Osmotic pump controlled release capsule case and preparation thereof
An osmotic pump controlled release and capsule shell technology, applied in the field of medicine, can solve the problems of no controlled release effect, prescription, complex process, etc., and achieve the effects of stable curative effect, slow release, and small toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Capsule Shell Composition:
[0029] Cellulose acetate 15g
[0030] Polyethylene glycol-200 1.5g
[0031] Tributyl Citrate 1.5g
[0032] Titanium dioxide 0.5g
[0033] Appropriate amount of gelatin
[0034]
[0035] Prepared capsule shells 100 capsules
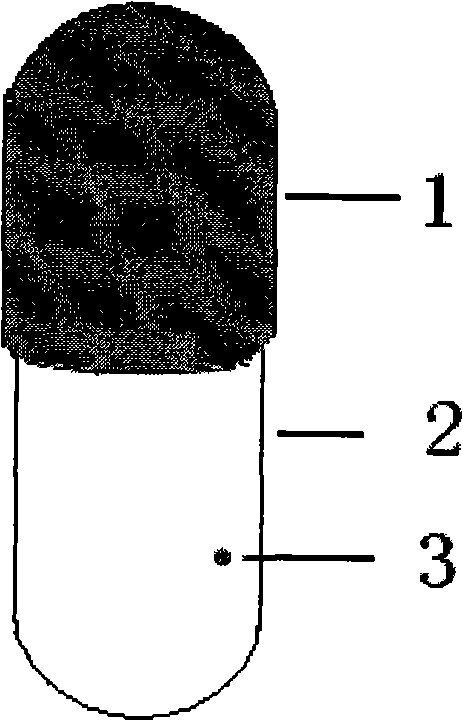
[0036] Preparation process: first dissolve cellulose acetate in acetone, add polyethylene glycol and tributyl citrate as plasticizers, put weighed titanium dioxide into it, stir well, and let stand to remove air bubbles. The blank is made by dipping in glue, dried, and a drug release hole with a diameter of 1.0 mm is prepared on the capsule body by mechanical drilling, sealed with gelatin solution, dried, pulled out, cut, and sorted to obtain the osmotic pump capsule shell.
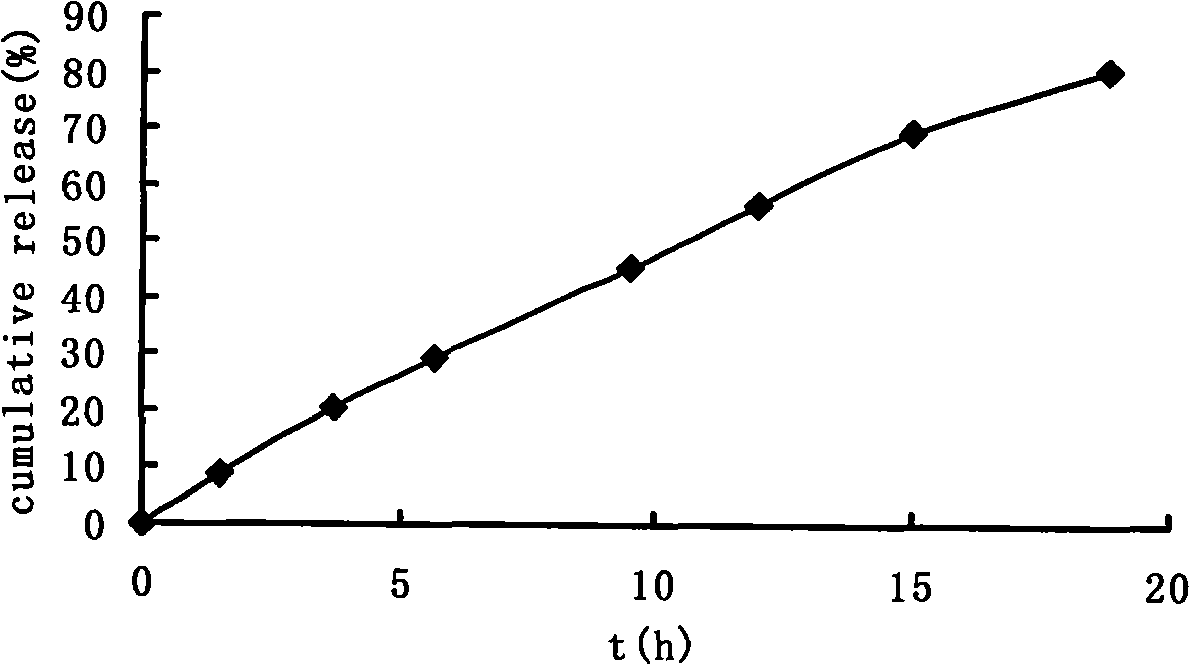
[0037] Take paracetamol as the model drug, paracetamol: sodium chloride = 2: 1 mixture, paracetamol is 100mg, fills in the capsule shell with powder form, carries out drug release test in water, release conditio...
Embodiment 2
[0039] Capsule Shell Composition:
[0040] Cellulose acetate 15g
[0041] Castor Oil 2.0g
[0042] Triethyl citrate 1.0g
[0043] Appropriate amount of gelatin
[0044]
[0045] Prepared capsule shells 100 capsules
[0046] Preparation process: first dissolve cellulose acetate in acetone, add castor oil and triethyl citrate as plasticizers, the preparation process is the same as Example 1, and prepare a diameter of 0.5 on the capsule body and capsule cap by mechanical punching method. mm drug release hole, seal the gelatin solution and dry, pull out the shell, cut, arrange, and obtain the capsule shell of the osmotic pump.
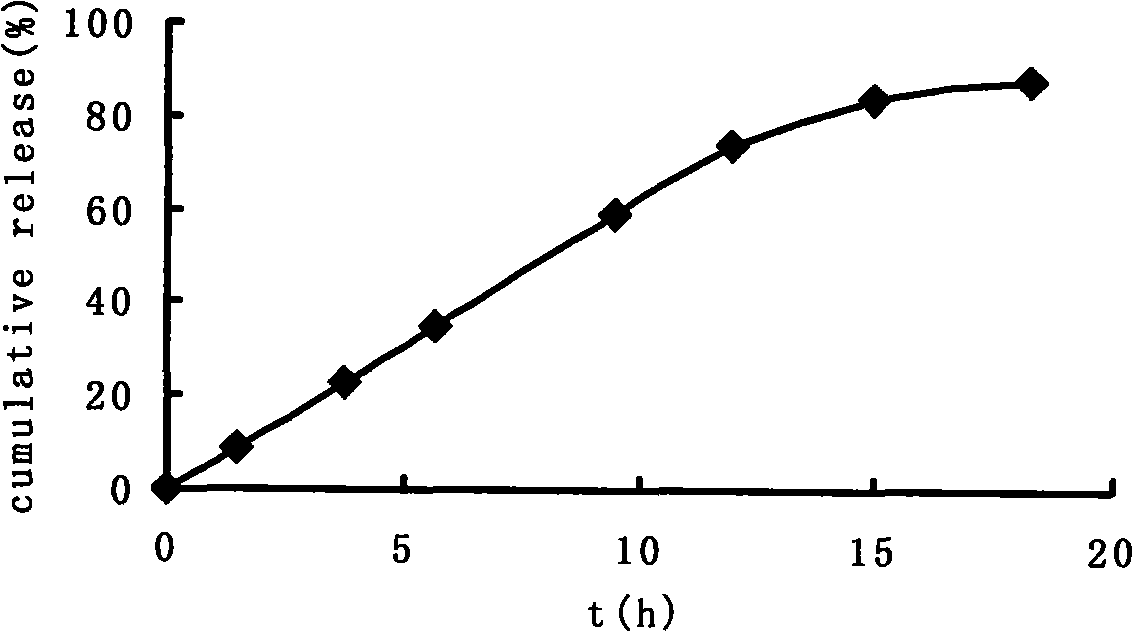
[0047] Take paracetamol as model medicine, paracetamol: sucrose=1: 1 mixes, paracetamol is 100mg, fills in this capsule, carries out drug release test in water, release condition is: distilled water 900ml, temperature 37 ℃ ± 0.5 ℃, rotating speed 100r / min, the drug release curve see image 3 .
Embodiment 3
[0049] Capsule Shell Composition:
[0050] Ethylcellulose 25g
[0051] Polyethylene glycol-400 2.0g
[0052] Dibutyl phthalate 1.5g
[0053] Titanium dioxide 0.5g
[0054] Appropriate amount of gelatin
[0055]
[0056] Prepared capsule shells 100 capsules
[0057] Preparation process: first dissolve ethyl cellulose in 95% ethanol, add polyethylene glycol-400 and dibutyl phthalate as plasticizers, add titanium dioxide and stir well, the preparation process is the same as Example 1, and mechanically beat Hole method A drug release hole with a diameter of 1.0 mm is prepared on the capsule body and sealed with gelatin solution.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com