Sinomenine hydrochloride sustained-released injection and preparation method thereof
A technology of sinomenine hydrochloride and slow-release injections, which is applied in anti-inflammatory agents, pharmaceutical formulas, non-central analgesics, etc., and can solve the problems of large side effects and frequent use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
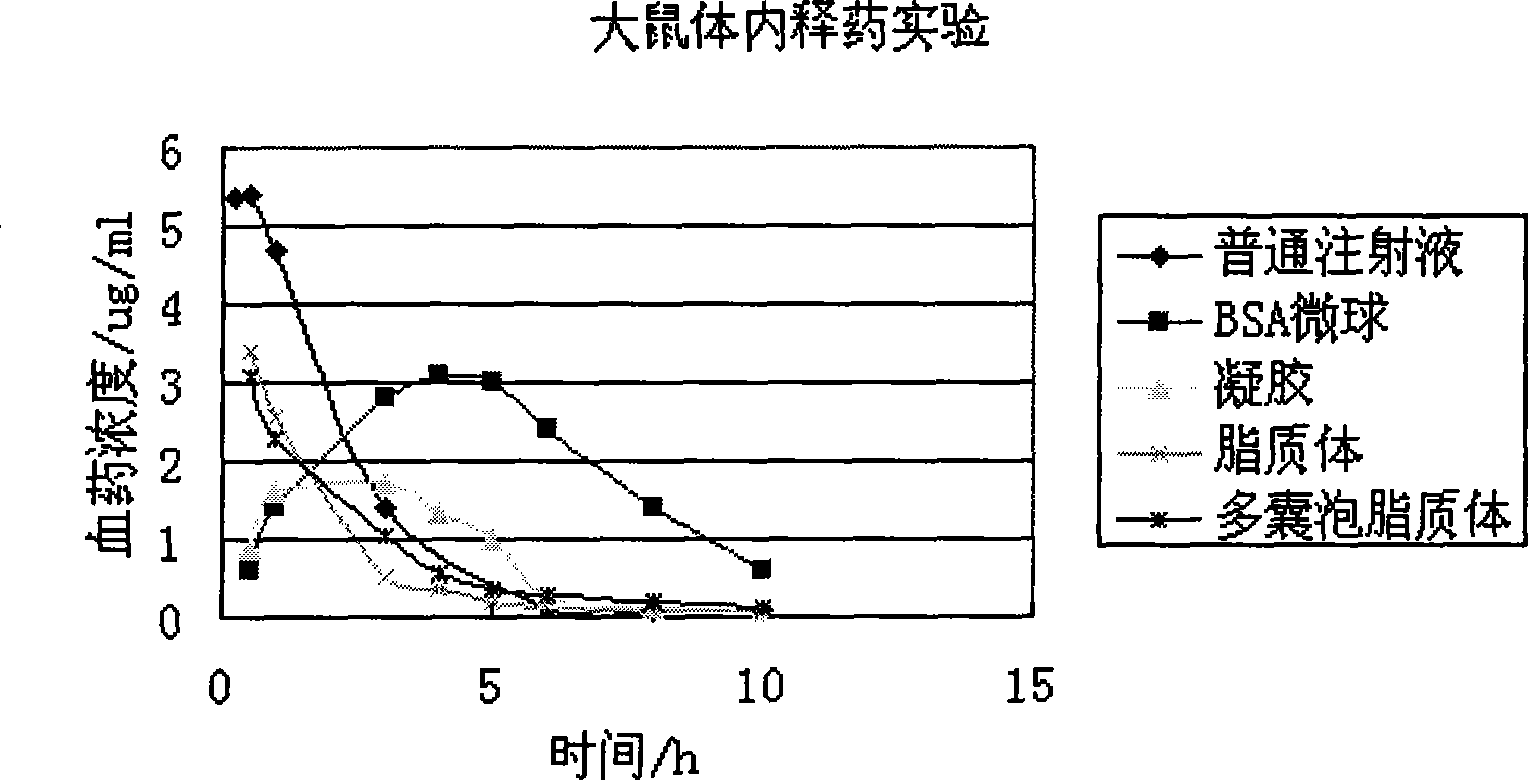
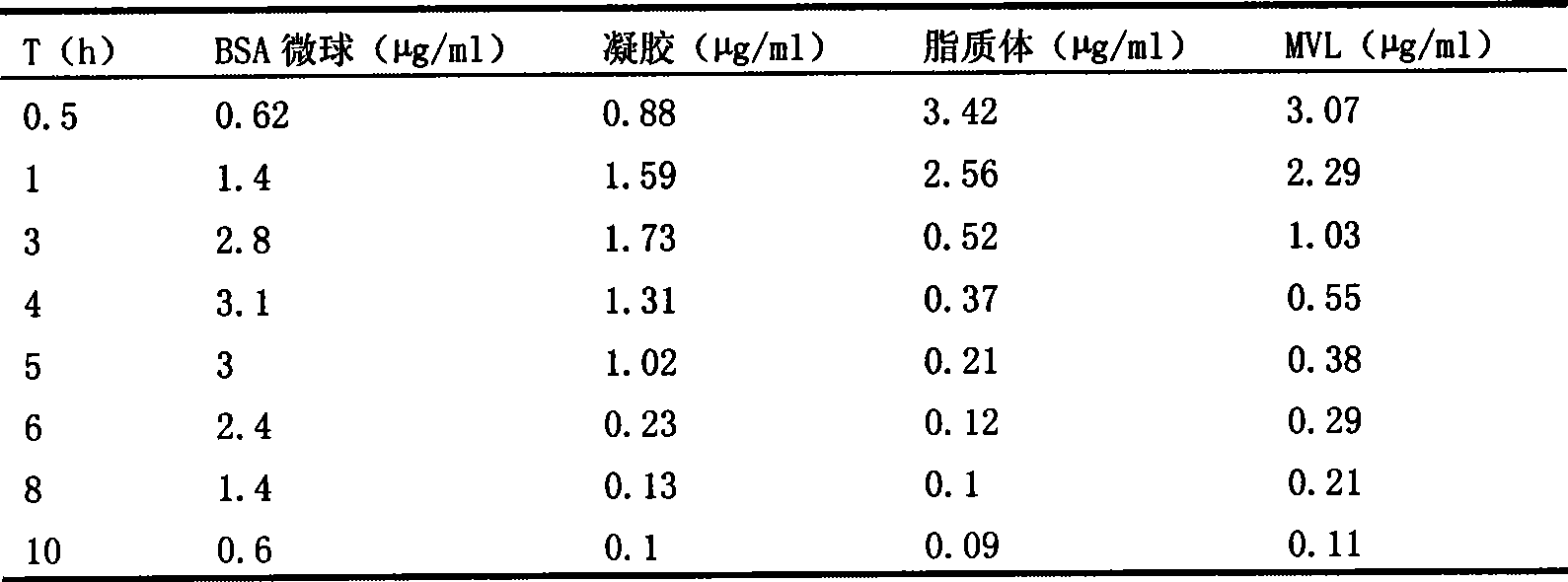
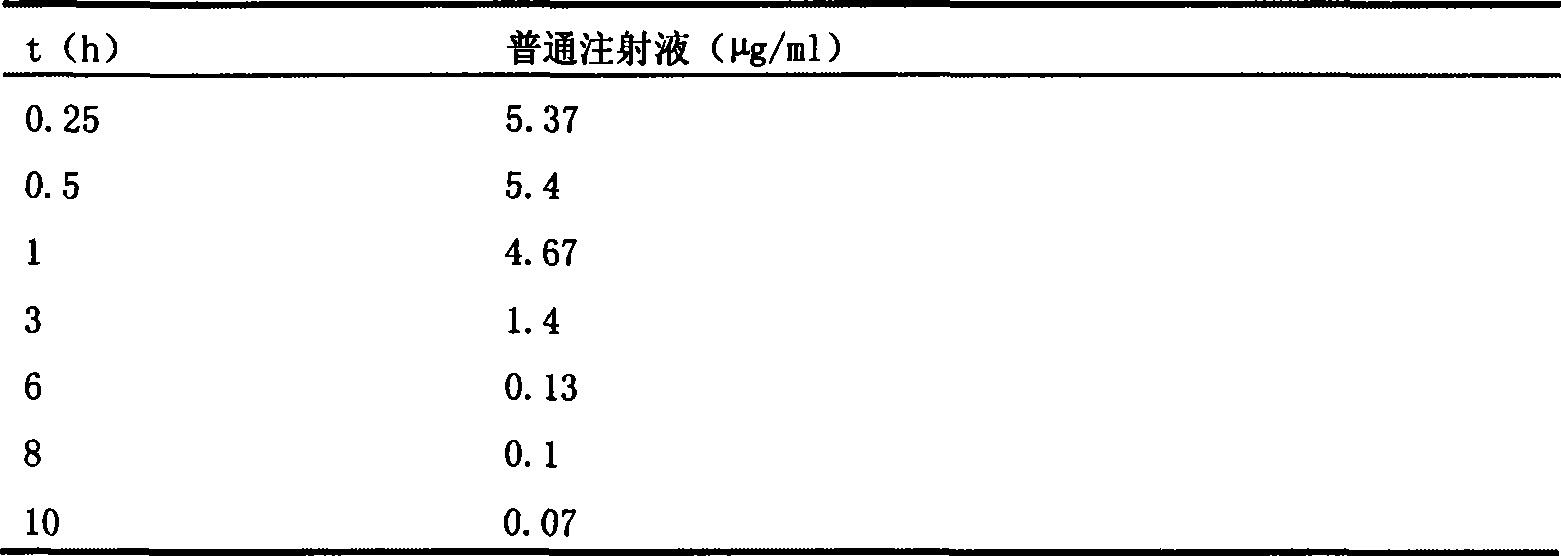
Image
Examples
Embodiment 1
[0059] Dissolve 10Kg of sinomenine hydrochloride in 0.2KL of water as the internal water phase, and dissolve 100Kg of lactide-glycolide copolymer 50 / 50 or lactide-glycolide copolymer 75 / 25 in 5KL of ethyl acetate as the organic phase, add the inner water phase to the outer water phase, and form water / oil type colostrum by high-speed shearing; add the formed colostrum drop by drop into 20KL aqueous solution containing 1% polyvinyl alcohol, and form water / oil type by high-speed shearing / water-type double emulsion; add this double emulsion to 100KL0.1% polyvinyl alcohol aqueous solution, stir magnetically at room temperature for 20 minutes, evaporate under reduced pressure to remove the organic solvent, centrifuge, wash with water, and dry to obtain synthetic polymer nanoparticles .
Embodiment 2
[0061] Dissolve 15Kg of sinomenine hydrochloride in 0.1KL of water as the internal water phase, and dissolve 140Kg of lactide-glycolide copolymer 50 / 50 or lactide-glycolide copolymer 75 / 25 in 4KL of ethyl acetate as the organic phase, add the inner water phase to the outer water phase, and form water / oil type colostrum by high-speed shearing; add the formed colostrum drop by drop into 30KL aqueous solution containing 0.5% polyvinyl alcohol, and form water / oil type by high-speed shearing / water-type double emulsion; add this double emulsion to 140KL0.08% polyvinyl alcohol aqueous solution, stir magnetically at room temperature for 25 minutes, evaporate under reduced pressure to remove the organic solvent, centrifuge, wash with water, and dry to obtain synthetic polymer nanoparticles .
Embodiment 3
[0063] Dissolve 8Kg of sinomenine hydrochloride in 0.3KL of water as the internal water phase, and dissolve 90Kg of lactide-glycolide copolymer 50 / 50 or lactide-glycolide copolymer 75 / 25 in 7KL of ethyl acetate as the organic phase, add the inner water phase to the outer water phase, and form water / oil type colostrum by high-speed shearing; add the formed colostrum drop by drop to 10KL aqueous solution containing 1.5% polyvinyl alcohol, and form water / oil type by high-speed shearing / water-type double emulsion; add this double emulsion to 90KL0.15% polyvinyl alcohol aqueous solution, stir magnetically at room temperature for 15 minutes, then evaporate under reduced pressure to remove the organic solvent, centrifuge, wash with water, and dry to obtain synthetic polymer nanoparticles .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com