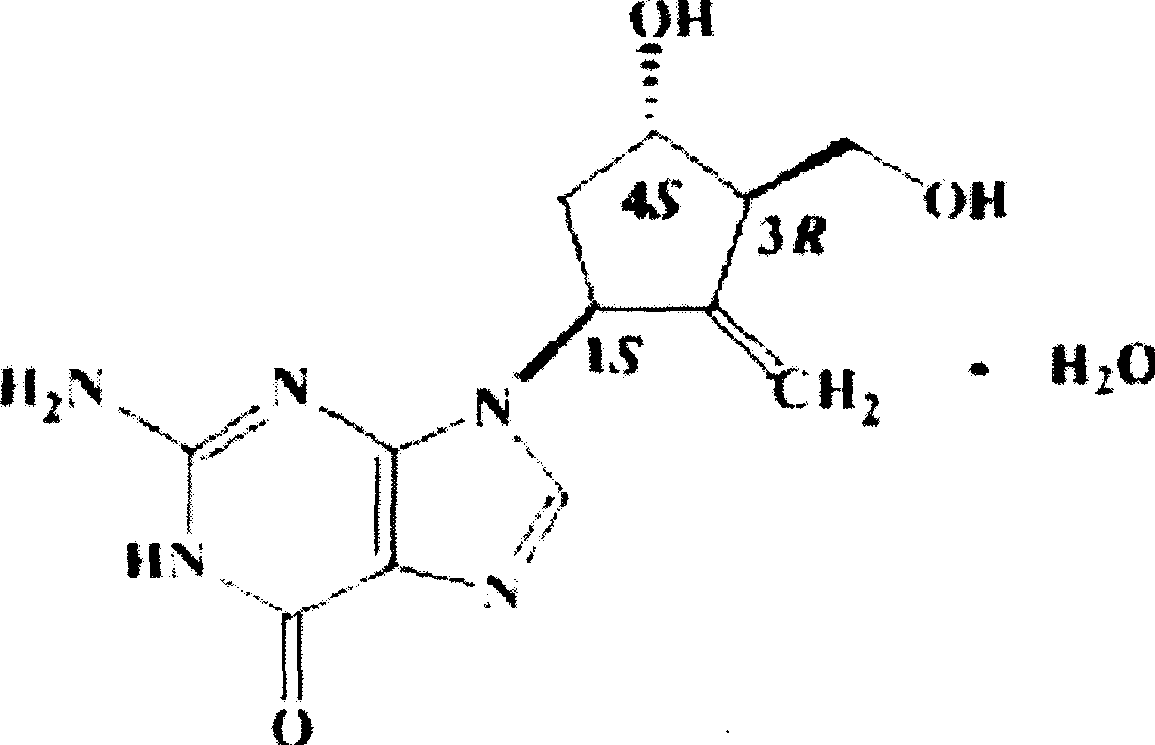
Enticawer release-controllable capsules and their preparation
A technology for entecavir and capsules, which is applied in the field of entecavir controlled-release capsules and their preparation, and can solve the problems of low variation rate and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Entecavir Controlled Release Capsules
[0025] 1. Ball core composition:
[0026] Name of raw material
[0027] Entecavir 1%
[0028] Lactose 46%
[0029] Starch 35%
[0030] Hypromellose 4%
[0031] 2. Membrane controlled release coating composition:
[0032] Ethylcellulose 5%
[0033] Methylcellulose 3%
[0034] Glycerin 1%
[0035] Talc 4%
[0036] 3. Capsule contents:
[0037] Coated core 90%
[0038] Polyethylene glycol 4%
[0039] Talc 6%
[0040] Operation method:
[0041] 1. According to the above formula amount, crush and sieve entecavir and filler, mix them evenly, make soft material with binder, granulate, dry and granulate, and use 12 mesh and 40 mesh sieves to sieve out 12 mesh to 40 mesh. The purpose is to round the particles as pellet cores.
[0042] 2. Prepare the controlled-release coating solution according to the above-mentioned film controlled-release coating composition prescription, put the prepared pellet cores in the coating pan, a...
Embodiment 2
[0046] Entecavir Controlled Release Capsules
[0047] 1. Ball core ingredients:
[0048] Name of raw material
[0049] Entecavir 1%
[0050] Microcrystalline Cellulose 46%
[0051] Starch 28%
[0052] Polyvinylpyrrolidone 5%
[0053] 2. Membrane controlled release coating composition:
[0054] Cellulose acetate 5%
[0055] Hypromellose 4%
[0056] Diethyl phthalate 1%
[0057] Talc 2%
[0058] 3. Capsule contents:
[0059] Coated core 92%
[0060] Polyethylene glycol 4%
[0061] Micronized silica gel 4%
[0062] Preparation method is the same as example 1.
[0063] time (small
Time)
4
8
12
16
24
Release
(%)
25.89
56.28
74.36
84.67
95.03
[0064]The stability test report of the Entecavir controlled-release capsules made by the method is as follows:
[0065] Storage stability data at room temperature
[0066] Sample packaging: Capsules are pa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com