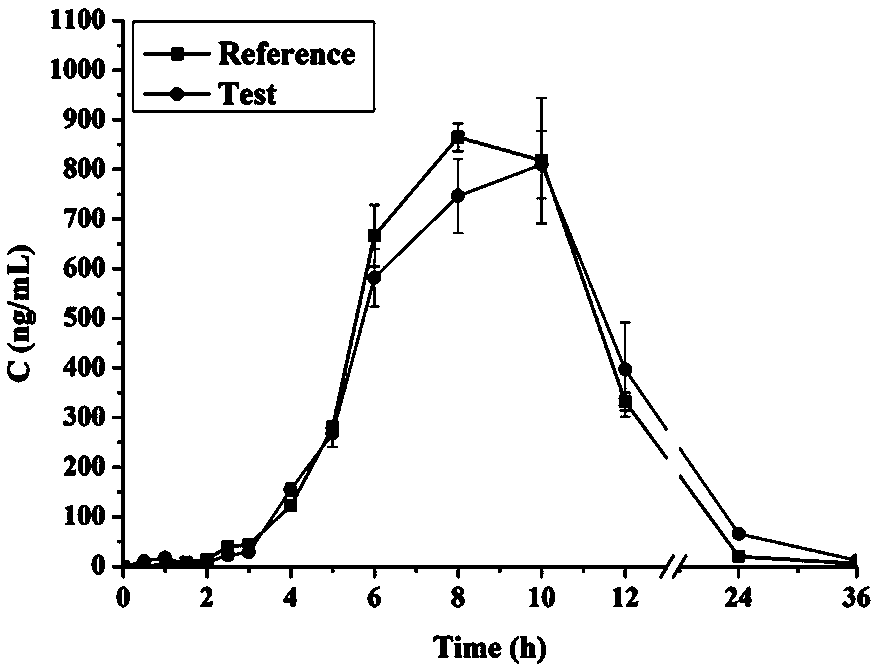
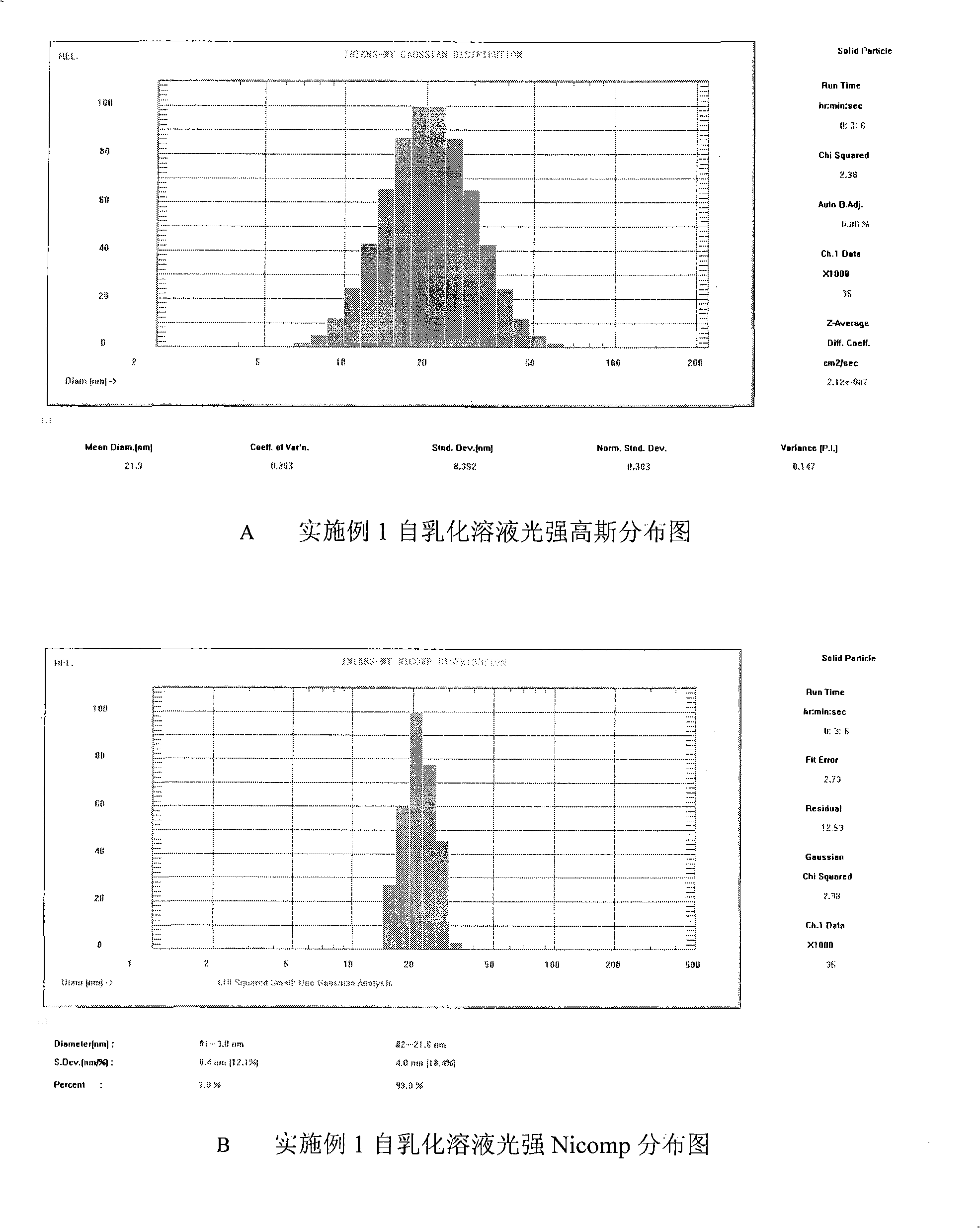
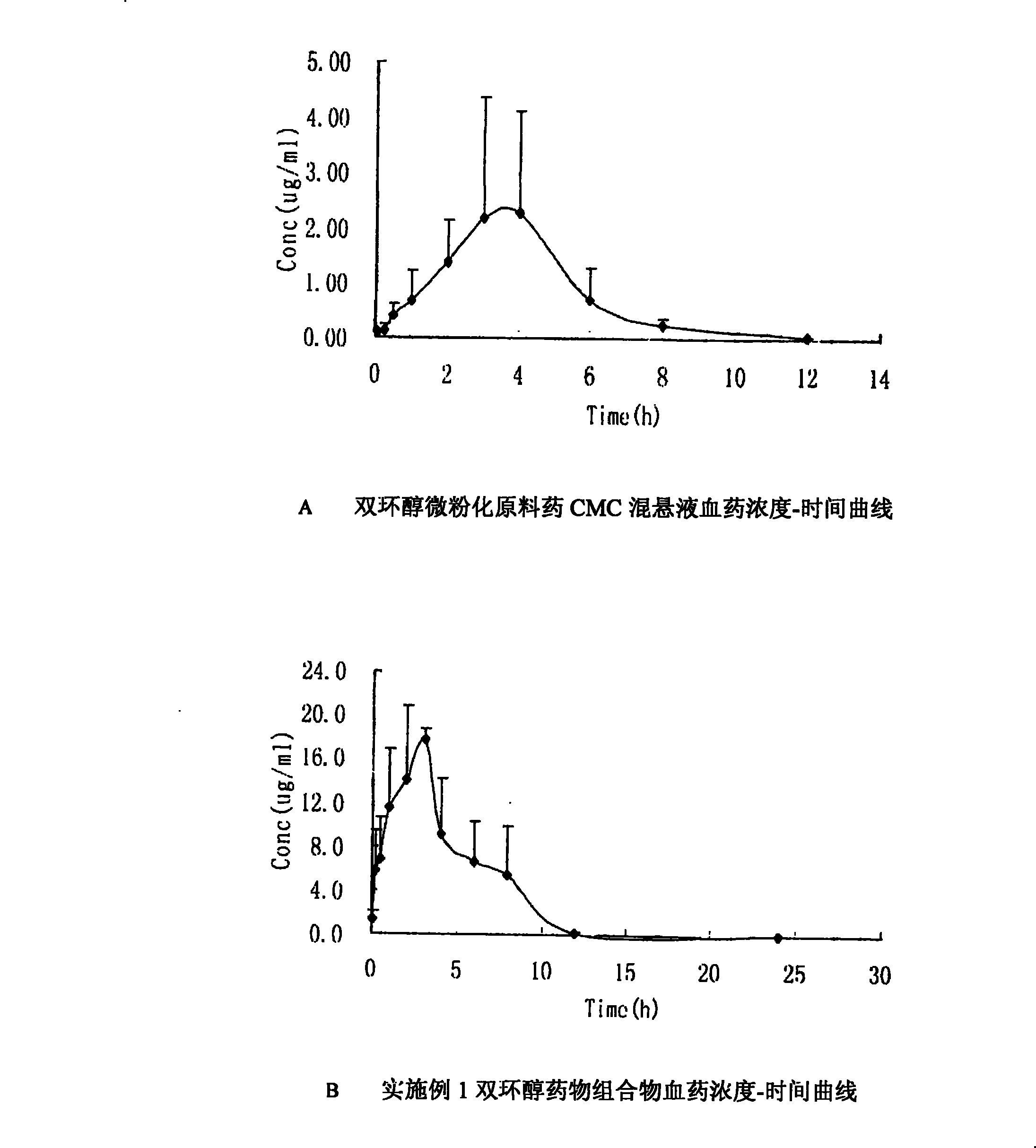
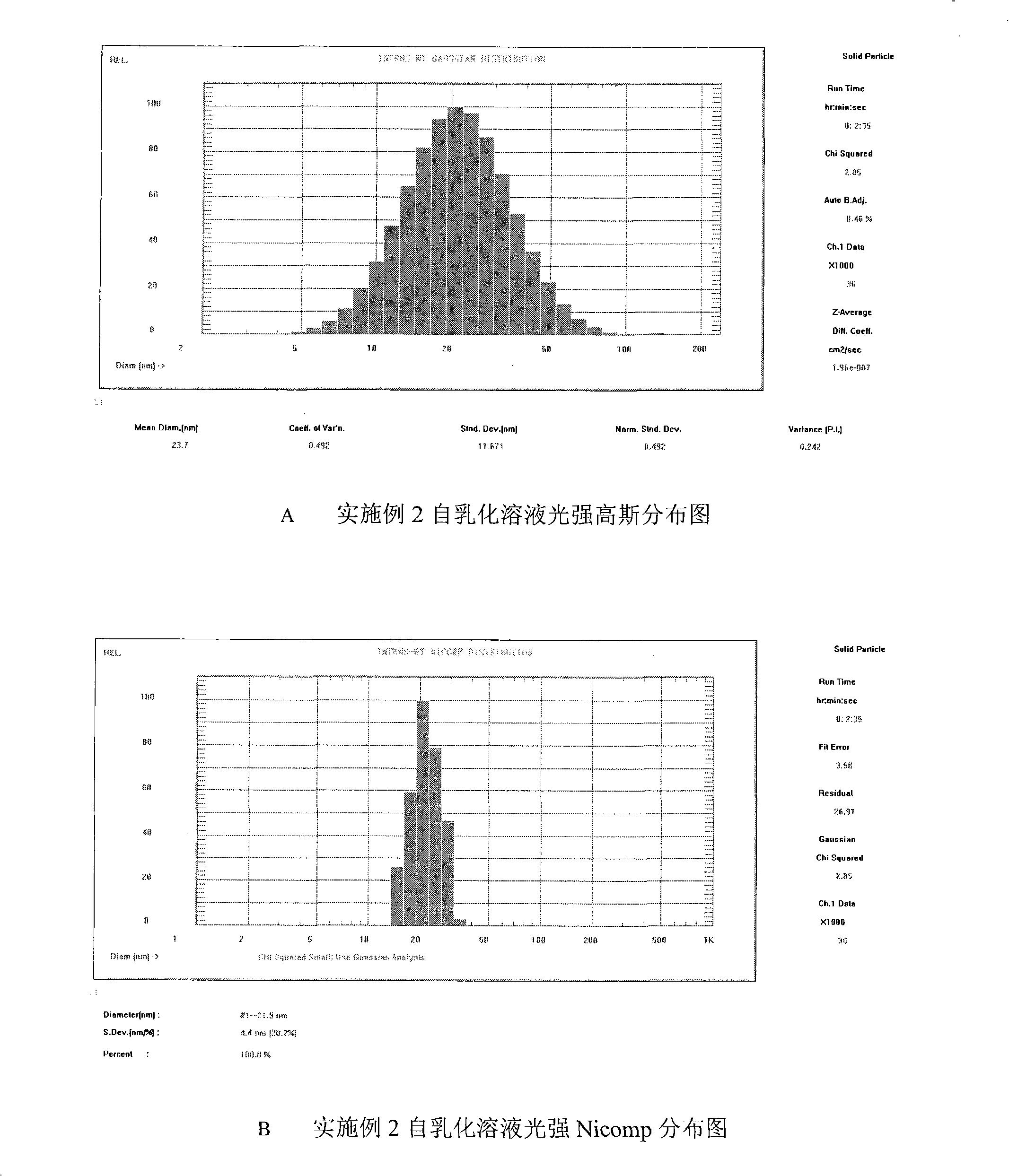
Double-cyclitol medicine composition containing surfactant and preparation method thereof
ActiveCN101390851AImprove bioavailabilityImprove clinical efficacyDigestive systemAntiviralsClinical efficacyAdditive ingredient
The invention discloses a bicyclo-ethanol medicine combination which contains surfactant, and the preparation thereof. Bicyclo-ethanol is dissolved in medicine loading substrate which is composed of one or more types of the following ingredients, surfactant, cosurfactant and oil, so as to form the medicine combination which is oily solution or semisolid. The medicine combination in different formulations can be added with water to form emulsion, microemulsion or transparent rapidly. When the medicine combination is taken orally, the absorption of difficult soluble bicyclo-ethanol is enhanced through self-emulsification, self micro-emulsification or solubilization, so as to reduce the individual difference and improve the bioavailability and clinical treatment effect of bicyclo-ethanol. The medicine combination has simple preparation process and is applicable to preparing various preparations. The invention also includes capsules, soft capsules, controlled-release capsule, osmotic pump capsules or other proper types of preparation which are prepared through the medicine combination.
Owner:BEIJING UNION PHARMA FACTORY
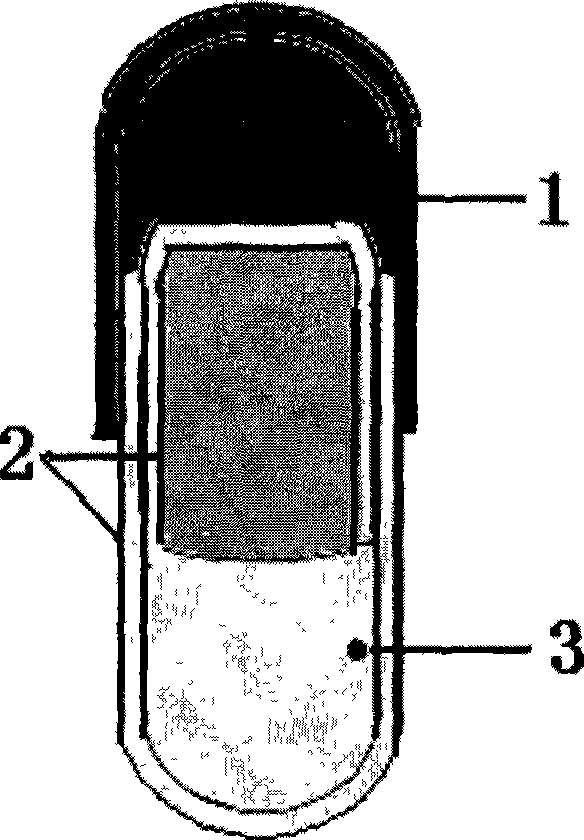
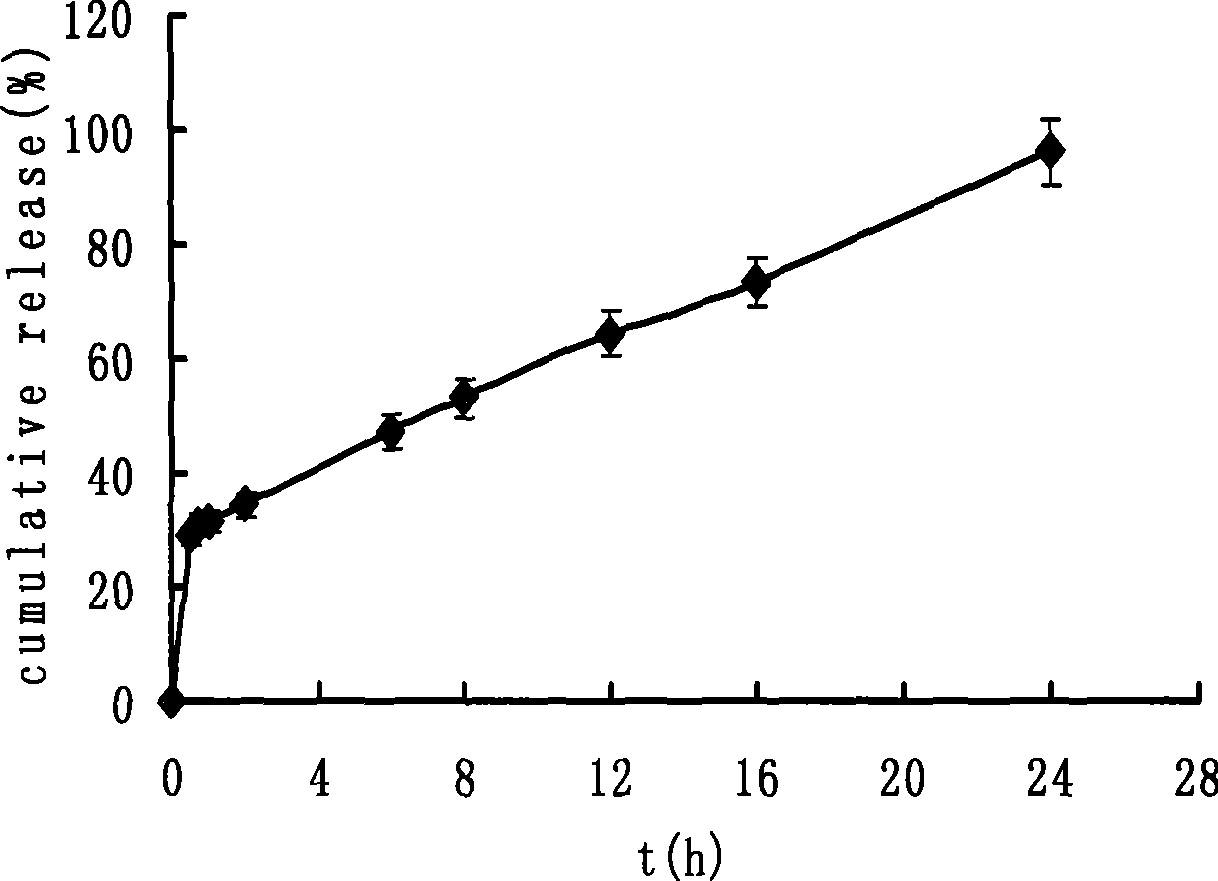
Novel controlled release capsule and preparation method thereof
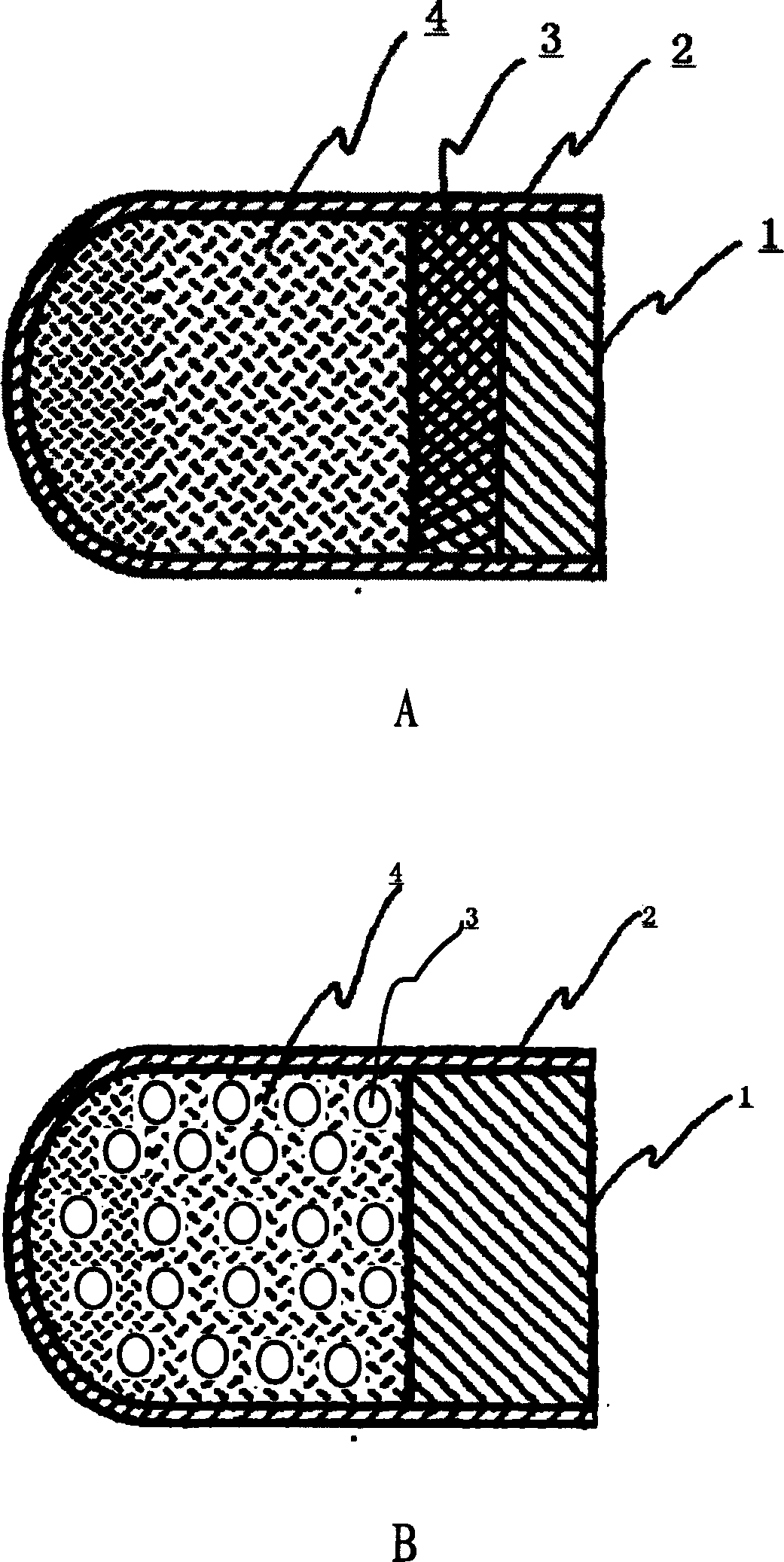
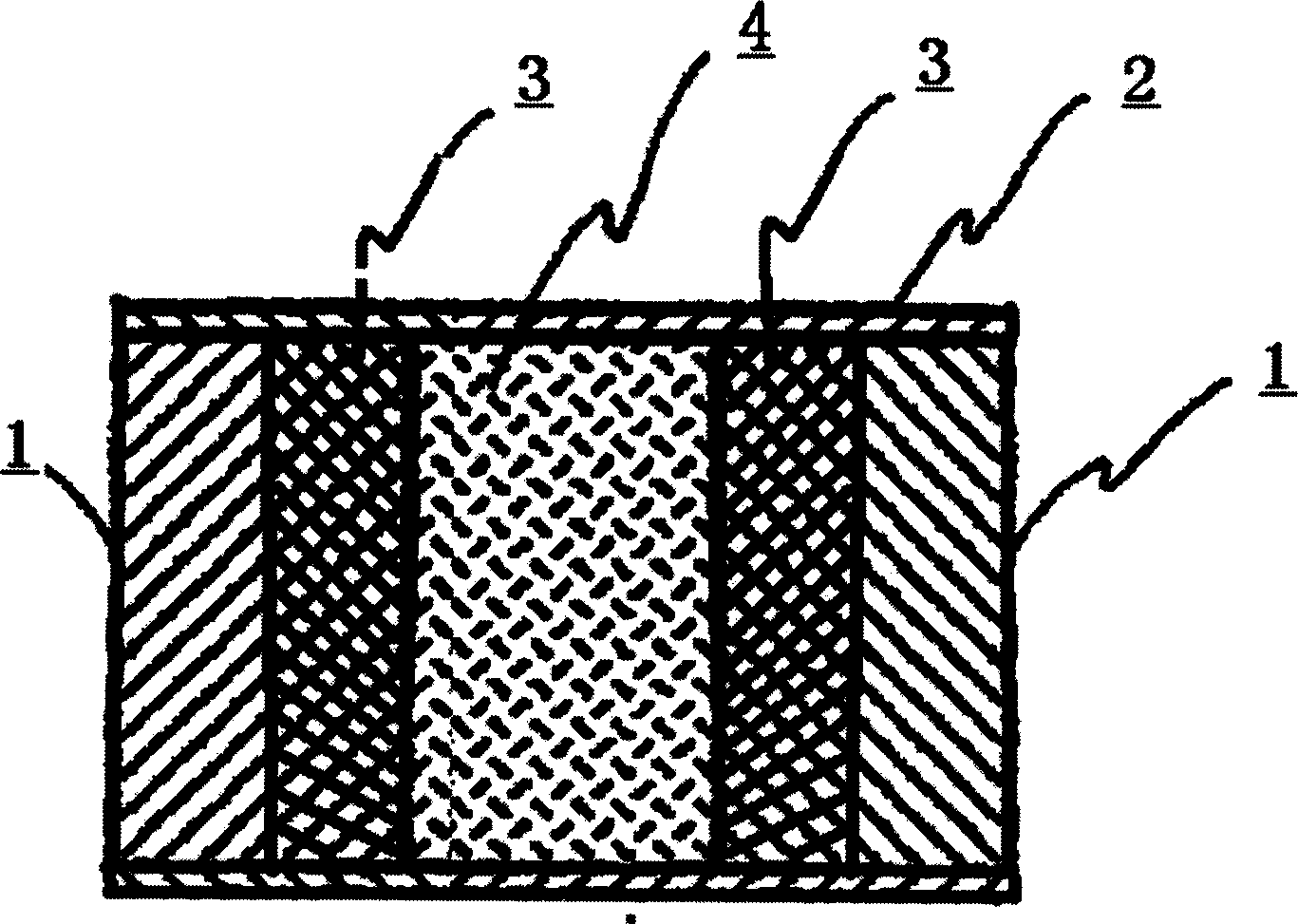
InactiveCN101485644AQuick effectBroaden your optionsPharmaceutical delivery mechanismOsmotic pumpControlled Release Capsule
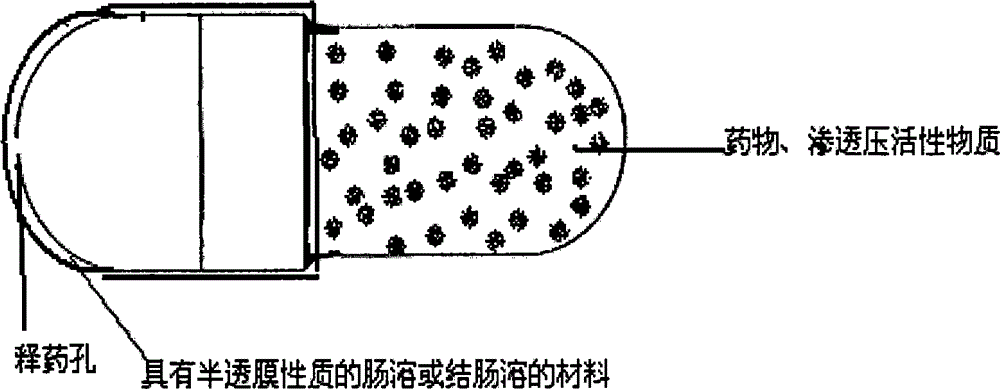
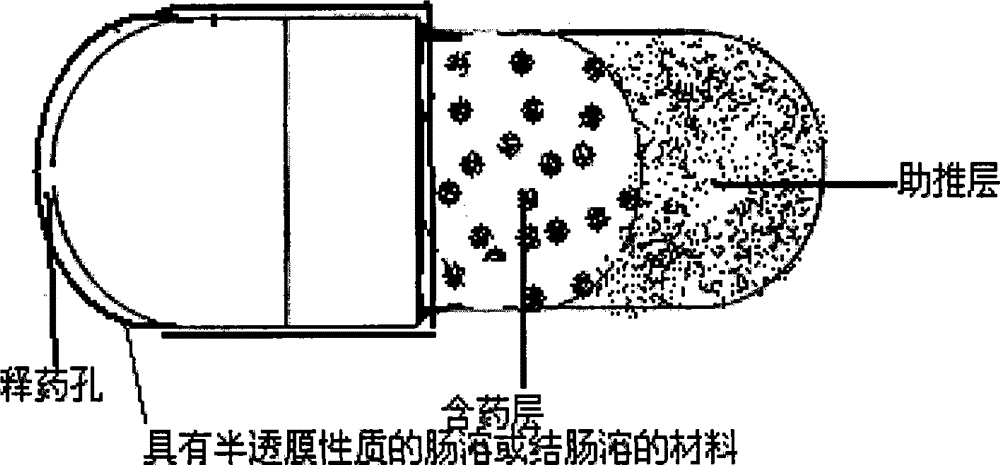
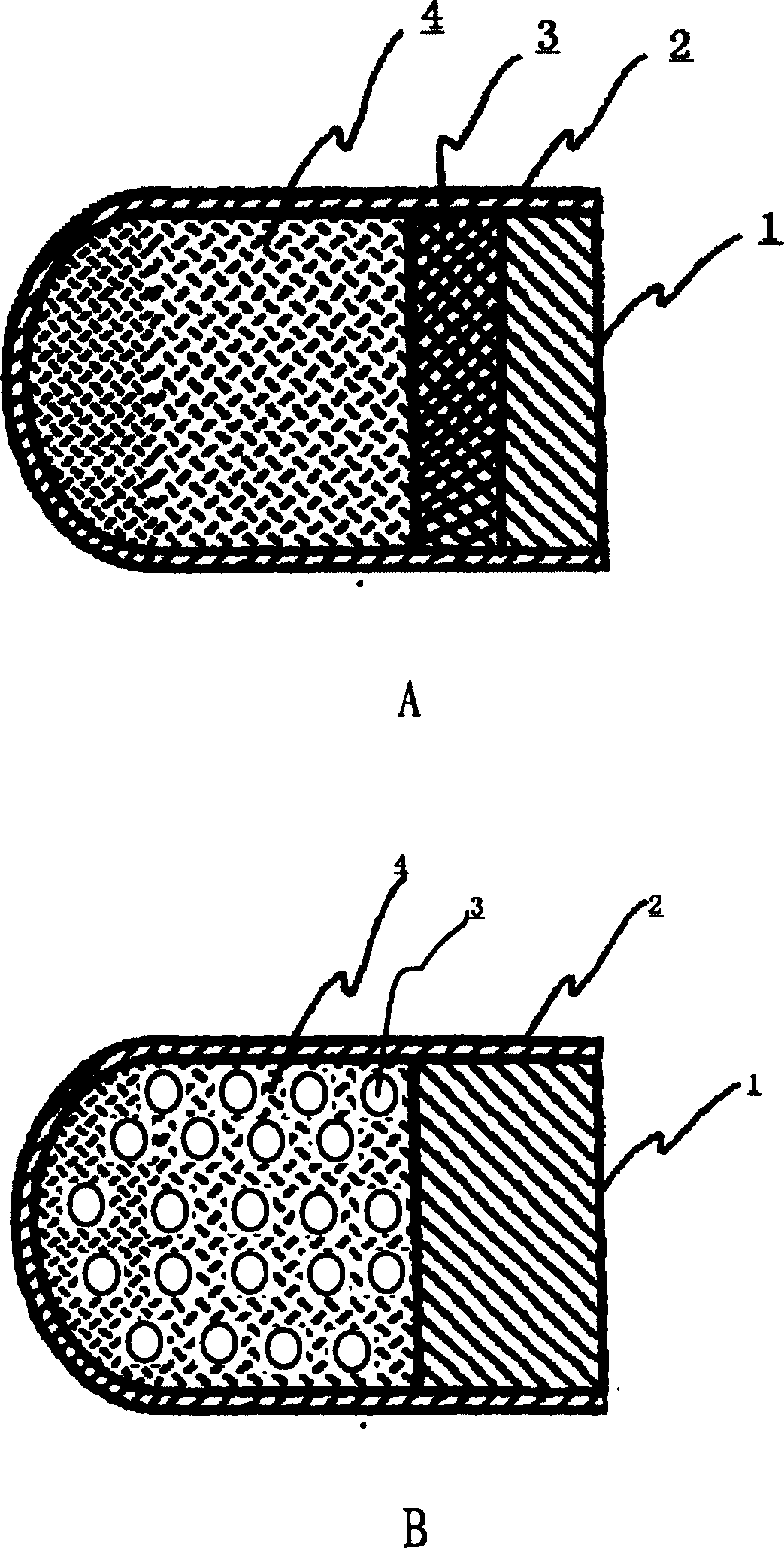
The invention relates to a novel controlled-release capsule and a method for preparing the same. The capsule has the advantages of simultaneously having a quick release part and a controlled-release part, along with simple preparation process and wide drug selection range. The novel controlled-release capsule consists of a capsule cap and a controlled-release capsule body, wherein the capsule cap is a common capsule shell sold in markets, the controlled-release capsule body is an osmotic pump controlled-release capsule which can be used to encapsulate chemicals, traditional Chinese medicines, biological product medicines and the like, and the medicines are packaged in the capsule cap and the controlled-release capsule body respectively. Tests show that the capsule preparation can quickly release the medicines, and the release rate of the remained medicines is constant.
Owner:WENZHOU MEDICAL UNIV
Lycium ruthenicum anthocyanin crude extract and controlled-release microcapsule thereof
ActiveCN105079281AStrong antioxidant activityAvoid purification processPharmaceutical delivery mechanismAntinoxious agentsDrug releaseControlled Release Capsule
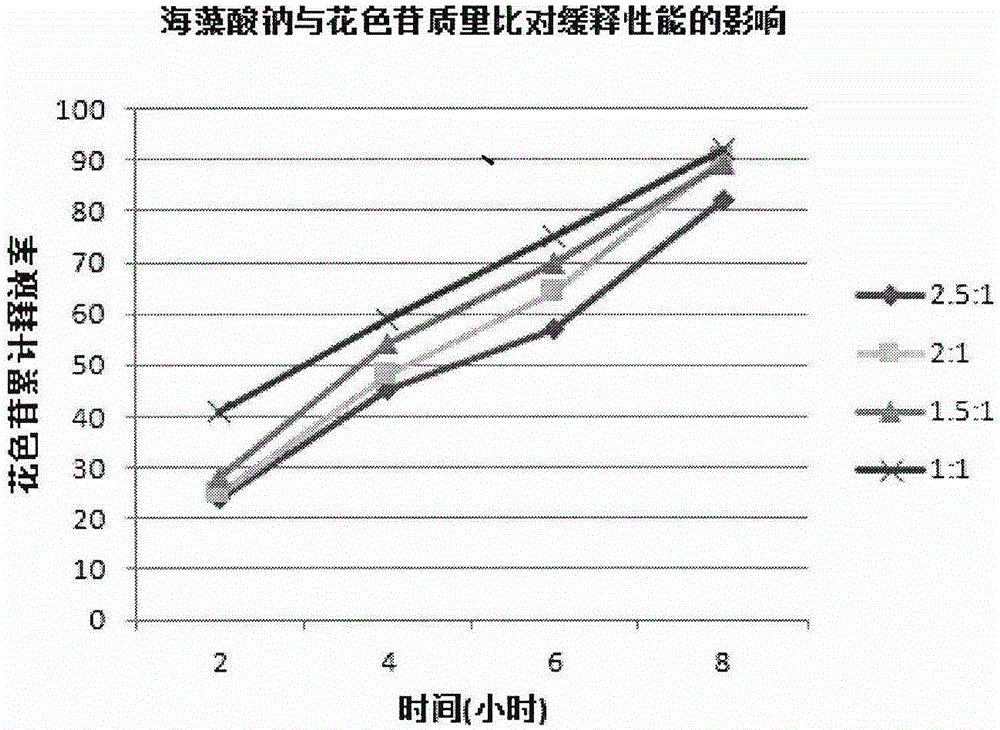
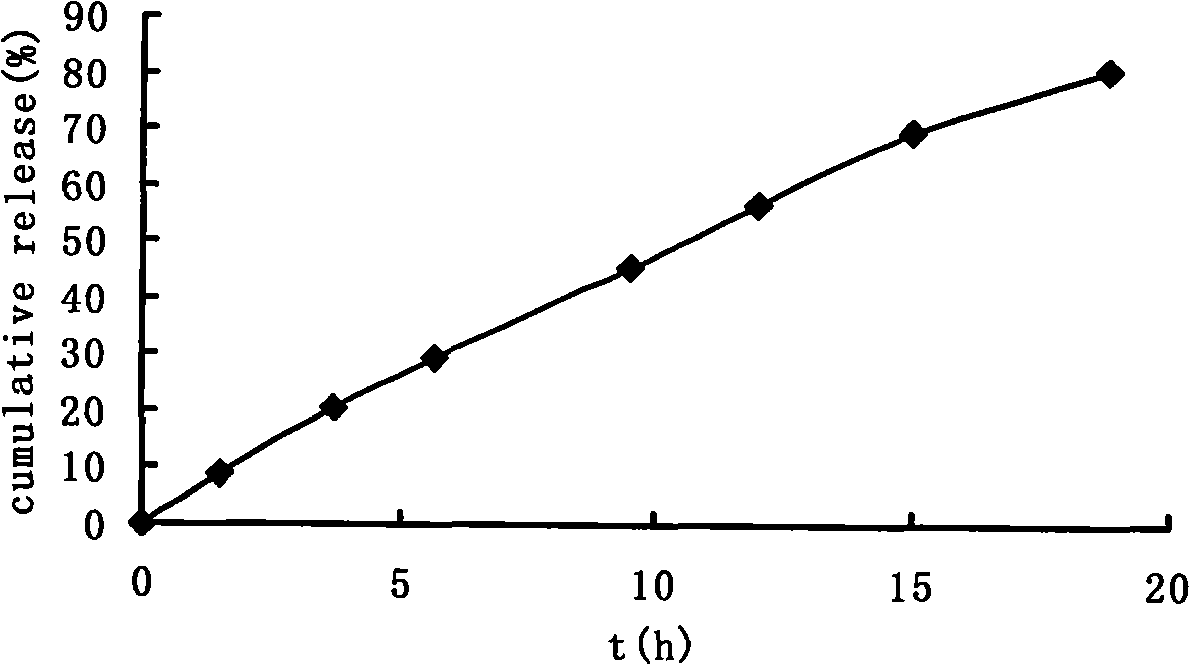
The invention provides a lycium ruthenicum anthocyanin crude extract and a controlled-release microcapsule thereof. The anthocyanin crude extract has good antioxidant activity, so that the further purification for an anthocyanin monomeric compound is prevented, and meanwhile, a preparation method is simple and convenient, production materials are easily available, and the production and use costs of the lycium ruthenicum anthocyanin are reduced. The controlled-release microcapsule is an eight-hour controlled-release capsule, the drug release is uniform in speed and complete, the bioavailability of the drug is improved, and the utilization ratio of the drug is guaranteed.
Owner:CHINA ACAD OF SCI NORTHWEST HIGHLAND BIOLOGY INST
Osmotic pump controlled release capsule case and preparation thereof
InactiveCN101301281ASmall side effectsRelease rate is slow and constantPharmaceutical non-active ingredientsCapsule deliveryControl releaseDrug release
The invention relates to an osmotic pump-controlled release capsule shell and a preparation method thereof. The capsule shell has a simple preparation technology and wide drug selection range, and can ensure that drugs achieve the controlled release effect. The osmotic pump-controlled release capsule shell consists of a capsule cap and a capsule body and is provided with a drug release hole for filling chemical drugs, traditional Chinese medicine, biological drugs and so on, can control the release rate of the drugs, and ensure that the release rate of the drugs is constant. The osmotic pump-controlled release capsule shell can adopt the prior capsule shell preparation method to prepare; the drug release hole with a certain hole diameter is prepared on the capsule shell by laser, mechanical or appropriate methods; as shown in a drug filling test, the capsule shell has the characteristic of ensuring that the release rate of the drugs is constant.
Owner:WENZHOU MEDICAL UNIV
Medicament compound adopting bicyclo-ethanol as active component and preparation thereof
ActiveCN102058577APromote absorptionSmall individual differencesDigestive systemAntiviralsEmulsionEthanol absorption
The invention discloses a medicament compound adopting bicyclo-ethanol as an active component and a preparation thereof. The medicament compound is formed by dissolving the bicyclo-ethanol in a medicament carrying substance consisting of one or more of surfactant, cosurfactant and oils, and is in the form of an oily solution or a semisolid object at room temperature. Through different formulas, the medicament compound can form emulsion and microemulsion quickly through gentle agitation. After being taken orally, the medicament compound can be used for enhancing the bicyclo-ethanol absorption of insoluble medicaments through self-emulsification and self-microemulsification, reducing the individual differences, and improving the bioavailability and clinical effects of the bicyclo-ethanol. The medicament compound is simple in preparation technology and is suitable for being prepared into various preparations. The invention further discloses a hard capsule, a soft capsule, a slow control and release type capsule, an osmotic pump type capsule and other suitable preparations, which are made from the medicament compound.
Owner:BEIJING UNION PHARMA FACTORY
Enteric sustained and controlled release capsule
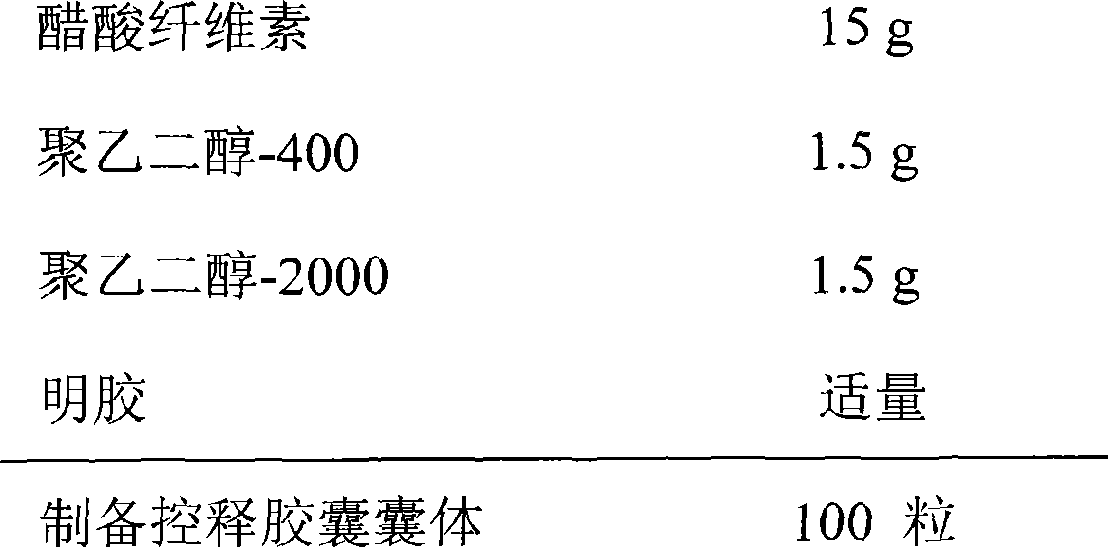
The invention relates to an enteric sustained and controlled release capsule which is characterized by comprising the following therapeutic medicines by the weight percent: 70 to 80 of berberis pruinosa and 20 to 30 of baikal skullcap roots, wherein the berberis pruinosa is extracted by alcohol, the baikal skullcap roots are extracted partially by water and are partially crushed into fine powder, after being mixed, alcohol extract liquid and water extract liquid are condensed into clear paste, the clear paste is mixed with the fine powder to prepare film coating medicine micro-capsules with grain diameters being 200 to 2000 micrometers, and the micro-capsules are divided into different shares and are respectively used for coating enteric target controlled release capsules which are dissolved by a solution with different pH values and formed by combining high molecular material enteric coatings and biodegradation controlled release structures, so the micro-capsules can evenly release in different enteric canals. The enteric sustained and controlled release capsule avoids the defect that the common preparation taken by a patient can generate a peak-valley phenomenon. Under the circulation of the enteric canal of a human body, the micro-capsules can release in different physiologic regions in a gradient way; and when arriving at the colon region, the micro-capsules can accelerate releasing under the degradation of specific enzyme to improve the curative effect.
Owner:ZIBO DEV ZONE YADA PHARMA
Diltiazem hydrochloride control release capsule and its preparing method
InactiveCN1554346AGood for controlling the concentrationFacilitate the control of its physiological effectsOrganic active ingredientsPharmaceutical delivery mechanismWater insolubleAdhesive
The present invention relates to controlled releasing diltiazem hydrochloride capsule as a kind of organic coated pill medicine preparation and its preparation process. Each capsule contains diltiazem hydrochloride pill in 90-180 mg. The medicine pill consists of: medicine pill containing 50-75 wt% of the medicine; one release controlling film layer, which is outside the pill, water insoluble and medicine permeable, comprises polymer, enteric soluble material, plasticizer and lubricant, and is in the amount of 5-20 wt% of the pills; and one fast releasing medicine layer, which is outside the release controlling film layer, comprises diltiazem hydrochloride and water soluble adhesive, and is in the amount of 5-35 wt% of the coated pill. The medicine preparation can maintain the concentration of diltiazem hydrochloride in blood plasma within the treating window for 24 hr.
Owner:GUANGZHOU PHARMACEUTICAL INDUSTRIAL RESEARCH INSTITUTE +1
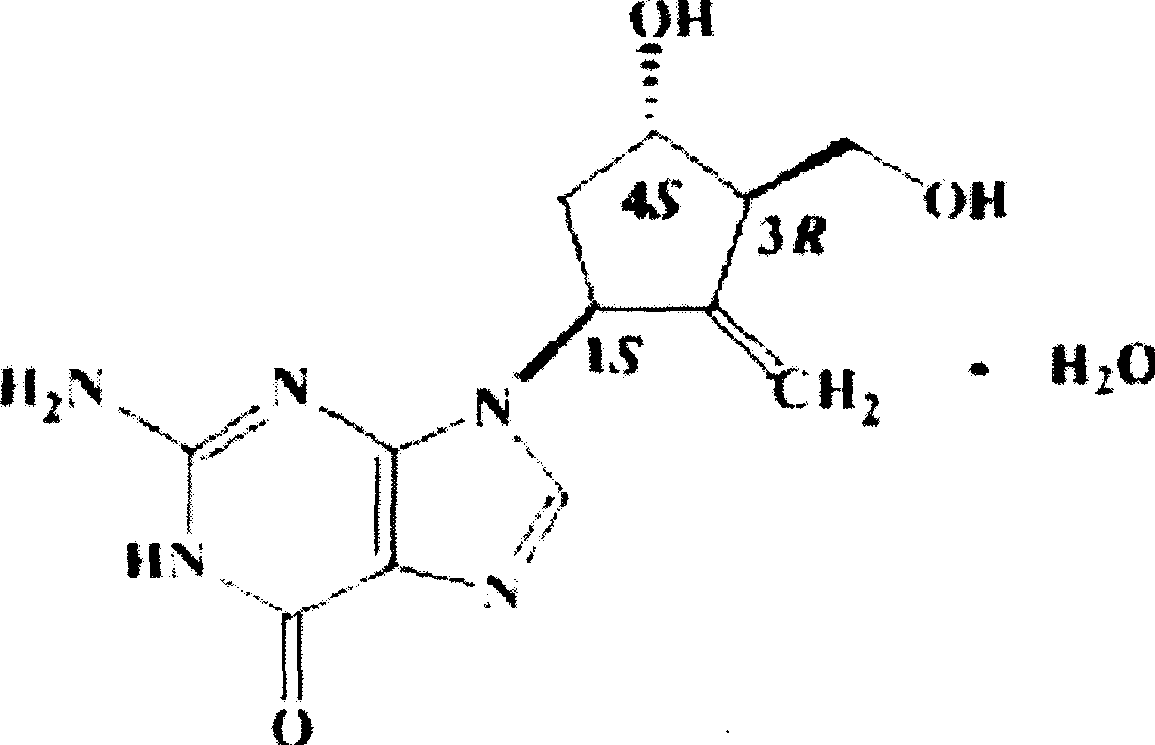
Enticawer release-controllable capsules and their preparation
A release-controlled capsule of entecavir for treating hepatitis B and its preparing process are disclosed. Its advantages are smooth release, stable blood concentration and low by-effect.
Owner:SUNSHINE LAKE PHARM CO LTD
Lipoic acid pellets
Lipoic acid pellets are described, obtained from inert cores externally coated with lipoic acid. The so obtained active cores are coated with a first layer of insulating polymeric material and then with a polymeric coat that is insoluble at the gastric pH. Pellet are then formulated pharmaceutically, for instance in jelly capsules or controlled release capsules or as oral suspensions, dispersible powders, sachets, etc.
Owner:ADARE PHARM SRL +1
Controlled release capsule with city appearance water body denitrification dephosphorization function
InactiveCN101343110AIncrease profitReduce pollutionBiological water/sewage treatmentDecompositionMixing tank
The invention relates to a controlled release capsule having nitrogen and phosphorus removal functions to urban landscape water, which is characterized in that the capsule comprises the following materials, in weight percentage: an algae sodium solution with concentration of between 3 percent and 10 percent, a mixed solution composed of aspartic acid, humic acids, glucose and water, the ratio of the algae sodium solution and the mixed solution is 1:1 to 1:3, and the preparation process thereof comprises that: the algae sodium solution and the mixed solution are put into a mixing tank and then into a pressurizing tank by artificial mixing, a compressed air tank inputs atmospheric pressure to the pressurizing tank, the solution is pressurized and extruded to enter a valve, the valve controls sizes of liquid droplets which are dripped into a storage tank filled with the calcium chloride solution, and the solution is agitated by a mechanical agitator to form elastic capsular particles. The controlled release capsule has the advantages of reducing environmental pollution and losses such as volatilization, losing and decomposition of active materials, and improving the physicochemical stability.
Owner:SHANGHAI BI & ENG TECH
Cordyceps sinensis sustained and controlled release capsule and preparation method thereof
InactiveCN101480415AHas a sustained release drug effectEasy to takeAntibacterial agentsNervous disorderCordycepsSide effect
The invention relates to a worm grass slowly controlled release capsule and a preparation method thereof. The capsule comprises 5 parts to 50 parts of quick release pillers and 50 parts to 95 parts of slow release pillers, each quick release piller comprises a core for carrying a pill and a protective layer, each slow release piller comprises a core for carrying a pill and a slowly controlled release coating layer, and the capsule has the dose of 30mg to 100mg. Medicine continuously released by the worm grass slowly controlled release capsule outside a body can reach more than 90 percent in 24 hours, a patient only needs to take medicine once every day, so times for taking medicine every day can be obviously reduced, the toxic side effect of medicine is lowered, the adaptability of the patient is enhanced and the abuse of medicine is not easy to occur.
Owner:JILIN UNIV
Drug slow control releaser and preparation method thereof
InactiveCN101926783ARelease will not affectLimit release rateOrganic active ingredientsNervous disorderTissue fluidControlled Release Capsule
The invention discloses a drug slow control releaser which comprises a drug controlled-release membrane, wherein a base material of the drug controlled-release membrane is a pharmaceutically acceptable first type polymer material, the drug controlled-release membrane comprises porogen molecules dispersed in the first type polymer material, the drug controlled-release membrane is a drug controlled-release capsule which can form a pore structure on the wall in vivo, and a drug-containing core containing a pharmaceutical effective dose of drug which can be uniformly distributed in a pharmaceutically acceptable second type polymer material and carry out drug administration in vivo is closed in the drug controlled-release capsule. The drug controlled-release membrane has good permeability, and the solid drug-containing core can be quickly and quantitatively placed into the hollow capsule prepared by the release membrane. Tissue fluid can enter into the drug slow control releaser after the drug slow control releaser is implanted into an organism, thereby leading the drug in the drug core to dissolve out rapidly, controlling the release speed of the drug by the drug controlled-release membrane, leading the drug to be released slowly for a long time and absorbed by the organism, and achieving the purpose of long-term drug delivery.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
Site-specific osmotic-pump controlled-release capsule shell and preparation method thereof
InactiveCN104784154ASmall side effectsRelease rate is slow and constantPharmaceutical non-active ingredientsCapsule deliveryDrug release rateIn vivo
The invention relates to a site-specific osmotic-pump controlled-release capsule shell and a preparation method thereof. The capsule shell is simple to prepare, has a wide application scope and realizes the effect of site-specific controlled-release of drugs. The site-specific osmotic-pump controlled-release capsule shell is composed of a capsule cap and a capsule body and provided with a drug release hole; and the capsule shell is used for filling of chemical drugs, traditional Chinese medicines, biological drugs, etc., can control drug release rates and enables the drug release rates to be constant. The site-specific osmotic-pump controlled-release capsule shell can be prepared by using a conventional capsule shell preparation method; the drug release hole with a certain pore size is formed in the capsule shell in virtue of laser, a machine or other proper methods; and test results of filled drugs prove that the capsule shell provided by the invention has the characteristic of capacity of allowing drugs to be constantly released at in-vivo specific sites (e.g., a position where small intestine or colon is located).
Owner:胡容峰
Floating type pulse release capsule in stomach
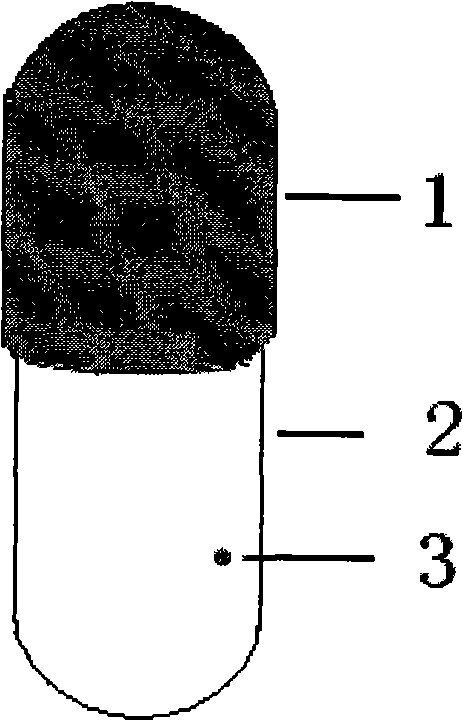
The present invention relates to medicine technology, and is a kind of stomach floating pulse releasing capsule. The capsule includes water insoluble capsule, controlled releasing capsule plug, floating aid and medicine set inside the capsule. Owing to the specific weight of the floating aid lower than 1, the capsule will be floated in gastric juice without entering intestinal tract. The capsule plug made of dissolving eroded or swelling material and lactose or other inertial material is dissolved or swelled in digestive fluid in some period for controlled release of medicine. The capsule may be opened in one or two ends, and may be designed based on different human rhythm time to prevent rhythm disease.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Arbidol sustained or controlled release capsule and preparation method thereof
ActiveCN102772392AImprove complianceMaintain blood levelsOrganic active ingredientsPharmaceutical delivery mechanismBlood concentrationMedicine
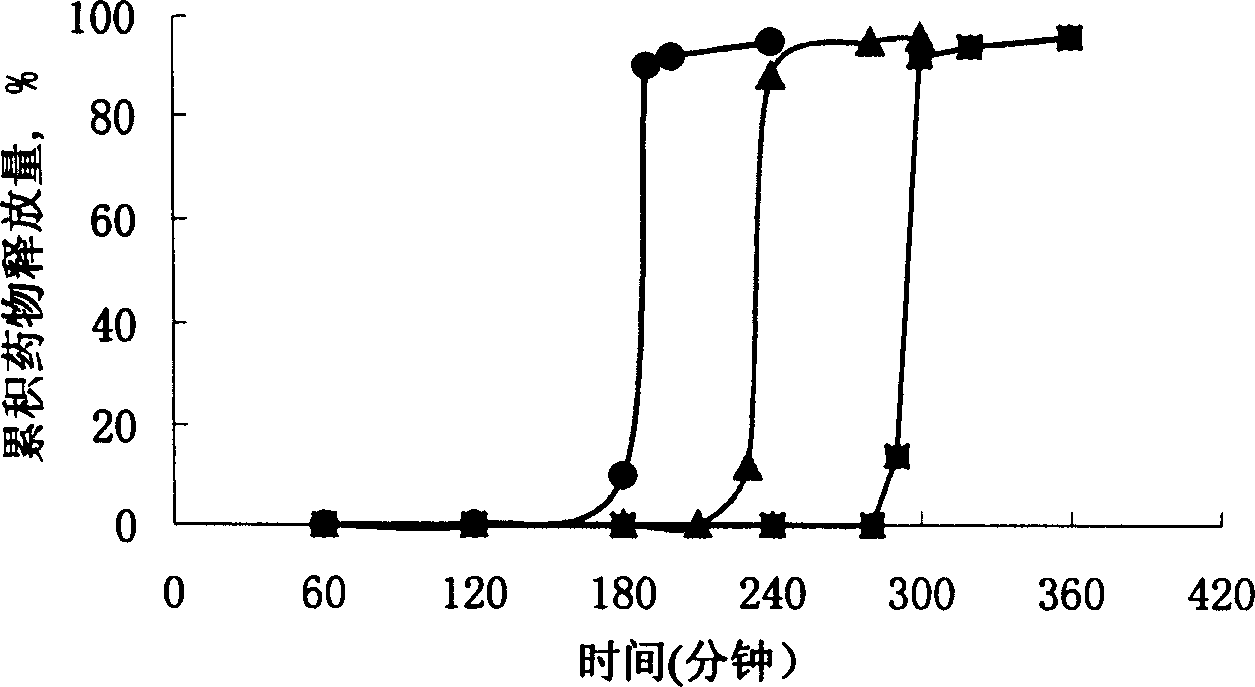
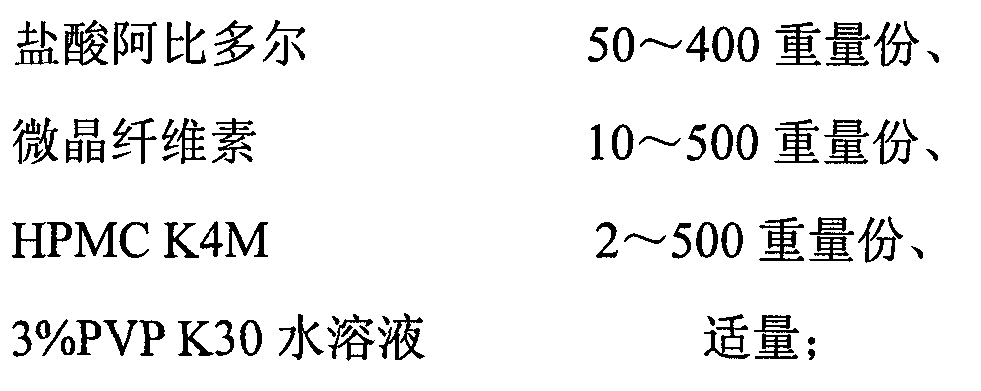
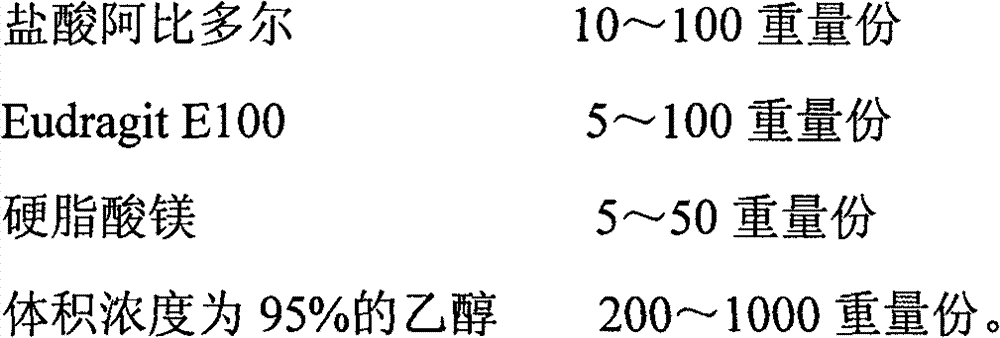
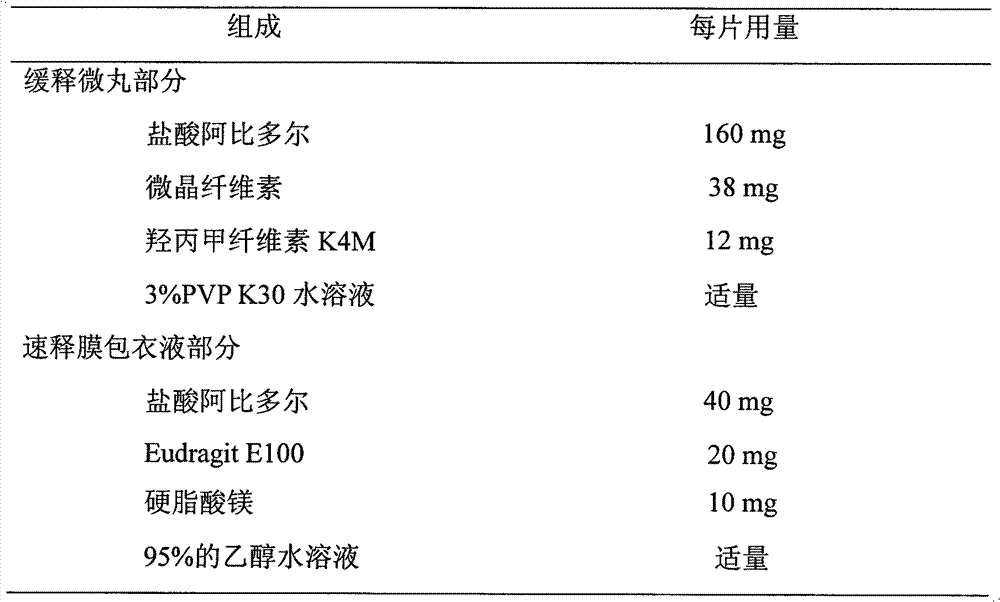
The invention discloses an arbidol sustained or controlled release capsule and a preparation method thereof. The content filled in the sustained or controlled release capsule is arbidol sustained release micro pills with quick release film lagging covers; the arbidol sustained release micro pill consists of the following raw materials in parts by weight: 50 to 400 parts of hydrochloric arbidol, 10 to 500 parts of microcrystalline cellulose, 2 to 100 parts of HPMC K4M and a proper amount of PVP K30 aqueous solution with the concentration of 3 percent; and the quick release film lagging cover consists of the following raw materials in parts by weight: 10 to 100 parts of hydrochloric arbidol, 50 to 100 parts of Eudragit E100, 5 to 50 parts of talcum powder and 500 parts of ethanol with the volumetric concentration of 95 percent. Compared with the prior art, the capsule has a quick response and can sustain a certain blood concentration; the working time of the medicament is prolonged; the medicine taking frequency is obviously reduced; the medicine taking compliance of patients is improved; and the preparation method is simple, easy to control and suitable for industrial production.
Owner:HUBEI BIO PHARMA IND TECHCAL INST
Floating type pulse release capsule in stomach
InactiveCN1473563AMeet the needs of circadian rhythmEasy accessCapsule deliveryDiseaseWater insoluble
The present invention relates to medicine technology, and is a kind of stomach floating pulse releasing capsule. The capsule includes water insoluble capsule, controlled releasing capsule plug, floating aid and medicine set inside the capsule. Owing to the specific weight of the floating aid lower than 1, the capsule will be floated in gastric juice without entering intestinal tract. The capsule plug made of dissolving eroded or swelling material and lactose or other inertial material is dissolved or swelled in digestive fluid in some period for controlled release of medicine. The capsulemay be opened in one or two ends, and may be designed based on different human rhythm time to prevent rhythm disease.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Slow and control release aspirin capsule formulation and method for making same
InactiveCN1522702ALittle side effectsFacilitated releaseSalicyclic acid active ingredientsAntipyreticSucroseAcrylic resin
The present invention relates to a slowly-controllable released capsule preparation of aspirin and its preparation method. It mainly includes aspirin, sucrose, starch, ethyl alcohol, Tween-80, acrylic resin and talcum powder. Every 1000 capsule pills includes 100-350g of micropill containing medicine, in which the aspirin is 50g, 75g or 150g and blank micropill 0-200g. Its preparation method includes the following steps: covering blank pill core with aspirin to obtain micropill containing aspirin, screening to obtain medicine-containing micropill with required grain size, then covering it with controllable release film and capsulizing so as to obtain the invented product. Said product can reduce side effect and can raise therapeutic effect.
Owner:TIANJIN PACIFIC PHARMA
Nifedipine controlled release capsule and preparation method thereof
InactiveCN111184699AWell mixedRelease constantOrganic active ingredientsPharmaceutical non-active ingredientsCelluloseDrug release rate
The invention provides a nifedipine controlled release capsule and a preparation method thereof. The controlled release capsule is prepared by filling a capsule shell with nifedipine, polyoxyethyleneand organic acid and coating the capsule shell. The mass ratio of nifedipine, polyoxyethylene and organic acid is (10-90):(1-15):(1-30); the coating is prepared from cellulose, a plasticizer and a pore-forming agent by mixing in a weight ratio of (10-50):(0-15):(1-25). Double-layer tabletting, laser or mechanical punching are not required, the preparation process is simple, the cost is low, the drug release rate is stable, zero-order release is basically achieved within 24 hours, and drug release is complete; the capsule is administered once a day, and thus, patient compliance is improved.
Owner:HEBEI UNIVERSITY
Double-cyclitol medicine composition containing surfactant and preparation method thereof
The invention discloses a bicyclo-ethanol medicine combination which contains surfactant, and the preparation thereof. Bicyclo-ethanol is dissolved in medicine loading substrate which is composed of one or more types of the following ingredients, surfactant, cosurfactant and oil, so as to form the medicine combination which is oily solution or semisolid. The medicine combination in different formulations can be added with water to form emulsion, microemulsion or transparent rapidly. When the medicine combination is taken orally, the absorption of difficult soluble bicyclo-ethanol is enhanced through self-emulsification, self micro-emulsification or solubilization, so as to reduce the individual difference and improve the bioavailability and clinical treatment effect of bicyclo-ethanol. The medicine combination has simple preparation process and is applicable to preparing various preparations. The invention also includes capsules, soft capsules, controlled-release capsule, osmotic pumpcapsules or other proper types of preparation which are prepared through the medicine combination.
Owner:BEIJING UNION PHARMA FACTORY
Trospium chloride controlled release capsule and preparation method thereof
ActiveCN102764246ALow priceGood treatment effectPharmaceutical delivery mechanismPharmaceutical non-active ingredientsBULK ACTIVE INGREDIENTFilm coating
The present invention provides a trospium chloride controlled release capsule and a preparation method thereof. A capsule shell is filled with pills; the pills are wrapped with film coatings; a pill cladded with the coating is provided with a hole with a diameter of 0.05 mm; the pills in the capsule shell comprise an active ingredient trospium chloride; controlled release carriers in the pills are sodium carboxymethyl cellulose and natural alginate; a weight ratio of trospium chloride and the controlled release carrier is 1:0.6; a weight ratio of sodium carboxymethyl cellulose sodium and natural alginate is 1:0.8; viscosity of the sodium carboxymethyl cellulose is 3000-5000 centipoise; and viscosity of the natural alginate is 300-500 centipoise. According to the invention, the trospium chloride can be released at a constant speed in 9-12 h, so as to reduce frequency of drug usage.
Owner:SHOUGUANG FUKANG PHARMA +1
Nifedipine controlled release micro-pill preparation and preparation method thereof
InactiveCN102038662AFully controlled releaseGood effectOrganic active ingredientsPharmaceutical delivery mechanismNifedipineAdhesive
The invention relates to a nifedipine controlled release micro-pill preparation and a preparation method thereof. The nifedipine controlled release micro-pill preparation comprises 30% to 70% of nifedipine, 10% to 30% of empty pill core, 1% to 5% of adhesive, 15% to 40% of controlled release material and 3% to 10% of anti-sticking agent. The preparation method comprises the steps that medical active components cover the surface of the empty pill core and then are covered with the controlled release coating to prepare a nifedipine controlled release micro-pill; and finally, the capsule is filled to prepare a nifedipine controlled release capsule or the controlled release micro-pill and a filling agent are mixed and tableted to prepare nifedipine controlled release tablets. The nifedipine controlled release micro-pill preparation has the advantages of complete controlled release and stable effect, and is suitable for mass production.
Owner:HANGZHOU SHARPLY PHARM R&D INSTIT +2
Ivermectin controlled-release capsule and preparation method and application thereof
InactiveCN107773554ARelease fullyHigh protection rateOrganic active ingredientsAntiparasitic agentsTherapeutic effectPhospholipid
The invention discloses an ivermectin controlled-release capsule and a preparation method and application thereof. The ivermectin controlled-release capsule comprises, by mass, 0.1-1% of ivermectin raw material, 20-60% of water-soluble carrier and 30-70% of enteric-soluble wrapping material, the water-soluble carrier is one or multiple of hydroxypropyl-beta-cyclodextrin, methyl-beta-cyclodextrin,HPMC, PVP, PEG, poloxamer 188, mannitol, D-alpha-tocopherol PEG 1000 succinate, cholate / phospholipid mixed micelle, polyethylenediamine dendritic polymer, phospholipid or cholesterol, and the enteric-soluble wrapping material is one or multiple of hydroxypropyl methyl cellulose phthalic acid, acrylic resin II, acrylic III, cellulose acetate phthalate, polyethylene diacetate phthalate or hydroxypropyl methyl cellulose acetate succinate. The ivermectin controlled-release capsule has the advantage of outstanding acid resistance, enables drug to reach intestinal tracts to be disintegrated and released, remarkably improves bioavailability and has remarkable treatment effect.
Owner:SOUTH CHINA AGRI UNIV
Naringin controlled release capsule and preparation method thereof
ActiveCN105560207AQuick releaseTo achieve the purpose of sustained and controlled releaseOrganic active ingredientsPharmaceutical non-active ingredientsNaringinBlood concentration
The invention discloses a naringin controlled release capsule and a preparation method thereof. Substances contained in the capsule are composed of one part of a quick release drug core and two parts of a slow release drug core; the quick release drug core is mainly prepared from naringin and a quick disintegration agent, and the slow release drug core is prepared from naringin and a hydrophilic gel slow release framework material; each part of the quick release drug core or the slow release drug core contains 1 / 3 the total mass of the naringin. The two types of drug cores are encapsulated according to the ratio of 1: 2, and the capsule is obtained. Compared with the prior art, the capsule is quick in effect onset, the blood concentration is maintained to be effective, stable and durable, and the side effect can be lowered; the production method is simple and applicable to large-scale industrial production.
Owner:广州中天康顺生物医药有限公司
Callicarpa nudiflora pellet capsule as well as preparation method thereof
InactiveCN103566055AEasy to takeImprove stabilityAntibacterial agentsDigestive systemCallicarpa nudifloraSide effect
The invention relates to a callicarpa nudiflora pellet (capsule) as well as a preparation method thereof. The pellet provided by the invention comprises the following components in parts by weight: 30-90 parts of a callicarpa nudiflora extract, 10-75 parts of a diluent, 0.5-10 parts of a disintegrating agent and an optimal binder. The invention further relates to a coating pellet comprising the pellet as well as the preparation method of the pellet. The pellet provided by the invention can be used for preparing a controlled release capsule or an enteric pellet capsule preparation by combining a controlled release or enteric pellet technology. The pellet preparation provided by the invention is reasonable in type and dosage in proportion of the selected main medicines and auxiliary materials, has good yield and has the advantages of stability and safety, high dissolving out rate, high bioavailability, small side effect, and / or convenience in delivery and the like.
Owner:许力宏 +1
Timed controlled-release amlodipine capsule and preparation method thereof
ActiveCN103690514AConvenient MedicationImprove complianceOrganic active ingredientsPharmaceutical delivery mechanismDrug activityDrug release
The invention relates to a timed controlled-release amlodipine capsule and a preparation method thereof. The amlodipine capsule comprises an impervious capsule body, a floating aid, amlodipine powder or tablet, a time-lag embolism tablet and a gastric soluble capsule cap, wherein the impervious capsule body has a structure with one closed end and one opened end and is filled with the floating aid and the amlodipine powder or tablet, the time-lag embolism tablet is encapsulated at an opening of the impervious capsule body, and the gastric soluble capsule cap is used for covering the impervious capsule body. The timed controlled-release amlodipine capsule can lastingly float in gastric juice for a set time and then quickly release active pharmaceutical ingredients, the seepage phenomenon is not caused within the lag time, the active pharmaceutical ingredients-amlodipine are released after a certain time lag, the lag time and the time-lag reproducibility are effectively controlled by the components and the component ratio of the time-lag embolism tablet, and a drug release mechanism is that the time lag is controlled by dissolving the time-lag embolism tablet. Therefore, a patient can conveniently take medicines for treatment and the compliance of the patient with the treatment is improved.
Owner:RUNZE PHARMACEUTICAL (SUZHOU) CO LTD
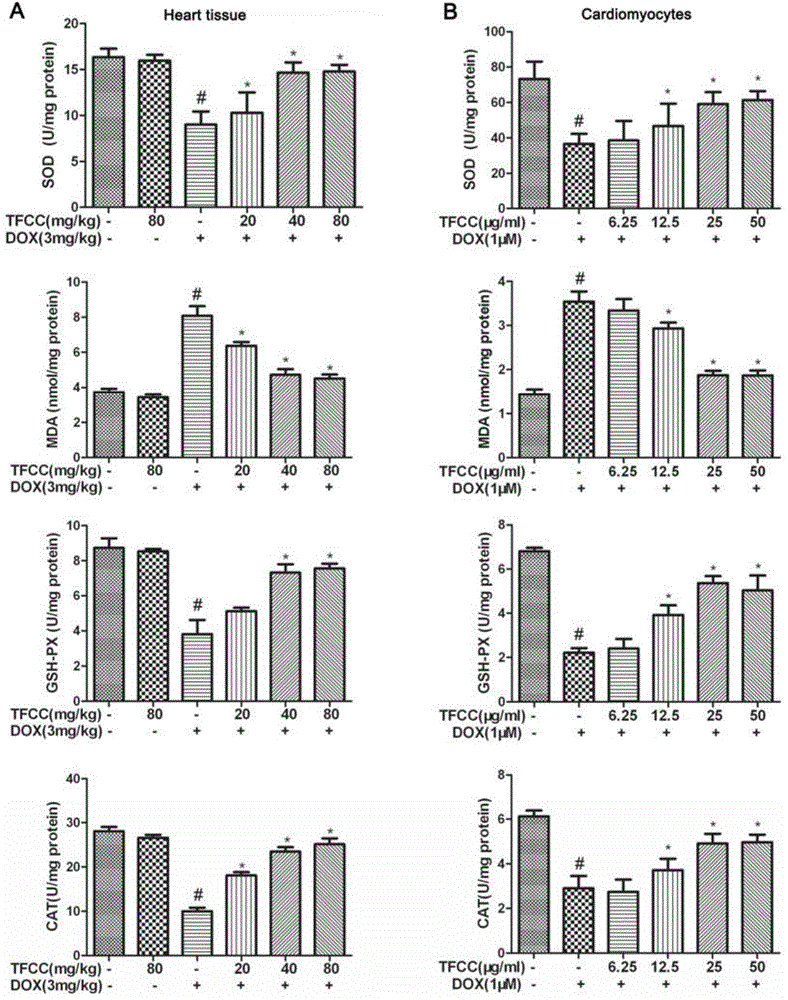
Application of clinopodium chinense total flavones in preparing medicines for protection effect of adriamycin-induced cardiotoxicity
InactiveCN103816218AOrganic active ingredientsAntineoplastic agentsSustained Release CapsuleClinopodium chinense
The invention discloses application of clinopodium chinense total flavones in preparing medicines for the protection effect of adriamycin-induced cardiotoxicity. The clinopodium chinense total flavones can be medicine compositions of clinopodium chinense total flavones, including various preparations of oral or parenteral administration forms. When being used in oral administration, the compositions can be tablets, capsules, soft capsules, oral liquid, syrup, granules, dropping pills, oral disintegrating tablets, sustained-release tablets, sustained-release capsules, controlled-release tablets and controlled-release capsules; when being used in parenteral administration, the compositions can be in the forms of water injection, lyophilized powder injection, sterile powder injection and infusion. Tablets and water injection are preferable dosage forms of the medicine compositions provided by the invention.
Owner:INST OF MEDICINAL PLANT DEV CHINESE ACADEMY OF MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com