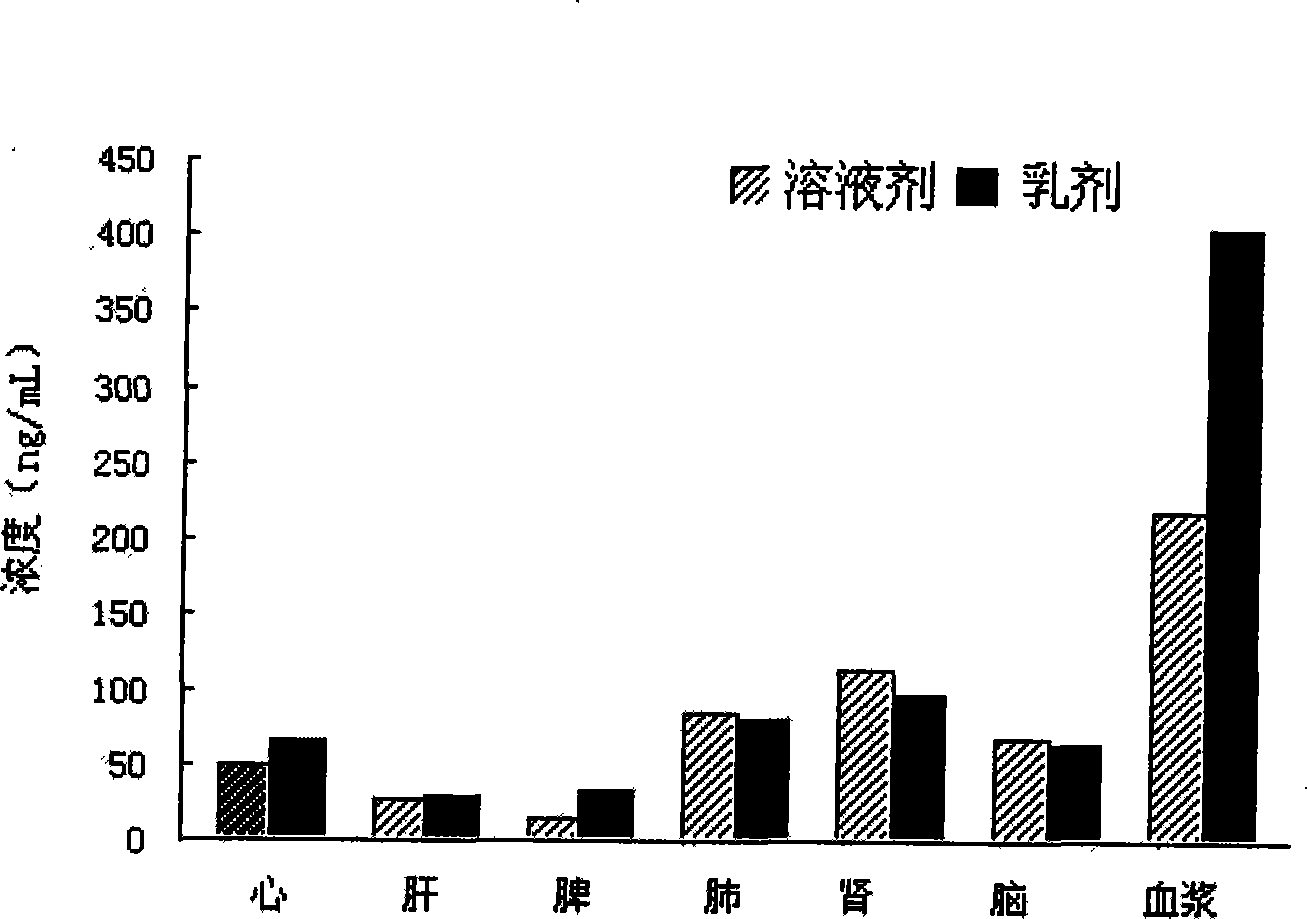
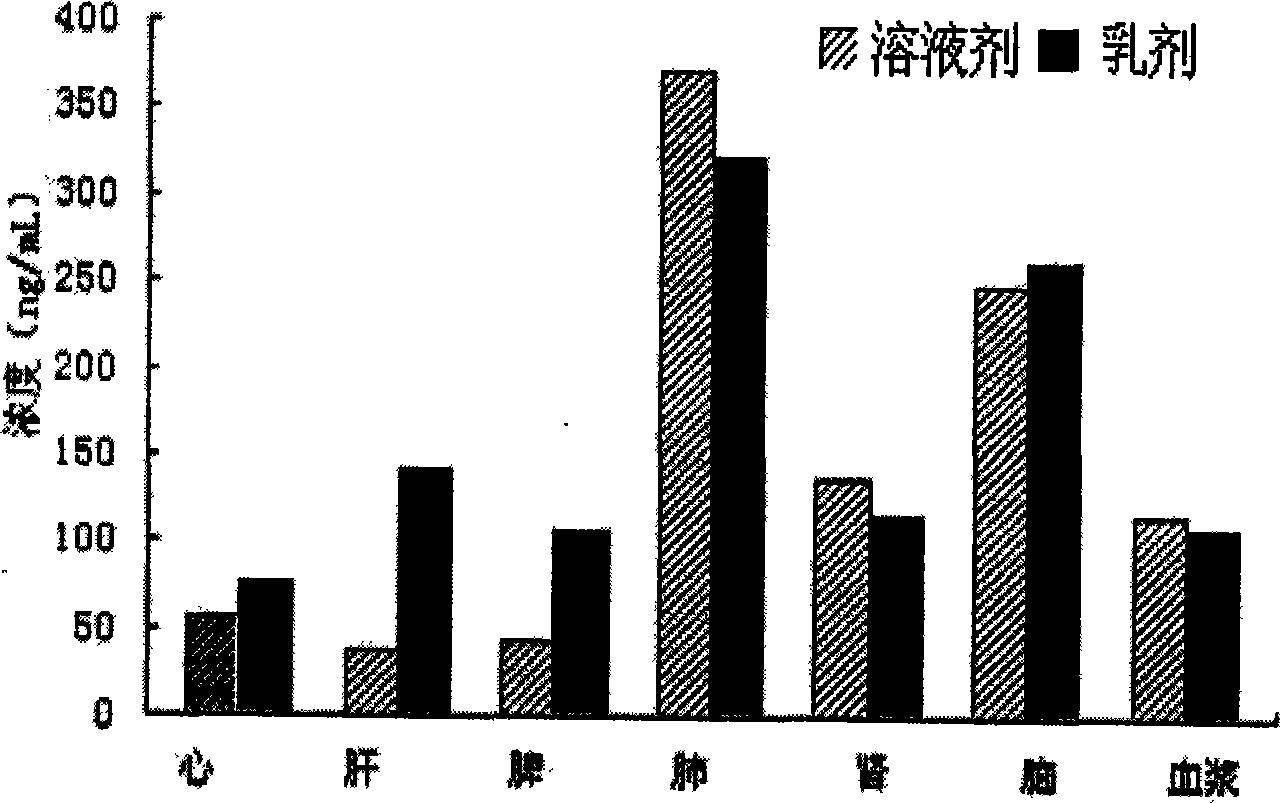
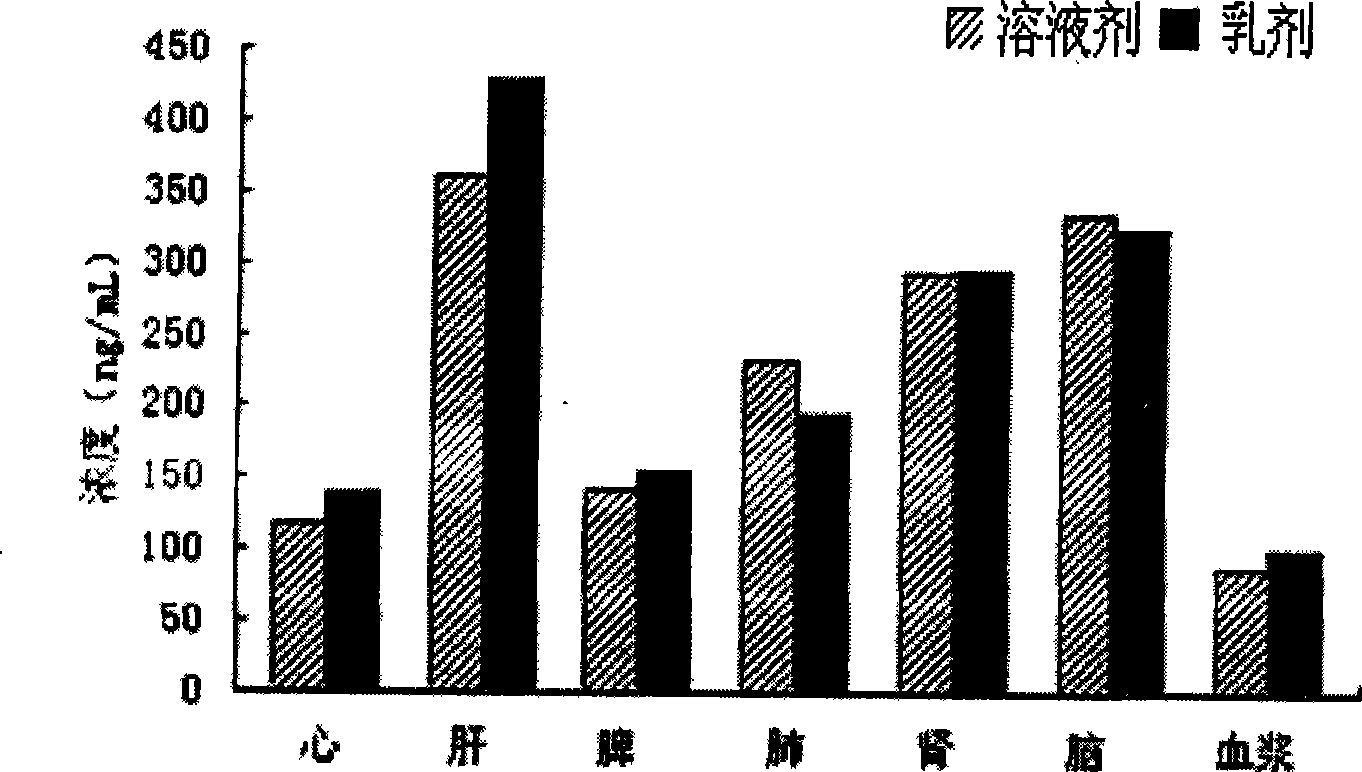
Nimodipine lipid microsphere injection and preparation method thereof
A nimodipine lipid and injection technology, which is applied in the direction of pharmaceutical formulations, oil/fat/wax non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of low solubility of nimodipine in the oil phase. To avoid the hepatic first-pass effect, improve bioavailability, and increase stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Taking the production of 1,000 bottles of the nimodipine lipid microsphere injection product of the present invention as an example, the used raw materials and auxiliary materials and their mass ratios are:
[0053] Nimodipine 4g
[0054] Lecithin for Injection 60g
[0055] Soybean Oil for Injection 250g
[0056] Medium Chain Fatty Acid for Injection 250g
[0057] Glycerin 125g
[0058] Tween-80 5g
[0059] Sodium Oleate 1.5g
[0060] Add water for injection to 5000ml
[0061] In the proportioning of the raw materials used in the present embodiment, the mass percentages of the medicinal active ingredients and the auxiliary materials are:
[0062] Nimodipine 0.08%
[0063] Lecithin for Injection 1.2%
[0064] Soybean Oil for Injection 5%
[0065] Medium Chain Fatty Acids for Injection 5%
[0066] Glycerin 2.5%
[0067] Tween-80 0.1%
[0068] Sodium Oleate 0.03%
[0069] Water for injection was added to 100%.
[0070] Its preparation method is as follows:
...
Embodiment 2
[0082] Taking the production of 1,000 bottles of the nimodipine lipid microsphere injection product of the present invention as an example, the used raw materials and auxiliary materials and their mass ratios are:
[0083] Nimodipine 4g
[0084] Lecithin for Injection 25g
[0085] Soybean Oil for Injection 100g
[0086] Medium Chain Fatty Acid for Injection 100g
[0087] Glycerin 50g
[0088] Tween-80 7.5g
[0089] Sodium Oleate 2g
[0090] Add water for injection to 5000ml
[0091] In the proportioning of the raw materials used in the present embodiment, the mass percentages of the medicinal active ingredients and the auxiliary materials are:
[0092] Nimodipine 0.08%
[0093] Lecithin for Injection 0.5%
[0094] Soybean Oil for Injection 2%
[0095] Medium Chain Fatty Acids for Injection 2%
[0096] Glycerin 1%
[0097] Tween-80 0.15%
[0098] Sodium Oleate 0.04%
[0099] Water for injection was added to 100%.
[0100] The preparation method is the same as that...
Embodiment 3
[0102] Taking the production of 1,000 bottles of the nimodipine lipid microsphere injection product of the present invention as an example, the used raw materials and auxiliary materials and their mass ratios are:
[0103] Nimodipine 4g
[0104] Lecithin for Injection 115g
[0105] Soybean Oil for Injection 400g
[0106] Medium Chain Fatty Acid for Injection 400g
[0107] Glycerin 150g
[0108] Tween-80 10g
[0109] Sodium Oleate 2.5g
[0110] Add water for injection to 5000ml
[0111] In the proportioning of the raw materials used in the present embodiment, the mass percentages of the medicinal active ingredients and the auxiliary materials are:
[0112] Nimodipine 0.08%
[0113] Lecithin for Injection 2.3%
[0114] Soybean Oil for Injection 8%
[0115] Medium Chain Fatty Acids for Injection 8%
[0116] Glycerin 3%
[0117]Tween-80 0.2%
[0118] Sodium Oleate 0.05%
[0119] Water for injection was added to 100%.
[0120] The preparation method is the same as tha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com